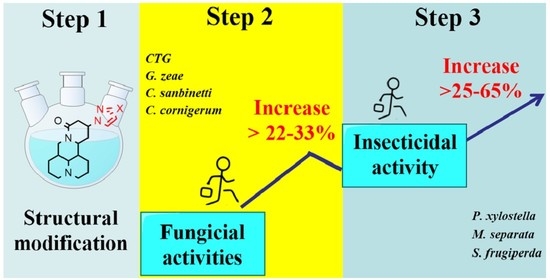
Synthesis of Halopyrazole Matrine Derivatives and Their Insecticidal and Fungicidal Activities
Abstract
:1. Introduction
2. Results and Discussion
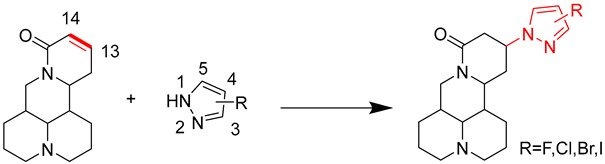
2.1. Chemical Synthesis
2.2. Spectroscopic Characterization
2.3. Structural Descriptions
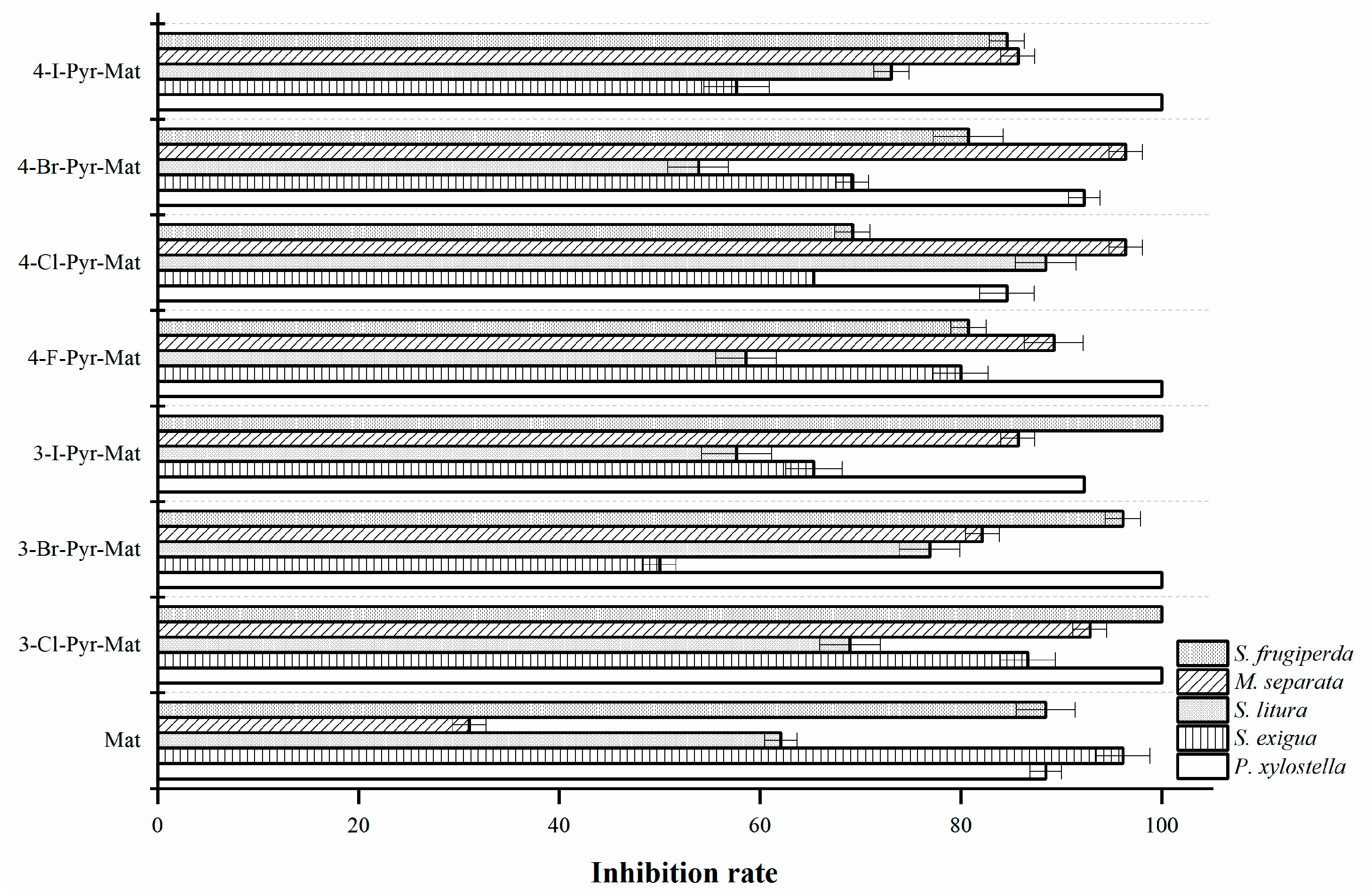
2.4. Insecticidal Activities
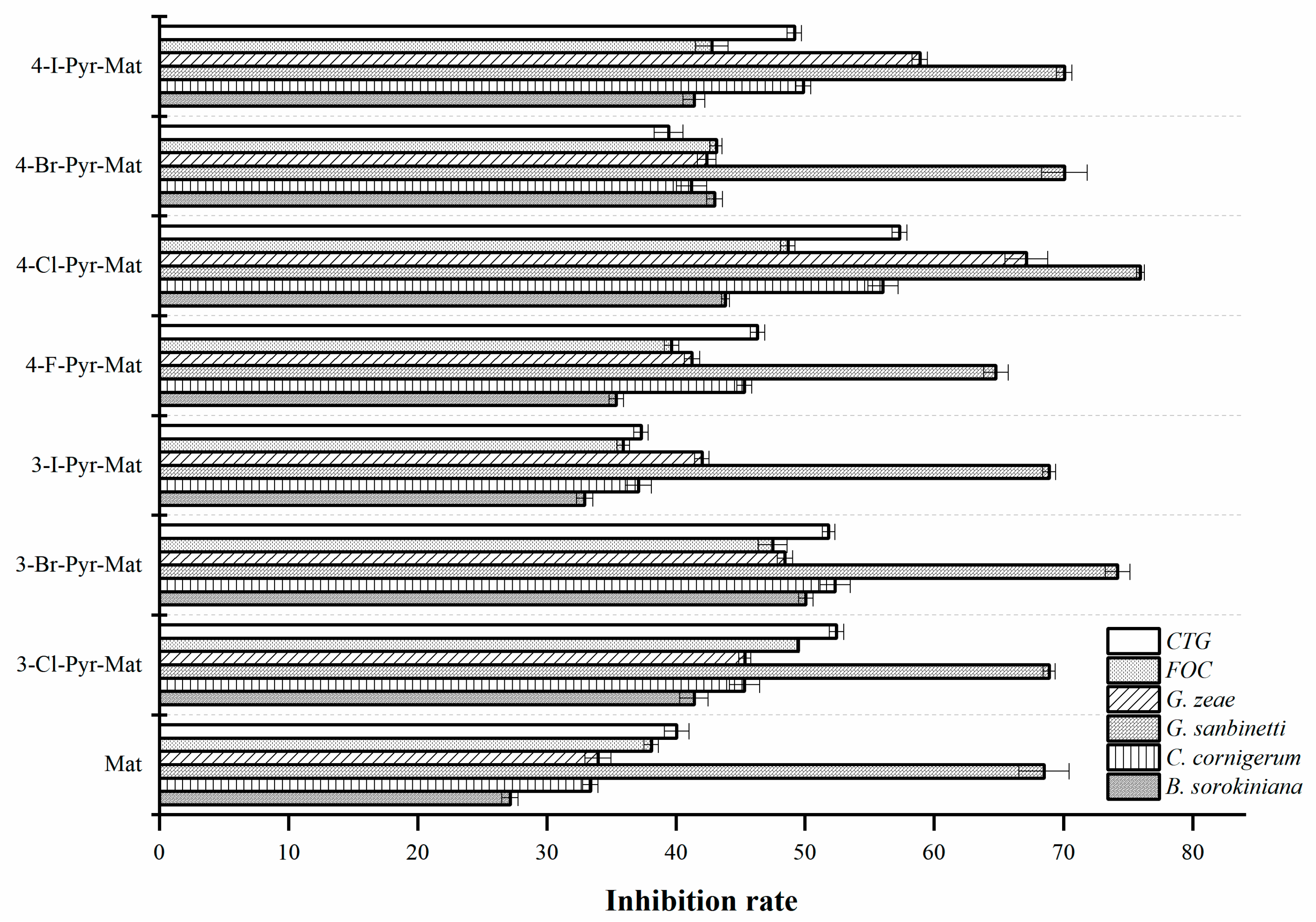
2.5. Fungcidal Activities
2.6. Degradability
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Compounds 1–7
3.3. Analytical Methods
3.4. Insecticidal Activity
3.5. Fungcidal Activity
3.6. Degradability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Popp, J.; Peto, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Z.C.; Zhen, D.Q.; Lin, Y.; Chen, J.; Ruan, J.; Chen, G. Determination of botanical insecticides residues in fish by liquid chromatography-electrospray tandem mass spectrometry. Food Anal. Methods 2011, 4, 601–607. [Google Scholar] [CrossRef]
- Isman, M.B. A renaissance for botanical insecticides? Pest Manag. Sci. 2015, 71, 1587–1590. [Google Scholar] [CrossRef]
- Schleier, J.J., III; Peterson, R.K.D. Chapter 3: Pyrethrins and pyrethroid insecticides. In Green Trends in Insect Control; RSC publishing: Philadelphia, PA, USA, 2011; pp. 94–131. [Google Scholar]
- Soderlund, D.M. Resmethrin, the first modern pyrethroid insecticide. Pest Manag. Sci. 2015, 71, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.R.; Beresford, N.; van der Woning, M.; Sumpter, J.P.; Tchorpe, K. Metabolism and environmental degradation of pyrethroid insecticides produce compounds with endocrine activities. Environ. Toxicol. Chem. 2000, 19, 801–809. [Google Scholar] [CrossRef]
- Brackmann, F.; Yufit, D.S.; Howard, J.; Es-Sayed, M.; de Meijere, A. Cyclopropyl building blocks for organic synthesis, part 106. Synthesis of spirocyclopropanated analogues of imidacloprid and thiacloprid. Eur. J. Org. Chem. 2005, 3, 600–609. [Google Scholar] [CrossRef]
