Antibacterial Activity of Aureonuclemycin Produced by Streptomyces aureus Strain SPRI-371
Abstract
:1. Introduction
2. Results
2.1. Identification of the Strain SPRI-371
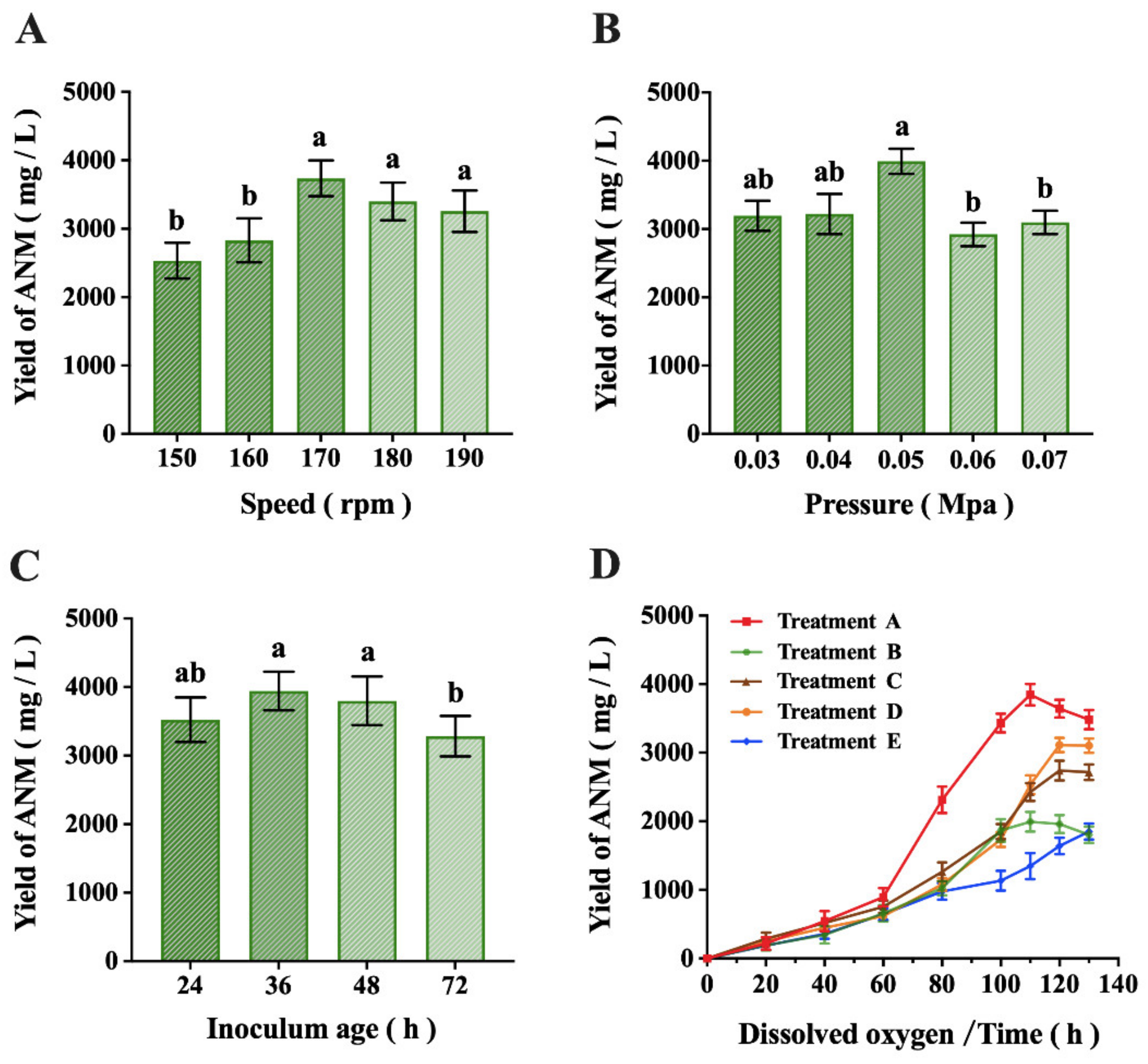
2.2. Results of Fermentation Process Optimization
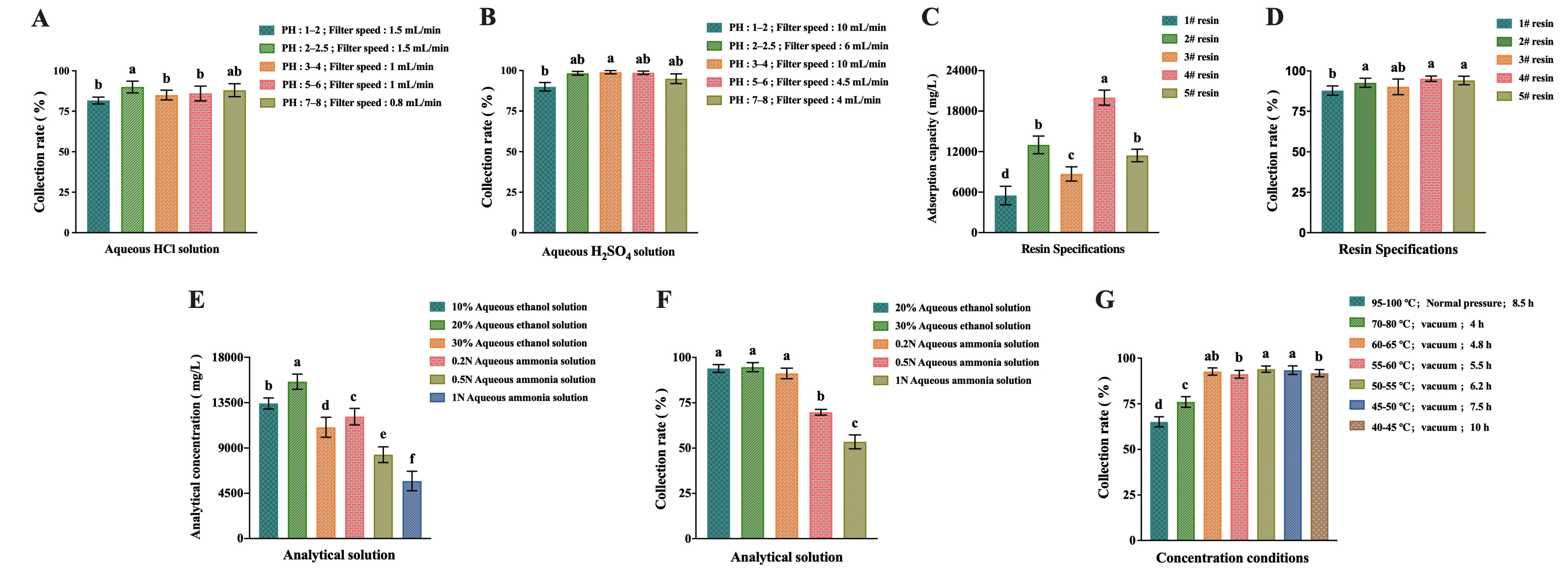
2.3. Results of Separation Process Optimization
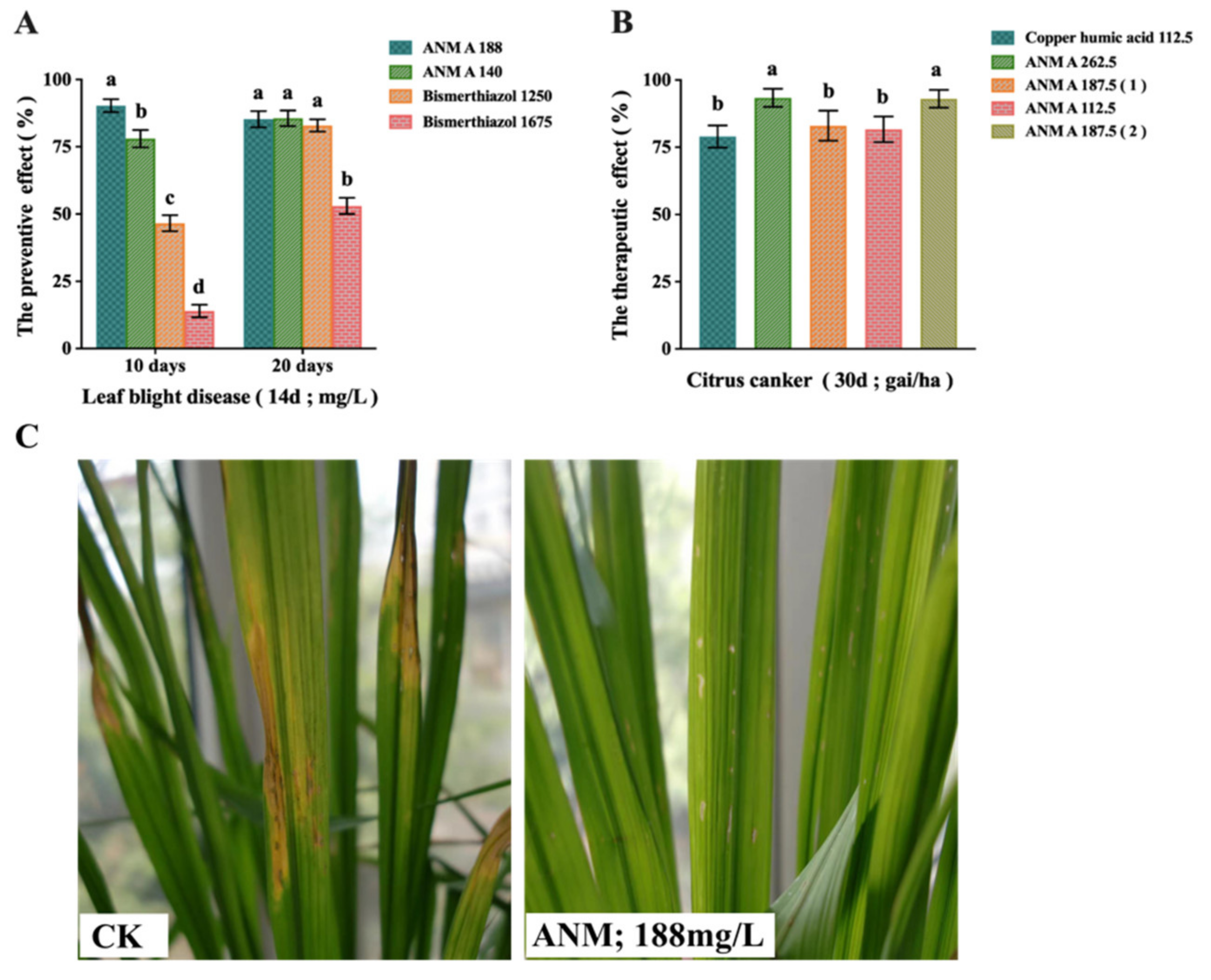
2.4. Evaluation of In Vivo Antibacterial Activity of ANM
2.4.1. Results of ANM A on the Preventive Effect of Rice Bacterial Leaf Blight Caused by X. oryzae pv. oryzae
2.4.2. Results of ANM A on the Therapeutic Effect of Citrus Canker Caused by X. citri
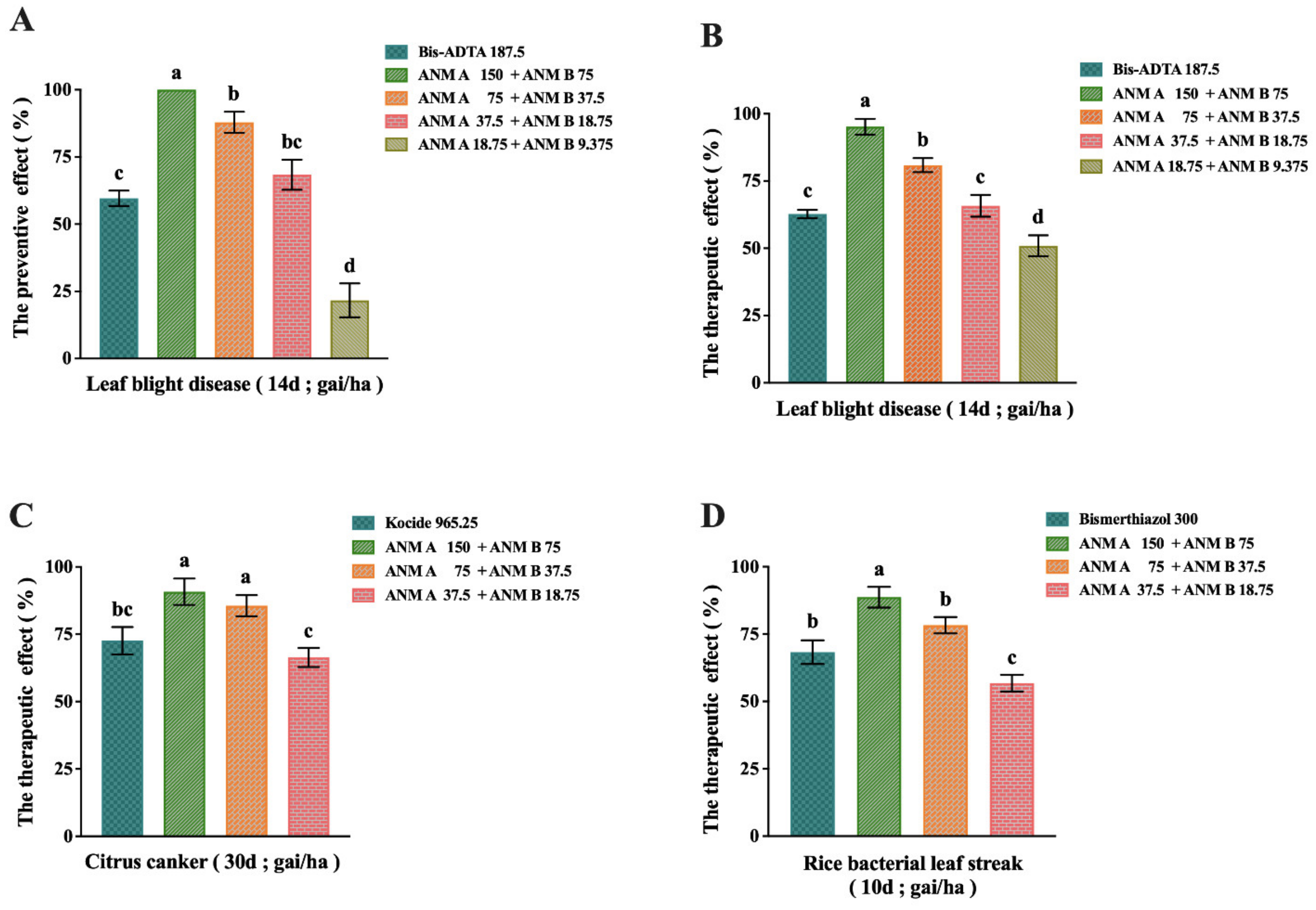
2.5. Evaluation of In Vivo Antibacterial Activity of ANM (A + B)
2.5.1. Results of ANM (A + B) on the Preventive and Therapeutic Effect of Rice Bacterial Leaf Blight Caused by X. oryzae pv. oryzae
2.5.2. Results of ANM (A + B) on the Preventive and Therapeutic Effect of Citrus Canker Caused by X. citri
2.5.3. Results of ANM (A + B) on the Preventive and Therapeutic Effect of Rice Bacterial Leaf Streak Caused by X. oryzae
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Antibiotic-Producing Organism the Strain SPRI-371
4.3. Taxonomic Studies
4.4. Culture Conditions for Antibiotic-Producing
4.5. Isolation and Purification of Antibiotic Substances in Laboratory
4.6. ANM A and ANM B Produced by Industrial Methods
4.7. Optimization of the Fermentation Process
4.8. Separation Process Optimization
4.9. ANM A Biological Activities Test
4.9.1. The Preventive Effect of ANM A on Rice Bacterial Leaf Blight Caused by X. oryzae pv. oryzae (Greenhouse)
