Restoration of Hepatic and Intestinal Integrity by Phyllanthus amarus Is Dependent on Bax/Caspase 3 Modulation in Intestinal Ischemia-/Reperfusion-Induced Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Preparation of Plant Extract
2.3. Phytochemical Analysis
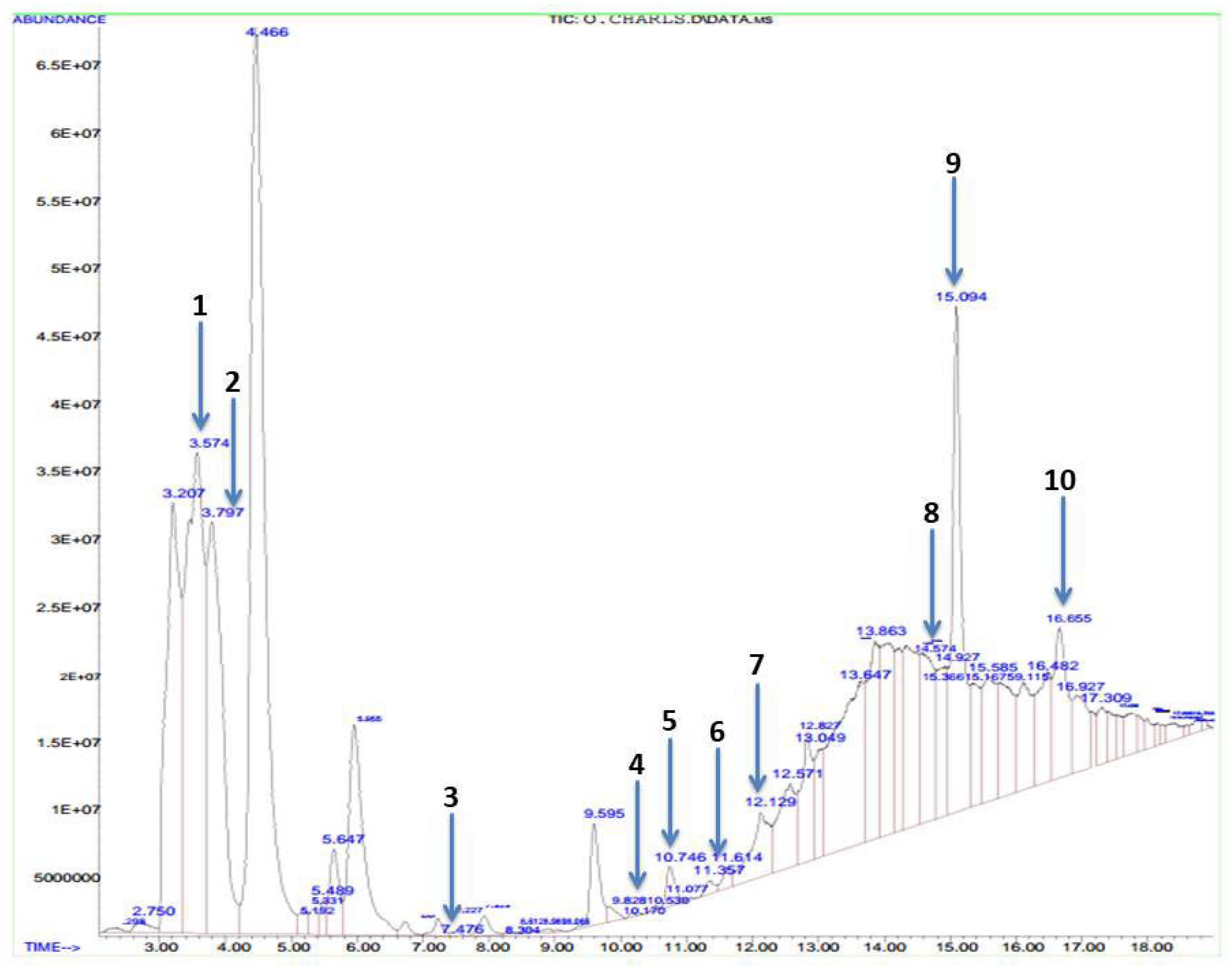
2.4. Gas Chromatography–Mass Spectrophotometric Analysis
2.5. Experimental Animals
2.6. Experimental Design
2.7. Sacrifice and Tissue Collection
2.8. Biochemical Analyses
2.8.1. Tissue Injury Markers
2.8.2. Markers of Oxidative Stress and Antioxidant Levels
2.8.3. Markers of Inflammation
2.8.4. Marker of Apoptosis
2.9. Microbiological Analysis
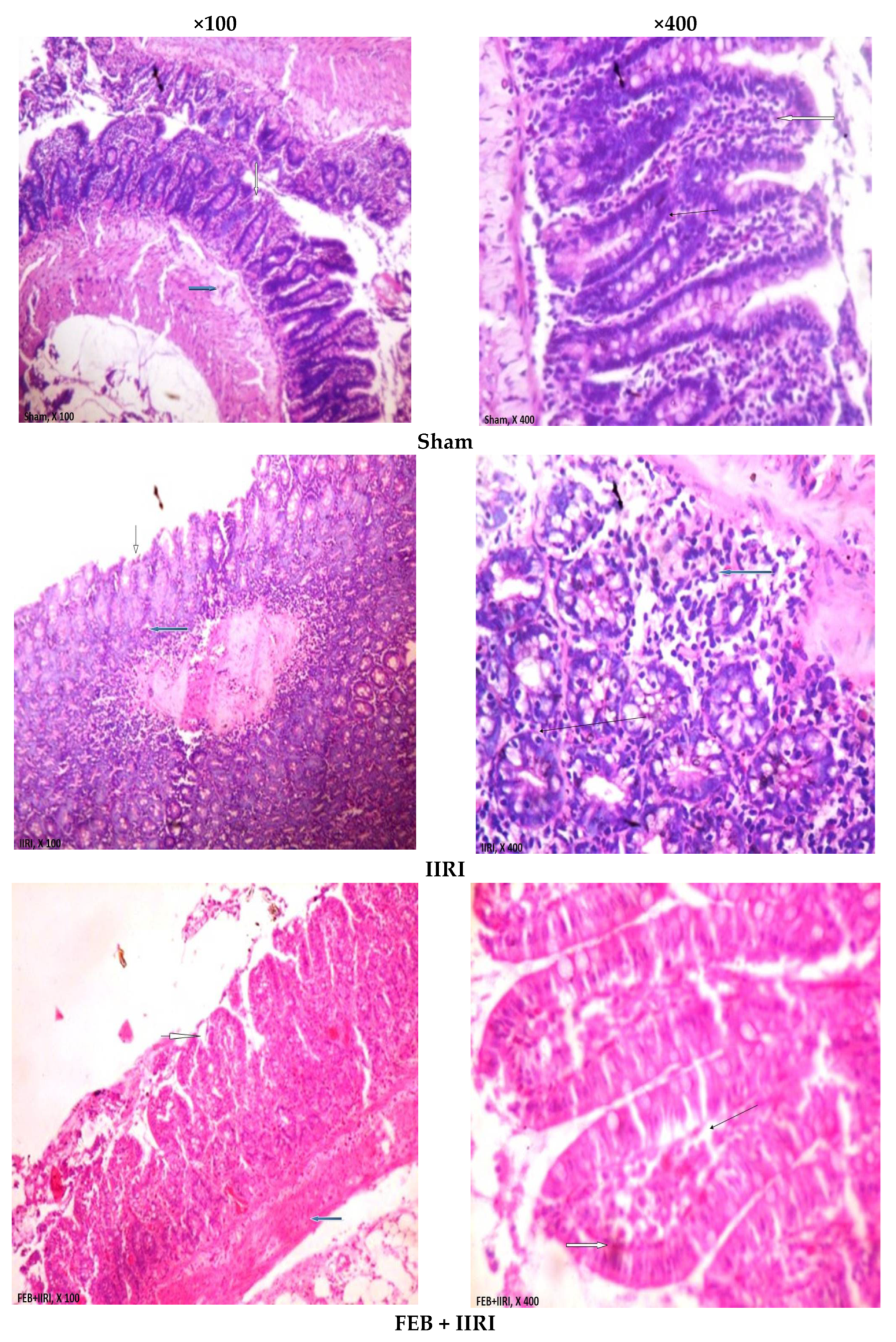
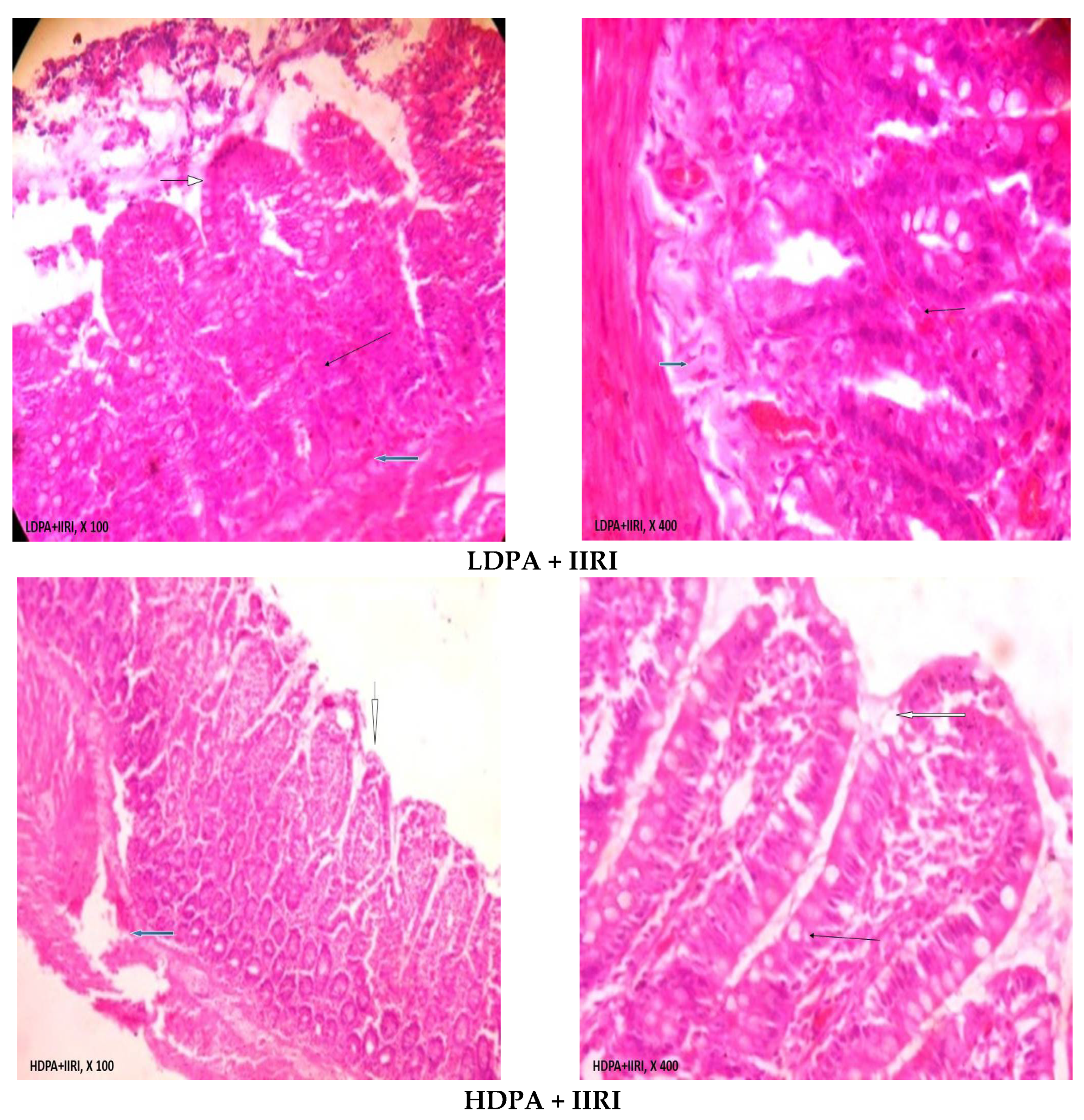
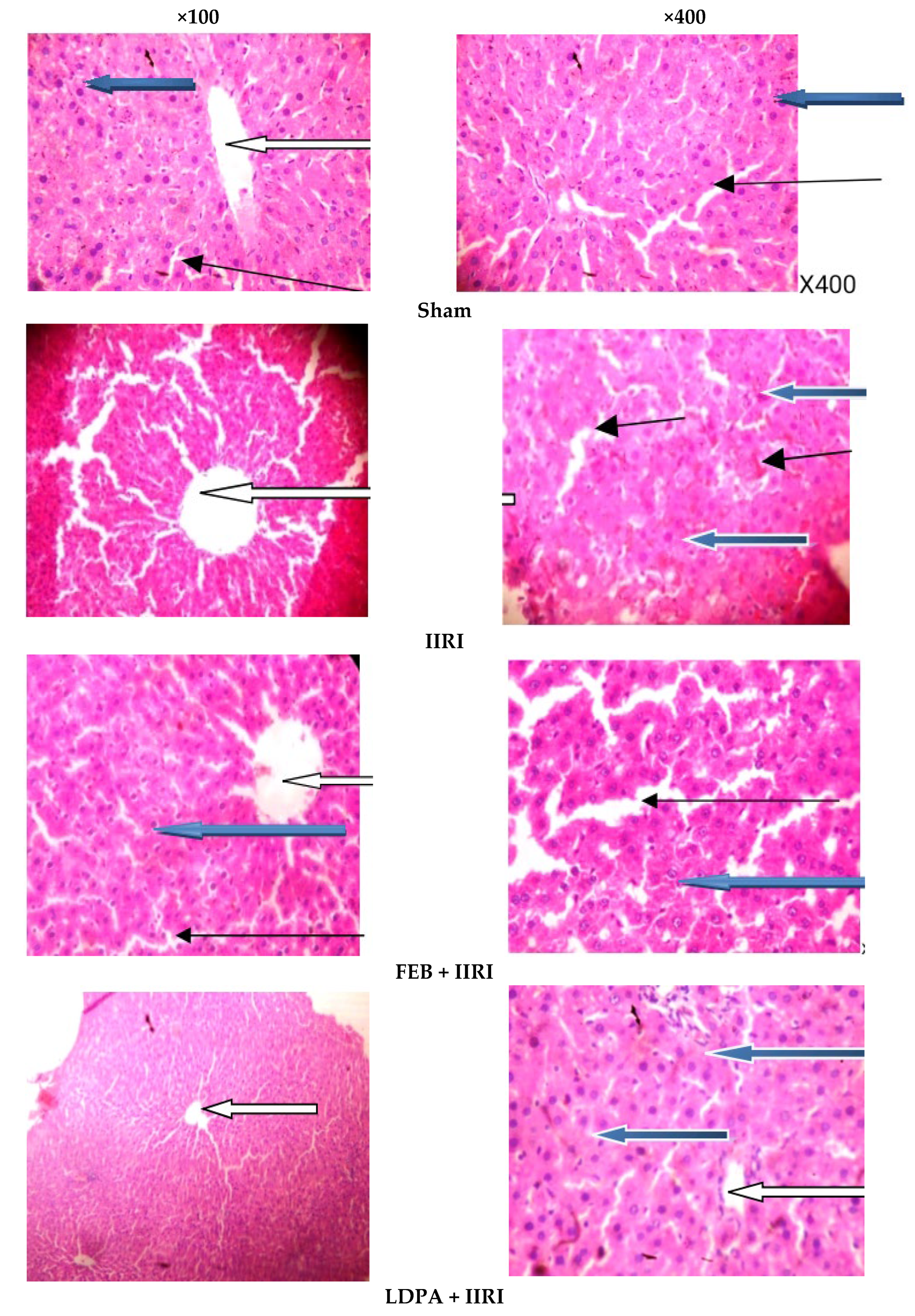
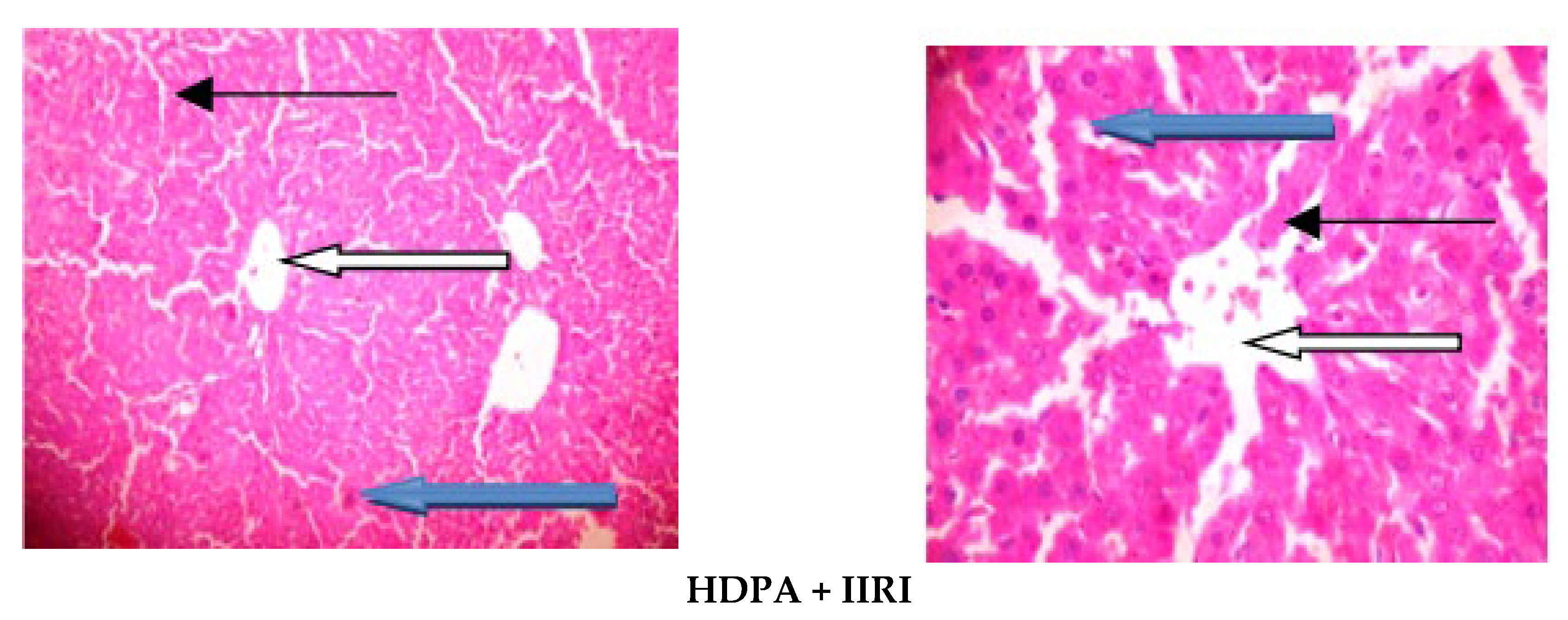
2.10. Histopathological Analysis
2.11. Statistical Analysis
3. Results
3.1. Bioactive Compounds of Methanolic Phyllanthus amarus Leaf Extract
3.2. Organ Weight
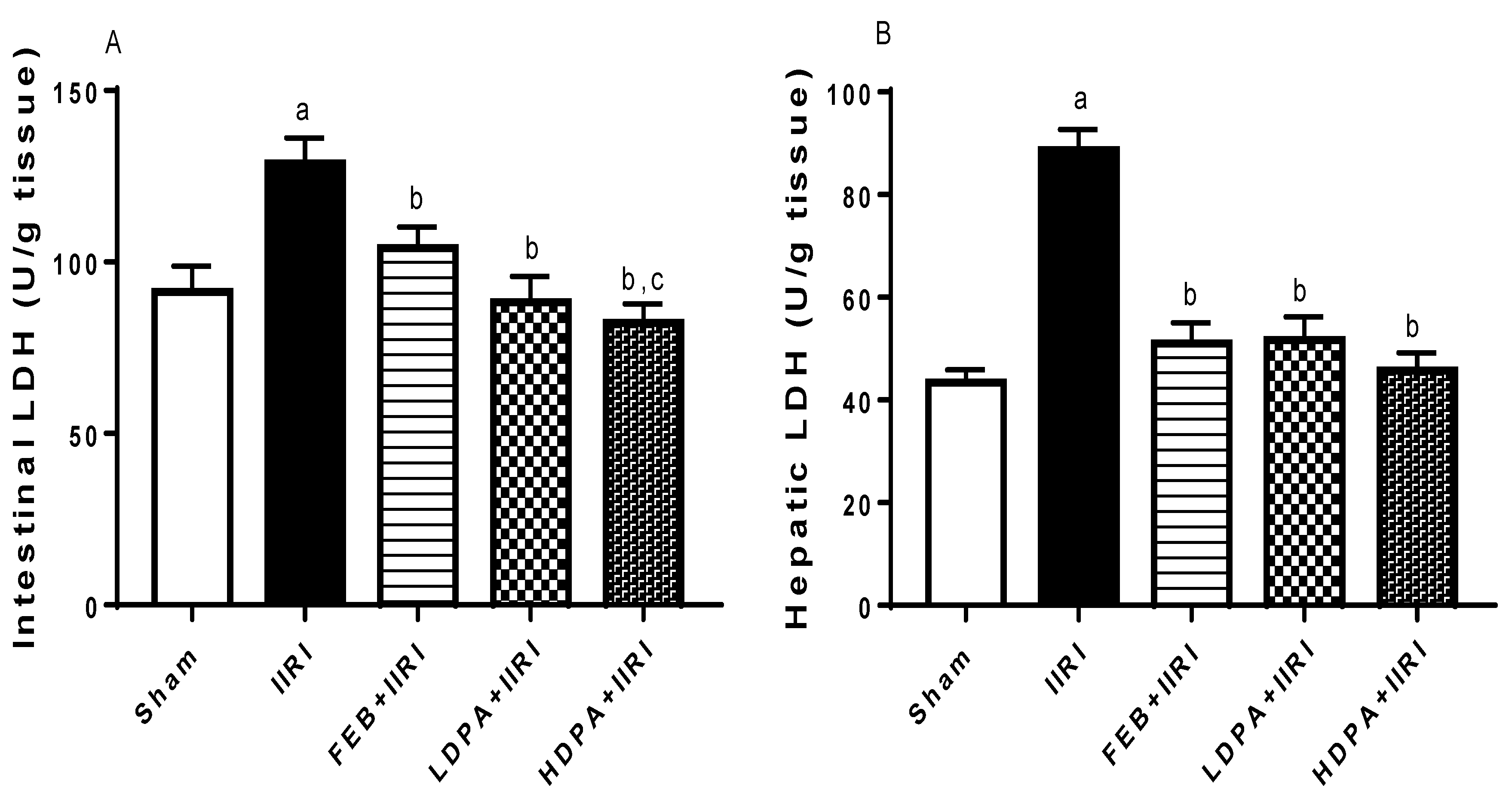
3.3. Hepatic Injury Markers
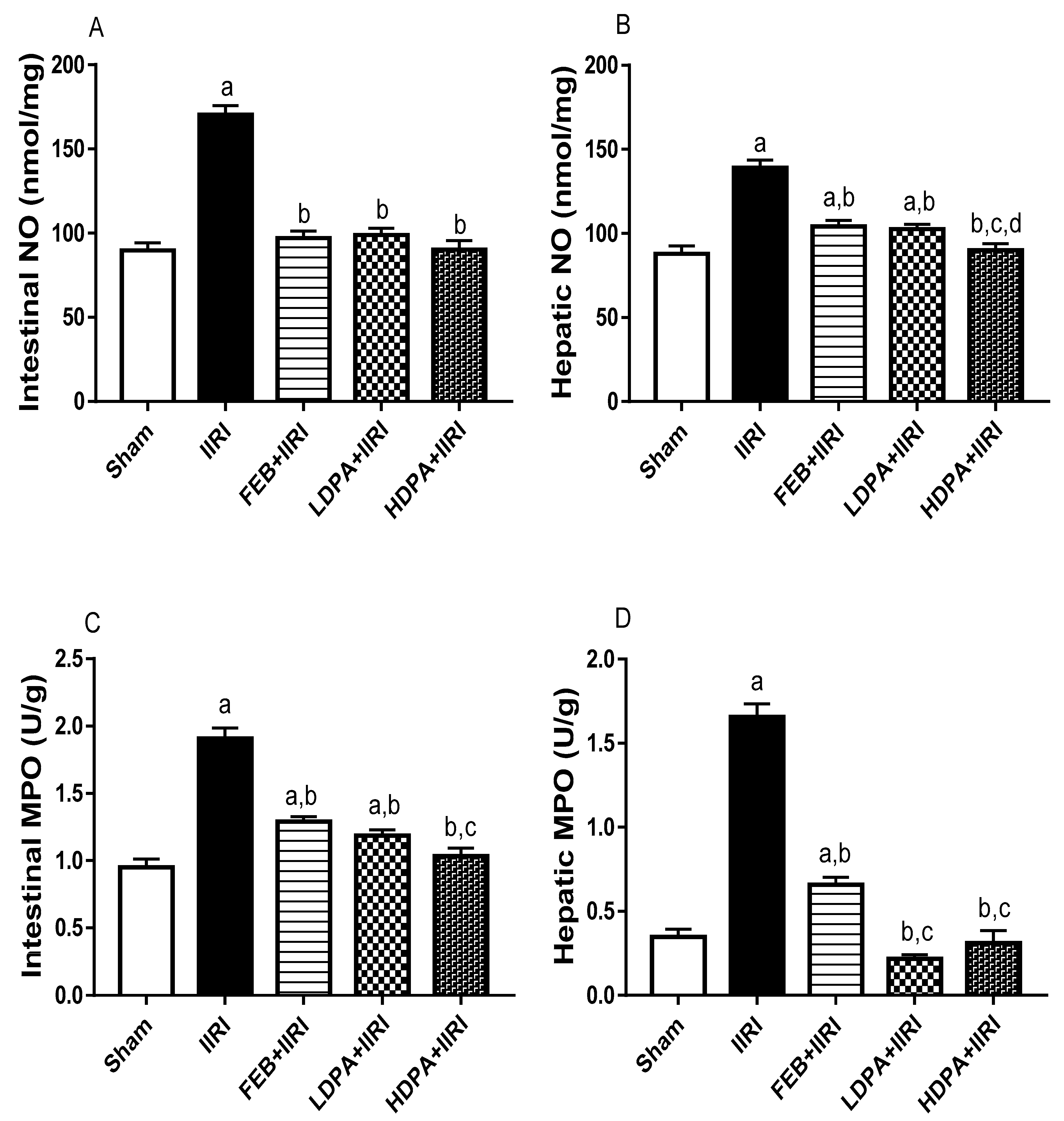
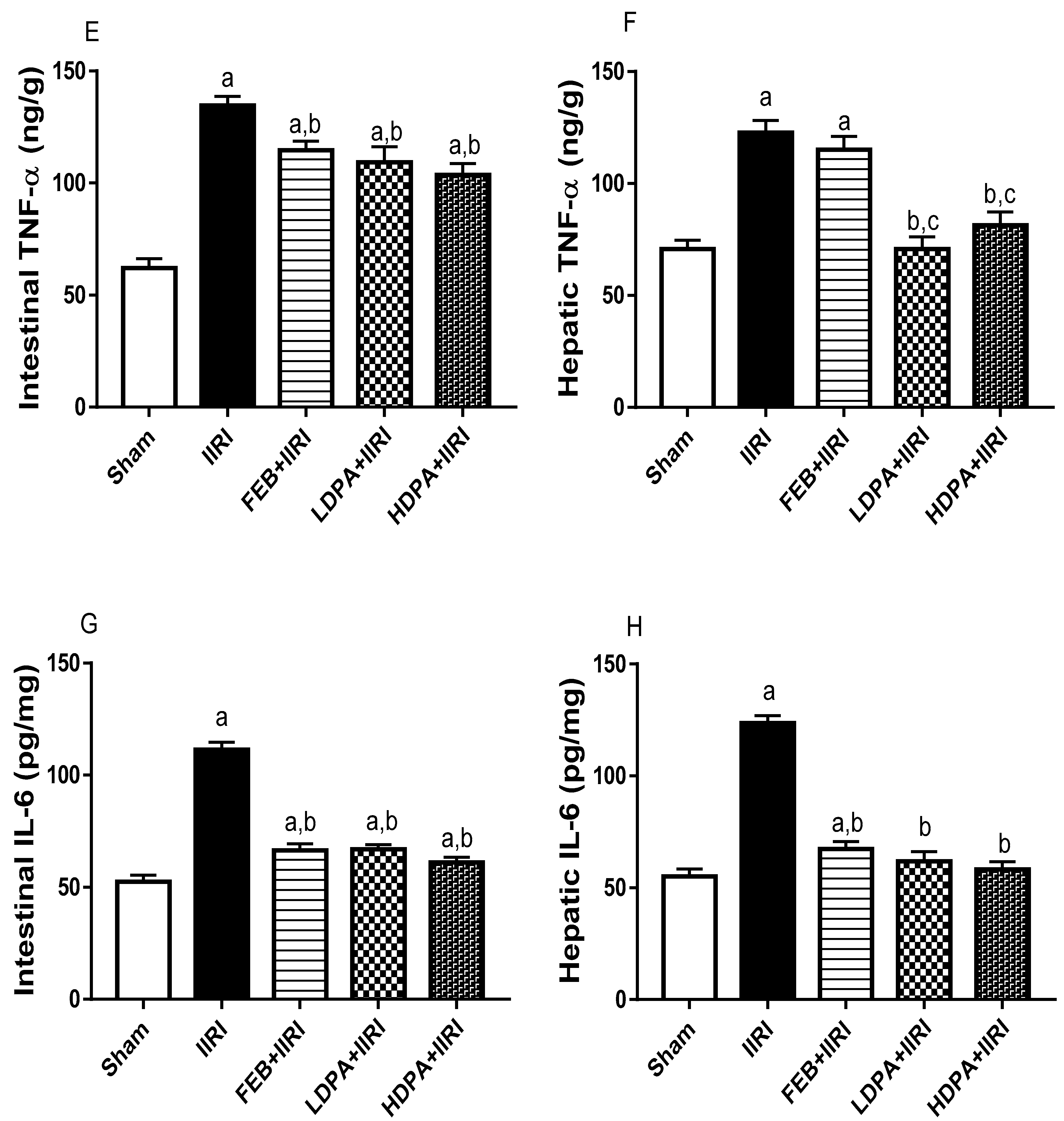
3.4. Markers of Oxidative Stress and Inflammation
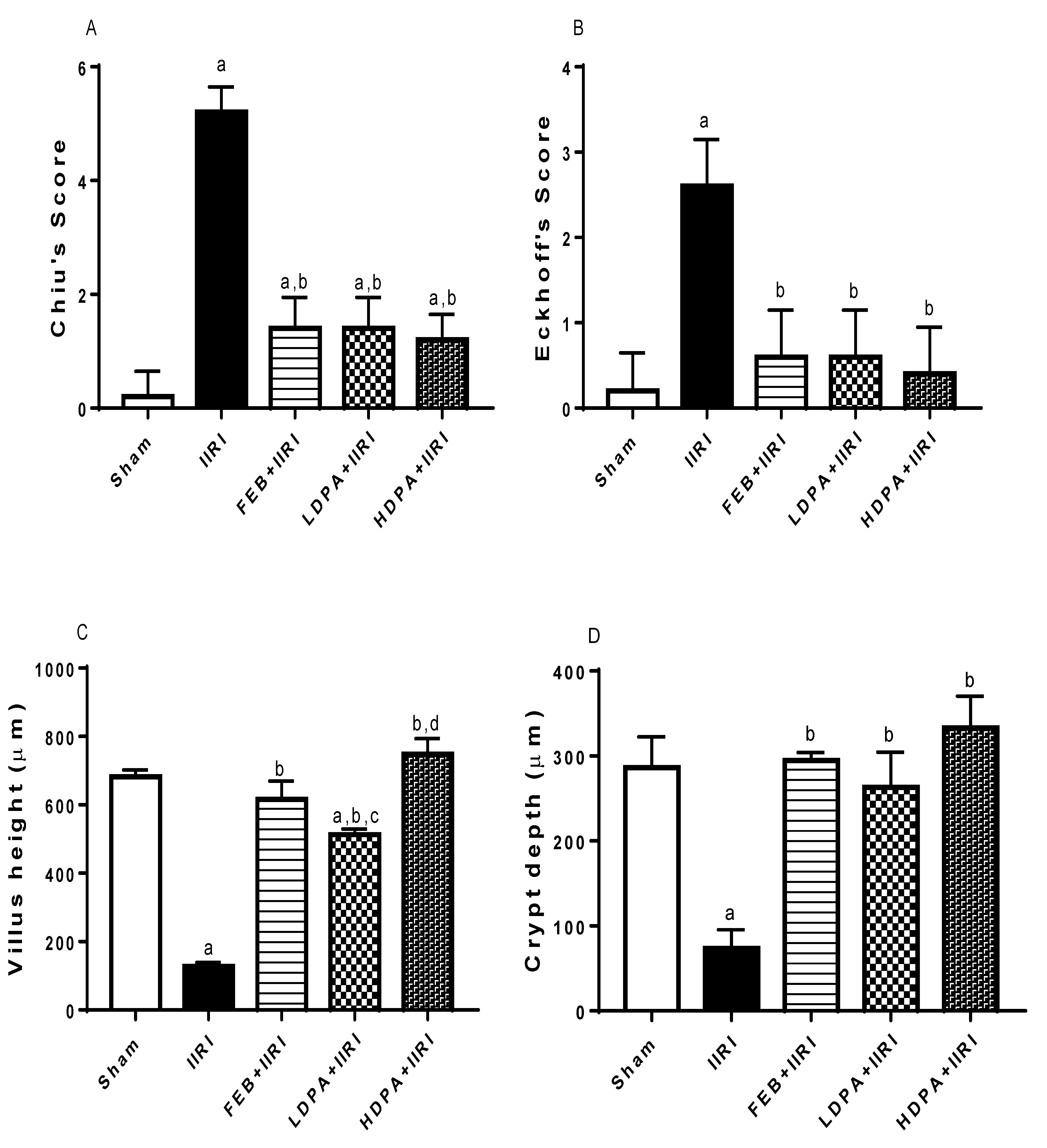
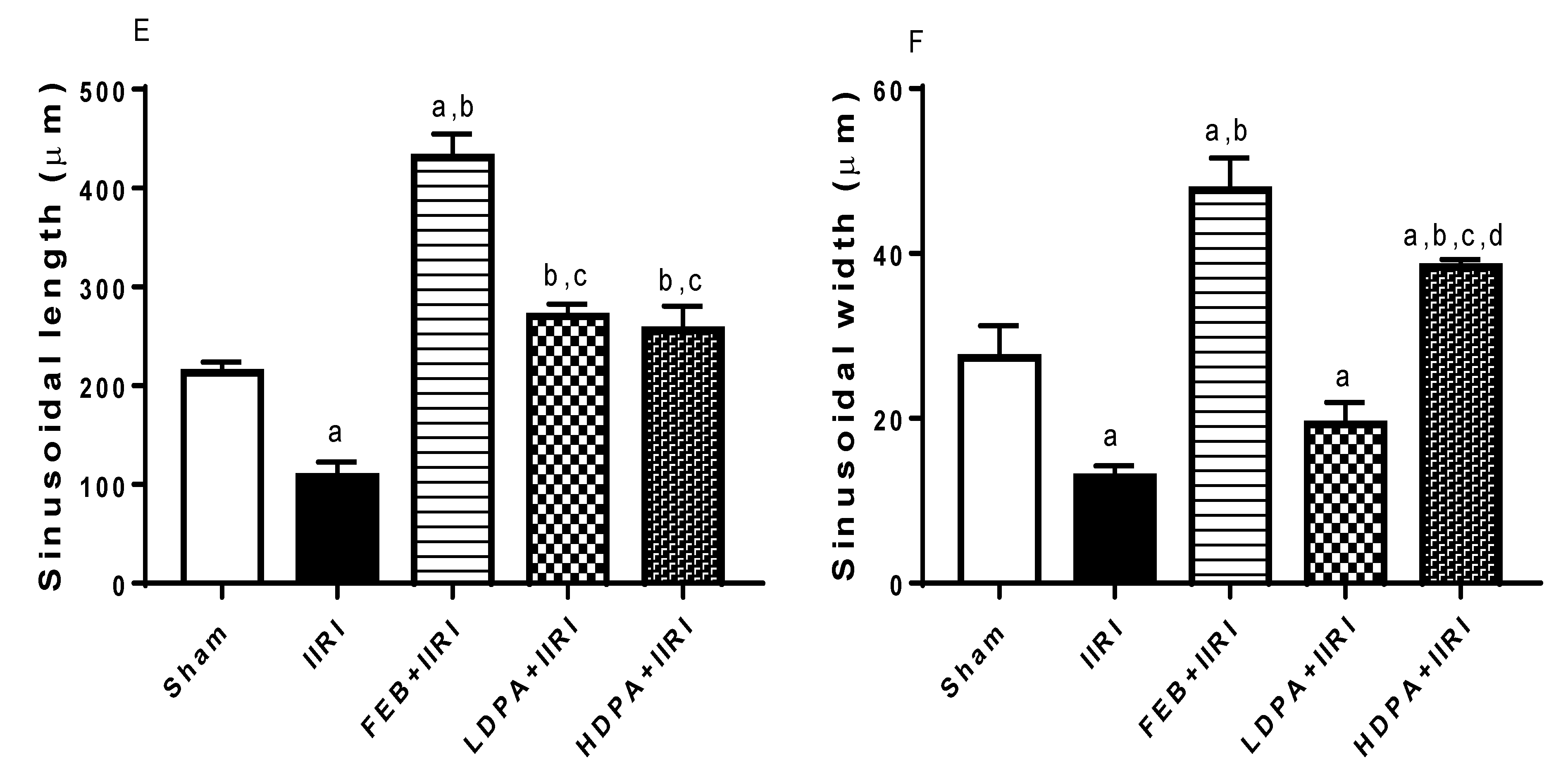
3.5. Intestinal and Hepatic Histoarchitecture
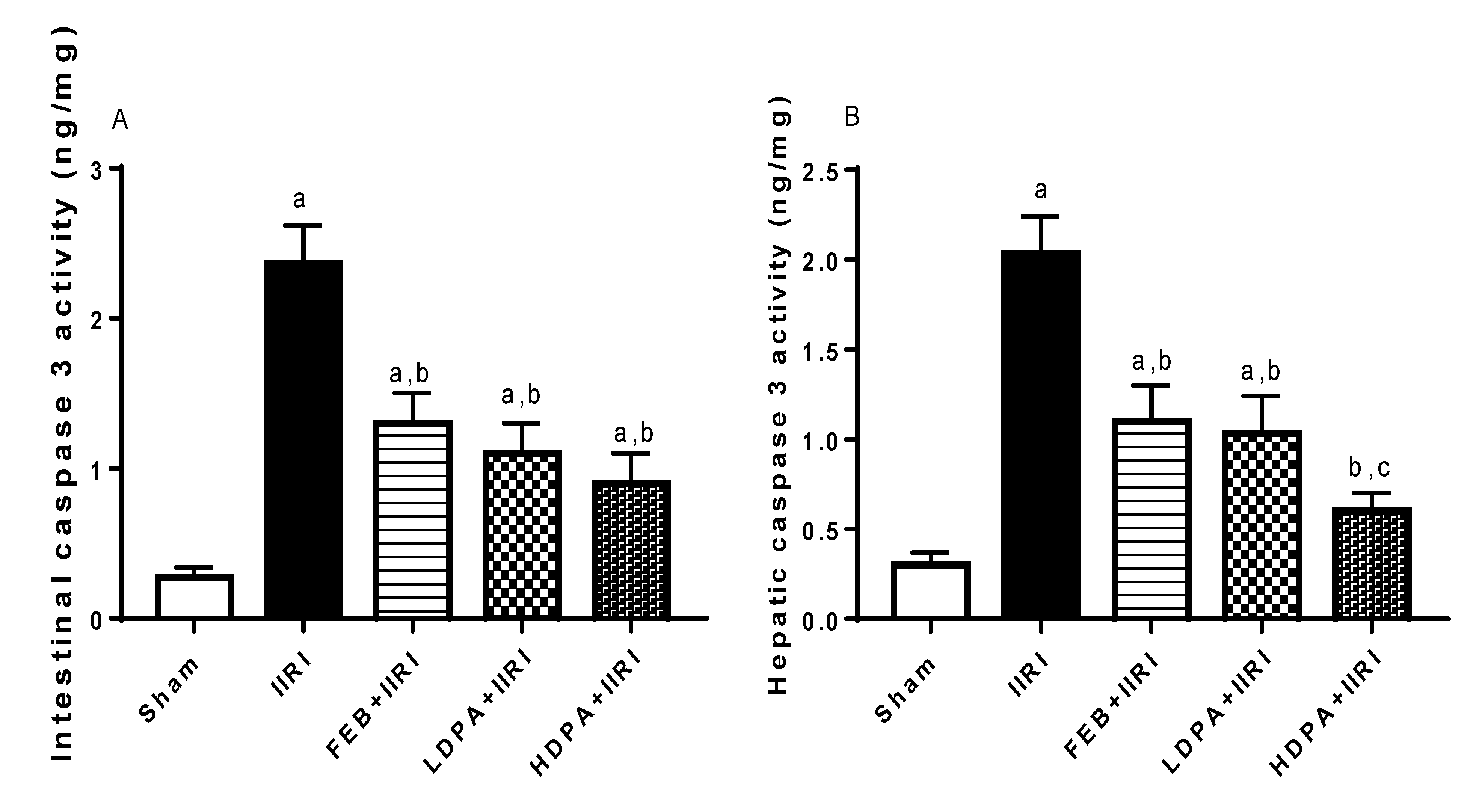
3.6. Apoptotic Markers
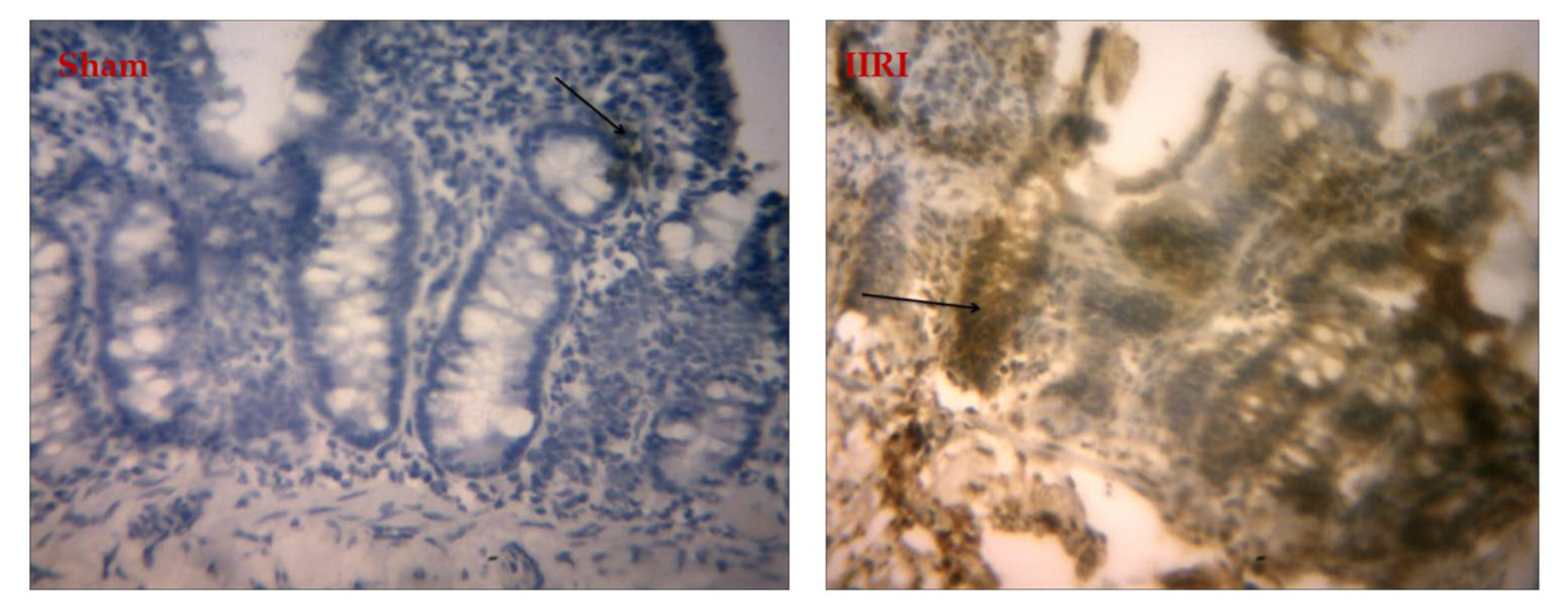
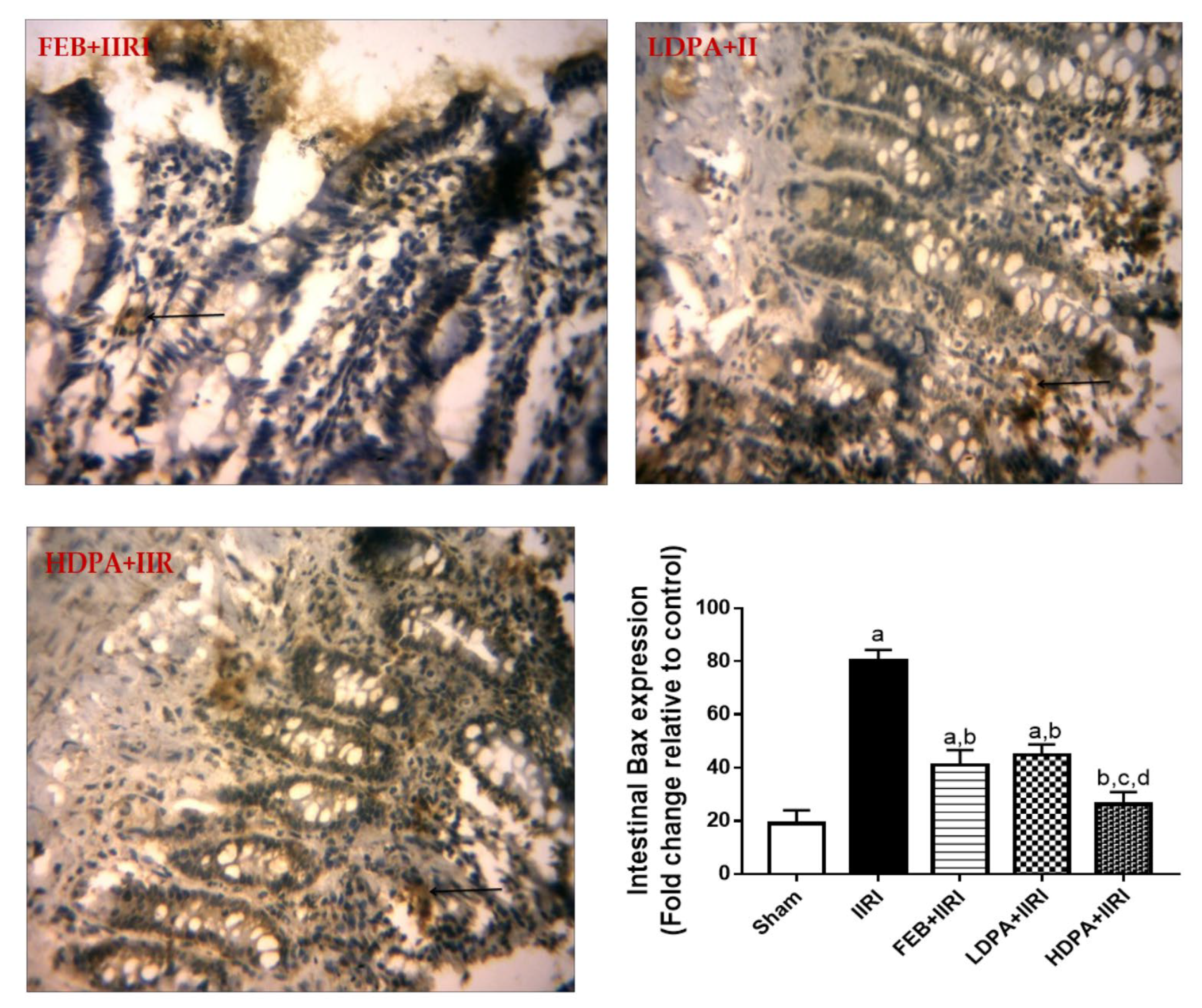
3.7. Bax Expression
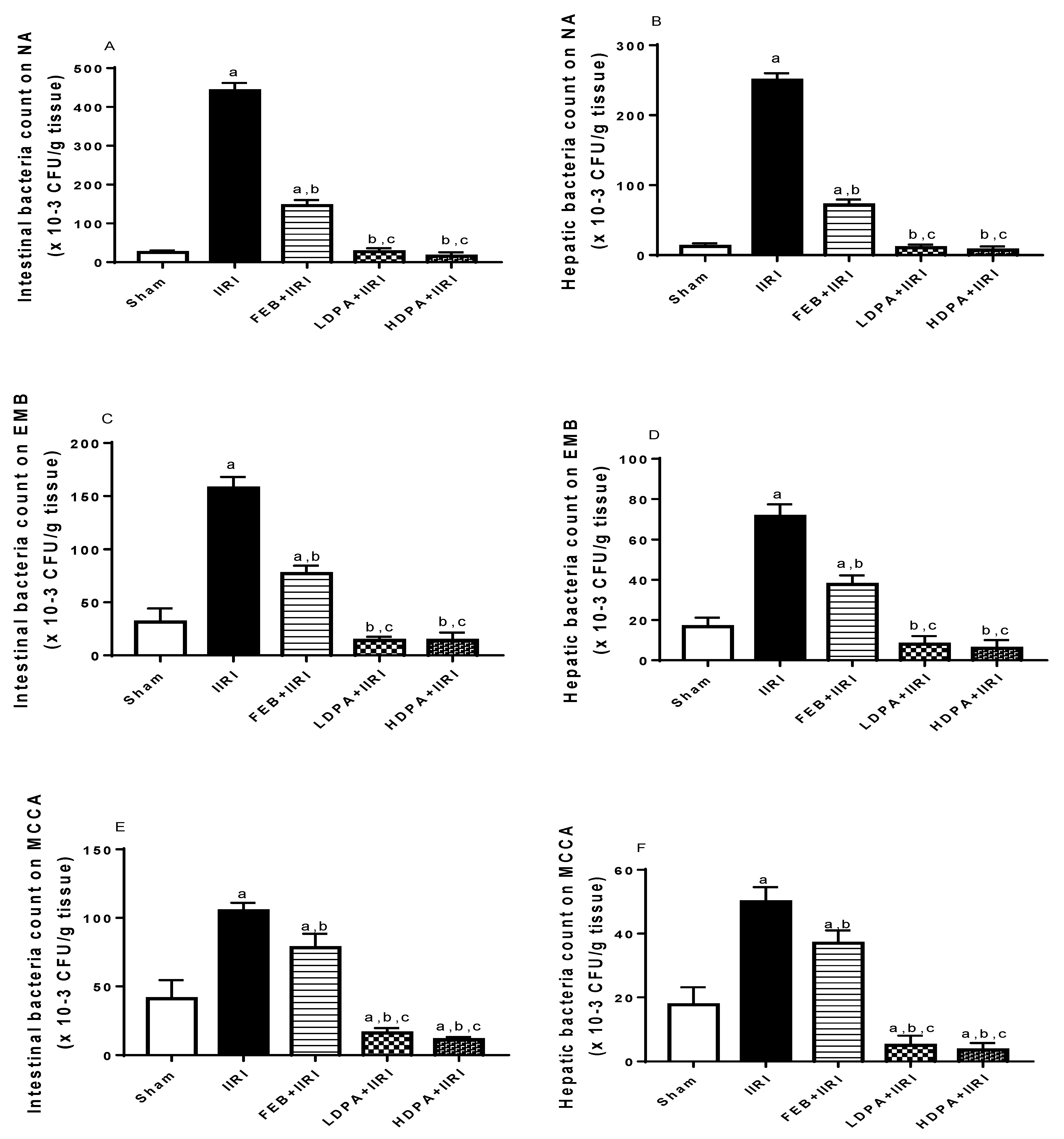
3.8. Bacterial Translocation
3.9. Correlation Study
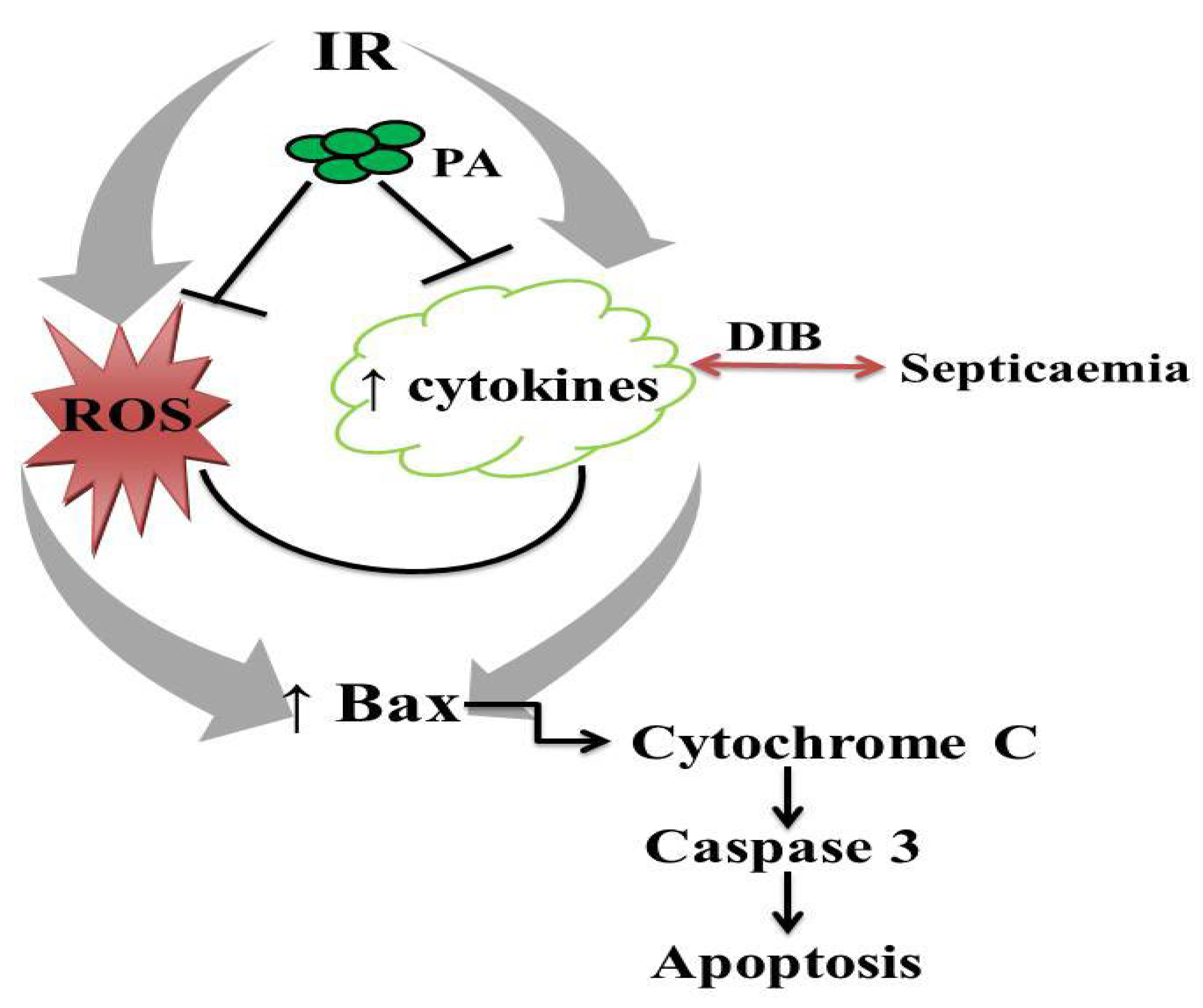
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IR | ischemia/reperfusion |
| IIRI | intestinal ischemia/reperfusion injury |
| MODS | multiple-organ dysfunction syndrome |
| MOF | multiple-organ failure |
| Bax | Bcl-2-associated X protein |
| Caspase 3 | cysteine-aspartic protease 3 |
| GC-MS | gas chromatography–mass spectrophotometric |
| AST | aspartate transaminase |
| ALT | alanine transferase |
| GGT | gamma-glutamyl transferase |
| LDH | lactate dehydrogenase |
| MDA | malondialdehyde |
| GSH | reduced glutathione |
| SOD | superoxide dismutase |
| GPx | glutathione peroxidase |
| NO | nitric oxide |
| MPO | myeloperoxidase |
| TNF-α | tumor necrotic factor-α |
| IL-6 | interleukin-6 |
| NA | nutrient agar |
| EMB | eosin-methylene blue |
| MCCA | Mac Conkey agar |
| Enos | endothelial nitric oxide synthase |
| ROS | reactive oxygen species |
| PPARγ | peroxisome-proliferator-activated receptors |
References
- Bertoni, S.; Ballabeni, V.; Barocelli, E.; Tognolini, M. Mesenteric ischemia-reperfusion: An overview of preclinical drug strategies. Drug Discov. Today 2018, 23, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, Y.-Y.; Lan, J.-N.; Liu, H.-M.; Li, W.; Wu, Y.; Leng, Y.; Tang, L.-H.; Hou, J.-B.; Sun, Q.; et al. Ischemic postconditioning alleviates intestinal ischemia-reperfusion injury by enhancing autophagy and suppressing oxidative stress through the Akt/GSK-3β/Nrf2 pathway in mice. Oxidative Med. Cell. Longev. 2020, 2020, 6954764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chen, T.; Lei, P.; Tang, X.; Huang, P. Exosomes released by bone marrow mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF-κB pathway. Int. J. Med. Sci. 2019, 16, 1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Fan, X.; Yang, B.; Chen, Y.; Liu, K.