- Kayser, H.; Lee, C.; Decock, A.; Baur, M.; Haettenschwiler, J.; Maienfisch, P. Comparative analysis of neonicotinoid binding to insect membranes, I. A structure-activity study of the mode of [3H] imidacloprid displacement in Myzus persicae and Aphis craccivora. Pest Manag. Sci. 2004, 60, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, K.P.; Martín, M.T.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Trace analysis of seven neonicotinoid insecticides in bee pollen by solid-liquid extraction and liquid chromatography coupled to electrospray ionization mass spectrometry. Food Anal. Methods 2014, 7, 490–499. [Google Scholar] [CrossRef]
- Zanardi, O.Z.; do Prado Ribeiro, L.; Ansante, T.F.; Santos, M.S.; Bordini, G.P.; Yamamoto, P.T.; Vendramim, J.D. Bioactivity of a matrine-based biopesticide against four pest species of agricultural importance. Crop Prot. 2015, 67, 160–167. [Google Scholar] [CrossRef]
- Liu, L.J.; Alam, M.S.; Hirata, K.; Matsuda, K.; Ozoe, Y. Actions of quinolizidine alkaloids on Periplaneta americana nicotinic acetylcholine receptors. Pest Manag. Sci. 2008, 64, 1222–1228. [Google Scholar] [CrossRef]
- Huang, J.; Xu, H. Matrine, bioactivities and structural modifications. Curr. Top. Med. Chem. 2016, 16, 3365–3378. [Google Scholar] [CrossRef]
- Yong, J.; Wu, X.; Lu, C. Anticancer advances of matrine and its derivatives. Curr. Pharm. Des. 2015, 21, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hu, H.; Tian, Y. Bioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosis. Hepatology 2014, 2, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, L.; Wang, D.; Jia, H.; Yu, M.; Gu, Y.; Shang, H.; Zou, Z. Design and synthesis of matrine derivatives as novel anti-pulmonary fibrotic agents via repression of the TGFβ/smad pathway. Molecules 2019, 24, 1108–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Ying, H.; Qu, Y.; Cai, X.B.; Xu, M.Y.; Lu, L.G. Novel matrine derivative MD-1 attenuates hepatic fibrosis by inhibiting EGFR activation of hepatic stellate cells. Protein Cell 2016, 7, 662–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Wang, S.; Zhang, C.; Wang, L.; Ding, L.; Zhang, J.; Wu, Q. Synthesis and in vitro inhibitory activity of matrine derivatives towards pro-inflammatory cytokines. Bioorganic Med. Chem. Lett. 2010, 20, 7537–7546. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ye, J.; He, H.; Liu, Z.; Xu, C.C.; Wu, B.; Xiong, X.; Shu, X.; Jiang, X.; Qin, X. Synthesis, characterization and in vitro biological evaluation of two matrine derivatives. Sci. Rep. 2018, 8, 15686. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; He, H.; Wang, W.X.; Dong, F.; Zhang, H.; Ye, J.; Tan, C.; Wu, Y.; Lv, X.; Jiang, X.; et al. Semi-synthesis and characterization of some new matrine derivatives as insecticidal agents. Pest Manag. Sci. 2020, 76, 2711–2719. [Google Scholar] [CrossRef]
- He, H.; Qin, X.; Dong, F.; Ye, J.; Xu, C.; Zhang, H.; Liu, Z.; Lv, X.; Wu, Y.; Jiang, X.; et al. Synthesis, characterization of two matrine derivatives and their cytotoxic effect on Sf9 cell of Spodoptera frugiperda. Sci. Rep. 2020, 10, 17999. [Google Scholar] [CrossRef]
- Ni, W.A.; Li, C.A.; Liu, Y.A.; Song, H.; Wang, L.; Song, H.; Wang, Q. Various bioactivity and relationship of structure-activity of matrine analogues. J. Agric. Food Chem. 2017, 10, 2039–2047. [Google Scholar] [CrossRef]
- Lv, M.; Liu, G.; Jia, M.; Xu, H. Synthesis of matrinic amide derivatives containing 1,3,4-thiadiazole scaffold as insecticidal/acaricidal agents. Bioorganic Chem. 2018, 81, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, Z.; Lv, M.; Xu, H. Semisynthesis of matrinic acid/alcohol/ester derivatives, their pesticidal activities and investigation of mechanisms of action against Tetranychus cinnabarinus. J. Agric. Food Chem. 2018, 66, 12898–12910. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, G.; Liu, S.; Wei, J.; Zhang, S.; Li, M.; Zhou, G.; Wang, L. Synthesis and biological evaluation of matrine derivatives containing benzo-alpha-pyrone structure as potent anti-lung cancer agents. Sci. Rep. 2016, 6, 35918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Liang, P.; Wu, L.; Xie, P.; Wang, H.; Zhang, S.; Wang, L.; Jiang, J. Design, synthesis, and biological evaluation of matrine derivatives possessing piperazine moiety as antitumor agents. Med. Chem. Res. 2019, 28, 1618–1627. [Google Scholar] [CrossRef]
