Mechanism of the Micellar Solubilization of Curcumin by Mixed Surfactants of SDS and Brij35 via NMR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
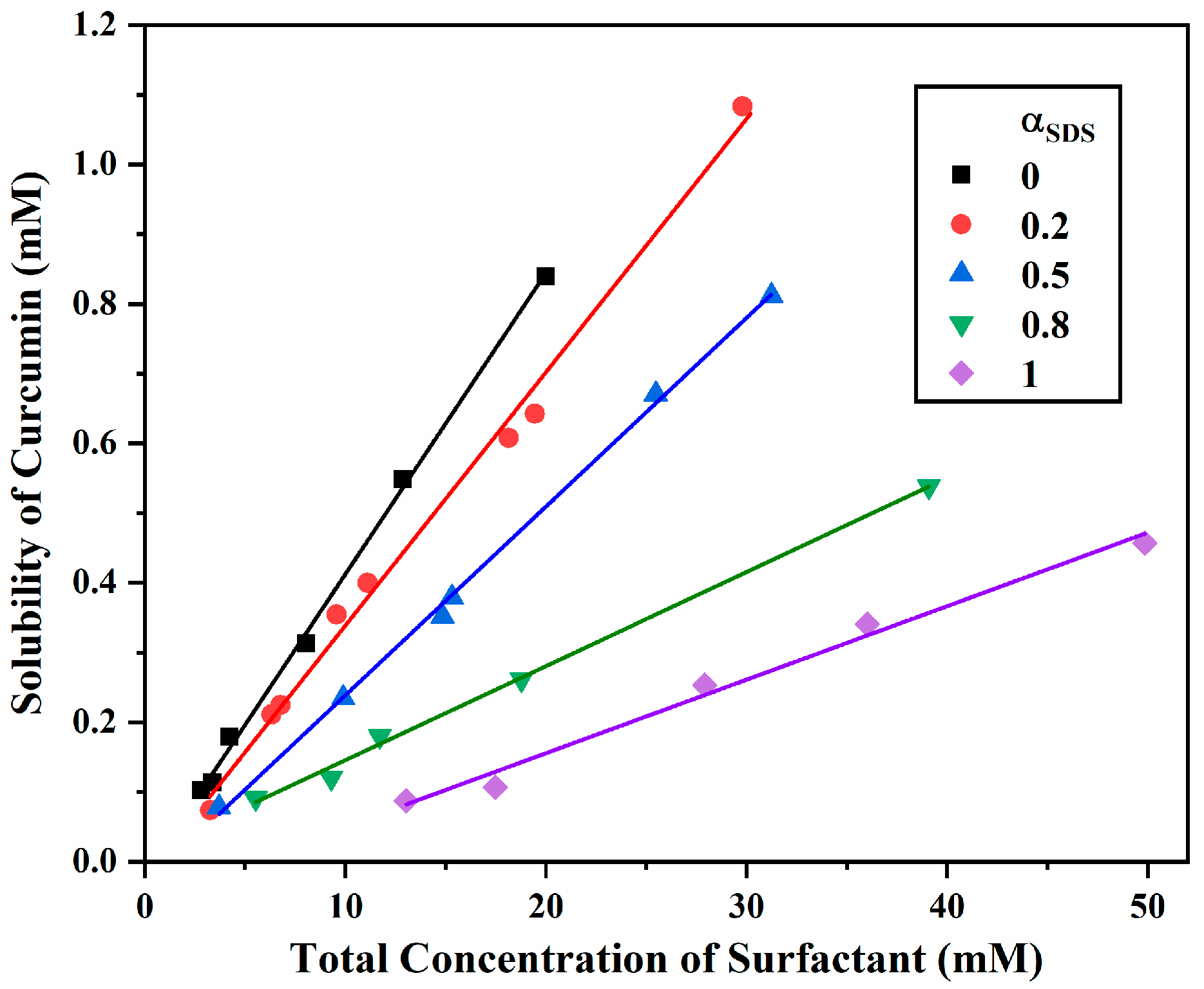
2.1. Apparent Solubility and Molar Solubilization Ratio (MSR)
2.2. The Formation Process of Mixed Micelles
2.3. The Composition and Morphology of Micelles
2.4. The Micellar Structure and Solubilization Sites of Curcumin
2.5. The Surfactant Aggregation Process (CMC) and the Solubilization of Curcumin
2.6. The Composition of Mixed Surfactants and the Micellar Structure
2.7. The Micellar Morphology and the Solubilization Sites
2.8. The Molar Ratio of Mixed Surfactants and Solubility
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. NMR Experiments
3.4. Surface Tension and pH Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Feng, J.; Wu, S.; Wang, H.; Liu, S. Stability of trianionic curcumin enhanced by gemini alkyl O-Glucosides and alkyl trimethyl ammonium halides mixed micelles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 190–200. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Lykova, A.; Kashapova, N.; Ziganshina, A.; Sergeeva, T.; Sapunova, A.; Voloshina, A.; Zakharova, L. Nanoencapsulation of food bioactives in supramolecular assemblies based on cyclodextrins and surfactant. Food Hydrocolloid. 2021, 113, 106449. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Jagtap, P.; Patil, K.; Dhatrak, P. Polyelectrolyte complex for drug delivery in biomedical applications: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1183, 012007. [Google Scholar] [CrossRef]
- Karashima, M.; Kimoto, K.; Yamamoto, K.; Kojima, T.; Ikeda, Y. A novel solubilization technique for poorly soluble drugs through the integration of nanocrystal and cocrystal technologies. Eur. J. Pharm. Biopharm. 2016, 107, 142–150. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, M.J. Surfactants and Interfacial Phenomena; John Wiley: New York, NY, USA, 1989. [Google Scholar]
- Moroi, Y. Micelles: Theoretical and applied aspects; Plenum: New York, NY, USA, 1992. [Google Scholar]
- Rubingh, D.N. Solution Chemistry of Surfactants; Mittal, K.L., Ed.; Plenum Press: New York, NY, USA, 1997; Volume 3, pp. 337–354. [Google Scholar]
- Clint, J.H. Micellization of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 1327–1334. [Google Scholar] [CrossRef]
- Aratono, M.; Ikeguchi, M.; Takiue, T.; Ikeda, N.; Motomura, K. Thermodynamic study on the miscibility of sodium perfluorooctanoate and sodium decyl sulfate in the adsorbed film and micelle. J. Colloid Interface Sci. 1995, 174, 156–161. [Google Scholar] [CrossRef]
