Application of Cleavable Linkers to Improve Therapeutic Index of Radioligand Therapies
Abstract
1. Introduction
2. Radioligand Therapy
3. Radiation-Induced Nephrotoxicity
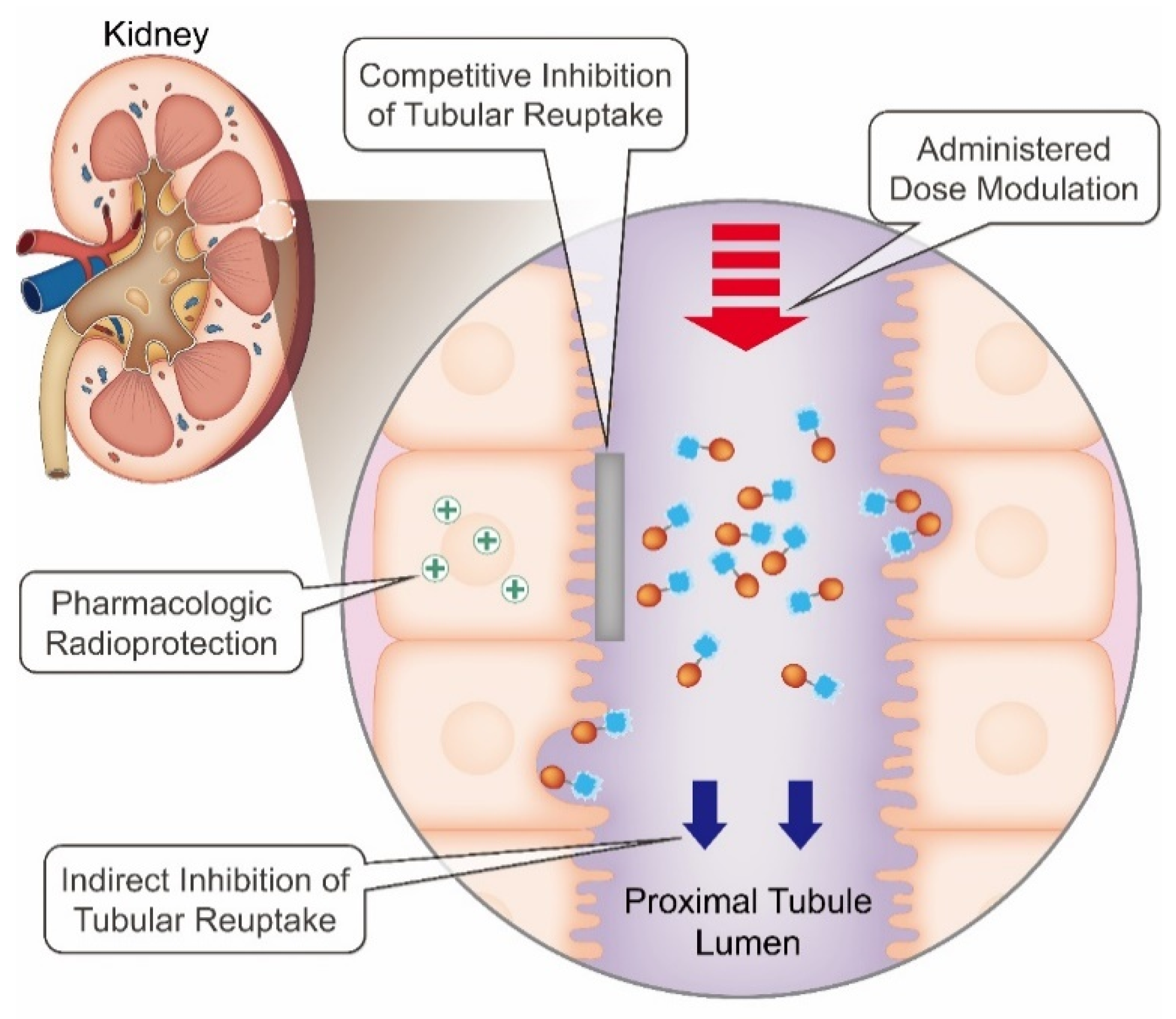
4. Strategies to Mitigate Radiation-Induced Nephrotoxicity
5. Cleavable Linkers
6. Perspective and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical Therapy in Cancer: Clinical Advances and Challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Jacobson, O.; Niu, G.; Lin, K.S.; Bénard, F.; Chen, X. Bench to Bedside: Albumin Binders for Improved Cancer Radioligand Therapies. Bioconjug. Chem. 2019, 30, 487–502. [Google Scholar] [CrossRef]
- Sia, J.; Szmyd, R.; Hau, E.; Gee, H.E. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front. Cell Dev. Biol. 2020, 8, 41. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177 Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Iravani, A.; Violet, J.; Azad, A.; Hofman, M.S. Lutetium-177 Prostate-Specific Membrane Antigen (PSMA) Theranostics: Practical Nuances and Intricacies. Prostate Cancer Prostatic Dis. 2020, 23, 38–52. [Google Scholar] [CrossRef]
- Cohen, E.P.; Premuzic, T.; Cohen, A.P. Radiation Nephropathy: Dose, Management, and Population Risk. J. Onco-Nephrol. 2021, 6, 23–28. [Google Scholar] [CrossRef]
- Vegt, E.; De Jong, M.; Wetzels, J.F.M.; Masereeuw, R.; Melis, M.; Oyen, W.J.G.; Gotthardt, M.; Boerman, O.C. Renal Toxicity of Radiolabeled Peptides and Antibody Fragments: Mechanisms, Impact on Radionuclide Therapy, and Strategies for Prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef]
- Beasley, M.; Driver, D.; Dobbs, H.J. Complications of Radiotherapy: Improving the Therapeutic Index. Cancer Imaging 2005, 5, 78–84. [Google Scholar] [CrossRef]
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Pacifici, M.; Grana, C.M.; Bartolomei, M.; Baio, S.M.; Sansovini, M.; Paganelli, G. Long-Term Evaluation of Renal Toxicity after Peptide Receptor Radionuclide Therapy with 90Y-DOTATOC and 177Lu-DOTATATE: The Role of Associated Risk Factors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1847–1856. [Google Scholar] [CrossRef]
- Das, S.; Al-Toubah, T.; El-Haddad, G.; Strosberg, J. 177Lu-DOTATATE for the Treatment of Gastroenteropancreatic Neuroendocrine Tumors. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1023–1031. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.K.V.; Press, O.W. Radioimmunotherapy of Human Tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Chakraborty, S.; Pillai, M.R.A.; Knapp, F.F.R. Peptide Receptor Radionuclide Therapy: An Overview. Cancer Biother. Radiopharm. 