4.9.2. The Therapeutic Effect of ANM A on Citrus Canker Caused by X. citri (Field)
4.10. ANM (A + B) Biological Activities Test
4.10.1. The Preventive and Therapeutic Effect of ANM (A + B) on Rice Bacterial Leaf Blight Caused by X. oryzae pv. oryzae (Field)
4.10.2. The Therapeutic Effect of ANM (A + B) on Citrus Canker Caused by X. citri (Field)
4.10.3. The Therapeutic Effect of ANM (A + B) on Rice Bacterial Leaf Streak Caused by X. oryzae pv. oryzicola (Field)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Silva, V.; Hans, G.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.C.; Delecluse, A.; Walton, W.E. Cyt1Ab1 and Cyt2Ba1 from Bacillus thuringiensis subsp medellin and B-thuringiensis subsp israelensis Synergize Bacillus sphaericus against Aedes aegypti and resistant Culex quinquefasciatus (Diptera: Culicidae). Appl. Environ. Microbiol. 2001, 67, 3280–3284. [Google Scholar] [CrossRef] [Green Version]
- Maurya, P.; Mohan, L.; Sharma, P.; Srivastava, C.N. Evaluation of larvicidal potential of certain insect pathogenic fungi extracts against Anopheles stephensi and Culex quinquefasciatus. Entomol. Res. 2011, 41, 211–215. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, N.S.; Heinrich, M. Plant made pharmaceuticals (PMPs)—Development of natural health products from bio-diversity. Indian J. Pharm. Educ. Res. 2008, 42, 113–121. [Google Scholar]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, H.Y.; Lee, S.; Kim, J.G.; Suh, J.W.; Lee, C.H. Metabolomics-Based Chemotaxonomic Classification of Streptomyces spp. and Its Correlation with Antibacterial Activity. J. Microbiol. Biotechnol. 2015, 25, 1265–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solecka, J.; Zajko, J.; Postek, M.; Rajnisz, A. Biologically active secondary metabolites from Actinomycetes. Cent. Eur. J. Biol. 2012, 7, 373–390. [Google Scholar] [CrossRef]
