Two New Picoline-Derived Meroterpenoids with Anti-Acetylcholinesterase Activity from Ascidian-Derived Fungus Amphichorda felina
Abstract
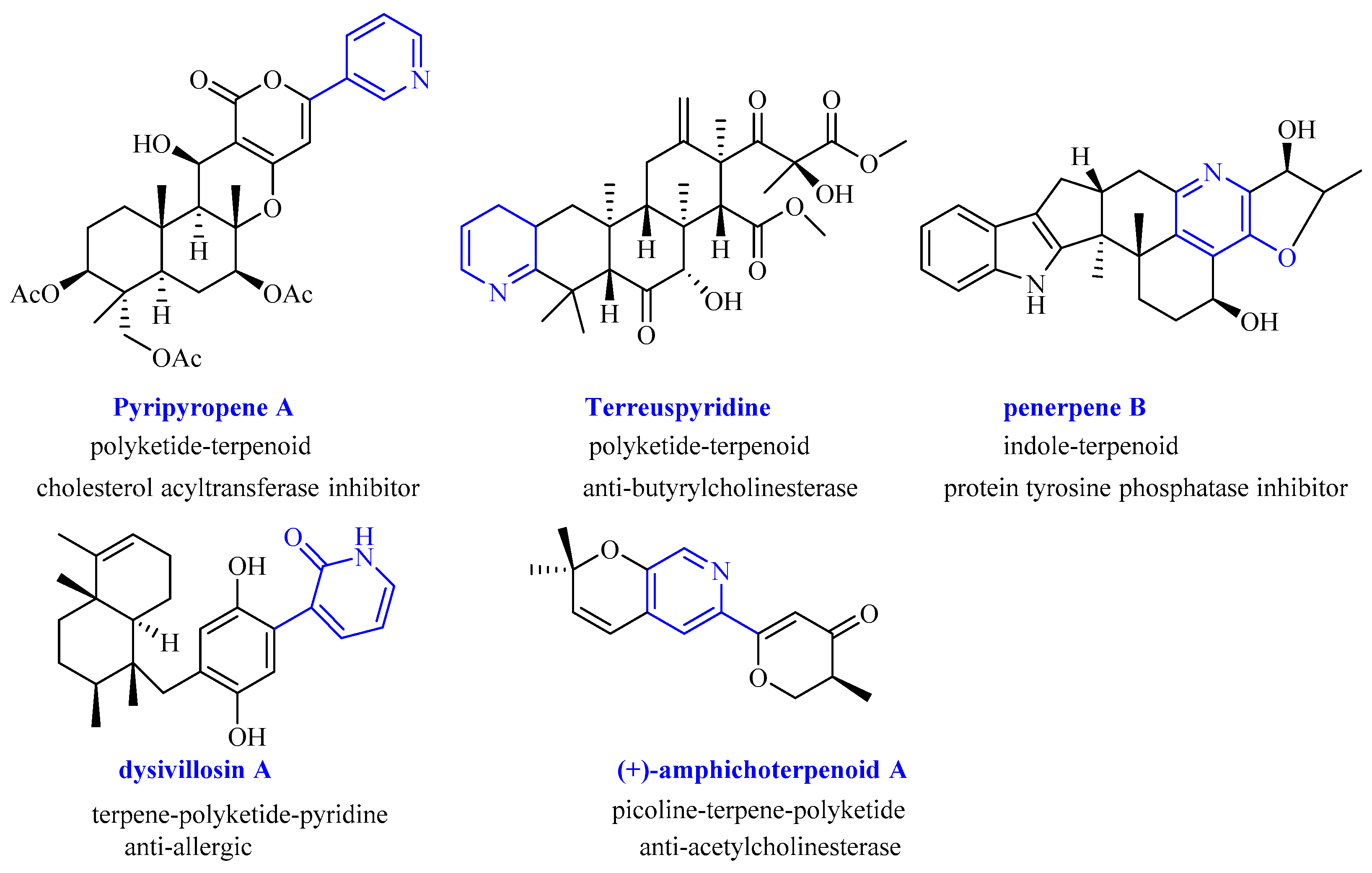
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. Calculation of the ECD Spectra
3.5. Anti-Acetylcholinesterase Activity
3.6. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jiang, M.; Wu, Z.; Liu, L.; Chen, S. The chemistry and biology of fungal meroterpenoids (2009–2019). Org. Biomol. Chem. 2021, 19, 1644–1704. [Google Scholar] [CrossRef] [PubMed]
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, Y.; Abe, I. Biosynthesis of fungal meroterpenoids. Nat. Prod. Rep. 2016, 33, 26–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, Y.; Abe, I. Fungal Meroterpenoids. In Comprehensive Natural Products III: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 445–478. [Google Scholar]
- Sintchak, M.D.; Fleming, M.A.; Futer, O.; Raybuck, S.A.; Chambers, S.P.; Caron, P.R.; Murcko, M.A.; Wilson, K.P. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell 1996, 85, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Widom, J.; Kemp, C.W.; Crews, C.M.; Clardy, J. Structure of human methionine aminopeptidase-2 complexed with fumagillin. Science 1998, 282, 1324–1327. [Google Scholar] [CrossRef]
- Molina, J.M.; Tourneur, M.; Sarfati, C.; Chevret, S.; de Gouvello, A.; Gobert, J.G.; Balkan, S.; Derouin, F.; Agence Natl Recherches, SIDA 090 St. Fumagillin treatment of intestinal microsporidiosis. N. Engl. J. Med. 2002, 346, 1963–1969. [Google Scholar] [CrossRef]
- Chen, M.C.; Cho, T.Y.; Kuo, Y.H.; Lee, T.H. Meroterpenoids from a Medicinal Fungus Antrodia cinnamomea. J. Nat. Prod. 2017, 80, 2439–2446. [Google Scholar] [CrossRef]
- Stierle, D.B.; Stierle, A.A.; Patacini, B. The berkeleyacetals, three meroterpenes from a deep water acid mine waste Penicillium. J. Nat. Prod. 2007, 70, 1820–1823. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.C. Acetylcholinesterase Inhibition by Territrem-B Derivatives. J. Nat. Prod. 1995, 58, 857–862. [Google Scholar] [CrossRef]
- Itoh, T.; Tokunaga, K.; Matsuda, Y.; Fujii, I.; Abe, I.; Ebizuka, Y.; Kushiro, T. Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases. Nat. Chem. 2010, 2, 858–864. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Wu, Q.; Yin, H.; Guo, H.; Yuan, S.; Liu, Z.; Chen, S.; Liu, L. Amphichoterpenoids A–C, unprecedented picoline-derived meroterpenoids from the ascidian-derived fungus Amphichorda felina SYSU-MS7908. Chin. Chem. Lett. 2021, 32, 1893–1896. [Google Scholar] [CrossRef]