- Barthe’le’my, P.; Tomao, V.; Selb, J.; Chaudier, Y.; Pucci, B. Fluorocarbon-hydrocarbon nonionic surfactants mixtures: A study of their miscibility. Langmuir 2002, 18, 2557–2563. [Google Scholar] [CrossRef]
- Nakano, T.Y.; Sugihara, G.; Nakashima, T.; Yu, S.C. Thermodynamic study of mixed hydrocarbon/fluorocarbon surfactant system by conductometric and fluorimetric techniques. Langmuir 2002, 18, 8777–8785. [Google Scholar] [CrossRef]
- Frotscher, E.; Hoering, J.; Durand, G.; Vargas, C.; Keller, S. Model-free analysis of critical micellar concentrations for detecting demixing in surfactant mixtures. Anal. Chem. 2017, 89, 3245–3249. [Google Scholar] [CrossRef] [PubMed]
- Agneta, M.; Li, Z.; Zhang, C.; Gerald, G. Investigating synergism and antagonism of binary mixed surfactants for foam efficiency optimization in high salinity. J. Petrol. Sci. Eng. 2019, 175, 489–494. [Google Scholar] [CrossRef]
- Srivastava, A.; Yanez, O.; Cantero-Lopez, P. Mixed micellization of bile salts and transglycosylated stevia and enhanced binding and solubility of non-steroidal anti-inflammatory drugs using mixed micelle. J. Mol. Liq. 2020, 311, 113341. [Google Scholar] [CrossRef]
- Singla, P.; Singh, O.; Chabba, S.; Mahajan, R.K. Pluronic-SAILs (surface active ionic liquids) mixed micelles as efficient hydrophobic quercetin drug carriers. J. Mol. Liq. 2018, 249, 294–303. [Google Scholar] [CrossRef]
- Ullrey, D.E. Biological availability of fat-soluble vitamins: Vitamin A and carotene. J. Anim. Sci. 1972, 35, 648–657. [Google Scholar] [CrossRef] [Green Version]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Mulrooney, S.L.; O’Neill, G.J.; Brougham, D.F.; O’Riordan, D. Vitamin D-3 bioaccessibility: Influence of fatty acid chain length, salt concentration and 1-alpha-phosphatidylcholine concentration on mixed micelle formation and delivery of vitamin D-3. Food Chem. 2021, 344, 128722. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Zou, L.; Zhang, R.; Salvia-Trujillo, L.; Kumosani, T.; Xiao, H. Enhancing nutraceutical performance using excipient foods: Designing food structures and compositions to increase bioavailability. Compr. Rev. Food Sci. Food Saf. 2015, 14, 824–847. [Google Scholar] [CrossRef]
- Srivastava, A.; Uchiyama, H.; Wada, Y.; Hatanaka, Y.; Shirakawa, Y.; Kadota, K.; Tozuka, Y. Mixed micelles of the antihistaminic cationic drug diphenhydramine hydrochloride with anionic and non-ionic surfactants show improved solubility, drug release and cytotoxicity of ethenzamide. J. Mol. Liq. 2019, 277, 349–359. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Sheelarani, B.; Joshi, R.G.; Dash, S. Solubilization and interaction of ciprofloxacin with pluronics and their mixed micelles. New J. Chem. 2019, 43, 16530–16537. [Google Scholar] [CrossRef]