-X.; Zhou, J. Irisin pretreatment ameliorates intestinal ischemia/reperfusion injury in mice through activation of the Nrf2 pathway. Int. Immunopharmacol. 2019, 73, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, R.E.; Hamed, M.A.; Odetayo, A.F.; Akhigbe, T.M.; Ajayi, A.F.; Ajibogun, F.A.H. Omega-3 fatty acid rescues ischaemia/perfusion-induced testicular and sperm damage via modulation of lactate transport and xanthine oxidase/uric acid signaling. Biomed. Pharmacother. 2021, 142, 111975. [Google Scholar] [CrossRef]
- Nadatani, Y.; Watanabe, T.; Shimada, S.; Otani, K.; Tanigawa, T.; Fujiwara, Y. Microbiome and intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 2018, 63, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Doudakmanis, C.; Bouliaris, K.; Kolla, C.; Efthimiou, M.; Koukoulis, G.D. Bacterial translocation in patients undergoing major gastrointestinal surgery and its role in postoperative sepsis. World J. Gastrointest. Pathophysiol. 2021, 12, 106. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Triantos, C.; Thomopoulos, K.; Fligou, F.; Maroulis, I.; Marangos, M.; Gogos, C.A. Gut-origin sepsis in the critically ill patient: Pathophysiology and treatment. Infection 2018, 46, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, J.; Li, Z.; Zu, G.; Feng, D.; Li, Y.; Qasim, W.; Zhang, S.; Li, T.; Zeng, H.; et al. miR-381-3p knockdown improves intestinal epithelial proliferation and barrier function after intestinal ischemia/reperfusion injury by targeting nurr1. Cell Death Dis. 2018, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, M.A.; Petroianu, A.; Barbosa, A.J.; Figueiredo, J.A.; Alberti, L.R.; Ribas Filho, J.M. Effects of different periods of gastric ischemia on liver as a remote organ. Acta Cir. Bras. 2018, 33, 964–974. [Google Scholar] [CrossRef]
- Ito, H.; Kimura, H.; Karasawa, T.; Hisata, S.; Sadatomo, A.; Inoue, Y.; Yamada, N.; Aizawa, E.; Hishida, E.; Kamata, R.; et al. NLRP3 inflammasome activation in lung vascular endothelial cells contributes to intestinal ischemia/reperfusion-induced acute lung injury. J. Immunol. 2020, 205, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Douzinas, E.E.; Apeiranthitis, A. Myocardial injury secondary to intestinal ischemia/reperfusion or microbiota disturbance: Preventive and therapeutic concerns. In Modulation of Oxidative Stress in Heart Disease; Springer: Singapore, 2019; pp. 555–564. [Google Scholar]
- Gorecki, A.M.; Dunlop, S.A.; Rodger, J.; Anderton, R.S. The gut-brain axis and gut inflammation in Parkinson’s disease: Stopping neurodegeneration at the toll gate. Expert Opin. Ther. Targets 2020, 24, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Ren, T.; Zhu, H.; Tian, L.; Yu, Q.; Li, M. Candida albicans infection disturbs the redox homeostasis system and induces reactive oxygen species accumulation for epithelial cell death. FEMS Yeast Res. 2020, 20, foz081. [Google Scholar] [CrossRef]
- Hamed, M.; Aremu, A.; Akhigbe, R. Concomitant administration of HAART aggravates anti-Koch-induced oxidative hepatorenal damage via dysregulation of glutathione and elevation of uric acid production. Biomed. Pharmacother. 2021, 137, 111309. [Google Scholar] [CrossRef]
