Classification of Japanese Pepper (Zanthoxylum piperitum DC.) from Different Growing Regions Based on Analysis of Volatile Compounds and Sensory Evaluation
Abstract
:1. Introduction
2. Results
2.1. Analysis of Volatile Compounds
2.2. Sensory Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction of Volatile Compounds
4.3. Analysis of Volatile Compounds
4.4. Sensory Evaluation
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jiang, L.; Kubota, K. Differences in the volatile components and their odor characteristics of green and ripe fruits and dried pericarp of Japanese pepper (Xanthoxylum piperitum DC.). J. Agric. Food Chem. 2004, 52, 4197–4203. [Google Scholar] [CrossRef] [PubMed]
- Sugai, E.; Morimitsu, Y.; Kubota, K. Quantitative analysis of sanshool compounds in Japanese pepper (Xanthoxylum piperitum DC.) and their pungent characteristics. Biosci. Biotechnol. Biochem. 2005, 69, 1958–1962. [Google Scholar] [CrossRef] [Green Version]
- Sakai, T.; Yoshihara, K.; Hirose, Y. Constituents of fruit oil from Japanese pepper. Bull. Chem. Soc. Jpn. 1968, 41, 1945–1950. [Google Scholar] [CrossRef]
- Sakai, T.; Yoshihara, K.; Hirose, Y. A comparative study of the constituents of volatile oils of Zanthoxylum. Bull. Chem. Soc. Jpn. 1970, 43, 484–487. [Google Scholar] [CrossRef] [Green Version]
- Small, D.M.; Gerber, J.C.; Mak, Y.E.; Hummel, T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 2005, 47, 593–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential oils and health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Wu, Y.; Zhang, Y.; Xie, G.; Zhao, A.; Pan, X.; Chen, T.; Hu, Y.; Liu, Y.; Cheng, Y.C.; Yao, L.; et al. The metabolic responses to aerial diffusion of essential oils. PLoS ONE 2012, 7, e44830. [Google Scholar] [CrossRef] [Green Version]
- Yadav, N.; Chandra, H. Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1,8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFκB. PLoS ONE 2017, 12, e0188232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, C.A.; Bidinotto, L.T.; Takahira, R.K.; Salvadori, D.M.; Barbisan, L.F.; Costa, M. Cholesterol reduction and lack of genotoxic or toxic effects in mice after repeated 21-day oral intake of lemongrass (Cymbopogon citratus) essential oil. Food Chem. Toxicol. 2011, 49, 2268–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Choi, K.C. R-Limonene Enhances Differentiation and 2-Deoxy-D-Glucose Uptake in 3T3-L1 Preadipocytes by Activating the Akt Signaling Pathway. Evid. Based Complement. Alternat. Med. 2018, 2018, 4573254. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.M.C.; Vieira, A.J.; Beserra, F.P.; Pellizzon, C.H.; Nóbrega, R.H.; Rozza, A.L. Gastroprotective effect of limonene in rats: Influence on oxidative stress, inflammation and gene expression. Phytomedicine 2019, 53, 37–42. [Google Scholar] [CrossRef] [PubMed]
