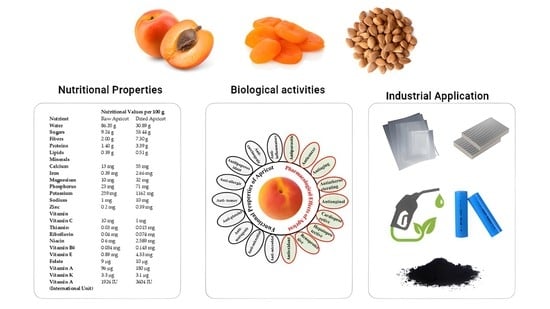
A Review with Updated Perspectives on Nutritional and Therapeutic Benefits of Apricot and the Industrial Application of Its Underutilized Parts
Abstract
:1. Introduction
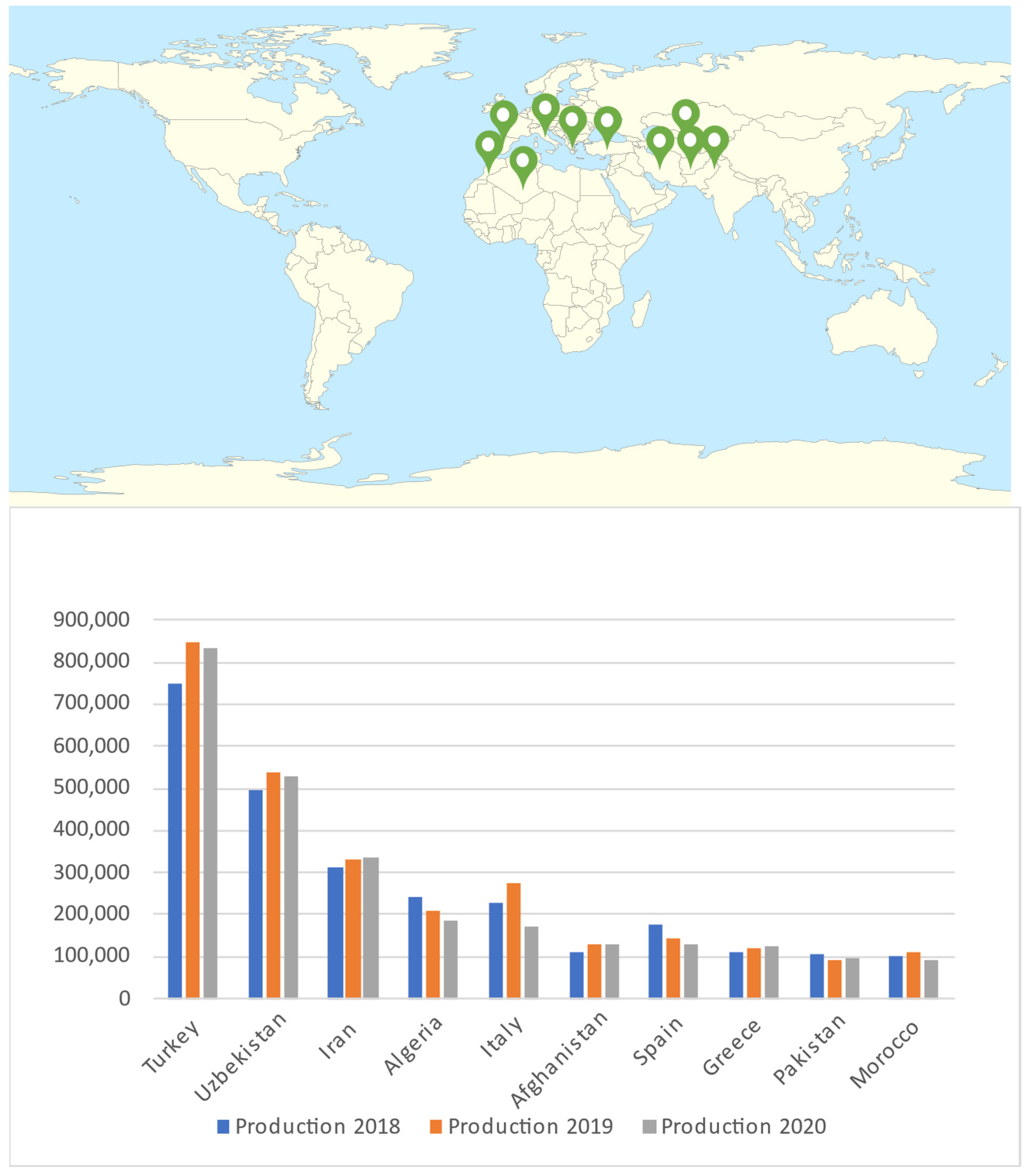
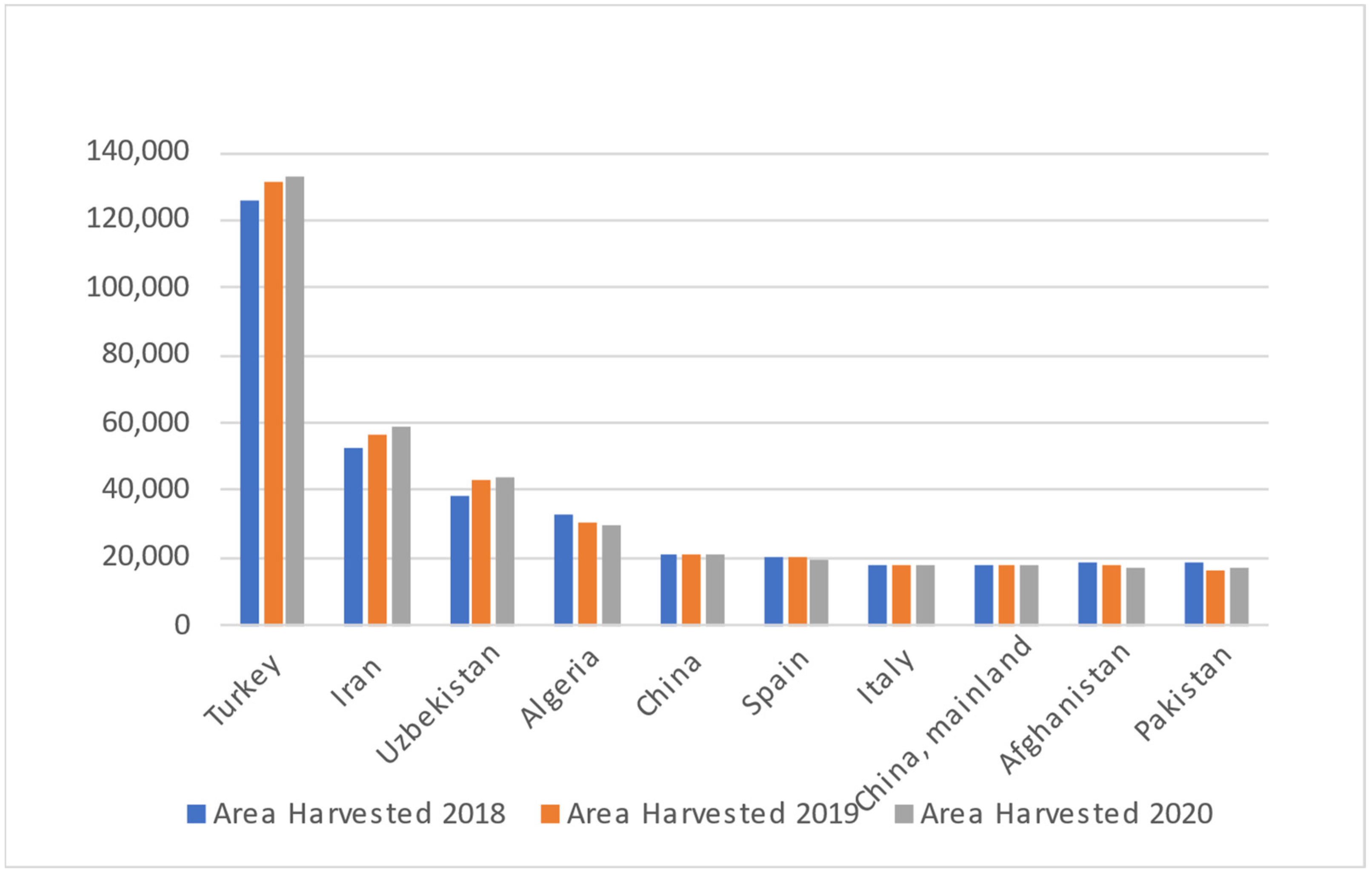
2. Apricot Production
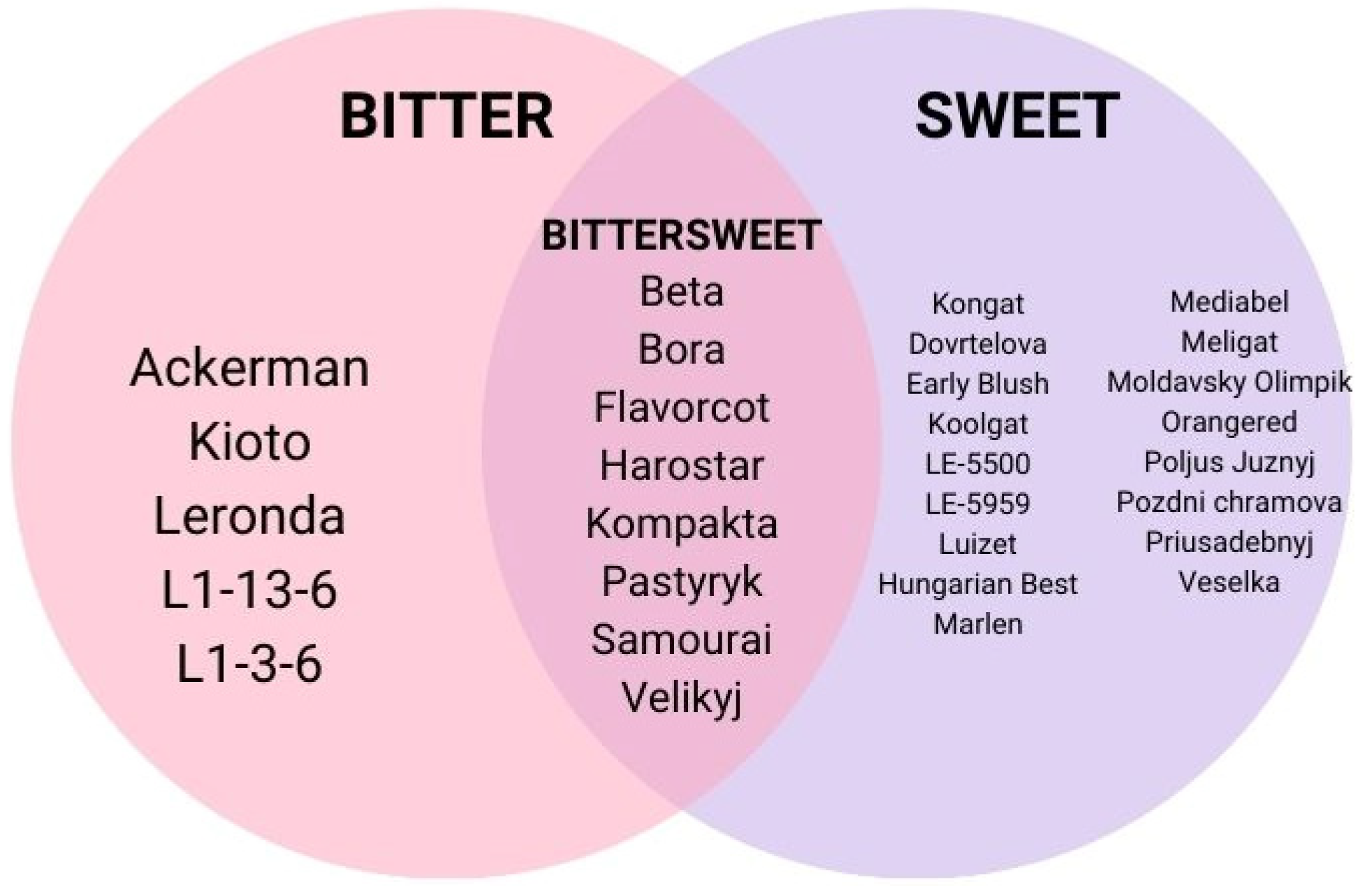
3. Physical Properties and Taste of Apricot Kernels
4. Nutritional Values of Apricots
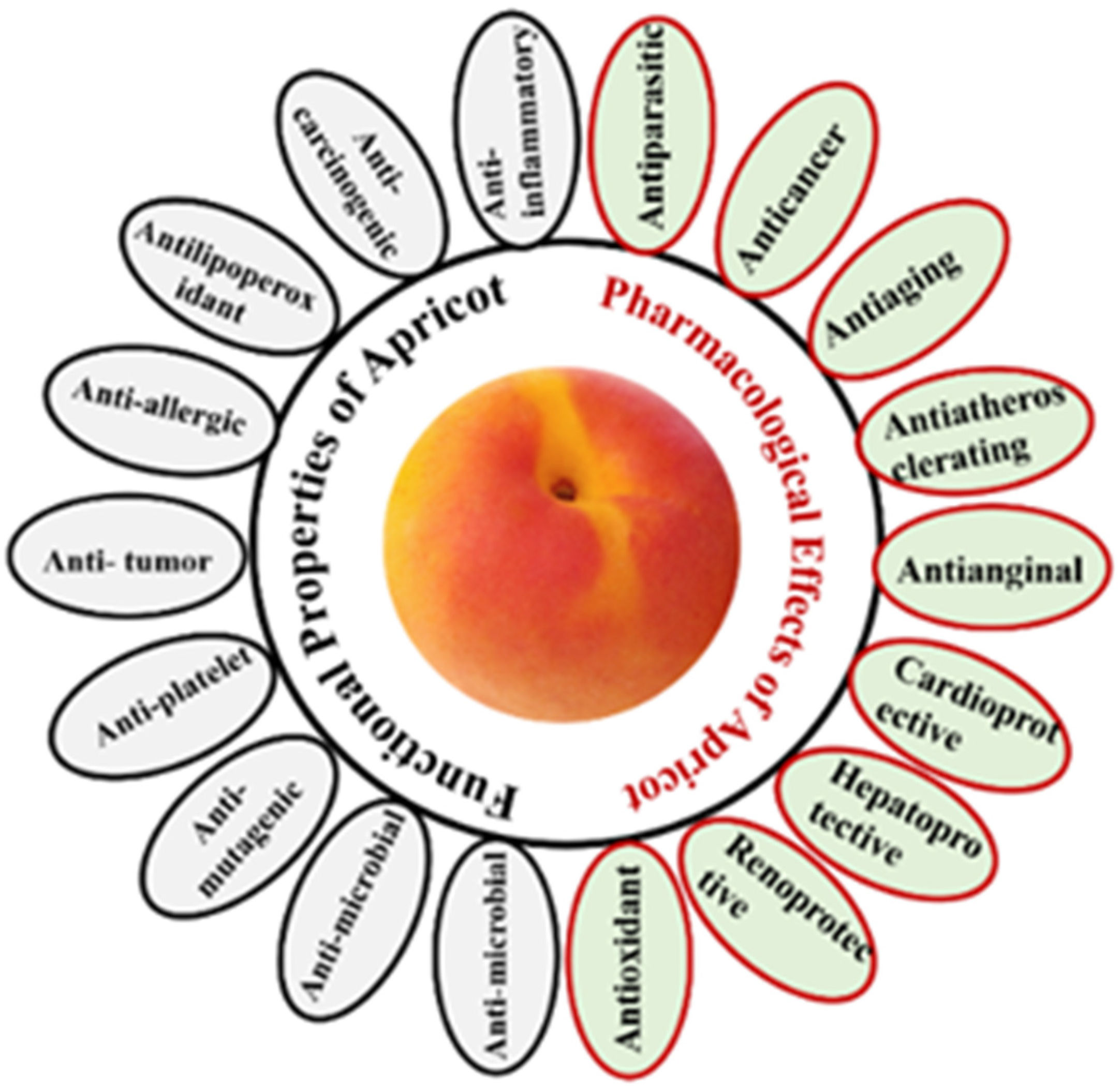
5. Biological Activities of Apricot
5.1. Antioxidants
5.2. Anti-Inflammatory Activity
5.3. Anticancer Effect of Apricot
5.4. Biological Activity of Apricot Leaves
6. Medicinal Properties of Japanese Apricot (Prunus mume)
6.1. Phytochemical Constituents
6.2. Medicinal Usage of P. mume Phenolic Compound
6.2.1. Antidiabetic
6.2.2. Helicobacter Pylori-Related Chronic Gastritis
6.2.3. Antimicrobial and Antiviral Activity
7. Industrial Utilization of Apricot Seed Waste and Kernels
7.1. Applications in Food Industry
7.2. Apricot Seed Waste in Polymer Production
7.2.1. Food Packaging
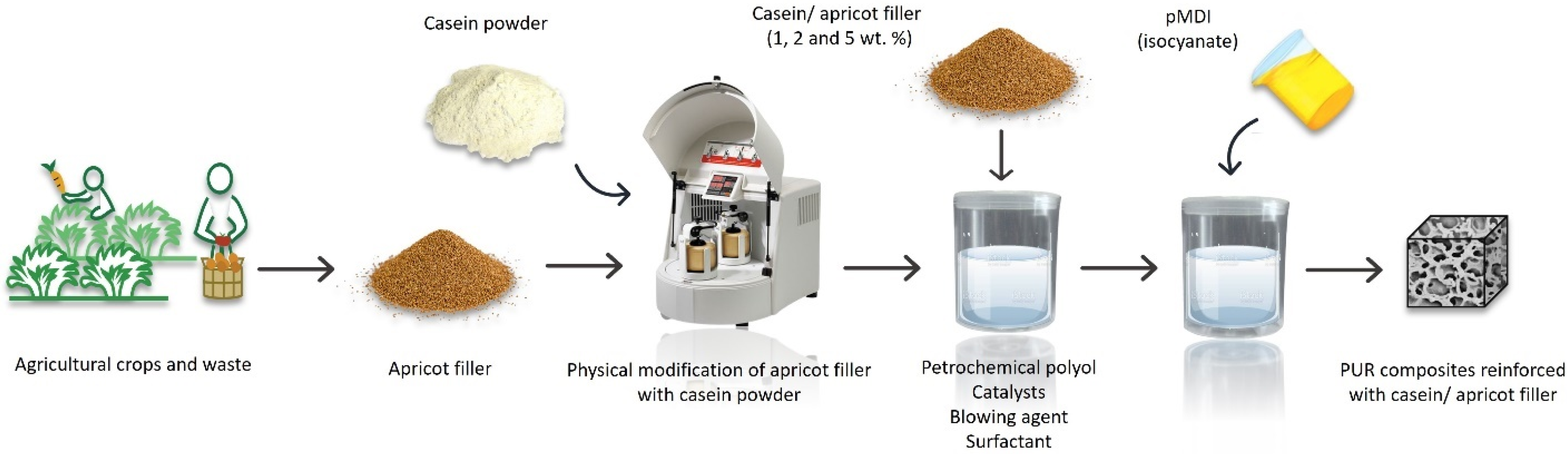
7.2.2. Polyurethane Composites
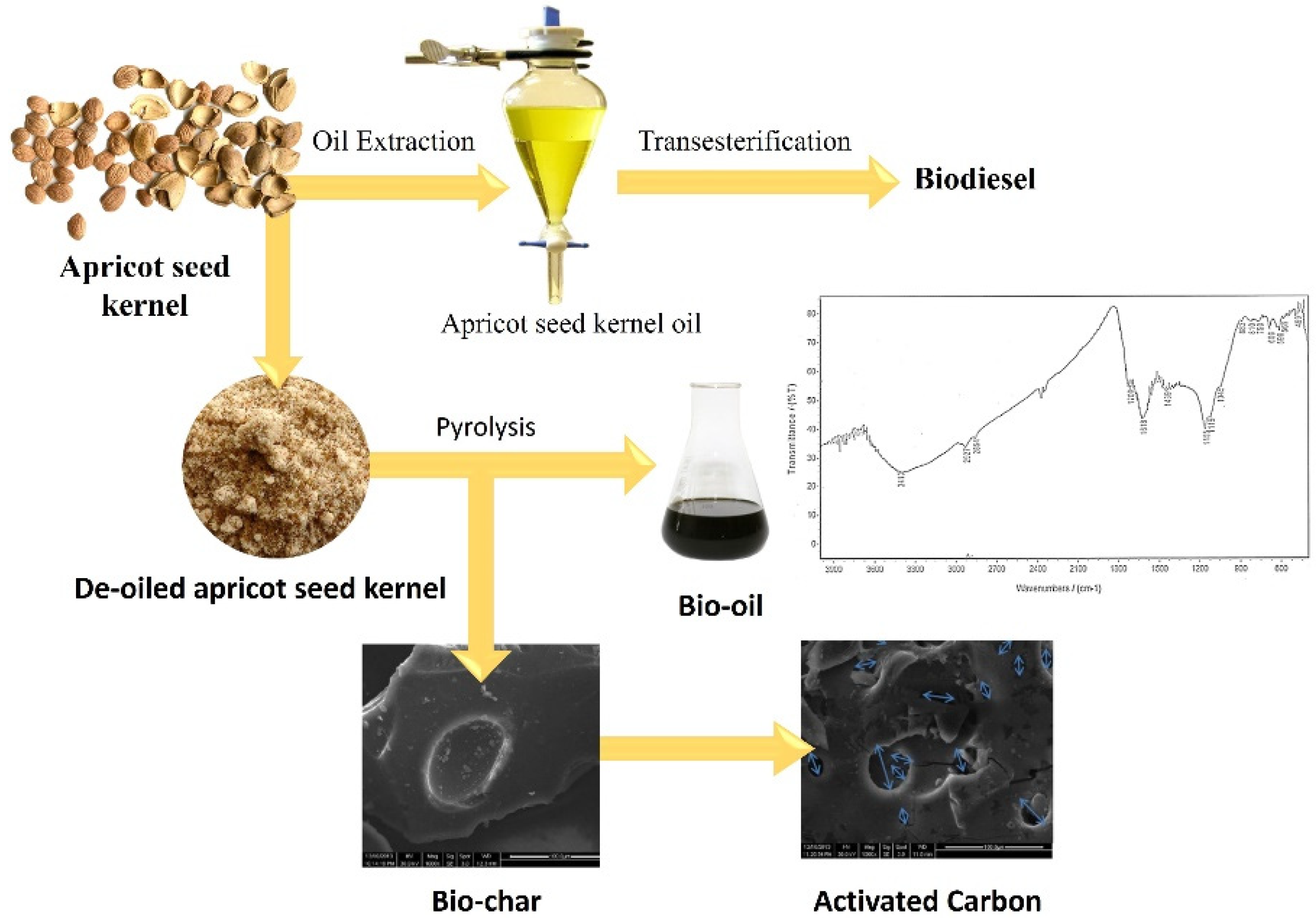
7.3. Apricot Seed Waste in Biofuel and Activated Carbon Production
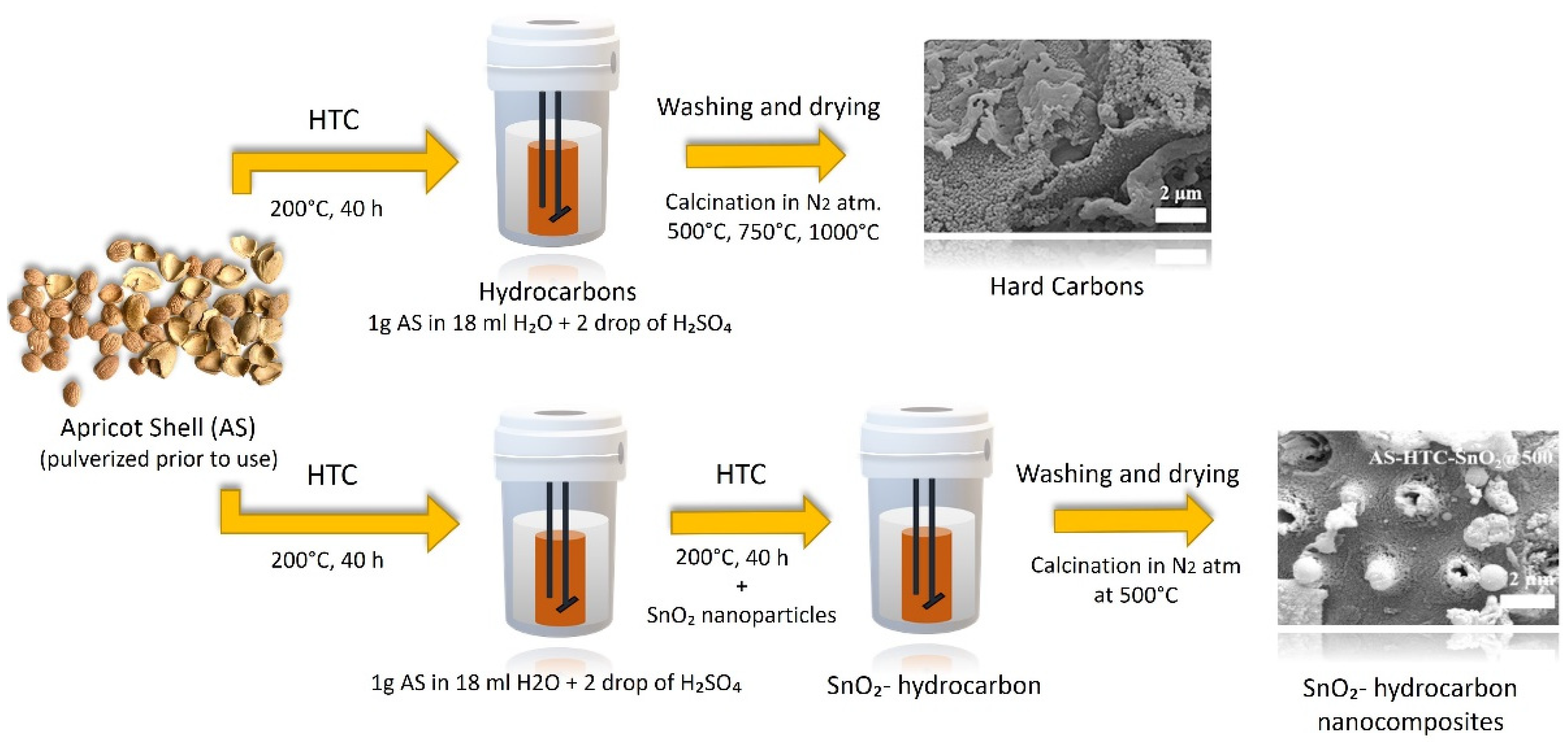
7.4. Apricot Seed Waste in Batteries
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasri, H.; Baradaran, A.; Shirzad, H.; Kopaei, M.R. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Verma, M.K.; Tomar, M.; Kumar, M.; Mekhemar, M. Nutritional and Phytochemical Traits of Apricots (Prunus armeniaca L.) for Application in Nutraceutical and Health Industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, H.J. Effects of Apricot and Apricot Kernels on Human Health and Nutrition: A Review of Recent Human Research. Tech. Biochem. 2021, 2, 139–162. [Google Scholar] [CrossRef]
- Mehlenbacher, S.A.; Cociu, V.; Hough, F.L. Apricots (Prunus). Genet. Resour. Temp. Fruit Nut Crop. 1991, 290, 65–110. [Google Scholar] [CrossRef]
- Gharaghani, A.; Solhjoo, S. Varietal Diversification of Stone Fruits. In Production Technology of Stone Fruits; Springer: Singapore, 2021; pp. 1–56. [Google Scholar]
- Yilmaz, I. The Biological and Pharmacological İmportance of Apricot. SOJ Pharm. Pharm. Sci. 2018, 5, 1–4. [Google Scholar] [CrossRef]
- Huang, W.; Bi, X.; Zhang, X.; Liao, X.; Hu, X.; Wu, J. Comparative Study of Enzymes, Phenolics, Carotenoids and Color of Apricot Nectars Treated by High Hydrostatic Pressure and High Temperature Short Time. Innov. Food Sci. Emerg. Technol. 2013, 18, 74–82. [Google Scholar] [CrossRef]