- Liu, E.S.; Wilkins, M.R. Process optimization and scale-up production of fungal aryl alcohol oxidase from genetically modified Aspergillus nidulans in stirred-tank bioreactor. Bioresour. Technol. 2020, 315, 123972. [Google Scholar] [CrossRef] [PubMed]
- Lajis, A.F.B. Biomanufacturing process for the production of bacteriocins from Bacillaceae family. Bioresour. Bioprocess. 2020, 7, 759536. [Google Scholar] [CrossRef]
- Burhan, M.; Sahi, S.T.; Ahmad, S. Screening of citrus cultivars for source of resistance against citrus canker under field conditions. Pak. J. Bot. 2007, 39, 1867–1871. [Google Scholar]
- Ference, C.M.; Gochez, A.M.; Behlau, F.; Wang, N.; Graham, J.H.; Jones, J.B. Recent advances in the understanding of Xanthomonas citri ssp. citri pathogenesis and citrus canker disease management. Mol. Plant Pathol. 2018, 19, 1302–1318. [Google Scholar] [CrossRef] [Green Version]
- De León, L.; Siverio, F.; López, M.M.; Rodríguez, A. Comparative efficiency of chemical compounds for in vitro and in vivo activity against Clavibacter michiganensis subsp. michiganensis, the causal agent of tomato bacterial canker. Crop Prot. 2008, 27, 1277–1283. [Google Scholar] [CrossRef]
- Fayette, J.; Roberts, P.D.; Pernezny, K.L. The role of cymoxanil and famoxadone in the management of bacterial spot on tomato and pepper and bacterial leaf spot on lettuce. Crop Prot. 2012, 31, 107–112. [Google Scholar] [CrossRef]