- D’ Alessio, P.A.; Ostan, R.; Bisson, J.-F.; Schulzke, J.D.; Ursini, M.V.; Béné, M.C. Oral administration of d-Limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013, 92, 1151–1156. [Google Scholar] [CrossRef]
- Miller, J.A.; Pappan, K.; Thompson, P.A.; Want, E.J.; Siskos, A.P.; Keun, H.C.; Wulff, J.; Hu, C.; Lang, J.E.; Chow, H.-H.S. Plasma metabolomic profiles of breast cancer patients after short-term limonene intervention. Cancer Prev. Res. 2015, 8, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.A.; Lang, J.E.; Ley, M.; Nagle, R.; Hsu, C.H.; Thompson, P.A.; Cordova, C.; Waer, A.; Chow, H.-H.S. Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer Prev. Res. 2013, 6, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Qiu, Y.; Liu, Y.; Huang, N.; Hua, C.; Wang, Q.; Wu, Z.; Lu, J.; Song, P.; Xu, J.; et al. Citronellal ameliorates doxorubicin-induced hepatotoxicity via antioxidative stress, antiapoptosis, and proangiogenesis in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22639. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, L.; Chen, J.; Sui, J.; Yi, G.; Wu, J.; Ma, Y. Correlation between chemical composition and antifungal activity of Clausena lansium essential oil against Candida spp. Molecules 2019, 24, 1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, F.; Yan, Q.; Zheng, Z.; Liu, J.; Chen, Y.; Zhang, G. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J. BUON 2018, 23, 346–352. [Google Scholar]
- Anderson, C.A.; Cobb, L.K.; Miller, E.R.; Woodward, M.; Hottenstein, A.; Chang, A.R.; Mongraw-Chaffin, M.; White, K.; Charleston, J.; Tanaka, T.; et al. Effects of a behavioral intervention that emphasizes spices and herbs on adherence to recommended sodium intake: Results of the SPICE randomized clinical trial. Am. J. Clin. Nutr. 2015, 102, 671–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hizume, A.; Yagi, T.; Aoyagi, M.; Harada, A.; Fushiki, T. Effectiveness of ripe and unripe sansho extracts on salt reduction. J. Cook. Sci. Jpn. 2021, 54, 215–223. [Google Scholar] [CrossRef]
- Premarathne, M.D.G.P.; Fukutome, N.; Yamasaki, K.; Hayakawa, F.; Nagano, A.J.; Mizuno, H.; Ibaragi, N.; Nagano, Y. Elucidation of Japanese pepper (Zanthoxylum piperitum De Candolle) domestication using RAD-Seq. Sci. Rep. 2021, 11, 6464. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.; Sandasi, M.; Tankeu, S.; van Vuuren, S.; Viljoen, A. Headspace analysis and characterisation of South African propolis volatile compounds using GCxGC–ToF–MS. Rev. Bras. Farmacogn. 2019, 29, 351–357. [Google Scholar] [CrossRef]