- Bourguiba, H.; Audergon, J.M.; Krichen, L.; Trifi-Farah, N.; Mamouni, A.; Trabelsi, S.; D’Onofrio, C.; Asma, B.M.; Santoni, S.; Khadari, B. Loss of Genetic Diversity as a Signature of Apricot Domestication and Diffusion into the Mediterranean Basin. BMC Plant Biol. 2012, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Campbell, O.E.; Merwin, I.A.; Padilla-Zakour, O.I. Characterization and the Effect of Maturity at Harvest on the Phenolic and Carotenoid Content of Northeast USA Apricot (Prunus armeniaca) Varieties. J. Agric. Food Chem. 2013, 61, 12700–12710. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Pospišil, J.; Levaj, B.; Delonga, K. The Study of Phenolic Profiles of Raw Apricots and Apples and Their Purees by HPLC for the Evaluation of Apricot Nectars and Jams Authenticity. Food Chem. 2005, 91, 373–383. [Google Scholar] [CrossRef]
- Iordanescu, O.A.; Alexa, E.; Lalescu, D.; Berbecea, A.; Camen, D.; Poiana, M.A.; Moigradean, D.; Bala, M. Chemical Composition and Antioxidant Activity of Some Apricot Varieties at Different Ripening Stages. Chil. J. Agric. Res. 2018, 78, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Layne, R.E.C.; Bailey, C.H.; Hough, L.F. Apricots. In Fruit Breeding; Janick, J., Moore, J.N., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1996; Volume 1, pp. 79–111. [Google Scholar]
- Wani, S.M.; Masoodi, F.A.; Wani, A.; Ahmad, M.; Gani, A.; Ganai, S.A.; Wani, T.A. Physical Characteristics, Mineral Analysis and Antioxidant Properties of Some Apricot Varieties Grown in North India Physical Characteristics, Mineral Analysis and Antioxidant Properties of Some Apricot Varieties Grown in North India Public Interest Statement. Cogent Food Agric. 2015, 1, 1118961. [Google Scholar] [CrossRef]
- Ali, S.; Masud, T.; Abbasi, K.S. Physico-Chemical Characteristics of Apricot (Prunus armeniaca L.) Grown in Northern Areas of Pakistan. Sci. Hortic. 2011, 130, 386–392. [Google Scholar] [CrossRef]
- Hussain, P.R.; Chatterjee, S.; Variyar, P.S.; Sharma, A.; Dar, M.A.; Wani, A.M. Bioactive Compounds and Antioxidant Activity of Gamma Irradiated Sun Dried Apricots (Prunus armeniaca L.). J. Food Compos. Anal. 2013, 30, 59–66. [Google Scholar] [CrossRef]
- Karayiannis, I. Inheritance of Sweet Kernel Taste in Apricot. Acta Hortic. 2010, 862, 73–76. [Google Scholar] [CrossRef]
- Rampáčková, E.; Göttingerová, M.; Gála, P.; Kiss, T.; Ercişli, S.; Nečas, T. Evaluation of Protein and Antioxidant Content in Apricot Kernels as a Sustainable Additional Source of Nutrition. Sustainability 2021, 13, 4742. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J. Soluble solids, acidity, phenolic content and antioxidant capacity of fruits and berries cultivated in Serbia. Fruits 2016, 71, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Fatima, T.; Bashir, O.; Gani, G.; Bhat, T.; Jan, N. Nutritional and Health Benefits of Apricots. Int. J. Unani Integr. Med. 2018, 2, 5–9. [Google Scholar]
- Moustafa, K.; Cross, J. Production, Pomological and Nutraceutical Properties of Apricot. J. Food Sci. Technol. 2019, 56, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Göttingerová, M.; Kumšta, M.; Rampáčková, E.; Kiss, T.; Nečas, T. Analysis of Phenolic Compounds and Some Important Analytical Properties in Selected Apricot Genotypes. HortScience 2021, 56, 1446–1452. [Google Scholar] [CrossRef]