- Liu, K.Y.; Nakatsu, C.H.; Jones-Hall, Y.; Kozik, A.; Jiang, Q. Vitamin E alpha-and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic. Biol. Med. 2021, 163, 180–189. [Google Scholar] [CrossRef]
- Rudel, T. Caspase inhibitors in prevention of apoptosis. Herz 1999, 24, 236–241. [Google Scholar] [CrossRef]
- Krajewska, M.; Wang, H.G.; Krajewski, S.; Zapata, J.M.; Shabaik, A.; Gascoyne, R.; Reed, J.C. Immunohistochemical analysis of in vivo patterns of expression of CPP32(caspase-3), a cell death protease. Cancer Res. 1997, 57, 1605–1613. [Google Scholar]
- Akhigbe, R.E.; Ajayi, L.O.; Adelakun, A.A.; Olorunnisola, O.S.; Ajayi, A.F. Codeine-induced hepatic injury is via oxido-inflammatory damage and caspase-3-mediated apoptosis. Mol. Biol. Rep. 2020, 47, 9521–9530. [Google Scholar] [CrossRef]
- Adeneye, A.A. Hypoglycemic and hypochloesterolemic activities of the leaf and seed extract of Phyllanthus amarus in mice. Fitoterapia 2006, 77, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, O.A.; Inya-Agha, S.I.; Ilori, O.O. Immune boosting herbs: Lipid per-oxidation in liver homogenate as index of activity. J. Pharm. Toxicol. 2007, 2, 190–195. [Google Scholar] [CrossRef]
- Oluwafemi, F.; Debiri, F. Antimicrobial effect of Phyllanthus amarus and paraquetina nigrescens on Salamonella typhi. Afr. J. Biomed. Res. 2008, 11, 215–219. [Google Scholar]
- Idowu, A.T.; Bola, O.O.; Peniel, N.F. Haematologcial properties of aqueous extracts of Phyllanthus amarus (Schum and Thonn) and Xylopia anethiopica (Dunal). Ethnomed 2009, 3, 99–103. [Google Scholar]
- Saka, W.A.; Akhigbe, R.E.; Ajayi, A.F.; Ajayi, L.O.; Nwabuzor, O.E. Anti-diabetic and antioxidant potentials of aqueous extract of Eucalyptus glubulus in experimentally-induced diabetic rats. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 20–26. [Google Scholar]
- Ogundola, A.F.; Akhigbe, R.E.; Saka, W.A.; Adeniyi, A.O.; Adeshina, O.S.; Babalola, D.O.; Akhigbe, T.M. Contraceptive potential of Andrographis paniculata is via androgen suppression and not induction of oxidative stress in male Wistar rats. Tissue Cell 2021, 73, 101632. [Google Scholar] [CrossRef]
- Sofowora, A. Medicinal Plant and Traditional Medcine in Africa; Chichester John Willey and Sons: New York, NY, USA, 1993; p. 256. [Google Scholar]
- Trease, G.E.; Evans, W.C. A Textbook of Pharmacognosy; Bailliere Tindall Ltd.: London, UK, 1989; p. 53. [Google Scholar]
- Saka, W.A.; Ayoade, T.E.; Akhigbe, T.M.; Akhigbe, R.E. Moringa oleifera seed oil partially abrogates 2,3-dichlorovinyl dimethyl phosphate (Dichlorvos)-induced cardiac injury in rats: Evidence for the role of oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 237–246. [Google Scholar] [CrossRef]
- Richardson, P.M.; Harborne, J.B. Phytochemical methods. Brittonia 1985, 37, 309. [Google Scholar] [CrossRef]
- Obadoni, B.O.; Ochuko, P.O. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in edo and delta states of nigeria. Glob. J. Pure Appl. Sci. 2002, 8, 203–208. [Google Scholar] [CrossRef]
- Okwu, D.E. Phytochemical and vitamin content of indigenous spices of South Eastern Nigeria. J. Sustain. Agric. Environ. 2004, 6, 30–34. [Google Scholar]
