Isolation and Characterization of Galloylglucoses Effective against Multidrug-Resistant Strains of Escherichia coli and Klebsiella pneumoniae
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Antibiotics
2.2. Study Organisms
2.3. Plant Material Collection, Preparation, and Extraction
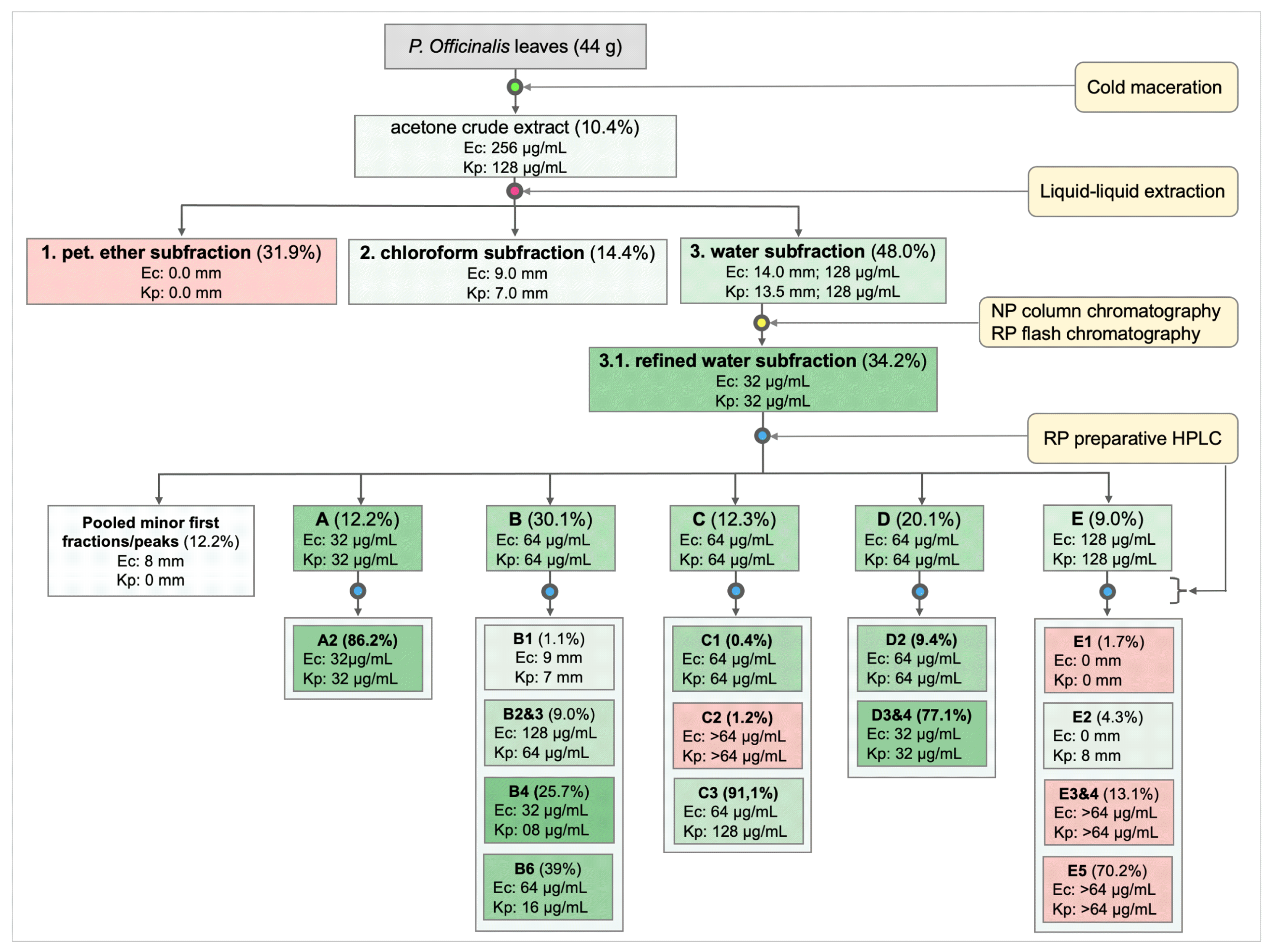
2.4. Isolation and Purification
2.4.1. Contact Bioautography
2.4.2. Liquid–Liquid Extraction
2.4.3. Open-Column and Flash Chromatography
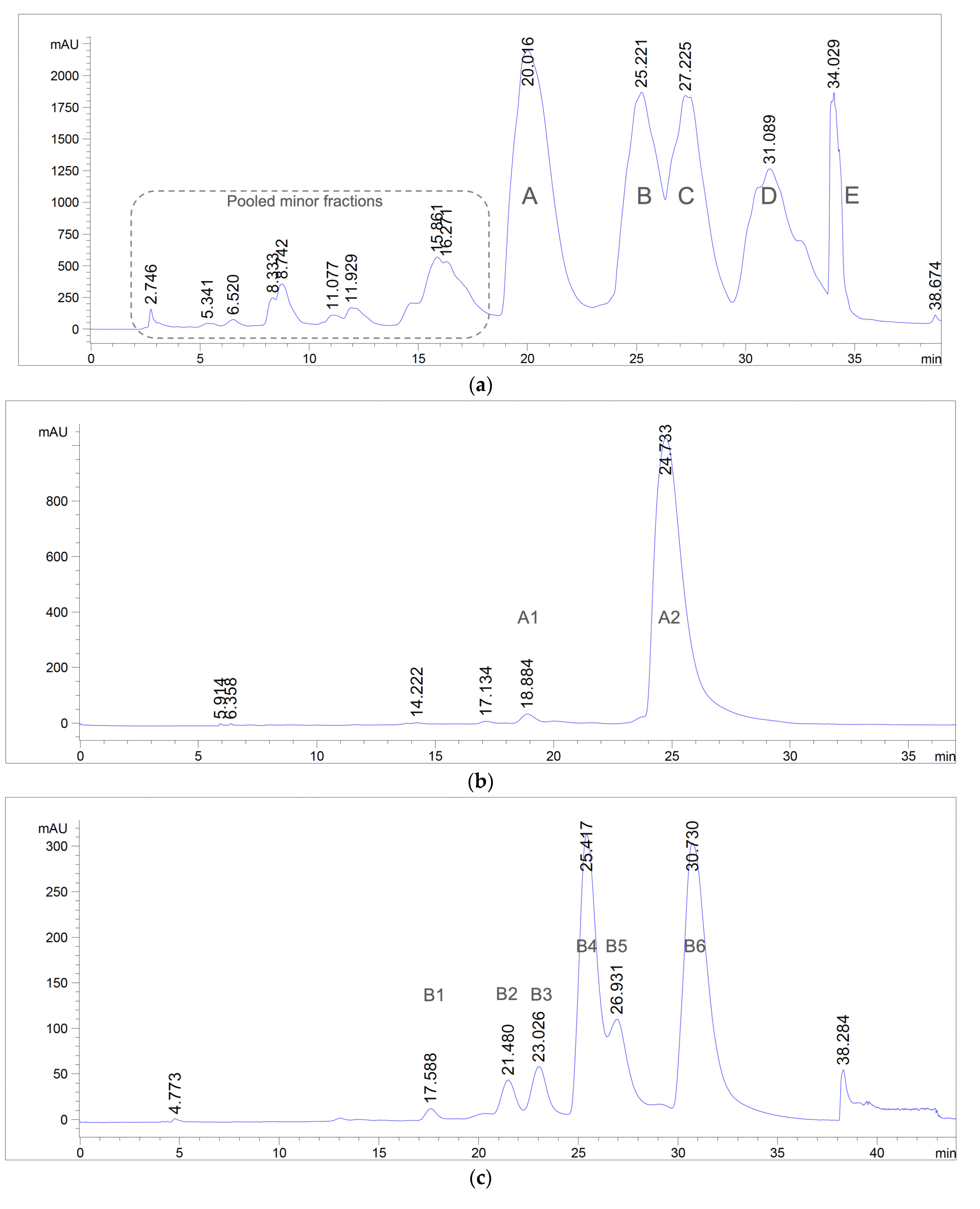
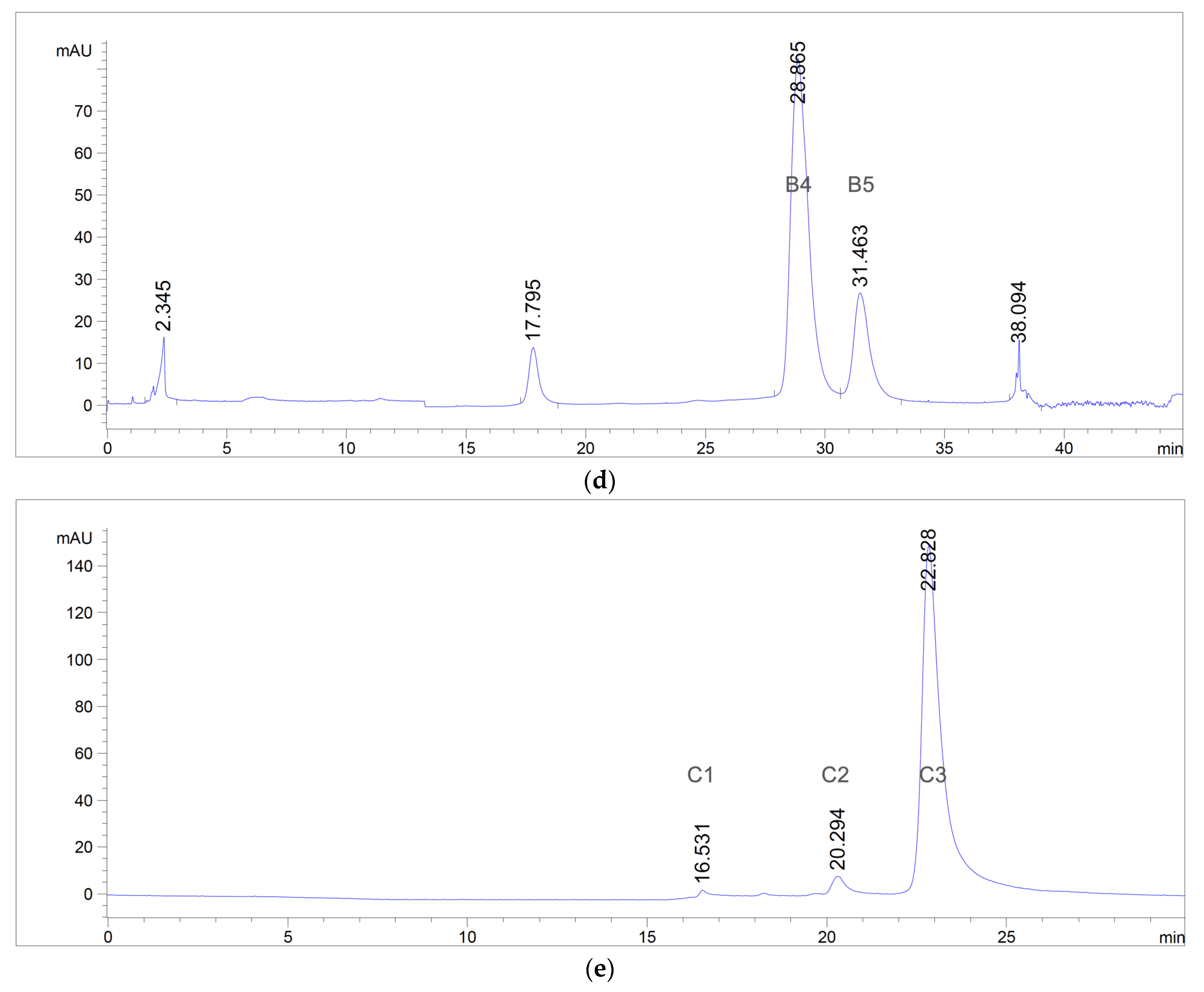
2.4.4. Preparative Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
2.5. Characterization and Structural Elucidation
2.5.1. Infrared Spectral Measurements
2.5.2. High-Resolution Mass Spectrometry
2.5.3. Nuclear Magnetic Resonance
2.6. Antibacterial Activity Testing
2.6.1. Disc Diffusion Assays
2.6.2. Broth Microdilution Assays for Determination of Minimum Inhibitory Concentration (MIC)
Preparation of Bacterial Suspensions
Loading of 96-Well Plates
2.6.3. Minimum Bactericidal Concentration (MBC) Testing
2.7. In Vivo Antibacterial Assay Using Galleria mellonella Larvae
2.8. Evaluation of the Amino Acid Composition of the Expressed Resistance Enzymes
3. Results
3.1. Extraction, Isolation, and Purification of Antibacterial Compounds
3.2. Characterization and Structural Elucidations of Selected Isolated Compounds
3.3. Antibacterial Activity Testing
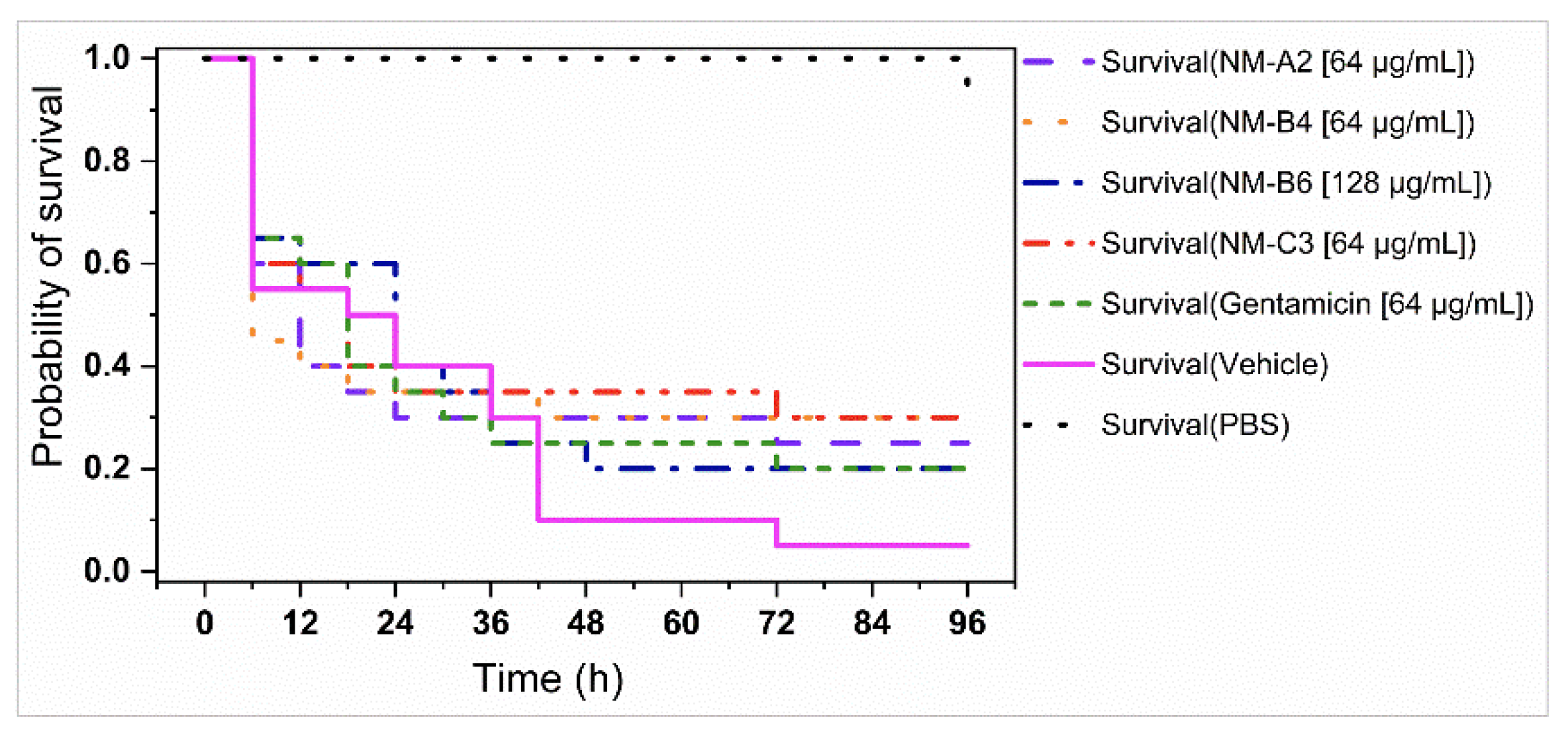
3.4. In Vivo Antibacterial Assays
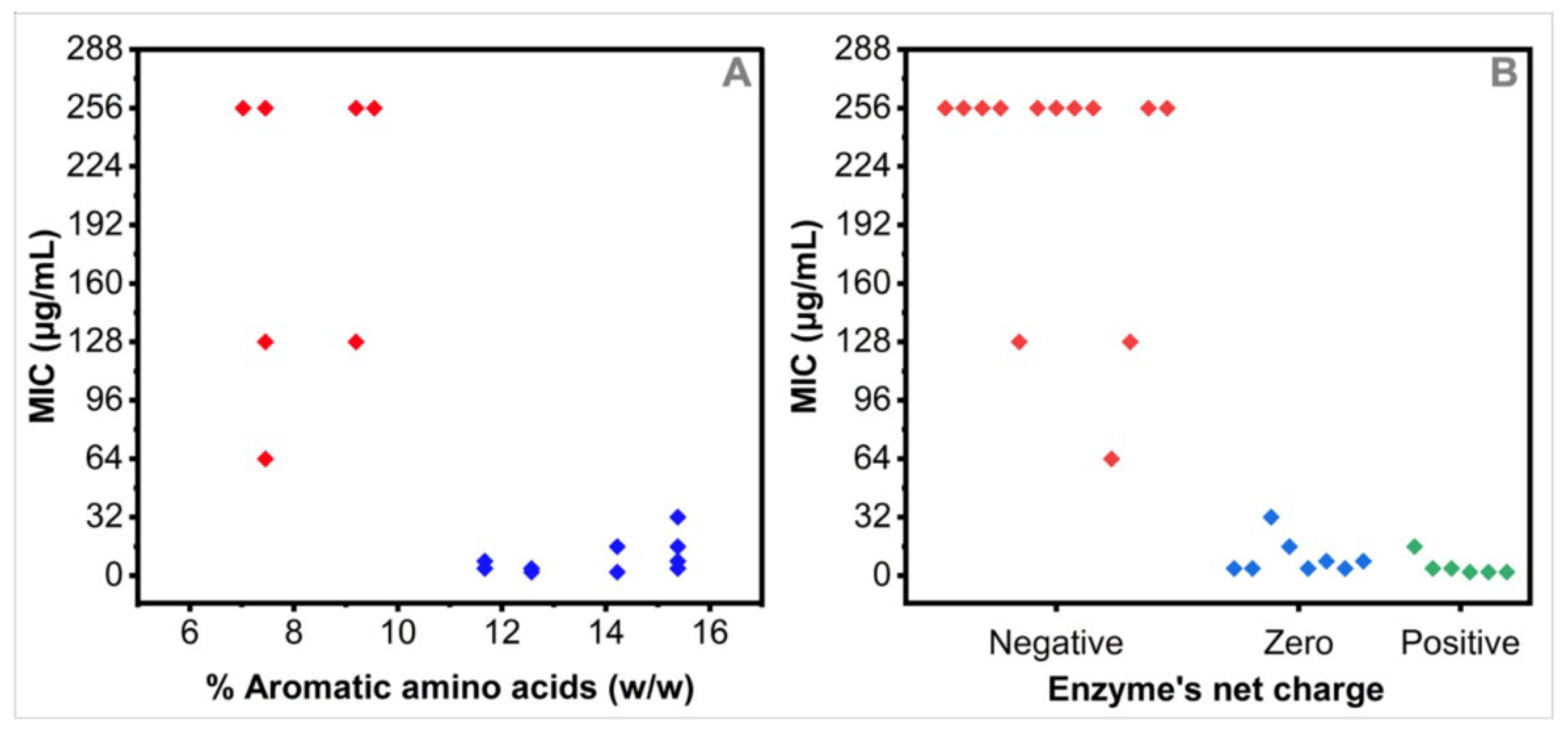
3.5. Relationships between the MIC Values and the Nature of Expressed Resistance Enzymes by the Tested MDR Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, P.S. Antibacterial discovery: 21st century challenges. Antibiotics 2020, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Aghapour, Z.; Gholizadeh, P.; Ganbarov, K.; Bialvaei, A.Z.; Mahmood, S.S.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Yousefi, B.; Kafil, H.S. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect. Drug Resist. 2019, 12, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Genilloud, O. Natural products discovery and potential for new antibiotics. Curr. Opin. Microbiol. 2019, 51, 81–87. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef]
- Ahmad, F.; Tabassum, N. Preliminary phytochemical, acute oral toxicity and antihepatotoxic study of roots of Paeonia officinalis Linn. Asian Pac. J. Trop. Biomed. 2013, 3, 64–68. [Google Scholar] [CrossRef]