- eddine Derardja, A.; Barkat, M. Effect of Traditional Sun-Drying and Oven-Drying on Carotenoids and Phenolic Compounds of Apricot (Prunus armeniaca L.). N. Afr. J. Food Nutr. Res. 2019, 3, 186–194. [Google Scholar] [CrossRef]
- Hasan, M.U.; Malik, A.U.; Ali, S.; Imtiaz, A.; Munir, A.; Amjad, W.; Anwar, R. Modern Drying Techniques in Fruits and Vegetables to Overcome Postharvest Losses: A Review. J. Food Process. Preserv. 2019, 43, 12. [Google Scholar] [CrossRef]
- Gupta, S.; Chhajed, M.; Arora, S.; Thakur, G.; Gupta, R. Medicinal Value of Apricot: A Review. Indian J. Pharm. Sci. 2018, 80, 790–794. [Google Scholar] [CrossRef]
- Akin, E.B.; Karabulut, I.; Topcu, A. Some Compositional Properties of Main Malatya Apricot (Prunus armeniaca L.) Varieties. Food Chem. 2008, 107, 939–948. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Tomás-Barberán, F.A.; Gil, M.I. Carotenoids from New Apricot (Prunus armeniaca L.) Varieties and Their Relationship with Flesh and Skin Color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Egea, J.; Gil, M.I.; Tomás-Barberán, F.A. Characterization and Quantitation of Phenolic Compounds in New Apricot (Prunus armeniaca L.) Varieties. J. Agric. Food Chem. 2005, 53, 9544–9552. [Google Scholar] [CrossRef] [PubMed]
- Sass-Kiss, A.; Kiss, J.; Milotay, P.; Kerek, M.M.; Toth-Markus, M. Differences in Anthocyanin and Carotenoid Content of Fruits and Vegetables. Food Res. Int. 2005, 38, 1023–1029. [Google Scholar] [CrossRef]
- Thompson, C.O.; Trenerry, V.C. A Rapid Method for the Determination of Total L-Ascorbic Acid in Fruits and Vegetables by Micellar Electrokinetic Capillary Chromatography. Food Chem. 1995, 53, 43–50. [Google Scholar] [CrossRef]
- Asma, B.M.; Kan, T.; Birhanlı, O. Characterization of Promising Apricot (Prunus armeniaca L.) Genetic Resources in Malatya, Turkey. Genet. Resour. Crop Evol. 2007, 54, 205–212. [Google Scholar] [CrossRef]
- Munzuroglu, O.; Karatas, F.; Geckil, H. The Vitamin and Selenium Contents of Apricot Fruit of Different Varieties Cultivated in Different Geographical Regions. Food Chem. 2003, 83, 205–212. [Google Scholar] [CrossRef]
- Theron, M.M.; Lues, J.F.R. Organic Acids and Meat Preservation: A Review. Food Rev. Int. 2007, 23, 141–158. [Google Scholar] [CrossRef]
- Karataş, N.; Şengül, M. Some Important Physicochemical and Bioactive Characteristics of the Main Apricot Cultivars from Turkey. Turkish J. Agric. For. 2020, 44, 651–661. [Google Scholar] [CrossRef]
- Siddiq, M. Handbook of Fruits and Fruit Processing; John Wiley & Sons: New York, NY, USA, 2006; Volume 697, pp. 279–292. [Google Scholar]
- Muratore, G.; Rizzo, V.; Licciardello, F.; Maccarone, E. Partial Dehydration of Cherry Tomato at Different Temperature, and Nutritional Quality of the Products. Food Chem. 2008, 111, 887–891. [Google Scholar] [CrossRef]