- Cazorla, F.M.; Arrebola, E.; Sesma, A.; Pérez-García, A.; Codina, J.C.; Murillo, J.; de Vicente, A. Copper resistance in Pseudomonas syringae strains isolated from mango is encoded mainly by plasmids. Phytopathology 2002, 92, 909–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Omar, A.A.; Orbovic, V. Biallelic Editing of the LOB1 YPromoter via CRISPR/Cas9 Creates Canker-Resistant ‘Duncan’ Grapefruit. Phytopathology 2022, 112, 308–314. [Google Scholar] [CrossRef]
- Nugroho, C.; Cumagun, C.J.R.; Oliva, R. Diversity of Xanthomonas oryzae pv. Oryzae on susceptible and resistant rice lines in bacterial blight hot spot areas of the Philippines. Eur. J. Plant Pathol. 2022, 163, 951–960. [Google Scholar] [CrossRef]
- Sosnowski, M.R.; Fletcher, J.D.; Daly, A.M.; Rodoni, B.C.; Viljanen, S. Techniques for the treatment, removal and disposal of host material during programmes for plant pathogen eradication. Plant Pathol. 2009, 58, 621–635. [Google Scholar] [CrossRef]
- Yang, T.-H.; Yuan, T.-H.; Hwang, Y.-H.; Lian, I.-B.; Meng, M.; Su, C.-C. Increased inflammation in rheumatoid arthritis patients living where farm soils contain high levels of copper. J. Formos. Med. Assoc. 2016, 115, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.X.; Chen, Z.; Zeng, T.; Jin, W.B.; Geng, Y.; Lin, G.M.; Zhao, J.; Li, W.-T.; Xiong, Z.; Huang, S.-X.; et al. Elucidation of the Herbicidin Tailoring Pathway Offers Insights into Its Structural Diversity. Org. Lett. 2019, 21, 1374–1378. [Google Scholar] [CrossRef]
- Cao, T.; Shen, Y.; Zhao, J.W.; Liu, C.; Zhao, X.; Jin, L.; Li, Y.; Wang, X.; Xiang, W. Streptomyces flavalbus sp. nov., an actinobacterium isolated from rhizosphere of maize (Zea mays L.). Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2018, 111, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, B.; Li, X.; Gan, L.; Long, X.; Zhang, Y.; Tian, Y. Streptomyces populi sp. nov., a novel endophytic actinobacterium isolated from stem of Populus adenopoda Maxim. Int. J. Syst. Evol. Microbiol. 2018, 68, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Henriques, T.M.; Pereira, S.R.; Serafim, L.S.; Xavier, A.M. Two-Stage Aeration Fermentation Strategy to Improve Bioethanol Production by Scheffersomyces stipitis. Fermentation 2018, 4, 97. [Google Scholar] [CrossRef] [Green Version]
- Ke, C.; Ren, Y.; Gao, P.; Han, J.; Tao, Y.; Huang, J.; Yang, X. Separation and purification of pyrroloquinoline quinone from fermentation broth by pretreatment coupled with macroporous resin adsorption. Sep. Purif. Technol. 2021, 257, 117962. [Google Scholar] [CrossRef]
- Ryu, H.W.; Kim, Y.M.; Wee, Y.J. Influence of Operating Parameters on Concentration and Purification of L-lactic Acid Using Electrodialysis. Biotechnol. Bioprocess Eng. 2012, 17, 1261–1269. [Google Scholar] [CrossRef]
- Goeller, F.; Heinemann, C.; Demuth, M. Investigations of cascade cyclizations of terpenoid polyalkenes via radical cations. A biomimetic-type synthesis of (+/−)-3-hydroxy-spongian-16-one. Synthesis 2001, 112, 1114–1116. [Google Scholar] [CrossRef]
- Okami, Y.; Okuda, T.; Takeuchi, T.; Nitta, K.; Umezawa, H. Studies on antitumor substances produced by microorganisms. IV. Sarkomycin-producing Streptomyces and other two Streptomyces producing the anti-tumor substance No. 289 and caryomycin. J. Antibiot. 1953, 6, 153–157. [Google Scholar]
- Uri, J. Menschen-pathogene Pilze in der Antibioticum-Forschung. II. Gegen menschen-pathogene Pilze wirksame, von Actinomyceten stammende Antibiotica. Arzneim. Forsch. 1958, 8, 687–692. [Google Scholar]