- Dienaite, L.; Pukalskiene, M.; Pukalskas, A.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Isolation of strong antioxidants from Paeonia Officinalis roots and leaves and evaluation of their bioactivities. Antioxidants 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Bee, G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal 2020, 14, 95–107. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.-Y.; Gan, R.-Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Cao, Y.; Himmeldirk, K.B.; Qian, Y.; Ren, Y.; Malki, A.; Chen, X. Biological and biomedical functions of penta-O-galloyl-d-glucose and its derivatives. J. Nat. Med. 2014, 68, 465–472. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Kim, S.H.; Hagerman, A.E.; Lu, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef]
- Girard, M.; Thanner, S.; Pradervand, N.; Hu, D.; Ollagnier, C.; Bee, G. Hydrolysable chestnut tannins for reduction of postweaning diarrhea: Efficacy on an experimental ETEC F4 model. PLoS ONE 2018, 13, e0197878. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.; Zhang, G.; Luo, Y. Identification and structure–activity relationship of gallotannins separated from Galla chinensis. LWT Food Sci. Technol. 2009, 42, 1289–1295. [Google Scholar] [CrossRef]
- Masota, N.E.; Vogg, G.; Ohlsen, K.; Holzgrabe, U. Reproducibility challenges in the search for antibacterial compounds from nature. PLoS ONE 2021, 16, e0255437. [Google Scholar] [CrossRef]
- Dewanjee, S.; Gangopadhyay, M.; Bhattacharya, N.; Khanra, R.; Dua, T.K. Bioautography and its scope in the field of natural product chemistry. J. Pharm. Anal. 2015, 5, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Wanp, M.C.; Brown, D.M.; Fullas, F.; Owald, J.B.; Josephson, F.F.; Thornton, N.M.; Pezzut, J.M.; Beecher, C.W.W.; Farnsworth, N.R.; et al. Effect of tannins on screening of plant extracts for enzyme inhibitory activity and techniques for their removal. Phytomedicine 1996, 3, 281–285. [Google Scholar] [CrossRef]
- Nishizawa, M.; Yamagishi, T. Tannins and Related Compounds. Part 5.1 Isolation and characterization of polygalloylglucoses from chinese gallotannin. J. Chem. Soc. Perkin Trans. 1982, 2963–2968. [Google Scholar] [CrossRef]
- Nishizawa, M.; Yamagishi, T. Tannins and Related Compounds. Part 9.1 Isolation and characterization of polygalloylglucoses from Turkish galls (Quercus infectoria). J. Chem. Soc. Perkin Trans. 1983, 961–965. [Google Scholar] [CrossRef]
- Hwang, J.; Kong, T.; Baek, N.; Pyun, Y. Alpha-Glycosidase inhibitory activity of hexagalloylglucose from the galls of Quercus infectoria. Planta Med. 2000, 66, 273–274. [Google Scholar] [CrossRef]
- Taiwo, B.J.; Popoola, T.D.; van Heerden, F.R.; Fatokun, A.A. Pentagalloylglucose, isolated from the leaf extract of Anacardium occidentale L., could elicit rapid and selective cytotoxicity in cancer cells. BMC Complement. Med. Ther. 2020, 20, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-S.; Bae, Y.-S. A Gallotannin from Cercidiphyllum japonicum leaves. J. Korean Wood Sci. Techol. 2015, 43, 558–565. [Google Scholar] [CrossRef][Green Version]