- Dahmani, M.; Et-Touhami, A.; Yahyi, A.; Harit, T.; Eddike, D.; Tillard, M.; Benabbes, R. Synthesis, characterization, X-ray structure and in vitro antifungal activity of triphenyltin complexes based on pyrazole dicarboxylic acid derivatives. J. Mol. Struct. 2021, 1225, 129137–129152. [Google Scholar] [CrossRef]
- Wing, K.D.; Sacher, M.; Kagaya, Y.; Tsurubuchi, Y.; Mulderig, L.; Connair, M.; Schnee, M. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Prot. 2000, 19, 537–545. [Google Scholar] [CrossRef]
- Yang, H.; Xue, B.; Li, L.; Zhou, S.; Tu, Y.; Lin, C. Hydrolysis and soil sorption of insecticide pyraclofos. J. Environ. Sci. Health 2008, 43, 219–223. [Google Scholar] [CrossRef]
- Victoria, N.K.; Kokoreva, A.A.; Belik, A.A.; Pletenev, P.A. Study of the behavior of the new insecticide cyantraniliprole in large lysimeters of the Moscow State University. Open Agric. 2019, 4, 599–607. [Google Scholar]
- Lima, D.B.; Monteiro, V.B.; Guedes, R.N.C.; Siqueira, H.A.; Pallini, A.; Gondim, M.G. Acaricide toxicity and synergism of fenpyroximate to the coconut mite predator Neoseiulus baraki. Biocontrol 2013, 58, 595–605. [Google Scholar] [CrossRef]
- Lai, T.; Li, J.; Su, J. Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pestic. Biochem. Physiol. 2011, 101, 198–205. [Google Scholar] [CrossRef]
- Tohnishi, M.; Nakao, H.; Furuya, T.; Seo, A.; Kodama, H.; Tsubata, K.; Fujioka, S.; Kodama, H.; Hirooka, T.; Nishimatsu, T. Flubendiamide, a novel insecticide highly active against Lepidopterous insect pests. J. Pestic. Sci. 2005, 30, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Motoba, K.; Nishizawa, H.; Suzuki, T.; Hamaguchi, H.; Uchida, M.; Funayama, S. Species-specific detoxification metabolism of fenpyroximate, a potent acaricide. Pestic. Biochem. Physiol. 2000, 67, 73–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Nie, J.; Zhang, F.G.; Ma, J.A. Development of cyanopyrazoles as building blocks to fungicide fluxapyroxad and analogues. J. Org. Chem. 2019, 84, 7148–7158. [Google Scholar] [CrossRef] [PubMed]
 | |||||
|---|---|---|---|---|---|
| Number | Entry | Material Ratio 1 | Solvent | Catalyst | Yield/% |