- Van Buren, J.P.; Robinson, W.B. Formation of complexes between protein and tannic acid. J. Agric. Food Chem. 1969, 17, 772–777. [Google Scholar] [CrossRef]
- Mamza, U.T.; Sodipo, O.A.; Khan, I.Z. Gas chromatography-mass spectrometry (gc-ms) analysis of bioactive components of Phyllanthus amarus leaves. Int. Res. J. Plant Sci. 2012, 3, 208–215. [Google Scholar]
- Bonnie, T.C. The wistar rat as a right choice: Establishing mammalian standards and the ideal of a standard mammal. J. Hist. Biol. 1993, 26, 329–349. [Google Scholar]
- Tsuda, H.; Kawada, N.; Kaimori, J.-Y.; Kitamura, H.; Moriyama, T.; Rakugi, H.; Takahara, S.; Isaka, Y. Febuxostat suppressed renal ischemia-reperfusion injury via reduced oxidative stress. Biochem. Biophys. Res. Commun. 2012, 427, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Saban-Ruiz, J.; Alonso-Pacho, A.; Fabregate-Fuente, M.; Gonzalez-Quevedo, C.D. Xanthine oxidase inhibitor febuxostat as a novel agent postulated to actagainst vascular inflammation. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2013, 12, 94–99. [Google Scholar] [CrossRef]
- Khames, A.; Khalaf, M.M.; Gad, A.M.; Abd El-Raouf, O.M. Ameliorative effects of Sildenafil and/or Febuxostat on Doxorubin-induced nephrotoxicity in rats. Eur. J. Pharmacol. 2017, 805, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kassuya, C.A.; Leite, D.F.; de Melo, L.V.; Rehder, V.L.; Calixto, J.B. Antiinflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus. Planta Med. 2005, 71, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Putakala, M.; Gujjala, S.; Nukala, S.; Desireddy, S. Beneficial effects of Phyllanthus amarus against high fructose diet induced insulin resistance and hepatic oxidative stress in male Wistar rats. Appl. Biochem. Biotechnol. 2017, 183, 744–764. [Google Scholar] [CrossRef]
- Yildiz, F.; Terzi, A.; Coban, S.; Çelik, H.; Aksoy, N.; Bitiren, M.; Ozdogan, M.K. Protective effects of resveratrol on small intestines against intestinal ischemia-reperfusion injury in rats. J. Gastroenterol. Hepatol. 2009, 24, 1781–1785. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. In vivo exposure to codeine induces reproductive toxicity: Role of HER2 and p53/Bcl-2 signaling pathway. Heliyon 2020, 6, e05589. [Google Scholar] [CrossRef]
- Saka, W.A.; Akhigbe, R.E.; Ishola, O.S.; Ashamu, E.A.; Olayemi, O.T.; Adeleke, G.E. Hepatotherapeutic effect of Aloe vera in alcohol-induced hepatic damage. Pak. J. Biol. Sci. 2011, 14, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, M.; Akhigbe, T.; Akhigbe, R.; Aremu, A.; Oyedokun, P.; Gbadamosi, J.; Anifowose, P.; Adewole, M.; Aboyeji, O.; Yisau, H.; et al. Glutamine restores testicular glutathione-dependent antioxidant defense and upregulates NO/cGMP signaling in sleep deprivation-induced reproductive dysfunction in rats. Biomed. Pharmacother. 2022, 148, 112765. [Google Scholar] [CrossRef] [PubMed]
- Jocelyn, P.C. Spectrophotometric assay of thiols. Methods Enzymol. 1987, 143, 44–67. [Google Scholar] [PubMed]
- Hu, M. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar]
- Ozkan, O.V.; Yuzbasioglu, M.F.; Ciralik, H.; Kurutas, E.B.; Yonden, Z.; Aydin, M.; Bulbuloglu, E.; Semerci, E.; Goksu, M.; Atli, Y.; et al. Resveratrol, a natural antioxidant, attenuates intestinal ischemia/reperfusion injury in rats. Tohoku J. Exp. Med. 2009, 218, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef]