- Nishizawa, M.; Yamagishi, T.; Nonaka, G.; Nishioka, I.; Nasagawa, T.; Oura, H. Tannins and related compounds. XII. Isolation and characterization of galloyglucoses from Paeoniae radix and their effect on urea-nitrogen concentration in rat serum. Chem. Pharm. Bull. 1983, 31, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Antimicrobial susceptibility testing—EUCAST disk diffusion method. Clin. Microbiol. Infect. 2022, 10, 1–22. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 9–15. [Google Scholar] [CrossRef]
- Saedtler, M.; Förtig, N.; Ohlsen, K.; Faber, F.; Masota, N.; Kowalick, K.; Holzgrabe, U.; Meinel, L. Antibacterial Anacardic Acid Derivatives. ACS Infect. Dis. 2020, 6, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Borisova, M.P.; Kataev, A.A.; Sivozhelezov, V.S. Action of tannin on cellular membranes: Novel insights from concerted studies on lipid bilayers and native cells. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1103–1111. [Google Scholar] [CrossRef]
- Gyemant, G.; Zajacz, A.; Becsi, B.; Ragunath, C.; Ramasubbu, N.; Erdodi, F.; Batta, G.; Kandra, L. Evidence for pentagalloyl glucose binding to human salivary alpha-amylase through aromatic amino acid residues. Biochim. Biophys. Acta 2009, 1794, 291–296. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, B.; Yao, K. Interactions of gallotannins with proteins, amino acids, phospholipids and sugars. Food Chem. 2006, 95, 250–254. [Google Scholar] [CrossRef]
- Chen, F.; Tang, Q.; Ma, H.; Bian, K.; Seeram, N.P.; Li, D. Hydrolyzable tannins are iron chelators that inhibit DNA repair enzyme ALKBH2. Chem. Res. Toxicol. 2019, 32, 1082–1086. [Google Scholar] [CrossRef]
- Deaville, E.R.; Green, R.J.; Mueller-Harvey, I.; Willoughby, I.; Frazier, R.A. Hydrolyzable tannin structures influence relative globular and random coil protein binding strengths. J. Agric. Food Chem. 2007, 44, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Oladoja, N.A.; Alliu, Y.B.; Ofomaja, A.E.; Unuabonah, I.E. Synchronous attenuation of metal ions and colour in aqua stream using tannin–alum synergy. Desalination 2011, 271, 34–40. [Google Scholar] [CrossRef]
- RCSB Protein Databank. Available online: https://www.rcsb.org/ (accessed on 7 March 2022).
- UniProt. Available online: https://www.uniprot.org/ (accessed on 7 March 2022).
- ExPASy Server. Available online: https://web.expasy.org/protparam/ (accessed on 10 March 2022).
- Lai, B.; Teixeira, G.; Moreira, I.; Correia, A.I.; Duarte, A.; Madureira, A.M. Evaluation of the antimicrobial activity in species of a Portuguese “Montado” ecosystem against multidrug resistant pathogens. J. Med. Plants Res. 2012, 6, 1846–1852. [Google Scholar]