- Leiva Díaz, E.; Giannuzzi, L.; Giner, S.A. Apple Pectic Gel Produced by Dehydration. Food Bioprocess Technol. 2009, 2, 194–207. [Google Scholar] [CrossRef]
- Hamid, A.A.; Aiyelaagbe, O.O.; Usman, L.A.; Ameen, O.M.; Lawal, A. Antioxidants: Its Medicinal and Pharmacological Applications. Afr. J. Pure Appl. Chem. 2010, 4, 142–151. [Google Scholar]
- Ozsahin, A.D.; Yilmaz, O. Fruit Sugar, Flavonoid and Phytosterol Contents of Apricot Fruits (Prunus armeniaca L. cv. Kabaasi) and Antioxidant Effects in the Free Radicals Environment. Asian J. Chem. 2010, 22, 6403–6412. [Google Scholar]
- Mohd Wani, S.; Rashid Hussain, P.; Ahmad Masoodi, F.; Ahmad, M.; Ahmad Wani, T.; Gani, A.; Ahmed Rather, S.; Suradkar, P. Evaluation of the Composition of Bioactive Compounds and Antioxidant Activity in Fourteen Apricot Varieties of North India. J. Agric. Sci. 2017, 9, 66–82. [Google Scholar] [CrossRef]
- Ramadan, A.; Kamel, G.; Awad, N.E.; Shokry, A.A.; Fayed, H.M. The Pharmacological Effect of Apricot Seeds Extracts and Amygdalin in Experimentally Induced Liver Damage and Hepatocellular Carcinoma. J. Herbmed Pharmacol. 2020, 9, 400–407. [Google Scholar] [CrossRef]
- Miyazawa, M.; Utsunomiya, H.; Inada, K.; Yamada, T.; Okuno, Y.; Tanaka, H.; Tatematsu, M. Inhibition of Helicobacter Pylori Motility by (+)-Syringaresinol from Unripe Japanese Apricot. Biol. Pharm. Bull. 2006, 29, 172–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, S.; Sawada, T.; Okada, T.; Ohsawa, T.; Adachi, M.; Keiichi, K. New Anti-Proliferative Agent, MK615, from Japanese Apricot “ Prunus Mume ” Induces Striking Autophagy in Colon Cancer Cells in Vitro. World J. Gastroenterol. 2007, 13, 6512. [Google Scholar] [CrossRef]
- Okada, T.; Sawada, T.; Osawa, T.; Adachi, M.; Kubota, K. MK615 Inhibits Pancreatic Cancer Cell Growth by Dual Inhibition of Aurora A and B Kinases. World J. Gastroenterol. 2008, 14, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, S.; Tada, K.; Kawahara, K.; Kawai, K.; Hashiguchi, T.; Maruyama, I.; Kanekura, T. Advanced Malignant Melanoma Responds to Prunus Mume Sieb. Et Zucc (Ume) extract: Case report and in vitro study. Exp. Ther. Med. 2010, 1, 569–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.; Asif, J.; Asif, M.; Saleem, U. Amygdalin from Apricot Kernels Induces Apoptosis and Causes Cell Cycle Arrest in Cancer Cells: An Updated Review. Anticancer. Agents Med. Chem. 2018, 18, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Kawahara, K.I.; Biswas, K.K.; Ando, K.; Mitsudo, K.; Nobuyoshi, M.; Maruyama, I. HMGB1 Expression by Activated Vascular Smooth Muscle Cells in Advanced Human Atherosclerosis Plaques. Cardiovasc. Pathol. 2007, 16, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.K.; Tyagi, S.M.; Singh, D. Pectinolytic Liquefaction of Apricot, Plum, and Mango Pulps for Juice Extraction. Int. J. Food Prop. 2001, 4, 103–109. [Google Scholar] [CrossRef]