- Clulow, A.J.; Barber, B.; Salim, M.; Ryan, T.; Boyd, B.J. Synergistic and antagonistic effects of non-ionic surfactants with bile salt plus phospholipid mixed micelles on the solubility of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119762. [Google Scholar] [CrossRef]
- Rupp, C.; Steckel, H.; Mueller, W.B. Solubilization of poorly water-soluble drugs by mixed micelles based on hydrogenated phosphatidylcholine. Int. J. Pharm. 2010, 395, 272–280. [Google Scholar] [CrossRef]
- Vinarov, Z.; Katev, V.; Burdzhiev, N.; Tcholakova, S.; Denkov, N. Effect of surfactant-bile interactions on the solubility of hydrophobic drugs in biorelevant dissolution media. Mol. Pharmaceut. 2018, 15, 5741–5753. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Sreenivasan, K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J. Colloid Interface Sci. 2011, 359, 318–325. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y. Effects of length and unsaturation of the alkyl chain on the hydrophobic binding of curcumin with Tween micelles. Food Chem. 2018, 246, 242–248. [Google Scholar] [CrossRef]
- Leung, M.H.M.; Colangelo, H.; Kee, T.W. Encapsulation of curcumin in cationic micelles suppresses alkaline hydrolysis. Langmuir 2008, 24, 5672–5675. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Fast-dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kaur, G.; Kansal, S.K.; Chaudhary, G.R.; Mehta, S.K. Enhanced solubilization of curcumin in mixed surfactant vesicles. Food Chem. 2016, 199, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, S.; Bilia, A.R.; Morris, G.A.; Nilsson, M. Novel artemisinin and curcumin micellar formulations: Drug solubility studies by NMR spectroscopy. J. Pharm. Sci. 2009, 98, 3666–3675. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kaur, G.; Kansal, S.K.; Chaudhary, G.R.; Mehta, S.K. (Cationic plus nonionic) mixed surfactant aggregates for solubilisation of curcumin. J. Chem. Thermodyn. 2016, 93, 115–122. [Google Scholar] [CrossRef]
- Jahed, V.; Zarrabi, A.; Bordbar, A.-K.; Hafezi, M.S. NMR (1H, ROESY) spectroscopic and molecular modelling investigations of supramolecular complex of beta-cyclodextrin and curcumin. Food Chem. 2014, 165, 241–246. [Google Scholar] [CrossRef]
- Awad, T.S.; Asker, D.; Romsted, L.S. Evidence of coexisting microemulsion droplets in oil-in-water emulsions revealed by 2D DOSY 1H NMR. J. Colloid Interface Sci. 2018, 514, 83–92. [Google Scholar] [CrossRef]