- Serrano, J.; Puupponen-Pimia, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef]
- Elham, A.; Arken, M.; Kalimanjan, G.; Arkin, A.; Iminjan, M. A review of the phytochemical, pharmacological, pharmacokinetic, and toxicological evaluation of Quercus infectoria galls. J. Ethnopharmacol. 2021, 273, 113592. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Yamagishi, T.; Nonaka, G.; Nishioka, I.; Bando, H. Novel hydrolyzable tannins from Nuphar japonicum DC. Chem. Pharm. Bull. 1882, 30, 1094–1097. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A. Scope of hydrolysable tannins as possible antimicrobial agent. Phytother. Res. 2016, 30, 1035–1045. [Google Scholar] [CrossRef]
- Shimozu, Y.; Kuroda, T.; Tsuchiya, T.; Hatano, T. Structures and antibacterial properties of isorugosins H-J, oligomeric ellagitannins from Liquidambar formosana with characteristic bridging groups between sugar Moieties. J. Nat. Prod. 2017, 80, 2723–2733. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Merkel, R.; Walker, S.; Tomita, G.; Anderson, R.C. Comparative antimicrobial activity of tannin extracts from perennial plants on mastitis pathogens. Sci. Res. Essays 2008, 3, 66–73. [Google Scholar]
- Engels, C.; Knodler, M.; Zhao, Y.Y.; Carle, R.; Ganzle, M.G.; Schieber, A. Antimicrobial activity of gallotannins isolated from mango (Mangifera indica L.) kernels. J. Agric. Food Chem. 2009, 57, 7712–7718. [Google Scholar] [CrossRef]
- Dettweiler, M.; Marquez, L.; Lin, M.; Sweeney-Jones, A.M.; Chhetri, B.K.; Zurawski, D.V.; Kubanek, J.; Quave, C.L. Pentagalloyl glucose from Schinus terebinthifolia inhibits growth of carbapenem-resistant Acinetobacter baumannii. Sci. Rep. 2020, 10, 15340. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, S.Y.; Ye, Y.B.; Zhao, W.H.; Sun, X.G.; Wang, Z.Q.; Li, R.; Sun, Y.H.; Tian, W.X.; Zhang, Y.X. The antibacterial efficacy of an aceraceous plant [Shantung maple (Acer truncatum Bunge)] may be related to inhibition of bacterial beta-oxoacyl-acyl carrier protein reductase (FabG). Biotechnol. Appl. Biochem. 2008, 51, 73–78. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Sohn, M.-J.; Lee, J.; Kim, W.-G. Isolation and identification of pentagalloylglucose with broad-spectrum antibacterial activity from Rhus trichocarpa Miquel. Food Chem. 2010, 123, 501–506. [Google Scholar] [CrossRef]
- Sylla, T.; Pouysegu, L.; Da Costa, G.; Deffieux, D.; Monti, J.P.; Quideau, S. Gallotannins and Tannic Acid: First Chemical Syntheses and In Vitro Inhibitory Activity on Alzheimer’s Amyloid beta-Peptide Aggregation. Angew. Chem. 2015, 54, 8217–8221. [Google Scholar] [CrossRef]