- Eckhoff, D.E.; Bilbao, G.; Frenette, L.; Thompson, J.A.; Contreras, J.L. 17-Beta-estradiol protects the liver against warm ischemia/reperfusion injury and is associated with increased serum nitric oxide and decreased tumor necrosis factor-alpha. Surgery 2002, 132, 302–309. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, R.; Wang, G.; Chen, Z.; Li, Y.; Zhao, Y.; Liu, D.; Zhao, H.; Zhang, F.; Yao, J.; et al. X.SIRT3-mediated deacetylation of PRDX3 alleviates mitochondrial oxidative damage and apoptosis induced by intestinal ischemia/reperfusion injury. Redox Biol. 2020, 28, 101343. [Google Scholar] [CrossRef]
- Grootjans, J.; Lenaerts, K.; Buurman, W.A.; Dejong, C.H.; Derikx, J.P. Life and death at the mucosal-luminal interface: New perspectives on human intestinal ischemia-reperfusion. World J. Gastroenterol. 2016, 22, 2760–2770. [Google Scholar] [CrossRef]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2011, 1813, 521–531. [Google Scholar] [CrossRef] [Green Version]
- Lalier, L.; Cartron, P.-F.; Juin, P.; Nedelkina, S.; Manon, S.; Bechinger, B.; Vallette, F. Bax activation and mitochondrial insertion during apoptosis. Apoptosis 2007, 12, 887–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.-T.; Wolter, K.G.; Youle, R.J. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 3668–3672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goping, I.S.; Gross, A.; Lavoie, J.N.; Nguyen, M.; Jemmerson, R.; Roth, K.; Korsmeyer, S.J.; Shore, G.C. Regulated targeting of BAX to mitochondria. J. Cell Biol. 1998, 143, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Youle, R.J.; Tjandra, N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell 2000, 103, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Zong, W.-X.; Li, C.; Hatzivassiliou, G.; Lindsten, T.; Yu, Q.-C.; Yuan, J.; Thompson, C.B. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003, 162, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Guha, G.; Mandal, T.; Rajkumar, V.; Kumar, R.A. Antimycin A-induced mitochondrial apoptotic cascade is mitigated by phenolic constituents of Phyllanthus amarus aqueous extract in Hep3B cells. Food Chem. Toxicol. 2010, 48, 3449–3457. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Zhang, X.; Wu, X.; Zhao, Y.; Ren, J. STING-dependent induction of lipid peroxidation mediates intestinal ischemia-reperfusion injury. Free Radic. Biol. Med. 2021, 163, 135–140. [Google Scholar] [CrossRef]
- Akhigbe, R.; Ajayi, A. Testicular toxicity following chronic codeine administration is via oxidative DNA damage and up-regulation of NO/TNF-α and caspase 3 activities. PLoS ONE 2020, 15, e0224052. [Google Scholar] [CrossRef] [Green Version]
- Ide, T.; Tsutsui, H.; Hayashidani, S.; Kang, D.; Suematsu, N.; Nakamura, K.-I.; Utsumi, H.; Hamasaki, N.; Takeshita, A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001, 88, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef]