2015, 30, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies—Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Ercan, M.T.; Caglar, M. Therapeutic Radiopharmaceuticals. Curr. Pharm. Des. 2005, 6, 1085–1121. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Pouget, J.P.; Navarro-Teulon, I.; Bardiès, M.; Chouin, N.; Cartron, G.; Pèlegrin, A.; Azria, D. Clinical Radioimmunotherapy—The Role of Radiobiology. Nat. Rev. Clin. Oncol. 2011, 8, 720–734. [Google Scholar] [CrossRef]
- Valentin, J.; Cox, R.; Kellerer, A.M. Relative Biological Effectiveness (RBE), Quality Factor (Q), and Radiation Weighting Factor (WR): ICRP Publication 92: Approved by the Commission in January 2003. Ann. ICRP 2016, 33, 1–121. [Google Scholar] [CrossRef]
- Humm, J.L. A Microdosimetric Model of Astatine-211 Labeled Antibodies for Radioimmunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 1767–1773. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. Efficacy and Safety of 225 Ac-DOTATATE Targeted Alpha Therapy in Metastatic Paragangliomas: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1595–1606. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, M.P.; Baylis, C.; Humes, H.D.; Glassock, R.J.; Robertson, C.R.; Brenner, B.M. Permselectivity of the Glomerular Capillary Wall. Facilitated Filtration of Circulating Polycations. J. Clin. Investig. 1978, 61, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Jarad, G.; Miner, J.H. Update on the Glomerular Filtration Barrier. Curr. Opin. Nephrol. Hypertens. 2009, 18, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Chigoho, D.M.; Bridoux, J.; Hernot, S. Reducing the Renal Retention of Low- to Moderate-Molecular-Weight Radiopharmaceuticals. Curr. Opin. Chem. Biol. 2021, 63, 219–228. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Barone, R.; Krenning, E.; Bernard, B.; Melis, M.; Visser, T.; Gekle, M.; Willnow, T.E.; Walrand, S.; Jamar, F.; et al. Megalin Is Essential for Renal Proximal Tubule Reabsorption of 111in-DTPA-Octreotide. J. Nucl. Med. 2005, 46, 1696–1700. [Google Scholar] [PubMed]
- Vegt, E.; Melis, M.; Eek, A.; De Visser, M.; Brom, M.; Oyen, W.J.G.; Gotthardt, M.; De Jong, M.; Boerman, O.C. Renal Uptake of Different Radiolabelled Peptides Is Mediated by Megalin: SPECT and Biodistribution Studies in Megalin-Deficient Mice. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 623–632. [Google Scholar] [CrossRef]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the Folate Receptor α in Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Itoh, K. 99mTc-MAG3: Review of Pharmacokinetics, Clinical Application to Renal Diseases and Quantification of Renal Function. Ann. Nucl. Med. 2001, 15, 179–190. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Bolton, R.C.D.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM Procedure Guidelines for Radionuclide Therapy with 177Lu-Labelled PSMA-Ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA-and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef]
- Sarikaya, I.; Sarikaya, A.; Alnafisi, N.; Alenezi, S. Significance of Splenic Uptake on Somatostatin Receptor Imaging Studies. Nucl. Med. Rev. 2018, 21, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Rolleman, E.J.; Valkema, R.; De Jong, M.; Kooij, P.P.M.; Krenning, E.P. Safe and Effective Inhibition of Renal Uptake of Radiolabelled Octreotide by a Combination of Lysine and Arginine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 9–15. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Krenning, E. New Advances in Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2002, 43, 617–620. [Google Scholar]
- Hope, T.A.; Abbott, A.; Colucci, K.; Bushnell, D.L.; Gardner, L.; Graham, W.S.; Lindsay, S.; Metz, D.C.; Pryma, D.A.; Stabin, M.G.; et al. NANETS/SNMMI Procedure Standard for Somatostatin Receptor–Based Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE. J. Nucl. Med. 2019, 60, 937–943. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The Joint IAEA, EANM, and SNMMI Practical Guidance on Peptide Receptor Radionuclide Therapy (PRRNT) in Neuroendocrine Tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; Teunissen, J.J.; Bakker, W.H.; Kooij, P.P.; De Herder, W.W.; Feelders, R.A.; Van Eijck, C.H.; Esser, J.P.; Kam, B.L.; Krenning, E.P. Radiolabeled Somatostatin Analog [177Lu-DOTA0, Tyr3]Octreotate in Patients with Endocrine Gastroenteropancreatic Tumors. J. Clin. Oncol. 2005, 23, 2754–2762. [Google Scholar] [CrossRef]
- Melis, M.; Krenning, E.P.; Bernard, B.F.; Barone, R.; Visser, T.J.; De Jong, M. Localisation and Mechanism of Renal Retention of Radiolabelled Somatostatin Analogues. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1136–1143. [Google Scholar] [CrossRef]
- Vegt, E.; Wetzels, J.F.M.; Russel, F.G.M.; Masereeuw, R.; Boerman, O.C.; Van Eerd, J.E.; Corstens, F.H.M.; Oyen, W.J.G. Renal Uptake of Radiolabeled Octreotide in Human Subjects Is Efficiently Inhibited by Succinylated Gelatin. J. Nucl. Med. 2006, 47, 432–436. [Google Scholar]