- Chen, J.J.; Rateb, M.E.; Love, M.S.; Xu, Z.; Yang, D.; Zhu, X.; Huang, Y.; Zhao, L.-X.; Jiang, Y.; Duan, Y.; et al. Herbicidins from Streptomyces sp. CB01388 Showing Anti-Cryptosporidium Activity. J. Nat. Prod. 2018, 81, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Osada, H. Discovery and applications of nucleoside antibiotics beyond polyoxin. J. Antibiot. 2019, 72, 855–864. [Google Scholar] [CrossRef]
- Zhang, J.; He, F.; Chen, J.; Wang, Y.; Yang, Y.; Hu, D.; Song, B. Purine Nucleoside Derivatives Containing a Sulfa Ethylamine Moiety: Design, Synthesis, Antiviral Activity, and Mechanism. J. Agric. Food Chem. 2021, 69, 5575–5582. [Google Scholar] [CrossRef]
- Gelin, M.; Poncet-Montange, G.; Assairi, L.; Morellato, L.; Huteau, V.; Dugué, L.; Dussurget, O.; Pochet, S.; Labesse, G. Screening and in Situ Synthesis Using Crystals of a NAD Kinase Lead to a Potent Antistaphylococcal Compound. Structure 2012, 20, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Roux, D.; Burger, P.B.; Niemand, J.; Grobler, A.; Urbán, P.; Fernàndez-Busquets, X.; Barker, R.H.; Serrano, A.E.; Louw, A.I.; Birkholtz, L.-M. Novel S-adenosyl-L-methionine decarboxylase inhibitors as potent antiproliferative agents against intraerythrocytic Plasmodium falciparum parasites. Int. J. Parasitol.-Drugs Drug Resist. 2014, 4, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slabaugh, M.B.; Howell, M.L.; Wang, Y.; Mathews, C.K. Deoxyadenosine reverses hydroxyurea inhibition of vaccinia virus growth. J. Virol. 