- Choi, J.; Yang, D.; Moon, M.Y.; Han, G.Y.; Chang, M.S.; Cha, J. The Protective Effect of Hamamelis virginiana Stem and Leaf Extract on Fine Dust-Induced Damage on Human Keratinocytes. Cosmetics 2021, 8, 119. [Google Scholar] [CrossRef]
- Fujimaki, T.; Sato, C.; Yamamoto, R.; Watanabe, S.; Fujita, H.; Kikuno, H.; Sue, M.; Matsushima, Y. Isolation of phenolic acids and tannin acids from Mangifera indica L. kernels as inhibitors of lipid accumulation in 3T3-L1 cells. Biosci. Biotechnol. Biochem. 2022, 86, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Islambekov, S.Y.; Mavyanov, S.M.; Kamaev, F.G.; Ismailov, A.I. Phenolic compounds of sumac. Chem. Nat. Compd. 1994, 30, 37–39. [Google Scholar] [CrossRef]
- Elisha, I.L.; Jambalang, A.R.; Botha, F.S.; Buys, E.M.; McGaw, L.J.; Eloff, J.N. Potency and selectivity indices of acetone leaf extracts of nine selected South African trees against six opportunistic Enterobacteriaceae isolates from commercial chicken eggs. BMC Complement. Altern. Med. 2017, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; He, Q.; Ying Jia, D.; Shi, B. The potential of wattle tannin extracts for fine use. Nat. Prod. Res. 2006, 20, 271–278. [Google Scholar] [CrossRef]
- Szabo, K.; Hamori, C.; Gyemant, G. Gallotannins are non-specific inhibitors of alpha-amylase: Aggregates are the active species taking part in inhibition. Chem. Biol. Drug Des. 2021, 97, 349–357. [Google Scholar] [CrossRef] [PubMed]



| Group | Pos. | 1,2,3,4,6-Penta-O-Galloyl-β-d-Glucose (NMA2) [23] | 3-O-Digalloyl-1,2,4,6-Tetra-O-Galloyl-β-d-Glucose (NMB4) [22] | ||||
|---|---|---|---|---|---|---|---|
| δC | δH, Multip., (J in Hz) | HMBC | δC | δH, Multip., (J in Hz) | HMBC | ||
| Glucose | 1 | 93.27 [93.8] | 6.26, d (8.2) [6.26] | 2, 3, 7a, 8a | 93.11 [93.77] | 6.21–6.25, m [6.14] | 3, 7a |
| 2 | 71.70 [70.8] | 5.62, dd (9.8, 8.3) [5.61] | 1, 3, 7b, 8b | 71.46 [72.14] | 5.57–5.62 a, m [5.81] | 1, 3, 4, 7b | |
| 3 | 73.26 [74.1] | 6.00, t (9.6) [5.65] | 1, 2, 4, 5, 7c, 8c | 73.44 [74.06] | 5.94–6.02, m [5.53] | 2, 4, 7c | |
| 4 | 69.34 [68.4] | 5.66, t (9.6) [5.90] | 3, 5, 6, 7d, 8d | 69.13 [69.75] | 5.62–5.68 a, m [5.48] | 3, 6, 7d | |
| 5 | 73.76 [72.2] | 4.55, oddd [4.42] | 1, 3, 4, 6 | 73.44 [74.35] | 4.52–4.4 a, m [4.30] | 1, 3, 4, 6 | |
| 6 | 62.94 [62.2] | 4.29, dd (12.3, 4.6) 4.58, odd [4.39] | 4, 5, 7e, 8e | 62.80 [63.08] | 4.25 a, m 4.54–4.56 a, m [4.28; 4.42] | 4, 5, 7e | |
| Galloyl A | 7a | 165.40 [166.2] | 165.20 [166.88] | ||||
| 8a | 119.09 [119.7] | 118.74 [119.68] | |||||
| 9a | 110.14 [110.3] | 7.06, s [7.06] | 7a, 8a, 10a, 11a | 110.05 [110.43] | 7.01–7.02 a, m [6.94] | 7a, 8a, 10a, 11a | |
| 10a | 146.14 [146.2] | 145.85 [146.44] | |||||
| 11a | 140.18 [140.0] | 140.11 [140.72] | |||||
| Galloyl B | 7b | 166.32 [166.9] | 166.21 [167.89] | ||||
