A Small Natural Molecule S3 Protects Retinal Ganglion Cells and Promotes Parkin-Mediated Mitophagy against Excitotoxicity
Abstract
:1. Introduction
2. Results
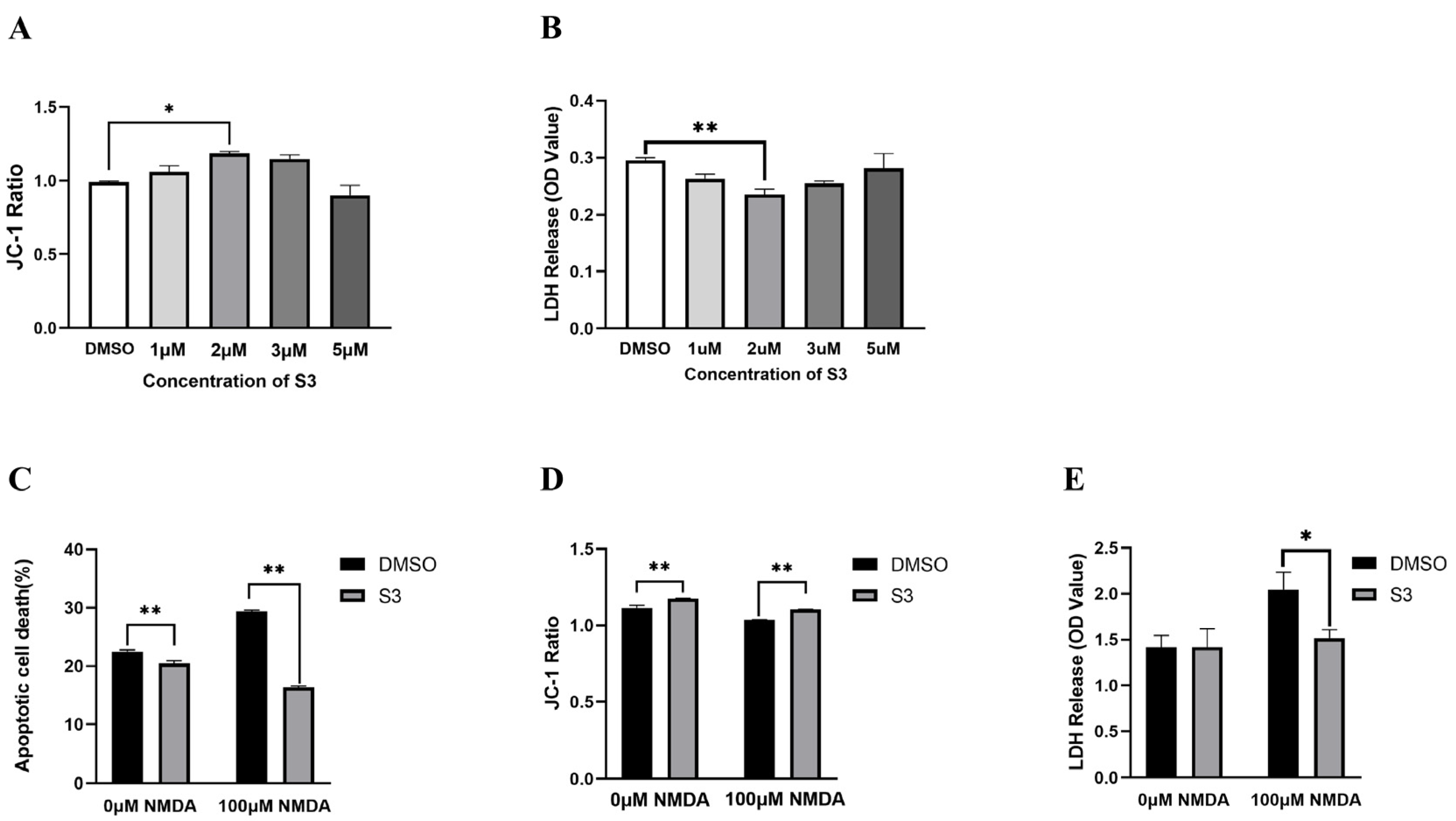
2.1. S3 Protected RGCs from NMDA-Induced Excitotoxicity
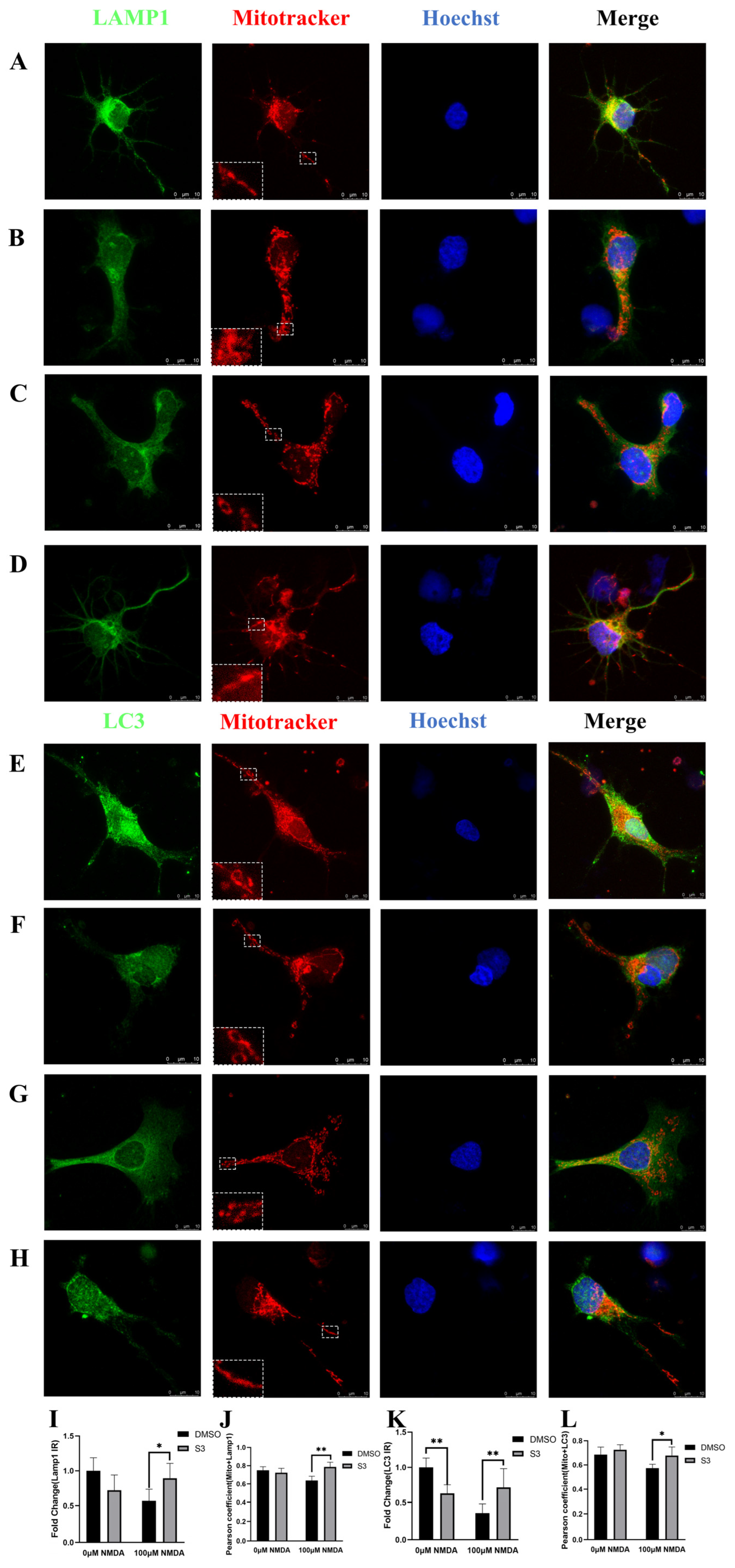
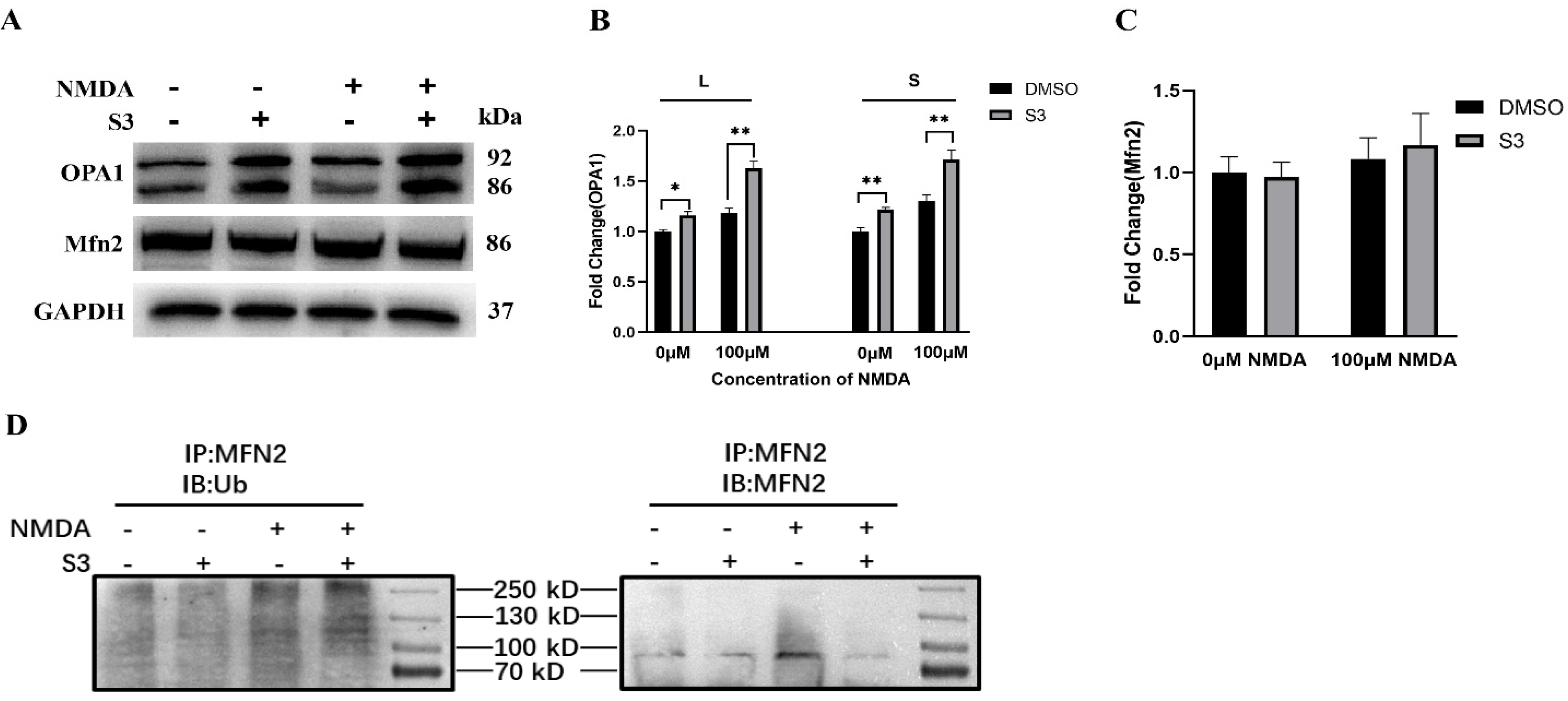
2.2. S3 Improved Mitochondrial Morphology and Mitophagy in RGCs under Excitotoxicity
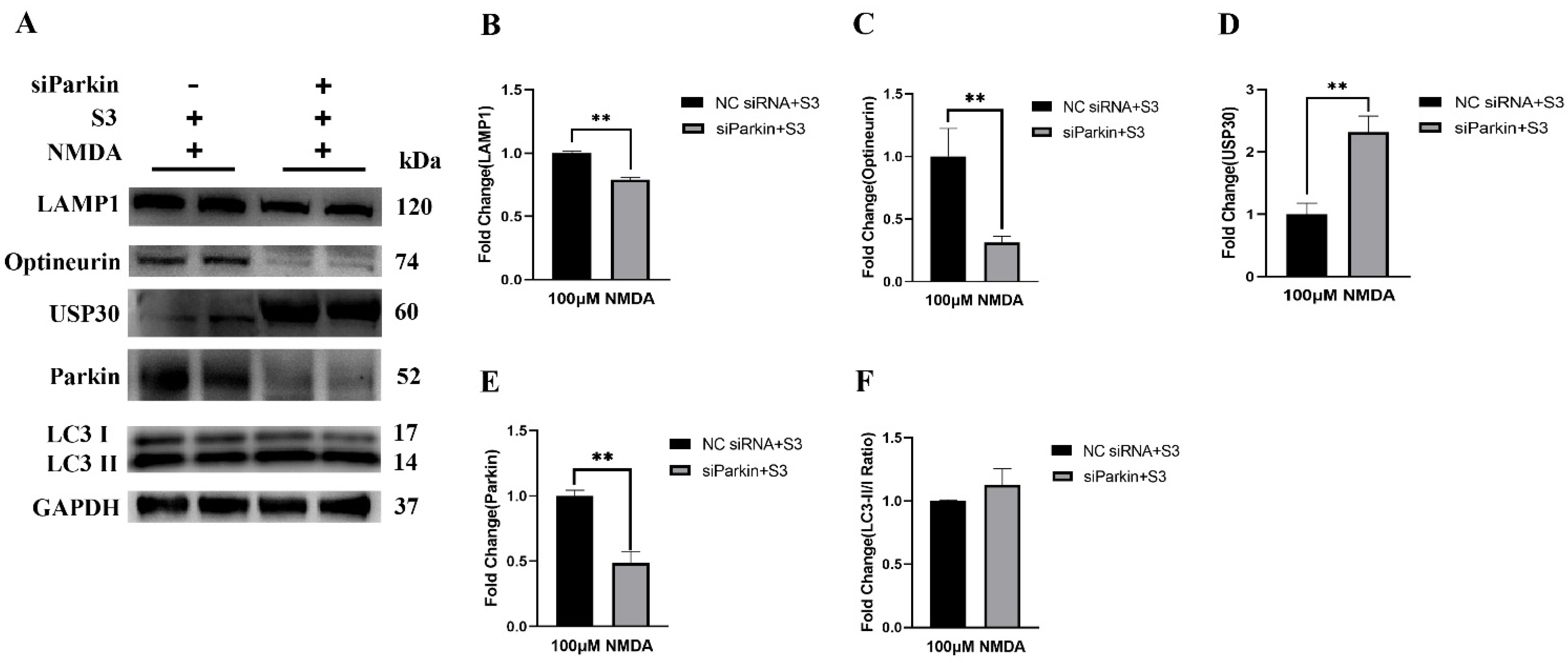
2.3. Parkin Knockdown Reduced the Effect of S3 on RGCs under Excitotoxicity
3. Discussion
4. Materials and Methods
4.1. RGCs Culture and Transfection
4.2. S3 Administration
4.3. Measurement of Mitochondrial Membrane Potential, Cytotoxicity and Apoptosis
4.4. Immunoprecipitation
4.5. Immunofluorescence and Mitotracker Staining
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Seki, M.; Lipton, S.A. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog. Brain Res. 2008, 173, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Lenaers, G.; Neutzner, A.; Le Dantec, Y.; Jüschke, C.; Xiao, T.; Decembrini, S.; Swirski, S.; Kieninger, S.; Agca, C.; Kim, U.S.; et al. Dominant optic atrophy: Culprit mitochondria in the optic nerve. Prog. Retin. Eye Res. 2021, 83, 100935. [Google Scholar] [CrossRef] [PubMed]