1991, 65, 2290–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flexner, C.; Owen, A.; Siccardi, M.; Swindells, S. Long-acting drugs and formulations for the treatment and prevention of HIV infection. Int. J. Antimicrob. Agents 2021, 57, 106220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, D.; Jiang, H.; Han, L.; Jiang, S.; Guo, X.; Wang, X.; Xiang, W. Streptomyces xiangluensis sp. nov., a novel actinomycete isolated from soil. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2018, 111, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Dehnad, A.; Hanifian, S.; Khani, S. Isolation and Molecular Identification of Streptomyces spp. with Antibacterial Activity from Northwest of Iran. Bioimpacts BI 2013, 3, 129–134. [Google Scholar] [CrossRef]
- Ke, Y.; Hui, S.; Yuan, M. Xanthomonas oryzae pv. oryzae Inoculation and Growth Rate on Rice by Leaf Clipping Method. Bio-Protocol 2017, 7, e2568. [Google Scholar] [CrossRef]
- Promnuan, Y.; Promsai, S.; Meelai, S. Antimicrobial activity of Streptomyces spp. isolated from Apis dorsata combs against some phytopathogenic bacteria. PEERJ 2020, 8, 10512. [Google Scholar] [CrossRef]
- Yang, R.; Li, S.; Li, Y.; Yan, Y.; Fang, Y.; Zou, L.; Chen, G. Bactericidal Effect of Pseudomonas oryziphila sp. nov., a Novel Pseudomonas Species Against Xanthomonas oryzae Reduces Disease Severity of Bacterial Leaf Streak of Rice. Front. Microbiol. 2021, 12, 759536. [Google Scholar] [CrossRef]
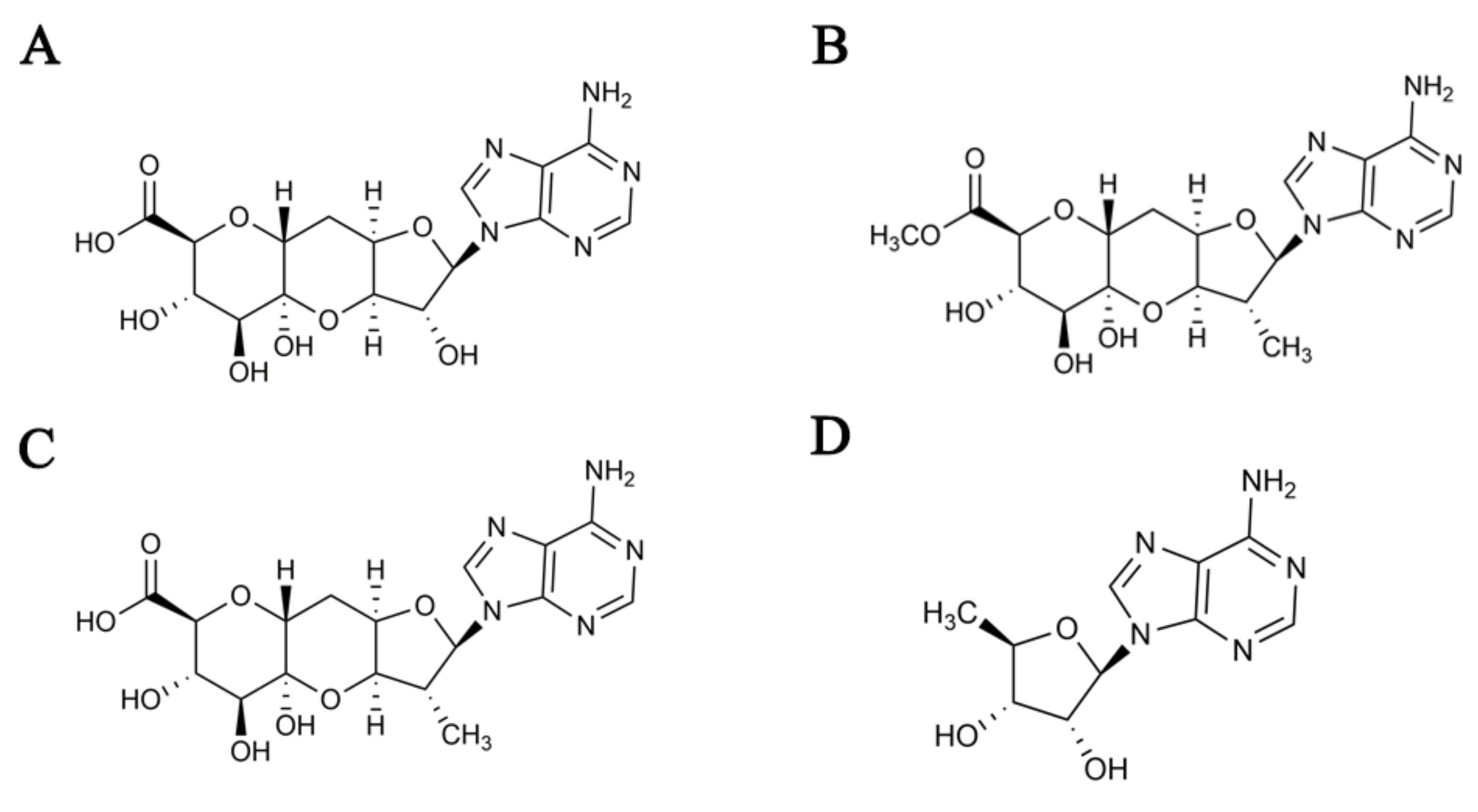
| Medium | Growth | Aerial Mycelium | Reverse | Soluble Pigment |
|---|---|---|---|---|
| Sucrose-nitrate agar | Poor | Scant, white | Yellowish | None |
| Asparagine-glucose agar ISP5 | Abundant | Good, grayish | White | None |
| Gao’s | Abundant | Good, grayish | White | Slight yellow |