| 8b | 119.70 [120.2] | 119.23 [120.31] | |||||
| 9b | 109.98 [110.3] | 6.99, s [6.94] | 7b, 8b, 10b, 11b | 109.88 [110.37] | 6.92–6.96, m [6.80] | 7b, 8b, 10b, 11b | |
| 10b | 146.00 [146.4] | 145.69 [146.32] | |||||
| 11b | 139.72 [140.1] | 139.65 [140.25] | |||||
| Galloyl C | 7c | 166.48 [167.9] | 165.77 [166.98] | ||||
| 8c | 119.80 [121.1] | 118.95-119.08 b [120.16] | |||||
| 9c | 109.96 [110.7] | 6.96, s [7.14] | 7c, 8c, 10c, 11c | 114.45 b; 117.21 b | 7.15, d (3.87) b; 7.27, d (2.03) b | 7c, 8c, 10c, 11c | |
| 109.35 c [110.00] | 6.99–7.00 ac, m [6.88] | ||||||
| 10c | 145.90 [146.5] | 145.69–145.83 b [146.42] | |||||
| 11c | 139.55 [140.8] | 139.39–139.49 b [139.96] | |||||
| Galloyl D | 7d | 166.14 [167] | 166.01 [167.25] | ||||
| 8d | 119.65 [120.2] | 119.23 [120.19] | |||||
| 9d | 110.06 [110.4] | 7.03, s [7.01] | 7d, 8d, 10d, 11d | 109.35 [110.00] | 6.99–7.00 a, m [6.85] | 7d, 8d, 10d, 11d | |
| 10d | 146.02 [146.4] | 140.11 [140.31] | |||||
| 11d | 139.75 [140.3] | 145.86 [146.50] | |||||
| Galloyl E | 7e | 166.82 [167.0] | 166.87 [169.00] | ||||
| 8e | 120.72 [120.2] | 120.37 [121.39] | |||||
| 9e | 109.91 [110.4] | 7.12, s [6.98] | 7e, 8e, 10e, 11e | 109.80 [110.30] | 7.07, d (6.21) [6.95] | 7e, 8e, 10e, 11e | |
| 10e | 145.98 [146.4] | 145.69 [146.23] | |||||
| 11e | 139.23 [140.3] | 139.14 [139.70] | |||||
| Galloyl C’ | 7c’ | 164.58–165.20 [166.18] | |||||
| 8c’ | 119.69–119.76 [121.00] | ||||||
| 9c’ | 110.28–110.33 [110.58] | 7.14, d (12.18) [7.01] | 7c’, 8c’, 10c’, 11c’ | ||||
| 10c’ | 145.69–145.83 [146.38] | ||||||
| 11c’ | 139.49–139.65 [140.07] | ||||||
| Group | Pos. | 6-O-Digalloyl-1,2,3,4-Tetra-O-Galloyl-β-d-Glucose (NMB6) [24] | 3,6-Bis-O-Digalloyl-1,2,4-Tri-O-Galloyl-β-d-Glucose (NMC3) [25] | ||||
|---|---|---|---|---|---|---|---|
| δC | δH, Multip.,(J in Hz) | HMBC | δC | δH, Multip. | HMBC | ||
| Glucose | 1 | 93.40 [93.87] | 6.31–6.34, m [6.23] | 3, 5, 7a, 8a | 93.25–93.33 [93.3] | 6.26–6.32, m | 2, 3, 5, 7a |
| 2 | 71.77–71.80 [72.25] | 5.59–5.62, om [5.58] | 1, 3, 4, 7b, 8b | 71.67 [71.7] | 5.63–5.66 a, m | 1, 3, 4, 7b | |
| 3 | 73.30–73.32 [74.09] | 5.98–6.03, m [5.91] | 1, 2, 4, 7c, 8c | 73.65–73.86 [73.9] | 6.01–6.07, m | 1, 2, 4, 5, 7c | |
| 4 | 69.22–69.43 [70.02] | 5.63–5.68, om [5.58] | 2, 3, 5, 6, 7d, 8d | 69.25–69.47 [69.3] | 5.66–5.72 a, m | 3, 5, 6, 7d | |
| 5 | 73.91–73.95 [74.38] | 4.55–4.60, om [4.52] | 1, 3, 4, 6 | 73.65–73.86 [73.9] | 4.56–4.64 a, m | 1, 3, 4, 6 | |
| 6 | 63.12–63.24 [63.60] | 4.45–4.54, om [4.43] | 4, 5, 7e | 63.25–63.43 [63.1] | 4.35–4.43, m 4.52–4.56 a, m | 4, 5, 7e | |
| Galloyl A | 7a | 164.97–165.05 [166.22] | 165.26–165.44 [164.9] | ||||
| 8a | 119.93–119.95 [119.78] | 119.20–119.84 | |||||