- Dvorakova, K.; Waltmire, C.N.; Payne, C.M.; Tome, M.E.; Briehl, M.M.; Dorr, R.T. Induction of mitochondrial changes in myeloma cells by imexon. Blood 2001, 97, 3544–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabulut, A.B.; Kirimlioglu, V.; Yilmaz, S.; Isik, B.; Isikgil, O.; Kirimlioglu, H. Protective effects of resveratrol on spleen and ileum in rats subjected to ischemia-reperfusion. Transplant. Proc. 2006, 38, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Roviezzo, F.; Cuzzocrea, S.; Di Lorenzo, A.; Brancaleone, V.; Mazzon, E.; Di Paola, R.; Bucci, M.; Cirino, G. Protective role of PI3-kinase-Akt-eNOS signaling pathway in intestinal injury associated with splanchnic artery occlusion shock. Br. J. Pharmacol. 2007, 151, 377–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhigbe, R.; Ajayi, A. The impact of reactive oxygen species in the development of cardiometabolic disorders: A review. Lipids Health Dis. 2021, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Oztürk, C.; Avlan, D.; Cinel, I.; Cinel, L.; Unlü, A.; Camdeviren, H.; Atik, U.; Oral, U. Selenium pretreatment prevents bacterial translocation in rat intestinal ischemia/reperfusion model. Pharmacol. Res. 2002, 46, 171–177. [Google Scholar] [CrossRef]
- Ho, K.Y.; Huang, J.S.; Tsai, C.C.; Lin, T.C.; Hsu, Y.F.; Lin, C.C. Antioxidant activity of tannin components from Vaccinium vitis-idaea L. J. Pharm. Pharmacol. 1999, 51, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Tunon, H.; Olavsdotter, C.; Bohlin, L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef] [Green Version]
- Muselík, J.; García-Alonso, M.; Martín-López, M.P.; Žemlička, M.; Rivas-Gonzalo, J.C. Measurement of antioxidant activity of wine catechins, procyanidins, anthocyanins and pyranoanthocyanins. Int. J. Mol. Sci. 2007, 8, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.K. Anthocyanins Increase Antioxidant Enzyme Activity in HT-29 Adenocarcinoma Cells. Master’s Thesis, University of Georgia, Athens, GA, USA, 2009. [Google Scholar]
- Yin, T.-P.; Cai, L.; Xing, Y.; Yu, J.; Li, X.-J.; Mei, R.-F.; Ding, Z.-T. Alkaloids with antioxidant activities from Aconitum handelianum. J. Asian Nat. Prod. Res. 2016, 18, 603–610. [Google Scholar] [CrossRef]
- Vattem, D.A.; Ghaedian, R.; Shetty, K. Enhancing health benefits of berries through phenolic antioxidant enrichment: Focus on cranberry. Asia Pac. J. Clin. Nutr. 2005, 14, 120–130. [Google Scholar] [PubMed]
- Baydar, N.G.; Özkan, G.; Sagdic, O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 2004, 15, 335–339. [Google Scholar] [CrossRef]
- Vaquero, M.R.; Alberto, M.; de Nadra, M.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Spina, L.; Cavallaro, F.; Fardowza, N.; Lagoussis, P.; Bona, D.; Ciscato, C.; Rigante, A.; Vecchi, M. Butyric acid: Pharmacological aspects and routes of administration. Dig. Liver Dis. Suppl. 2007, 1, 7–11. [Google Scholar] [CrossRef]
- Lo, T.S.; Hammer, K.D.; Zegarra, M.; Cho, W.C. Methenamine: A forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era. Expert Rev. Anti-Infect. Ther. 2014, 12, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Antonsen, S.; Østby, R.B.; Stenstrøm, Y. Naturally occurring cyclobutanes: Their biological significance and synthesis. Stud. Nat. Prod. Chem. 2018, 57, 1–40. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tsai, T.-H.; Chuang, L.-T.; Li, Y.-Y.; Zouboulis, C.C.; Tsai, P.-J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2013, 73, 232–240. [Google Scholar] [CrossRef]