- Rolleman, E.J.; Bernard, B.F.; Breeman, W.A.P.; Forrer, F.; De Blois, E.; Hoppin, J.; Gotthardt, M.; Boerman, O.C.; Krenning, E.P.; De Jong, M. Molecular Imaging of Reduced Renal Uptake of Radiolabelled [DOTA 0,Tyr3]Octreotate by the Combination of Lysine and Gelofusine in Rats. NuklearMedizin 2008, 47, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Rolleman, E.J.; De Jong, M.; Valkema, R.; Kwekkeboom, D.; Kam, B.; Krenning, E.P. Inhibition of Kidney Uptake of Radiolabeled Somatostatin Analogs: Amino Acids or Gelofusine? J. Nucl. Med. 2006, 47, 1730–1731. [Google Scholar] [PubMed]
- Van Eerd, J.E.M.; Vegt, E.; Wetzels, J.F.M.; Russel, F.G.M.; Masereeuw, R.; Corstens, F.H.M.; Oyen, W.J.G.; Boerman, O.C. Gelatin-Based Plasma Expander Effectively Reduces Renal Uptake of 111in-Octreotide in Mice and Rats. J. Nucl. Med. 2006, 47, 528–533. [Google Scholar] [PubMed]
- Vegt, E.; Eek, A.; Oyen, W.J.G.; De Jong, M.; Gotthardt, M.; Boerman, O.C. Albumin-Derived Peptides Efficiently Reduce Renal Uptake of Radiolabelled Peptides. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 226. [Google Scholar] [CrossRef] [PubMed]
- Rolleman, E.J.; Krenning, E.P.; Van Gameren, A.; Bernard, B.F.; De Jong, M. Uptake of [111in-DTPA0]Octreotide in the Rat Kidney Is Inhibited by Colchicine and Not by Fructose. J. Nucl. Med. 2004, 45, 709–713. [Google Scholar]
- De Jong, M.; Rolleman, E.J.; Bernard, B.F.; Visser, T.J.; Bakker, W.H.; Breeman, W.A.P.; Krenning, E.P. Inhibition of Renal Uptake of Indium-111-DTPA-Octreotide in Vivo. J. Nucl. Med. 1996, 37, 1388–1392. [Google Scholar]
- Stahl, A.R.; Wagner, B.; Poethko, T.; Perutka, M.; Wester, H.J.; Essler, M.; Heemann, U.; Schwaiger, M.; Lutz, J. Renal Accumulation of [111In]DOTATOC in Rats: Influence of Inhibitors of the Organic Ion Transport and Diuretics. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2129–2134. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. The Efficacy and Safety of Amifostine for the Acute Radiation Syndrome. Expert Opin. Drug Saf. 2019, 18, 1077–1090. [Google Scholar] [CrossRef]
- Hensley, M.L.; Hagerty, K.L.; Kewalramani, T.; Green, D.M.; Meropol, N.J.; Wasserman, T.H.; Cohen, G.I.; Emami, B.; Gradishar, W.J.; Mitchell, R.B.; et al. American Society of Clinical Oncology 2008 Clinical Practice Guideline Update: Use of Chemotherapy and Radiation Therapy Protectants. J. Clin. Oncol. 2009, 27, 127–145. [Google Scholar] [CrossRef]
- Rolleman, E.J.; Forrer, F.; Bernard, B.; Bijster, M.; Vermeij, M.; Valkema, R.; Krenning, E.P.; De Jong, M. Amifostine Protects Rat Kidneys during Peptide Receptor Radionuclide Therapy with [177Lu-DOTA0,Tyr3]Octreotate. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 763–771. [Google Scholar] [CrossRef]