| 9a | 110.39 [110.66] | 7.10, d (8.42) [7.04] | 7a, 8a, 10a, 11a | 110.12–110.18 | 7.05–7.07 a, m | 7a, 8a, 10a, 11a | |
| 10a | 146.12 [146.59] | 146.05–146.13 | |||||
| 11a | 139.84 [140.80] | 139.46–140.08 | |||||
| Galloyl B | 7b | 165.74–165.75 [167.01] | 166.25–166.31 [165.0] | ||||
| 8b | 120.53 [120.50] | 119.72–119.84 | |||||
| 9b | 110.17 [110.50] | 7.00, d (6.23) [6.97] | 7b, 8b, 10b, 11b | 110.03 | 6.99–7.00, m | 7b, 8b, 10b, 11b | |
| 10b | 145.90 [146.47] | 145.98–146.00 | |||||
| 11b | 139.33 [140.39] | 139.61–139.63 | |||||
| Galloyl C | 7c | 165.93–165.98 [167.34] | 165.80 b [165.9] | ||||
| 8c | 120.64–120.67 [120.40] | 119.72 b; 120.66 b | |||||
| 9c | 110.05–110.11 [110.41] | 6.96, d (3.63) [6.89] | 7c, 8c, 10c, 11c | 114.68 b; 117.43 b | 7.31–7.34 b, m 7.19–7.21 b, m | 7c, 8c, 10c, 11c | |
| 109.55 c | 7.03, d (7.11)c | ||||||
| 10c | 145.89 [146.32] | 144.00 b; 144.35 b | |||||
| 11c | 139.18–139.20 [140.28] | 139.63 b; 139.73 b | |||||
| Galloyl D | 7d | 165.74–165.75 [167.07] | 166.16–166.21 [165.6] | ||||
| 8d | 120.55 [120.28] | 119.21–119.79 | |||||
| 9d | 110.29 [110.44] | 7.05, d (6.43) [6.94] | 7d, 8d, 10d, 11d | 110.12–110.18 | 7.04–7.05 c, m | 7d, 8d, 10d, 11d | |
| 10d | 145.97 [146.42] | 145.98–146.07 | |||||
| 11d | 139.35 [140.34] | 139.48–139.61 | |||||
| Galloyl E | 7e | 165.93–165.98 [167.23] | 166.16 b [165.8] | ||||
| 8e | 121.30 [121.13] | 119.72 b; 120.66 | |||||
| 9e | 114.77 b; 117.71 b [115.09; 117.60] | 7.40, d (1.99) b; 7.51, d (1.99) b | 7e, 8e, 10e, 11e | 114.68 b;117.34 b | 7.47–7.48 b, m 7.31–7.34 b, m | 7e, 8e, 10e, 11e | |
| 110.05 c [-] | 7.25, s c [7.29] | 109.55 c | 7.17–7.21 c, m | ||||
| 10e | 143.86–146.96 [144.62; 147.55] | 144.00 b; 144.35 b | |||||
| 11e | 139.86–139.95 [140.39] | 139.73–139.79 b | |||||
| Galloyl E’ | 7e’ | 164.97–165.98 [166.70] | 164.54–165.26 | ||||
| 8e’ | 120.88–128.58 [120.54] | 120.07–120.33 | |||||
| 9e’ | 110.71–110.78 [110.91] | 7.28, d (7.41) [7.23] | 7e’, 8e’, 10e’, 11e’ | 110.42–110.46 | 7.23–7.24, m | 7e’, 8e’, 10e’, 11e’ | |
| 10e’ | 146.16–151.44 [146.64] | 146.03–146.11 | |||||
| 11e’ | 132.67–139.43 [140.54] | 139.48–139.67 | |||||
| Galloyl C’ | 7c’ | 164.55–165.14 | |||||
| 8c’ | 120.07–120.18 | ||||||
| 9c’ | 110.38–110.42 | 7.17–7.19, m | 7c’, 8c’, 10c’, 11c’ | ||||
| 10c’ | 145.99–146.05 | ||||||
| 11c’ | 139.38–139.67 | ||||||
| Bacteria and Resistance Phenotypes and MICs (μg/mL) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | K. pneumoniae | ||||||||||||||
| Antibiotic/ Compound | ATCC 25922 | OXA-48 | VIM 1 | ESBL | KPC-3 | Vim1/ 4TEM-1 | KPC-2 MCR-1 | ATCC 10031 | OXA-48 | TEM-/ SHV-/ CTX-M-pos | NDM-1 | DHA-1 (AmpC) | KPC-2 | OXA-48 | SHV-4 |
| Cefotaxime | S | R | R | R | R | R | R | R | R | R | R | R | R * | ||