- Adachi, M.; Suzuki, Y.; Mizuta, T.; Osawa, T.; Suzuki, K.; Shiojima, K.; Arai, Y.; Masuda, K.; Uchiyama, M.; Oyamada, T. The Japanese Apricot ‘Prunus Mume Sieb. Zucc’ is a rich Natural Source of Novel Anti-Cancer Substance. Int. J. Food Prop. 2007, 10, 375–384. [Google Scholar] [CrossRef]
- Houghton, A.; Polsky, D.F. Focus on Melanoma. Cancer Cell 2002, 2, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Al-Ghamdi, A.A.; Elshikh, M.S.; Shah, M.H.; Al-Dosary, M.A.; Abbasi, A.M. Phytochemical Profiling, Antioxidant and HepG2 Cancer Cells’ Antiproliferation Potential in the Kernels of Apricot Cultivars. Saudi J. Biol. Sci. 2020, 27, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bortiri, E.; Sang-Hun, O.H.; Jiang, U.O.; Baggett, S.; Granger, A.; Weeks, C.; Buckingham, M.; Potter, D.; Parfitt, D.E. Phylogeny and Systematics of Prunus (Rosaceae) as Determined by Sequence Analysis of ITS and the Chloroplast TrnL-TrnF Spacer DNA. Syst. Bot. 2001, 26, 797–807. [Google Scholar]
- Gong, X.P.; Tang, Y.; Song, Y.Y.; Du, G.; Li, J. Comprehensive Review of Phytochemical Constituents, Pharmacological Properties, and Clinical Applications of Prunus Mume. Front. Pharmacol. 2021, 12, 679378. [Google Scholar] [CrossRef]
- Kim, Y.-S.P. Antimicrobial Activity of Prunus Mume and Schizandra Chinenis H-20 Extracts and Their Effects on Quality of Functional Kochujang. Korean J. Food Sci. Technol. 2003, 35, 893–897. [Google Scholar]
- Enomoto, S.; Yanaoka, K.; Utsunomiya, H.; Niwa, T.; Inada, K.; Deguchi, H.; Ueda, K.; Mukoubayashi, C.; Inoue, I.; Maekita, T.; et al. Inhibitory Effects of Japanese Apricot (Prunus Mume Siebold et Zucc.; UME) on Helicobacter Pylori-Related Chronic Gastritis. Nat. News 2010, 64, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Femenia, A.; Rosselló, C.; Mulet, A.; Cañellas, J. Chemical Composition of Bitter and Sweet Apricot Kernels. J. Agric. Food Chem. 1995, 43, 356–361. [Google Scholar] [CrossRef]
- Fogarasi, S. Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes. Foods 2022, 11, 2184. [Google Scholar]
- Hyun, S.-W.; Kim, J.; Park, B.; Jo, K.; Lee, T.G.; Kim, J.S.; Kim, C.-S. Molecules Apricot Kernel Extract and Amygdalin Inhibit Urban Particulate Matter-Induced Keratoconjunctivitis Sicca. Molecules 2019, 24, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, D.; Rawat, N.; Negi, T.; Barthwal, R.; Sharma, S. Utilization, Valorization and Functional Properties of Wild Apricot Kernels. J. Pharmacogn. Phytochem. 2021, 10, 119–126. [Google Scholar]
- Raj, V.; Mishra, A.K.; Mishra, A.; Khan, N.A. Hepatoprotective Effect of Prunus armeniaca L. (Apricot) Leaf Extracts on Paracetamol Induced Liver Damage in Wistar Rats. Pharmacogn. J. 2016, 8, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Tang, X.; Liu, Z.; Huang, W.; Ye, Y. Enzymes-Dependent Antioxidant Activity of Sweet Apricot Kernel Protein Hydrolysates. LWT 2022, 154, 112825. [Google Scholar] [CrossRef]