- Kong, F.D.; Fan, P.; Zhou, L.M.; Ma, Q.Y.; Xie, Q.Y.; Zheng, H.Z.; Zheng, Z.H.; Zhang, R.S.; Yuan, J.Z.; Dai, H.F.; et al. Penerpenes A-D, Four Indole Terpenoids with Potent Protein Tyrosine Phosphatase Inhibitory Activity from the Marine-Derived Fungus Penicillium sp. KFD28. Org. Lett. 2019, 21, 4864–4867. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, W.; Li, X.; Kang, X.; Yan, S.; Chao, M.; Mo, S.; Sun, W.; Lu, Y.; Chen, C.; et al. Terreuspyridine: An Unexpected Pyridine-Fused Meroterpenoid Alkaloid with a Tetracyclic 6/6/6/6 Skeleton from Aspergillus terreus. Org. Lett. 2020, 22, 7041–7046. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.H.; Cheng, B.H.; Shi, G.H.; Chen, G.D.; Gu, B.B.; Zhou, Y.J.; Hong, L.L.; Yang, F.; Liu, Z.Q.; Qiu, S.Q.; et al. Dysivillosins A-D, Unusual Anti-allergic Meroterpenoids from the Marine Sponge Dysidea villosa. Sci. Rep. 2017, 7, 8947–8956. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Hou, Y.L.; Shen, X.Y.; Pan, X.D.; Zhang, X.; Yao, X.S. Monoterpene pyridine alkaloids and phenolics from Scrophularia ningpoensis and their cardioprotective effect. Fitoterapia 2013, 88, 44–49. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among US FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Lin, S.X.; Curtis, M.A.; Sperry, J. Pyridine alkaloids with activity in the central nervous system. Bioorg. Med. Chem. 2020, 28, 115820–115841. [Google Scholar] [CrossRef]
- Yan, Y.J.; Ma, Y.T.; Yang, J.; Horsman, G.P.; Luo, D.; Ji, X.; Huang, S.X. Tropolone Ring Construction in the Biosynthesis of Rubrolone B, a Cationic Tropolone Alkaloid from Endophytic Streptomyces. Org. Lett. 2016, 18, 1254–1257. [Google Scholar] [CrossRef]
- Luo, F.; Hong, S.; Chen, B.; Yin, Y.; Tang, G.; Hu, F.; Zhang, H.; Wang, C. Unveiling of Swainsonine Biosynthesis via a Multibranched Pathway in Fungi. ACS Chem. Biol. 2020, 15, 2476–2484. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A Review of Terpenes from Marine-Derived Fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, S.; Li, J.; Liu, L. The biological and chemical diversity of tetramic acid compounds from marine-derived microorganisms. Mar. Drugs 2020, 18, 114. [Google Scholar] [CrossRef] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef]
- Chen, S.; Shen, H.; Deng, Y.; Guo, H.; Jiang, M.; Wu, Z.; Yin, H.; Liu, L. Roussoelins A and B: Two phenols with antioxidant capacity from ascidian-derived fungus Roussoella siamensis SYSU-MS4723. Mar. Life Sci. Technol. 2020, 3, 69–76. [Google Scholar] [CrossRef]