- Viet, N.K.; Prevost, S.; Seidel, K.; Maier, W.; Marguerre, K.A.; Oetter, G.; Tadros, T.; Gradzielski, M. Solubilization of active ingredients of different polarity in Pluronic micellar solutions-correlations between solubilizate polarity and solubilization site. J. Colloid Interface Sci. 2016, 477, 94–102. [Google Scholar]
- Wang, M.; Wu, C.; Tang, Y.; Fan, Y.; Han, Y.; Wang, Y. Interactions of cationic trimeric, gemini and monomeric surfactants with trianionic curcumin in aqueous solution. Soft Matter 2014, 10, 3432–3440. [Google Scholar] [CrossRef]
- Pigliacelli, C.; Belton, P.; Wilde, P.; Qi, S. Probing the molecular interactions between pharmaceutical polymeric carriers and bile salts in simulated gastrointestinal fluids using NMR spectroscopy. J. Colloid Interface Sci. 2019, 551, 147–154. [Google Scholar] [CrossRef]
- Edwards, D.A.; Luthy, R.G.; Liu, Z.B. Solubilization of polycyclic aromatic-hydrocarbons in micellar nonionic surfactant solutions. Environ. Sci. Technol. 1991, 25, 127–133. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, L. Solubilization of pyrene by anionic-nonionic mixed surfactants. J. Hazard. Mater. 2004, 109, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadabi, B.A.; Aoudia, M. Surfactant-surfactant and surfactant-solute interactions in SLES-Brij35 mixed micelles: Effect of the degree of ethoxylation on pyrene solubilization enhancement in water. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 82–89. [Google Scholar] [CrossRef]
- Blandamer, M.J.; Cullis, P.M.; Soldi, L.G.; Engberts, J.; Kacperska, A.; Vanos, N.M.; Subha, M.C.S. Thermodynamics of micellar systems: Comparison of mass action and phase equilibrium models for the calculation of standard Gibbs energies of micelle formation. Adv. Colloid Interface Sci. 1995, 58, 171–209. [Google Scholar] [CrossRef] [Green Version]
- Wettig, S.D.; Verrall, R.E. Thermodynamic studies of aqueous m-s-m gemini surfactant systems. J. Colloid Interface Sci. 2001, 235, 310–316. [Google Scholar] [CrossRef]
- Cui, X.; Jiang, Y.; Yang, C.; Lu, X.; Chen, H.; Mao, S.; Liu, M.; Yuan, H.; Luo, P.; Du, Y. Mechanism of the mixed surfactant micelle formation. J. Phys. Chem. B 2010, 114, 7808–7816. [Google Scholar] [CrossRef]
- Acharjee, A.; Rakshit, A.; Chowdhury, S.; Ali, M.A.; Singh, B.; Saha, B. Mixed anionic-nonionic micelle catalysed oxidation of aliphatic alcohol in aqueous medium. J. Mol. Liq. 2020, 303, 112655. [Google Scholar] [CrossRef]
- Al-Soufi, W.; Piñeiro, L.; Novo, M. A model for monomer and micellar concentrations in surfactant solutions: Application to conductivity, NMR, diffusion, and surface tension data. J. Colloid Interface Sci. 2012, 370, 102–110. [Google Scholar] [CrossRef]