| Carbamazepine | S | R | R | R | R | R | R | R | R | R | R | R | R | ||
| Ertapenem | R | S | S | R | R | R | R | S | R | R | R | R | S | ||
| Imipenem | S | S | S | R | R | R | R | S | R | R * | R | R * | S | ||
| Meropenem | S | S | S | R * | R | R | R | S | R | R * | R | R * | S | ||
| Ciprofloxacin | R | S | R | S | R | R | R | R | R | R | R | R | S | ||
| Piperacillin-Taz | R | R | R | R | R | R | R | I | R | R | R | R | R | ||
| NMA2 | 32 | >256 | >256 | >256 | 4 | 256 | >256 | 32 | 32 | 128 | 256 | >256 | 2 | 8 | 256 |
| NMB4 | 32 | 256 | >256 | >256 | 4 | >256 | >256 | 08 | 16 | 64 | 256 | >256 | 4 | 4 | 256 |
| NMB6 | 64 | >256 | 256 | 32 | 4 | 128 | >256 | 16 | 32 | 64 | 256 | 2 | 4 | 8 | 256 |
| NMC3 | 64 | >256 | >256 | 16 | 8 | >256 | >256 | 128 | 16 | >256 | 256 | 16 | 2 | 8 | 256 |
| Gentamicin | <0.25 | 1 | 4 | 1 | 1 | 2 | 128 | <0.25 | 128 | 64 | >128 | >128 | 2 | <0.5 | 8 |
| Protein Code | Enzyme Class | PDB Code | % Aromatic AA (w/w) | Net Charge |
|---|---|---|---|---|
| OXA-48 | Beta-lactamase | 7KHQ | 15.90 | 0 |
| VIM-1 | Metallo-beta-lactamase | 5N5I | 9.20 | −15 |
| KPC-3 | Beta-lactamase | 6QWD | 11.67 | 0 |
| TEM-1 | Beta-lactamase | 1M40 | 7.45 | −7 |
| KPC-2 | Beta-lactamase | 3DW0 | 12.57 | 1 |
| MCR-1 | Phosphoethanolamine transferase | 5GRR | 14.43 | −12 |
| CTX-M 9 | Beta-lactamase | 1YLJ | 7.09 | 1 |
| SHV-1 | Beta-lactamase | 2ZD8 | 7.02 | −1 |
| NDM-1 | Metallo-beta-lactamase | 4EXY | 9.54 | −6 |
| DHA-1 or AmpC | Beta-lactamase | Q84AE1 (uniprot) | 14.22 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masota, N.E.; Ohlsen, K.; Schollmayer, C.; Meinel, L.; Holzgrabe, U. Isolation and Characterization of Galloylglucoses Effective against Multidrug-Resistant Strains of Escherichia coli and Klebsiella pneumoniae. Molecules 2022, 27, 5045. https://doi.org/10.3390/molecules27155045
Masota NE, Ohlsen K, Schollmayer C, Meinel L, Holzgrabe U. Isolation and Characterization of Galloylglucoses Effective against Multidrug-Resistant Strains of Escherichia coli and Klebsiella pneumoniae. Molecules. 2022; 27(15):5045. https://doi.org/10.3390/molecules27155045
Chicago/Turabian StyleMasota, Nelson E., Knut Ohlsen, Curd Schollmayer, Lorenz Meinel, and Ulrike Holzgrabe. 2022. "Isolation and Characterization of Galloylglucoses Effective against Multidrug-Resistant Strains of Escherichia coli and Klebsiella pneumoniae" Molecules 27, no. 15: 5045. https://doi.org/10.3390/molecules27155045
APA StyleMasota, N. E., Ohlsen, K., Schollmayer, C., Meinel, L., & Holzgrabe, U. (2022). Isolation and Characterization of Galloylglucoses Effective against Multidrug-Resistant Strains of Escherichia coli and Klebsiella pneumoniae. Molecules, 27(15), 5045. https://doi.org/10.3390/molecules27155045