- Kristiansson, A.; Ahlstedt, J.; Holmqvist, B.; Brinte, A.; Tran, T.A.; Forssell-Aronsson, E.; Strand, S.E.; Gram, M.; Akerström, B. Protection of Kidney Function with Human Antioxidation Protein A1-Microglobulin in a Mouse 177Lu-DOTATATE Radiation Therapy Model. Antioxid. Redox Signal. 2019, 30, 1746–1759. [Google Scholar] [CrossRef]
- Ilhan, H.; Wang, H.; Gildehaus, F.J.; Wängler, C.; Herrler, T.; Todica, A.; Schlichtiger, J.; Cumming, P.; Bartenstein, P.; Hacker, M.; et al. Nephroprotective Effects of Enalapril after [177Lu]-DOTATATE Therapy Using Serial Renal Scintigraphies in a Murine Model of Radiation-Induced Nephropathy. EJNMMI Res. 2016, 6, 64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cremonesi, M.; Ferrari, M.; Bodei, L.; Tosi, G.; Paganelli, G. Dosimetry in Peptide Radionuclide Receptor Therapy: A Review. J. Nucl. Med. 2006, 47, 1467–1475. [Google Scholar] [PubMed]
- Rolleman, E.J.; Krenning, E.P.; Bernard, B.F.; De Visser, M.; Bijster, M.; Visser, T.J.; Vermeij, M.; Lindemans, J.; De Jong, M. Long-Term Toxicity of [177Lu-DOTA0,Tyr3]Octreotate in Rats. Eur. J. Nucl. Med. Mol. Imaging 2006, 34, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Sandström, M.; Garske-Román, U.; Johansson, S.; Granberg, D.; Sundin, A.; Freedman, N. Kidney Dosimetry during 177Lu-DOTATATE Therapy in Patients with Neuroendocrine Tumors: Aspects on Calculation and Tolerance. Acta Oncol. 2018, 57, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Arano, Y. Renal Brush Border Strategy: A Developing Procedure to Reduce Renal Radioactivity Levels of Radiolabeled Polypeptides. Nucl. Med. Biol. 2021, 92, 149–155. [Google Scholar] [CrossRef]
- Arano, Y.; Fujioka, Y.; Akizawa, H.; Ono, M.; Uehara, T.; Wakisaka, K.; Nakayama, M.; Sakahara, H.; Konishi, J.; Saji, H. Chemical Design of Radiolabeled Antibody Fragments for Low Renal Radioactivity Levels. Cancer Res. 1999, 59, 128–134. [Google Scholar]
- Akizawa, H.; Imajima, M.; Hanaoka, H.; Uehara, T.; Satake, S.; Arano, Y. Renal Brush Border Enzyme-Cleavable Linkages for Low Renal Radioactivity Levels of Radiolabeled Antibody Fragments. Bioconjug. Chem. 2013, 24, 291–299. [Google Scholar] [CrossRef]