- Aizat, M.; Aziz, F. Nanotechnology in Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–265. [Google Scholar]
- Priyadarshi, R.; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y. Chitosan Films Incorporated with Apricot (Prunus armeniaca) Kernel Essential Oil as Active Food Packaging Material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F. Polyurethane: An Introduction. In Polyurethane; Zafar, F., Sharmin, E., Eds.; IntechOpen: London, UK, 2012. [Google Scholar]
- Heath, R. Isocyanate-Based Polymers: Polyurethanes, Polyureas, Polyisocyanurates, and Their Copolymers; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780323358248. [Google Scholar]
- Członka, S.; Kairytė, A.; Miedzińska, K.; Strąkowska, A. Casein/Apricot Filler in the Production of Flame-Retardant Polyurethane Composites. Materials 2021, 14, 3620. [Google Scholar] [CrossRef]
- Wolska, A.; Goździkiewicz, M.; Ryszkowska, J. Thermal and Mechanical Behaviour of Flexible Polyurethane Foams Modified with Graphite and Phosphorous Fillers. J. Mater. Sci. 2012, 47, 5627–5634. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Kaskel, S. KOH Activation of Carbon-Based Materials for Energy Storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Fadhil, A. Evaluation of Apricot (Prunus armeniaca L.) Seed Kernel as a Potential Feedstock for the Production of Liquid Bio-Fuels and Activated Carbons. Energy Convers. Manag. 2017, 133, 307–317. [Google Scholar] [CrossRef]
- Lang, X.; Dalai, A.; Bakhshi, N.; Reaney, M.; Hertz, P. Preparation and Characterization of Bio-Diesels from Various Bio-Oils. Bioresour. Technol. 2001, 80, 53–62. [Google Scholar] [CrossRef]
- Janković, B.; Manić, N.; Dodevski, V.; Radović, I.; Pijović, M.; Katnić, Đ.; Tasić, G. Physico-Chemical Characterization of Carbonized Apricot Kernel Shell as Precursor for Activated Carbon Preparation in Clean Technology Utilization. J. Clean. Prod. 2019, 236, 117614. [Google Scholar] [CrossRef]
- Demir, E.; Aydin, M.; Arie, A.A.; Demir-Cakan, R. Apricot Shell Derived Hard Carbons and Their Tin Oxide Composites as Anode Materials for Sodium-Ion Batteries. J. Alloys Compd. 2019, 788, 1093–1102. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.; Antonietti, M.; Titirici, M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]


| Nutrient | Nutritional Values per 100 g | ||
|---|---|---|---|
| Raw Apricot | Dried Apricot | Kernel | |
| Water | 86.35 g | 30.89 g | - |
| Sugars | 9.24 g | 53.44 g | 17.5–35.6 g |
| Fibres | 2.00 g | 7.30 g | 11.85–13.6 g |
| Proteins | 1.40 g | 3.39 g | 14.6–27.1 g |
| Lipids | 0.39 g | 0.51 g | 2.1–3 g |
| Minerals | |||
| Calcium | 13 mg | 55 mg | 0.0076 g |
| Iron | 0.39 mg | 2.66 mg | 0.0042 g |
| Magnesium | 10 mg | 32 mg | 0.003 g |
| Phosphorus | 23 mg | 71 mg | 0.0028 g |