- Niaz, S.I.; Zhang, P.; Shen, H.; Li, J.; Chen, B.; Chen, S.; Liu, L.; He, J. Two new isochromane derivatives penisochromanes A and B from ascidian-derived fungus Penicillium sp. 4829. Nat. Prod. Res. 2019, 33, 1262–1268. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, M.; Chen, B.; Salaenoi, J.; Niaz, S.I.; He, J.; Liu, L. Penicamide A, a unique N,N’-ketal quinazolinone alkaloid from ascidian-derived fungus Penicillium sp. 4829. Mar. Drugs 2019, 17, 522. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Shen, H.; Zhang, P.; Cheng, H.; Dai, X.; Liu, L. Anti-glioma trichobamide A with an unprecedented tetrahydro-5H-furo [2,3-b]pyrrol-5-one functionality from ascidian-derived fungus Trichobotrys effuse 4729. Chem. Commun. 2019, 55, 1438–1441. [Google Scholar] [CrossRef]
- Chen, S.; Guo, H.; Jiang, M.; Wu, Q.; Li, J.; Shen, H.; Liu, L. Mono- and dimeric xanthones with anti-glioma and anti-inflammatory activities from the ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Mar. Drugs 2022, 20, 51. [Google Scholar] [CrossRef]
- Pan, N.; Li, Z.C.; Li, Z.H.; Chen, S.H.; Jiang, M.H.; Yang, H.Y.; Liu, Y.S.; Hu, R.; Zeng, Y.W.; Dai, L.H.; et al. Antiplatelet and antithrombotic effects of isaridin E isolated from the marine-derived fungus via downregulating the PI3K/Akt signaling pathway. Mar. Drugs 2022, 20, 23. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, L.; Wu, Q.; Jiang, M.; Guo, H.; Hu, Z.; Chen, S.; Liu, L.; Gao, Z. Genome Mining of α-Pyrone Natural Products from Ascidian-Derived Fungus Amphichorda felina SYSU-MS7908. Mar. Drugs 2022, 20, 298. [Google Scholar] [CrossRef]
- Zaki, A.G.; El-Sayed, E.R.; Abd Elkodous, M.; El-Sayyad, G.S. Microbial acetylcholinesterase inhibitors for Alzheimer’s therapy: Recent trends on extraction, detection, irradiation-assisted production improvement and nano-structured drug delivery. Appl. Microbiol. Biotechnol. 2020, 104, 4717–4735. [Google Scholar] [CrossRef]
- Houghton, P.J.; Ren, Y.; Howes, M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef]
- Berkov, S.; Atanasova, M.; Georgiev, B.; Bastida, J.; Doytchinova, I. The Amaryllidaceae alkaloids: An untapped source of acetylcholinesterase inhibitors. Phytochem. Rev. 2021. [Google Scholar] [CrossRef]
- Kong, Y.R.; Tay, K.C.; Su, Y.X.; Wong, C.K.; Tan, W.N.; Khaw, K.Y. Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. Molecules 2021, 26, 728. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Kucukaydin, S.; Yeskaliyeva, B.; Ozturk, M.; Dinica, R.M. Non-Alkaloid Cholinesterase Inhibitory Compounds from Natural Sources. Molecules 2021, 26, 5582. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bartolucci, C.; Perola, E.; Pilger, C.; Fels, G.; Lamba, D. Three-dimensional structure of a complex of galanthamine (Nivalin (R)) with acetylcholinesterase from Torpedo californica: Implications for the design of new anti-Alzheimer drugs. Proteins 2001, 42, 182–191. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]





| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH, Mult (J in Hz) | δC, Type | δH, Mult (J in Hz) | |
| 2 | 140.3, CH | 8.22, s | 140.3, CH | 8.22, s |
| 3 | 152.4, C | 152.4, C | ||
| 4 | 127.7, C | 127.6, C | ||
| 5 | 122.7, CH | 7.57, s | 122.7, CH | 7.57, s |
| 6 | 142.3, C | 142.3, C | ||
| 7 | 168.2, C | 167.9, C | ||
| 8 | 101.6, CH | 6.40, s | 101.6, CH | 6.40, s |
| 9 | 196.1, C | 196.1, C | ||
| 10 | 39.27, CH | 2.66, m | 39.27, CH | 2.67, m |
| 11 | 73.68, CH2 | a:4.65, dd (11.1, 5.0); b: 4.25, t (10.8) | 73.69, CH2 | a:4.64, dd (11.1, 5.0); b: 4.25, t (10.8) |
| 12 | 11.34, CH3 | 1.18, d (7.0) | 11.37, CH3 | 1.18, d (7.0) |
| 1′ | 30.9, CH2 | a: 3.08 dd (17.4, 4.5); b: 2.81 dd (17.4, 4.5) | 30.9, CH2 | a: 3.08 dd (17.4, 4.5); b: 2.81 dd (17.4, 4.5) |
| 2′ | 68.9, CH | 3.88, t (9.9) | 68.9, CH | 3.89, t (9.9) |
| 3′ | 78.7, C | 78.6, C | ||
| 4′ | 25.0, CH3 | 1.36, s | 25.0, CH3 | 1.36, s |
| 5′ | 22.1, CH3 | 1.40, s | 22.1, CH3 | 1.40, s |
| Compound | log (FBE), kcal/mol | Targeting Residues (H bond Å) | Hydrophobic Interaction Residues |
|---|---|---|---|
| 1 | −9.3 | Arg289(3.13,3.11),Phe288(2.89) | Trp279,Tyr121, Phe330, Phe288,Phe290,Ile287, Tyr334, Phe331 |
| 2 | −9.3 | Arg289(3.24,3.06),Tyr121(2.85) | Trp279,Tyr121, Phe330, Phe288,Phe290,Ile287, Tyr334, Phe331,Ser286 |
| 3 | −7.9 | Leu305(2.97), Glu306(2.84) | Leu305,Glu306,Ser235,Ser304,Pro232,Trp524,Pro529, His398,Asn525,Asn230 |
| 4 | −6.8 | none | Trp279, Phe290,Phe284,Leu282,Ser286, Phe331 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Guo, H.; Wu, Q.; Yuan, S.; Liu, L. Two New Picoline-Derived Meroterpenoids with Anti-Acetylcholinesterase Activity from Ascidian-Derived Fungus Amphichorda felina. Molecules 2022, 27, 5076. https://doi.org/10.3390/molecules27165076
Jiang M, Guo H, Wu Q, Yuan S, Liu L. Two New Picoline-Derived Meroterpenoids with Anti-Acetylcholinesterase Activity from Ascidian-Derived Fungus Amphichorda felina. Molecules. 2022; 27(16):5076. https://doi.org/10.3390/molecules27165076
Chicago/Turabian StyleJiang, Minghua, Heng Guo, Qilin Wu, Siwen Yuan, and Lan Liu. 2022. "Two New Picoline-Derived Meroterpenoids with Anti-Acetylcholinesterase Activity from Ascidian-Derived Fungus Amphichorda felina" Molecules 27, no. 16: 5076. https://doi.org/10.3390/molecules27165076
APA StyleJiang, M., Guo, H., Wu, Q., Yuan, S., & Liu, L. (2022). Two New Picoline-Derived Meroterpenoids with Anti-Acetylcholinesterase Activity from Ascidian-Derived Fungus Amphichorda felina. Molecules, 27(16), 5076. https://doi.org/10.3390/molecules27165076