- Zhou, Z.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. An Efficient Method for Labeling Single Domain Antibody Fragments with 18F Using Tetrazine- Trans-Cyclooctene Ligation and a Renal Brush Border Enzyme-Cleavable Linker. Bioconjug. Chem. 2018, 29, 4090–4103. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Kang, C.M.; McDougald, D.; Minn, I.; Brummet, M.; Pomper, M.G.; Zalutsky, M.R. Brush Border Enzyme-Cleavable Linkers: Evaluation for Reducing Renal Uptake of Radiolabeled Prostate-Specific Membrane Antigen Inhibitors. Nucl. Med. Biol. 2018, 62–63, 18. [Google Scholar] [CrossRef]
- Yim, C.B.; Mikkola, K.; Fagerholm, V.; Elomaa, V.V.; Ishizu, T.; Rajander, J.; Schlesinger, J.; Roivainen, A.; Nuutila, P.; Solin, O. Synthesis and Preclinical Characterization of [64Cu]NODAGA-MAL-Exendin-4 with a Nε-Maleoyl-l-Lysyl-Glycine Linkage. Nucl. Med. Biol. 2013, 40, 1006–1012. [Google Scholar] [CrossRef]
- Barros, N.M.T.; Campos, M.; Bersanetti, P.A.; Oliveira, V.; Juliano, M.A.; Boileau, G.; Juliano, L.; Carmona, A.K. Neprilysin Carboxydipeptidase Specificity Studies and Improvement in Its Detection with Fluorescence Energy Transfer Peptides. Biol. Chem. 2007, 388, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, C.; Uehara, T.; Kanazawa, N.; Wada, S.; Suzuki, H.; Arano, Y. Preferential Cleavage of a Tripeptide Linkage by Enzymes on Renal Brush Border Membrane to Reduce Renal Radioactivity Levels of Radiolabeled Antibody Fragments. J. Med. Chem. 2018, 61, 5257–5268. [Google Scholar] [CrossRef]
- Uehara, T.; Yokoyama, M.; Suzuki, H.; Hanaoka, H.; Arano, Y. A Gallium-67/68-Labeled Antibody Fragment for Immuno-SPECT/PET Shows Low Renal Radioactivity Without Loss of Tumor Uptake. Clin. Cancer Res. 2018, 24, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jacobson, O.; Kiesewetter, D.O.; Ma, Y.; Wang, Z.; Lang, L.; Tang, L.; Kang, F.; Deng, H.; Yang, W.; et al. Improving the Theranostic Potential of Exendin 4 by Reducing the Renal Radioactivity through Brush Border Membrane Enzyme-Mediated Degradation. Bioconjug. Chem. 2019, 30, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
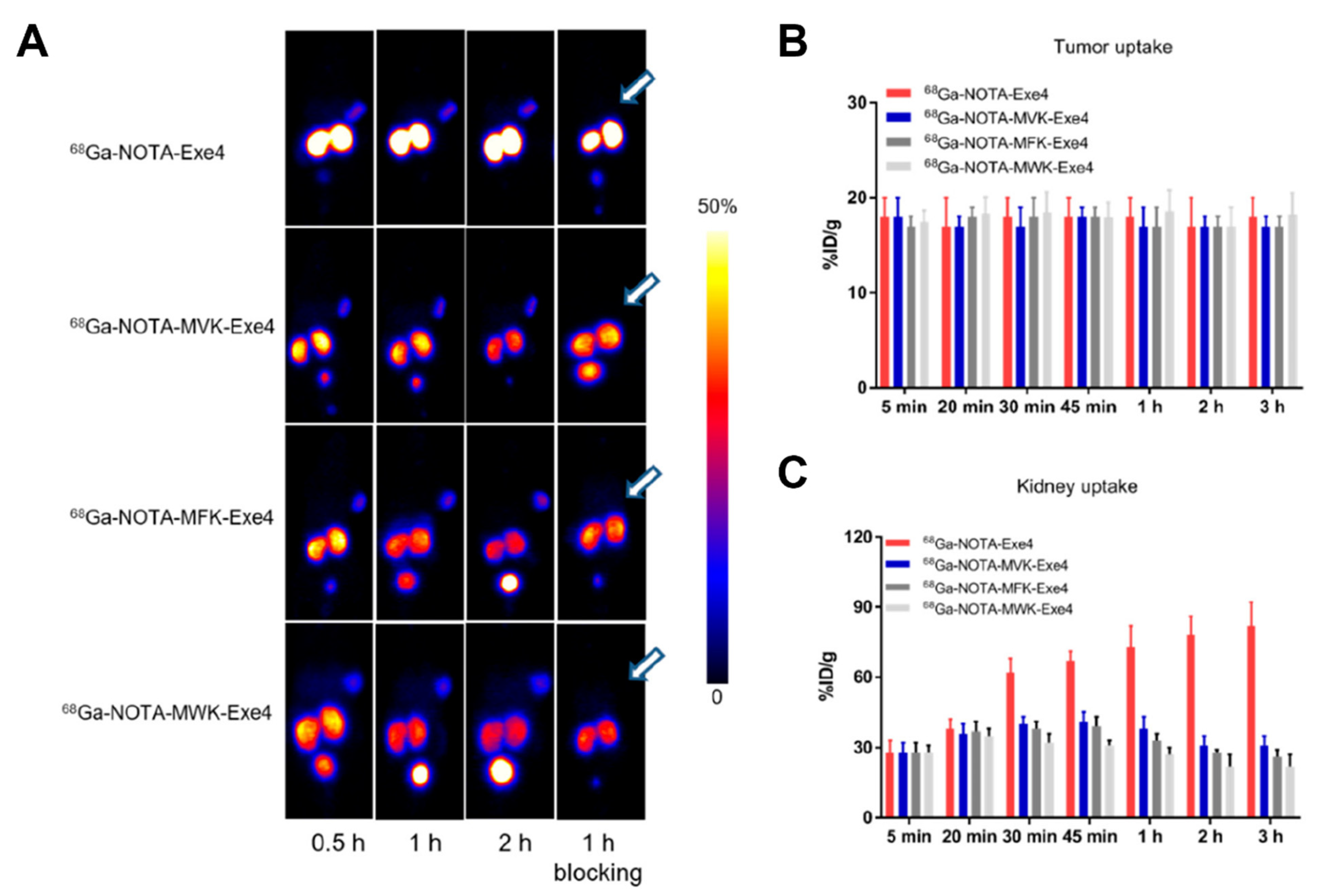
- Bendre, S.; Zhang, Z.; Kuo, H.T.; Rousseau, J.; Zhang, C.; Merkens, H.; Roxin, Á.; Bénard, F.; Lin, K.S. Evaluation of Met-Val-Lys as a Renal Brush Border Enzyme-Cleavable Linker to Reduce Kidney Uptake of 68Ga-Labeled DOTA-Conjugated Peptides and Peptidomimetics. Molecules 2020, 25, 3854. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kise, S.; Kaizuka, Y.; Watanabe, R.; Sugawa, T.; Furukawa, T.; Fujii, H.; Uehara, T. Copper-64-Labeled Antibody Fragments for Immuno-PET/Radioimmunotherapy with Low Renal Radioactivity Levels and Amplified Tumor-Kidney Ratios. ACS Omega 2021, 6, 21556–21562. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.T.; Pan, J.; Zhang, Z.; Lau, J.; Merkens, H.; Zhang, C.; Colpo, N.; Lin, K.S.; Bénard, F. Effects of Linker Modification on Tumor-to-Kidney Contrast of 68 Ga-Labeled PSMA-Targeted Imaging Probes. Mol. Pharm. 2018, 15, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Usmani, B.A.; Harden, B.; Maitland, N.J.; Turner, A.J. Differential Expression of Neutral Endopeptidase-24.11 (Neprilysin) and Endothelin-Converting Enzyme in Human Prostate Cancer Cell Lines. Clin. Sci. 2002, 103, 314S–317S. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, J.; Xie, Z.; Yan, Y.; Wang, J.; Chen, X. Optimization of Enzymolysis Clearance Strategy to Enhance Renal Clearance of Radioligands. Bioconjug. Chem. 2021, 32, 2108–2116. [Google Scholar] [CrossRef]
- Rousseau, J.; Lau, J.; Bénard, F. Radiolabeled Antibodies for Cancer Radioimmunotherapy. In Nuclear Medicine and Immunology; Springer International Publishing: Cham, Switzerland, 2022; pp. 297–345. [Google Scholar]
- Nuttall, S.D.; Walsh, R.B. Display Scaffolds: Protein Engineering for Novel Therapeutics. Curr. Opin. Pharmacol. 2008, 8, 609–615. [Google Scholar] [CrossRef]
- Ganju, R.K.; Sunday, M.; Tsarwhas, D.G.; Card, A.; Shipp, M.A. CD10/NEP in Non-Small Cell Lung Carcinomas. Relationship to Cellular Proliferation. J. Clin. Investig. 1994, 94, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, H.; Shibata, K.; Terauchi, M.; Morita, T.; Ino, K.; Mizutani, S.; Kikkawa, F. Neutral Endopeptidase 24.11/CD10 Suppresses Progressive Potential in Ovarian Carcinoma In Vitro and In Vivo. Clin. Cancer Res. 2005, 11, 1798–1808. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, J.; Lee, H.; Rousseau, J.; Bénard, F.; Lin, K.-S. Application of Cleavable Linkers to Improve Therapeutic Index of Radioligand Therapies. Molecules 2022, 27, 4959. https://doi.org/10.3390/molecules27154959
Lau J, Lee H, Rousseau J, Bénard F, Lin K-S. Application of Cleavable Linkers to Improve Therapeutic Index of Radioligand Therapies. Molecules. 2022; 27(15):4959. https://doi.org/10.3390/molecules27154959
Chicago/Turabian StyleLau, Joseph, Hwan Lee, Julie Rousseau, François Bénard, and Kuo-Shyan Lin. 2022. "Application of Cleavable Linkers to Improve Therapeutic Index of Radioligand Therapies" Molecules 27, no. 15: 4959. https://doi.org/10.3390/molecules27154959
APA StyleLau, J., Lee, H., Rousseau, J., Bénard, F., & Lin, K.-S. (2022). Application of Cleavable Linkers to Improve Therapeutic Index of Radioligand Therapies. Molecules, 27(15), 4959. https://doi.org/10.3390/molecules27154959