| Potassium | 259 mg | 1162 mg | - |
| Sodium | 1 mg | 10 mg | 0.0011 g |
| Zinc | 0.2 mg | 0.39 mg | - |
| Vitamins | |||
| Vitamin C | 10 mg | 1 mg | - |
| Thiamin | 0.03 mg | 0.015 mg | - |
| Riboflavin | 0.04 mg | 0.074 mg | - |
| Niacin | 0.6 mg | 2.589 mg | - |
| Vitamin B6 | 0.054 mg | 0.143 mg | - |
| Vitamin E | 0.89 mg | 4.33 mg | 0.003–0.040 g |
| Folate | 9 µg | 10 µg | - |
| Vitamin A | 96 µg | 180 µg | - |
| Vitamin K | 3.3 µg | 3.1 µg | - |
| Vitamin A (International Unit) | 1926 IU | 3604 IU | - |
| Varities | Hacıhloğlu | Hasanbey | Soğancı | Kabaası | Cöloğlu | Cataloğlu | |
|---|---|---|---|---|---|---|---|
| Quality | |||||||
| Total phenolics | 5341.29 g | 5827.98 g | 4965.99 g | 5822.03 g | 5674.25 g | 6107.21 g | |
| β-carotene | 21.87 g | 22.02 g | 9.18 g | 26.18 g | 5.74 g | 17.53 g | |
| Total Carotenoids | 21.87 g | 50.78 g | 23.29 g | 40.00 g | 14.83 g | 32.08 g | |
| Sucrose | 22.96 g | 35.96 g | 25.69 g | 39.00 g | 34.89 g | 24.98 g | |
| Glucose | 19.21 g | 14.72 g | 17.16 g | 18.64 g | 18.95 g | 21.40 g | |
| Fructoses | 13.56 g | 12.16 g | 13.99 g | 13.05 g | 15.68 g | 15.02 g | |
| Sorbitol | 26.80 g | 16.91 g | 22.66 g | 19.14 g | 24.35 g | 26.84 g | |
| Citric Acid | 776.2 g | 739.7 g | 725.7 g | 923.1 g | 431.8 g | 436.5 g | |
| Malic Acid | 1814.8 g | 2341.1 g | 1092.4 g | 1279.0 g | 1312.9 g | 973.4 g | |
| Ascorbic Acid | 37.7 g | 49.3 g | 28.5 g | 41.6 g | 20.6 g | 27.9 g | |
| Potassium | 1849 g | 1811 g | 1879 g | 1880 g | 1227 g | 1377 g | |
| Calcium | 102.3 g | 100.7 g | 110.0 g | 105.7 g | 87.0 g | 140.8 g | |
| Sodium | 10.9 g | 8.8 g | 8.9 g | 12.6 g | 14.0 g | 13.9 g | |
| Phosphor | 107.0 g | 118.6 g | 97.9 ± 4.5 | 97.0 g | 72.0 g | 88.9 g | |
| Magnesium | 134.7 g | 152.2 g | 110.4 g | 131.0 g | 120.4 g | 131.7 g | |
| Iron | 2.98 g | 2.80 g | 3.48 g | 2.34 g | 3.73 g | 2.73 g | |
| Selenium | 0.150 g | 0.190 g | 0.115 g | 0.150 g | 0.230 g | 0.145 g | |
| Zinc | 1.38 g | 1.41 g | 1.90 g | 2.63 g | 1.61 g | 2.19 g | |
| Manganese | 1.41 g | 1.59 g | 1.24 g | 1.66 g | 1.71 g | 1.58 g | |
| Nickel | 0.325 g | 0.440 g | 0.325 g | 0.430 g | 0.390 g | 0.350 g | |
| Varities | Hacıkız | Tokaloğlu | Alyanak | Iğdır | Bursa | |
|---|---|---|---|---|---|---|
| Quality | ||||||
| Total phenolics | 6592.38 g | 4233.70 g | 6773.43 g | 5823.76 g | 8180.49 g | |
| β-carotene | 13.05 g | 21.59 g | 48.69 g | 13.44 g | 42.18 g | |
| Total Carotenoids | 22.81 g | 50.07 g | 91.75 g | 25.26 g | 91.89 g | |
| Sucrose | 30.07 g | 56.83 g | 41.27 g | 34.83 g | 49.87 g | |
| Glucose | 23.67 g | 11.38 g | 18.33 g | 17.06 g | 9.47 g | |
| Fructoses | 11.03 g | 7.77 g | 6.53 g | 11.95 g | 6.34 g | |
| Sorbitol | 19.87 g | 5.05 g | 2.47 g | 6.37 g | 3.38 g | |
| Citric Acid | 443.9 g | 2055.2 g | 3524.5 g | 7697.3 g | 9997.1 g | |
| Malic Acid | 1347.8 g | 2656.2 g | 3888.9 g | 3030.4 g | 3930.0 g | |