- Jassim, A.H.; Inman, D.M.; Mitchell, C.H. Crosstalk Between Dysfunctional Mitochondria and Inflammation in Glaucomatous Neurodegeneration. Front. Pharmacol. 2021, 12, 699623. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.N.; Baughman, J.M.; Phu, L.; Tea, J.S.; Yu, C.; Coons, M.; Kirkpatrick, D.S.; Bingol, B.; Corn, J.E. USP30 and parkin homeostatically regulate atypical ubiquitin chains on mitochondria. Nat. Cell Biol. 2015, 17, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Serricchio, M.; Jauregui, M.; Shanbhag, R.; Stoltz, T.; Di Paolo, C.T.; Kim, P.K.; McQuibban, G.A. Deubiquitinating enzymes regulate PARK2-mediated mitophagy. Autophagy 2015, 11, 595–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gersch, M.; Gladkova, C.; Schubert, A.F.; Michel, M.A.; Maslen, S.; Komander, D. Mechanism and regulation of the Lys6-selective deubiquitinase USP30. Nat. Struct. Mol. Biol. 2017, 24, 920–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Hu, X.; Sun, X. Overexpression of parkin protects retinal ganglion cells in experimental glaucoma. Cell Death Dis. 2018, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Zhuang, D.; Zhang, R.; Sun, X.; Lu, Q.; Dai, Y. The small molecule inhibitor PR-619 protects retinal ganglion cells against glutamate excitotoxicity. Neuroreport 2020, 31, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Bingol, B.; Tea, J.S.; Phu, L.; Reichelt, M.; Bakalarski, C.E.; Song, Q.; Foreman, O.; Kirkpatrick, D.S.; Sheng, M. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 2014, 510, 370–375. [Google Scholar] [CrossRef] [PubMed]
- He, H.P.; Shen, Y.M.; Zhang, J.X.; Zuo, G.Y.; Hao, X.J. New diterpene alkaloids from the roots of Spiraea japonica. J. Nat. Prod. 2001, 64, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Chen, Z.; Liu, H.; Yan, C.; Chen, M.; Feng, D.; Yan, C.; Wu, H.; Du, L.; Wang, Y.; et al. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 2014, 24, 482–496. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, W.; Li, R.; Yang, H. Mitophagy in Parkinson’s Disease: From Pathogenesis to Treatment. Cells 2019, 8, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Eldeeb, M.; Wang, X. Beyond Deubiquitylation: USP30-Mediated Regulation of Mitochondrial Homeostasis. Adv. Exp. Med. Biol. 2017, 1038, 133–148. [Google Scholar] [CrossRef]
- Xian, H.; Liou, Y.C. Functions of outer mitochondrial membrane proteins: Mediating the crosstalk between mitochondrial dynamics and mitophagy. Cell Death Differ. 2021, 28, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Dai, Y.; Zhang, R.; Shang, K.; Sun, X. Overexpression of Optic Atrophy Type 1 Protects Retinal Ganglion Cells and Upregulates Parkin Expression in Experimental Glaucoma. Front. Mol. Neurosci. 2018, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Müller-Rischart, A.K.; Pilsl, A.; Beaudette, P.; Patra, M.; Hadian, K.; Funke, M.; Peis, R.; Deinlein, A.; Schweimer, C.; Kuhn, P.-H.; et al. The E3 ligase parkin maintains mitochondrial integrity by increasing linear ubiquitination of NEMO. Mol. Cell 2013, 49, 908–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Dai, Y.; Sun, X. Parkin overexpression protects retinal ganglion cells against glutamate excitotoxicity. Mol. Vis. 2017, 23, 447–456. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, D.; Zhang, R.; Liu, H.; Dai, Y. A Small Natural Molecule S3 Protects Retinal Ganglion Cells and Promotes Parkin-Mediated Mitophagy against Excitotoxicity. Molecules 2022, 27, 4957. https://doi.org/10.3390/molecules27154957
Zhuang D, Zhang R, Liu H, Dai Y. A Small Natural Molecule S3 Protects Retinal Ganglion Cells and Promotes Parkin-Mediated Mitophagy against Excitotoxicity. Molecules. 2022; 27(15):4957. https://doi.org/10.3390/molecules27154957
Chicago/Turabian StyleZhuang, Dongli, Rong Zhang, Haiyang Liu, and Yi Dai. 2022. "A Small Natural Molecule S3 Protects Retinal Ganglion Cells and Promotes Parkin-Mediated Mitophagy against Excitotoxicity" Molecules 27, no. 15: 4957. https://doi.org/10.3390/molecules27154957
APA StyleZhuang, D., Zhang, R., Liu, H., & Dai, Y. (2022). A Small Natural Molecule S3 Protects Retinal Ganglion Cells and Promotes Parkin-Mediated Mitophagy against Excitotoxicity. Molecules, 27(15), 4957. https://doi.org/10.3390/molecules27154957