- Malapaka, R.R.V.; Khoo, S.; Zhang, J.; Choi, J.H.; Zhou, X.E.; Xu, Y.; Gong, Y.; Li, J.; Yong, E.L.; Chalmers, M.J.; et al. Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. J. Biol. Chem. 2012, 287, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Cui, T.; Schopfer, F.J.; Zhang, J.; Chen, K.; Ichikawa, T.; Paul, R.B.; Batthyany, C.; Chacko, B.K.; Feng, X.; Patel, R.P.; et al. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J. Biol. Chem. 2006, 281, 35686–35698. [Google Scholar] [CrossRef] [Green Version]
- Dubuquoy, L.; Rousseaux, C.; Thuru, X.; Peyrin-Biroulet, L.; Romano, O.; Chavatte, P.; Chamaillard, M.; Desreumaux, P. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut 2006, 55, 1341–1349. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Salam, O.M.; Youness, E.; Mohammed, N.A.; Morsy, S.; Omara, E.; Sleem, A. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J. Med. Food 2014, 17, 588–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdes, D.S.; So, D.; Gill, P.A.; Kellow, N.J. Effect of dietary acetic acid supplementation on plasma glucose, lipid profiles, and body mass index in human adults: A systematic review and meta-analysis. J. Acad. Nutr. Diet. 2021, 121, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.E.A.; Araujo, S.G.; Morais, M.I.; Sa, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L. Antifungal and antioxidant activity of fatty acid methy esters from vegetable oils. An. Acad. Bras. Ciênc. 2017, 89, 1671–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Phytochemicals | Values |
|---|---|
| Saponins | 0.747 ± 0.017 |
| Tannins | 1.850 ± 0.030 |
| Phenolics | 1.130 ± 0.014 |
| Anthocyanin | 1.820 ± 0.020 |
| Alkaloids | 1.607 ± 0.013 |
| Triterpenoids | 0.140 ± 0.010 |
| Glycosides | 0.863 ± 0.015 |
| S/N | Compound | Molecular Weight (g/mol) | Molecular Formula | Area (%) | Retention Time | Quality |
|---|---|---|---|---|---|---|
| 1 | Butanoic acid | 88.11 | CH4H8O2 | 10.75 | 3.574 | 9 |
| 2 | Methenamine | 140.186 | C6H12N4 | 7.58 | 3.797 | 5 |
| 3 | Cyclobutane | 56.107 | C4H8 | 0.04 | 7.476 | 9 |
| 4 | Octanoic acid | 144.211 | C8H16O2 | 0.17 | 9.828 | 89 |
| 5 | Thiazole | 85.12 | C3H3NS | 0.43 | 10.746 | 28 |
| 6 | Oleic acid | 282.468 | C18H24O2 | 0.14 | 11.357 | 30 |
| 7 | Citric acid | 192.124 | C6H8O7 | 1.53 | 12.129 | 25 |
| 8 | Acetic acid | 128.942 | C2H2CL2O2 | 3.04 | 14.578 | 9 |
| 9 | Hexadeconoic acid, methyl ester | 270.45 | C17H34O2 | 6.00 | 15.094 | 98 |
| 10 | Methyl stearate | 298.5 | C19H38O2 | 2.41 | 16.655 | 95 |
| Sham | IIRI | FEB + IIRI | LDPA + IIRI | HDPA + IIRI | |
|---|---|---|---|---|---|
| AST (U/g tissue) | 35.40 ± 4.27 | 84.80 ± 4.55 a | 46.40 ± 1.52 a,b | 40.51 ± 4.89 b | 37.40 ± 1.67 b,c |
| ALT (U/g tissue) | 54.67 ± 9.86 | 87.67 ± 9.29 a | 58.33 ± 2.88 b | 47.67 ± 4.50 b | 56.33 ± 3.51 b |
| ALP (U/g tissue) | 50.80 ± 2.58 | 86.34 ± 8.33 a | 59.60 ± 7.89 b | 57.52 ± 8.42 b | 54.01 ± 4.06 b |
| GGT (U/g tissue) | 123.51 ± 5.86 | 178.30 ± 7.70 a | 142.12 ± 4.40 a,b | 147.35 ± 7.59 a,b | 137.41 ± 9.10 b |
| Sham | IIRI | FEB + IIRI | LDPA + IIRI | HDPA + IIRI | |
|---|---|---|---|---|---|
| MDA (mM) | |||||
| Intestinal | 0.683 ± 0.056 | 1.257 ± 0.051 a | 0.790 ± 0.036 b | 0.513 ± 0.032 a,b,c | 0.510 ± 0.037 a,b,c |
| Hepatic | 1.967 ± 0.305 | 5.567 ± 0.473 a | 1.967 ± 0.513 b | 2.400 ± 0.4359 b | 2.167 ± 0.305 b |