- Denkova, P.S.; van Lokeren, L.; Verbruggen, I.; Willem, R. Self-aggregation and supramolecular structure investigations of triton X-100 and SDP2S by NOESY and diffusion ordered NMR spectroscopy. J. Phys. Chem. B 2008, 112, 10935–10941. [Google Scholar] [CrossRef]
- Giorgio, G.; Colafemmina, G.; Mavelli, F.; Murgia, S.; Palazzo, G. The impact of alkanes on the structure of Triton X100 micelles. RSC Adv. 2016, 6, 825–836. [Google Scholar] [CrossRef]
- Arkhipov, V.P.; Arkhipov, R.V.; Kuzina, N.A.; Filippov, A. Study of the premicellar state in aqueous solutions of sodium dodecyl sulfate by nuclear magnetic resonance diffusion. Magn. Reson. Chem. 2021, 59, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, L.Z. Effect of SDBS-Tween 80 mixed surfactants on the distribution of polycyclic aromatic hydrocarbons in soil-water system. J. Soil. Sediment. 2010, 10, 1123–1130. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, M.; Guo, C.; Abel, S.; Yi, X.; Lu, G.; Yang, C.; Dang, Z. Competitive solubilization of low-molecular-weight polycyclic aromatic hydrocarbons mixtures in single and binary surfactant micelles. Chem. Eng. J. 2014, 244, 522–530. [Google Scholar] [CrossRef]
- Yuan, S.; Shu, Z.; Wan, J.; Lu, X. Enhanced desorption of hexachlorobenzene from kaolin by single and mixed surfactants. J. Colloid Interface Sci. 2007, 314, 167–175. [Google Scholar] [CrossRef] [PubMed]







| SDS | Brij35 | ||
|---|---|---|---|
| αSDS | CMC (CT) /mM | CMC1 (CT) /mM | CMC2 (CT) /mM |
| 1 | 6.91 (8.1 mM a) | ||
| 0.8 | 1.60 (2.00) | 0.0632(0.316, 0.254 ST) | 0.388 (1.94, 1.92 ST) |
| 0.5 | 0.384(0.768) | 0.0611(0.122, 0.173 ST) | 0.916(1.83, 1.82 ST) |
| 0.2 | 0.0532(0.266) | 0.131(0.164, 0.167 ST) | 2.15(2.69) |
| 0 | 0.240 (0.066 mM b) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, X.; Wu, Z.; Chen, Z.; Cui, X. Mechanism of the Micellar Solubilization of Curcumin by Mixed Surfactants of SDS and Brij35 via NMR Spectroscopy. Molecules 2022, 27, 5032. https://doi.org/10.3390/molecules27155032
Zhan X, Wu Z, Chen Z, Cui X. Mechanism of the Micellar Solubilization of Curcumin by Mixed Surfactants of SDS and Brij35 via NMR Spectroscopy. Molecules. 2022; 27(15):5032. https://doi.org/10.3390/molecules27155032
Chicago/Turabian StyleZhan, Xiao, Zhaoxia Wu, Zhong Chen, and Xiaohong Cui. 2022. "Mechanism of the Micellar Solubilization of Curcumin by Mixed Surfactants of SDS and Brij35 via NMR Spectroscopy" Molecules 27, no. 15: 5032. https://doi.org/10.3390/molecules27155032
APA StyleZhan, X., Wu, Z., Chen, Z., & Cui, X. (2022). Mechanism of the Micellar Solubilization of Curcumin by Mixed Surfactants of SDS and Brij35 via NMR Spectroscopy. Molecules, 27(15), 5032. https://doi.org/10.3390/molecules27155032