| Ascorbic Acid | 37.1 g | 45.8 g | 38.3 g | 68.4 g | 96.8 g | |
| Potassium | 1605 g | 1926 g | 2319 g | 3219 g | 3455 g | |
| Calcium | 173.6 g | 113.6 g | 240.5 g | 233.7 g | 230.2 g | |
| Sodium | 15.9 g | 8.0 g | 10.1 g | 17.8 g | 11.7 g | |
| Phosphor | 104.6 g | 144.1 g | 157.2 g | 237.9 g | 177.6 g | |
| Magnesium | 146.7 g | 148.8 | 160.4 g | 222.0 g | 284.4 g | |
| Iron | 3.51 g | 5.09 g | 7.74 g | 7.94 g | 11.3 g | |
| Selenium | 0.185 g | 0.250 g | 0.335 g | 0.310 g | 0.400 g | |
| Zinc | 2.02 g | 2.05 g | 2.54 g | 4.24 g | 3.38 g | |
| Manganese | 2.29 g | 1.97 g | 2.85 g | 2.67 g | 2.85 g | |
| Nickel | 0.645 g | 0.510 g | 0.715 g | 0.425 g | 0.575 g | |
| Reference Foam | 1 wt.% | 2 wt.% | 5 wt.% | |
|---|---|---|---|---|
| Mechanical strength | ||||
| Compressive strength (Measured parallel) (Measured perpendicular) | +~5% +~7% | +~10% +~9% | −~11% −~18% | |
| Flexural strength | +~3% | +~6% | −~6% | |
| Impact strength | +~2% | +~4% | −~6% | |
| Flammability | ||||
| Tmax (°C) at stages of thermal decomposition: 1st 2nd 3rd | 205 °C 313 °C 587 °C | 221 °C 315 °C 587 °C | 219 °C 317 °C 589 °C | 217 °C 313 °C 591 °C |
| Char residue (wt.%) at 600 °C | 27.6 | 30.3 | 31.0 | 31.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Soufi, M.H.; Alshwyeh, H.A.; Alqahtani, H.; Al-Zuwaid, S.K.; Al-Ahmed, F.O.; Al-Abdulaziz, F.T.; Raed, D.; Hellal, K.; Mohd Nani, N.H.; Zubaidi, S.N.; et al. A Review with Updated Perspectives on Nutritional and Therapeutic Benefits of Apricot and the Industrial Application of Its Underutilized Parts. Molecules 2022, 27, 5016. https://doi.org/10.3390/molecules27155016
Al-Soufi MH, Alshwyeh HA, Alqahtani H, Al-Zuwaid SK, Al-Ahmed FO, Al-Abdulaziz FT, Raed D, Hellal K, Mohd Nani NH, Zubaidi SN, et al. A Review with Updated Perspectives on Nutritional and Therapeutic Benefits of Apricot and the Industrial Application of Its Underutilized Parts. Molecules. 2022; 27(15):5016. https://doi.org/10.3390/molecules27155016
Chicago/Turabian StyleAl-Soufi, Maryam Haroon, Hussah Abdullah Alshwyeh, Haifa Alqahtani, Safa Khalil Al-Zuwaid, Fatimah Othman Al-Ahmed, Fatima Taher Al-Abdulaziz, Daniya Raed, Khaoula Hellal, Nurul Hidayah Mohd Nani, Siti Norliyana Zubaidi, and et al. 2022. "A Review with Updated Perspectives on Nutritional and Therapeutic Benefits of Apricot and the Industrial Application of Its Underutilized Parts" Molecules 27, no. 15: 5016. https://doi.org/10.3390/molecules27155016
APA StyleAl-Soufi, M. H., Alshwyeh, H. A., Alqahtani, H., Al-Zuwaid, S. K., Al-Ahmed, F. O., Al-Abdulaziz, F. T., Raed, D., Hellal, K., Mohd Nani, N. H., Zubaidi, S. N., Asni, N. S. M., Hamezah, H. S., Kamal, N., Al-Muzafar, H., & Mediani, A. (2022). A Review with Updated Perspectives on Nutritional and Therapeutic Benefits of Apricot and the Industrial Application of Its Underutilized Parts. Molecules, 27(15), 5016. https://doi.org/10.3390/molecules27155016