| Starch agar ISP4 | Good | Good, white | Milky | Slight yellow |
| Tyrosine agar ISP7 | Abundant | Good, gray | Brown | Yellow brown |
| Malic calcium agar | Abundant | Good, grayish | Brown | Brown yellow |
| Yeast-glucose agar | Good | Good, white | Deep brown | Deep brown |
| Glycine-asparagine agar | Abundant | Good, gray, brown | Cuticolor, brown | Brown yellow |
| Oatmeal agar ISP3 | Poor | Poor, grayish | Yellow, brown | None |
| Ke’s | Abundant | Abunt, gray, crapy | White | Slight yellow |
| Potato | Abundant | Good, grayish, | Brown | Deep brown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Feng, M.; Li, X.; Chen, F.; Zhang, Z.; Yang, W.; Shao, C.; Tao, L.; Zhang, Y. Antibacterial Activity of Aureonuclemycin Produced by Streptomyces aureus Strain SPRI-371. Molecules 2022, 27, 5041. https://doi.org/10.3390/molecules27155041
Wang W, Feng M, Li X, Chen F, Zhang Z, Yang W, Shao C, Tao L, Zhang Y. Antibacterial Activity of Aureonuclemycin Produced by Streptomyces aureus Strain SPRI-371. Molecules. 2022; 27(15):5041. https://doi.org/10.3390/molecules27155041
Chicago/Turabian StyleWang, Weiguo, Minkang Feng, Xiaomeng Li, Feiyu Chen, Zhihao Zhang, Wenlong Yang, Chen Shao, Liming Tao, and Yang Zhang. 2022. "Antibacterial Activity of Aureonuclemycin Produced by Streptomyces aureus Strain SPRI-371" Molecules 27, no. 15: 5041. https://doi.org/10.3390/molecules27155041
APA StyleWang, W., Feng, M., Li, X., Chen, F., Zhang, Z., Yang, W., Shao, C., Tao, L., & Zhang, Y. (2022). Antibacterial Activity of Aureonuclemycin Produced by Streptomyces aureus Strain SPRI-371. Molecules, 27(15), 5041. https://doi.org/10.3390/molecules27155041