| Compound 1 | 3-Cl-Pyr-Mat | 1:1 | Acetonitrile | K3PO4 | 82.8 |
| Compound 2 | 3-Br-Pyr-Mat | 1:1 | Ultrapure water | Cs2CO3 | 84.5 |
| Compound 3 | 3-I-Pyr-Mat | 1:1 | Acetonitrile | K3PO4 | 78.1 |
| Compound 4 | 4-F-Pyr-Mat | 2:1 | Dioxane | K3PO4 | 85.6 |
| Compound 5 | 4-Cl-Pyr-Mat | 2:1 | Dioxane | Cs2CO3 | 87.0 |
| Compound 6 | 4-Br-Pyr-Mat | 4:3 | Dioxane | Cs2CO3 | 83.0 |
| Compound 7 | 4-I-Pyr-Mat | 1:1 | Acetonitrile | Cs2CO3 | 81.3 |
| P. xylostella | S. exigua | S. litura | M. separata | S. frugiperda | |
|---|---|---|---|---|---|
| Mat | 0.052 | 0.043 | 0.215 | >1.00 | <0.01 |
| 3-Cl-Pyr-Mat | <0.01 | <0.01 | 0.189 | <0.01 | <0.01 |
| 3-Br-Pyr-Mat | <0.01 | 0.75 | 0.198 | <0.01 | <0.01 |
| 3-I-Pyr-Mat | <0.01 | 0.652 | 0.444 | <0.01 | <0.01 |
| 4-F-Pyr-Mat | <0.01 | 0.058 | 0.316 | 0.012 | <0.01 |
| 4-Cl-Pyr-Mat | 0.035 | 0.385 | 0.095 | 0.020 | 0.083 |
| 4-Br-Pyr-Mat | 0.014 | 0.297 | >1.00 | <0.01 | 0.032 |
| 4-I-Pyr-Mat | 0.01 | 0.414 | 0.228 | <0.01 | 0.058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; He, H.; Dong, F.; Xu, C.C.; Zhang, H.; Liu, Z.; Lv, X.; Wu, Y.; Jiang, X.; Qin, X. Synthesis of Halopyrazole Matrine Derivatives and Their Insecticidal and Fungicidal Activities. Molecules 2022, 27, 4974. https://doi.org/10.3390/molecules27154974
Cheng X, He H, Dong F, Xu CC, Zhang H, Liu Z, Lv X, Wu Y, Jiang X, Qin X. Synthesis of Halopyrazole Matrine Derivatives and Their Insecticidal and Fungicidal Activities. Molecules. 2022; 27(15):4974. https://doi.org/10.3390/molecules27154974
Chicago/Turabian StyleCheng, Xingan, Huiqing He, Fangyun Dong, Chunbao Charles Xu, Hanhui Zhang, Zhanmei Liu, Xiaojing Lv, Yuehua Wu, Xuhong Jiang, and Xiangjing Qin. 2022. "Synthesis of Halopyrazole Matrine Derivatives and Their Insecticidal and Fungicidal Activities" Molecules 27, no. 15: 4974. https://doi.org/10.3390/molecules27154974
APA StyleCheng, X., He, H., Dong, F., Xu, C. C., Zhang, H., Liu, Z., Lv, X., Wu, Y., Jiang, X., & Qin, X. (2022). Synthesis of Halopyrazole Matrine Derivatives and Their Insecticidal and Fungicidal Activities. Molecules, 27(15), 4974. https://doi.org/10.3390/molecules27154974



_Xu.png)