| GSH (nmoL/g tissue) | |||||
| Intestinal | 57.470 ± 3.522 | 32.430 ± 3.017 a | 58.800 ± 3.297 b | 78.600 ± 3.396 a,b,c | 78.570 ± 3.247 a,b,c |
| Hepatic | 63.130 ± 2.259 | 33.100 ± 3.751 a | 63.470 ± 2.721 b | 83.600 ± 3.396 a,b,c | 83.230 ± 2.673 a,b,c |
| SOD (U/g) | |||||
| Intestinal | 17.330 ± 0.493 | 12.500 ± 0.458 a | 24.470 ± 0.566 a,b | 16.130 ± 0.611 b,c | 18.130 ± 0.611 b,c,d |
| Hepatic | 8.333 ± 0.251 | 3.900 ± 0.435 a | 7.533 ± 0.404 b | 7.433 ± 0.321 b | 8.167 ± 0.305 b |
| Catalase (mU/mg) | |||||
| Intestinal | 37.000 ± 2.400 | 23.000 ± 2.000 a | 36.000 ± 2.210 b | 34.330 ± 2.517 b | 43.670 ± 2.517 a,b,c,d |
| Hepatic | 33.000 ± 2.000 | 20.000 ± 2.646 a | 29.670 ± 2.082 b | 30.000 ± 2.646 b | 32.670 ± 2.082 b |
| GPx (nm/min/mg protein) | |||||
| Intestinal | 161.900 ± 11.360 | 92.310 ± 5.473 a | 153.900 ± 12.590 b | 144.800 ± 7.333 b | 144.800 ± 8.951 b |
| Hepatic | 185.600 ± 19.240 | 108.900 ± 7.001 a | 158.400 ± 8.445 a,b | 148.300 ± 11.960 a,b | 157.000 ± 9.123 a,b |
| Thiol protein (U/mg protein) | |||||
| Intestinal | 16.430 ± 0.503 | 7.500 ± 0.360 a | 15.500 ± 0.400 b | 16.400 ± 0.625 b | 15.570 ± 0.416 b |
| Hepatic | 18.430 ± 0.503 | 6.500 ± 0.361 a | 17.830 ± 0.945 b | 18.400 ± 0.625 b | 17.800 ± 0.360 b |
| Non-thiol protein (U/mg protein) | |||||
| Intestinal | 28.370 ± 1.097 | 18.030 ± 0.650 a | 32.230 ± 0.776 a,b | 22.070 ± 0.802 a,b,c | 24.240 ± 0.686 a,b,c |
| Hepatic | 30.700 ± 1.082 | 18.370 ± 1.350 a | 34.230 ± 0.776 a,b | 25.070 ± 0.802 a,b,c | 27.240 ± 0.686 a,b,c |
| Eckhoff’s Score | AST | ALT | GGT | ||
|---|---|---|---|---|---|
| Chiu’s score | Pearson correlation | 0.8551 | 0.933 | 0.8822 | 0.2406 |
| R2 | 0.7312 | 0.8704 | 0.7784 | 0.0578 | |
| Sig. (2-tailed) | <0.0001 * | <0.001 * | <0.001 * | 0.388 | |
| N | 50 | 50 | 50 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afolabi, A.O.; Akhigbe, T.M.; Odetayo, A.F.; Anyogu, D.C.; Hamed, M.A.; Akhigbe, R.E. Restoration of Hepatic and Intestinal Integrity by Phyllanthus amarus Is Dependent on Bax/Caspase 3 Modulation in Intestinal Ischemia-/Reperfusion-Induced Injury. Molecules 2022, 27, 5073. https://doi.org/10.3390/molecules27165073
Afolabi AO, Akhigbe TM, Odetayo AF, Anyogu DC, Hamed MA, Akhigbe RE. Restoration of Hepatic and Intestinal Integrity by Phyllanthus amarus Is Dependent on Bax/Caspase 3 Modulation in Intestinal Ischemia-/Reperfusion-Induced Injury. Molecules. 2022; 27(16):5073. https://doi.org/10.3390/molecules27165073
Chicago/Turabian StyleAfolabi, Ayobami Oladele, Tunmise Maryanne Akhigbe, Adeyemi Fatai Odetayo, Davinson Chuka Anyogu, Moses Agbomhere Hamed, and Roland Eghoghosoa Akhigbe. 2022. "Restoration of Hepatic and Intestinal Integrity by Phyllanthus amarus Is Dependent on Bax/Caspase 3 Modulation in Intestinal Ischemia-/Reperfusion-Induced Injury" Molecules 27, no. 16: 5073. https://doi.org/10.3390/molecules27165073
APA StyleAfolabi, A. O., Akhigbe, T. M., Odetayo, A. F., Anyogu, D. C., Hamed, M. A., & Akhigbe, R. E. (2022). Restoration of Hepatic and Intestinal Integrity by Phyllanthus amarus Is Dependent on Bax/Caspase 3 Modulation in Intestinal Ischemia-/Reperfusion-Induced Injury. Molecules, 27(16), 5073. https://doi.org/10.3390/molecules27165073