- Gad, H.A.; Mamadalieva, N.Z.; Bohmdorfer, S.; Rosenau, T.; Zengin, G.; Mamadalieva, R.Z.; AI Musayeib, N.M.; Ashour, M.L. GC-MS based identification of the volatile components of six Astragalus species from Uzbekistan and their biological activity. Plants 2021, 10, 124. [Google Scholar] [CrossRef]
- Aros, D.; Garrido, N.; Rivas, C.; Medel, M.; Müller, C.; Rogers, H.; Úbeda, C. Floral scent evaluation of three cut flowers through sensorial and gas chromatography analysis. Agronomy 2010, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Sawamura, M.; Thi Minh Tu, N.; Onishi, Y.; Ogawa, E.; Choi, H.S. Characteristic odor components of Citrus reticulata Blanco (ponkan) cold-pressed oil. Biosci. Biotechnol. Biochem. 2004, 68, 1690–1697. [Google Scholar] [CrossRef] [Green Version]
- Wijaya, C.H.; Hadiprodjo, I.T.; Apriyantono, A. Identification of volatile compounds and key aroma compounds of andaliman fruit (Zanthoxylum acanthopodium DC). Food Sci. Biotechnol. 2002, 2, 680–683. [Google Scholar]
- Kitamoto, T.; Matsumoto, T.; Roppongi, M.; Watanabe, S.; Soeta, Y.; Hasegawa, H. Effect of Temperature and Density on Evaluation of Lemon Aroma. Trans. Jpn. Soc. Kansei Eng. 2017, 16, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Buttery, R.G.; Ling, L.C.; Light, D.M. Tomato leaf volatile aroma components. J. Agric. Food Chem. 1987, 35, 1039–1042. [Google Scholar] [CrossRef]
- Jang, H.J.; Son, H.H.; Lee, D.S. Optimization of disk sorptive extraction based on monolithic material for the determination of aroma compounds from Lantana camara L. by gas chromatography-mass spectrometry. Bull. Korean Chem. Soc. 2011, 32, 4275–4280. [Google Scholar] [CrossRef] [Green Version]
- Risvik, E.; McEwan, J.A.; Colwill, J.S.; Rogers, R.; Lyon, D.H. Projective mapping: A tool for sensory analysis and consumer research. Food Qual. Prefer. 1994, 5, 263–269. [Google Scholar] [CrossRef]
- Valentin, D.; Chollet, S.; Lelièvre, M.; Abdi, H. Quick and dirty but still pretty good: A review of new descriptive methods in food science. Int. J. Food Sci. Technol. 2012, 47, 1563–1578. [Google Scholar] [CrossRef]
- Le, S.; Le, T.M.; Cadoret, M. Napping and sorted Napping as a sensory profiling technique. In Rapid Sensory Profiling Techniques and Related Methods; Delarue, J., Lawlor, J.B., Rogeaux, M., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 197–213. [Google Scholar]
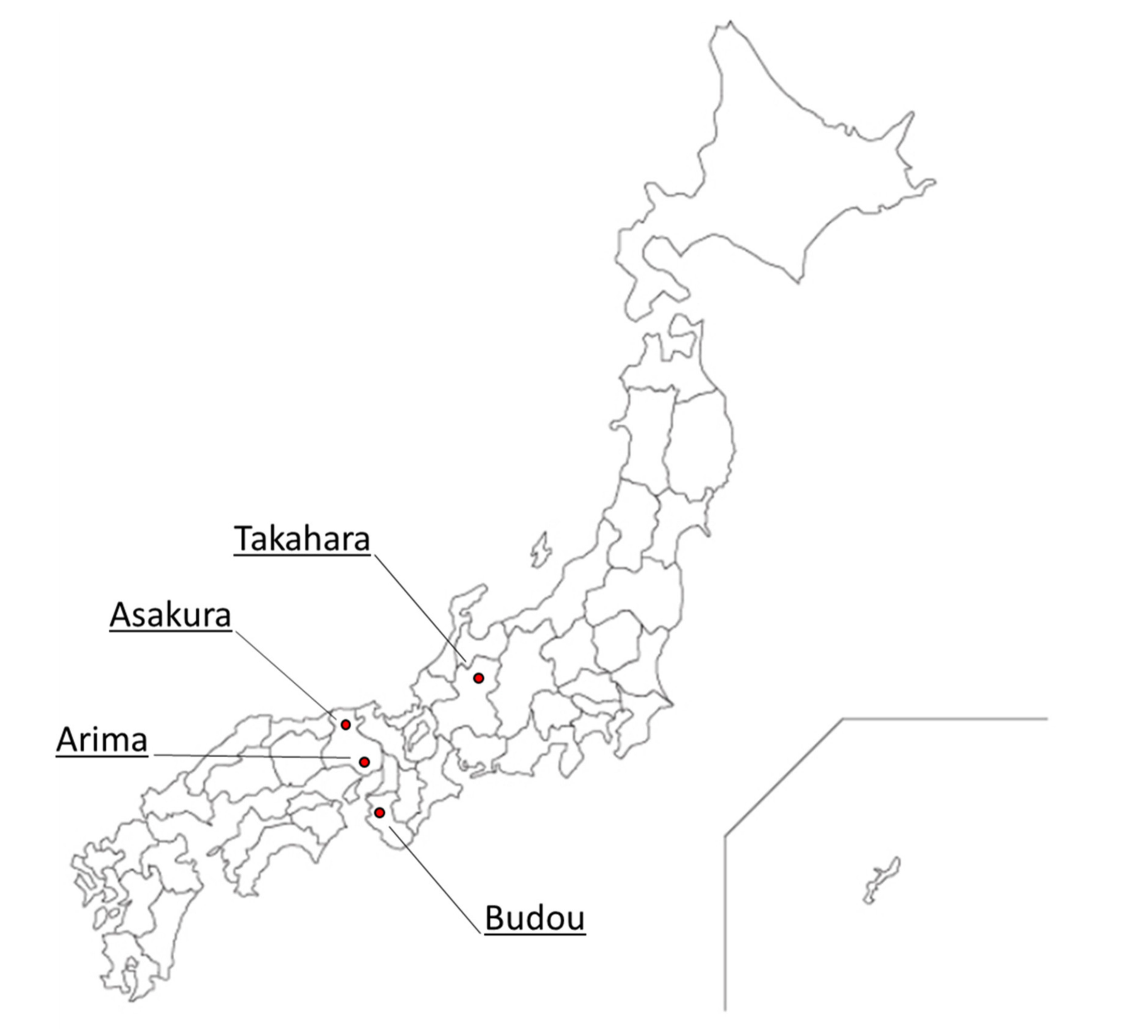
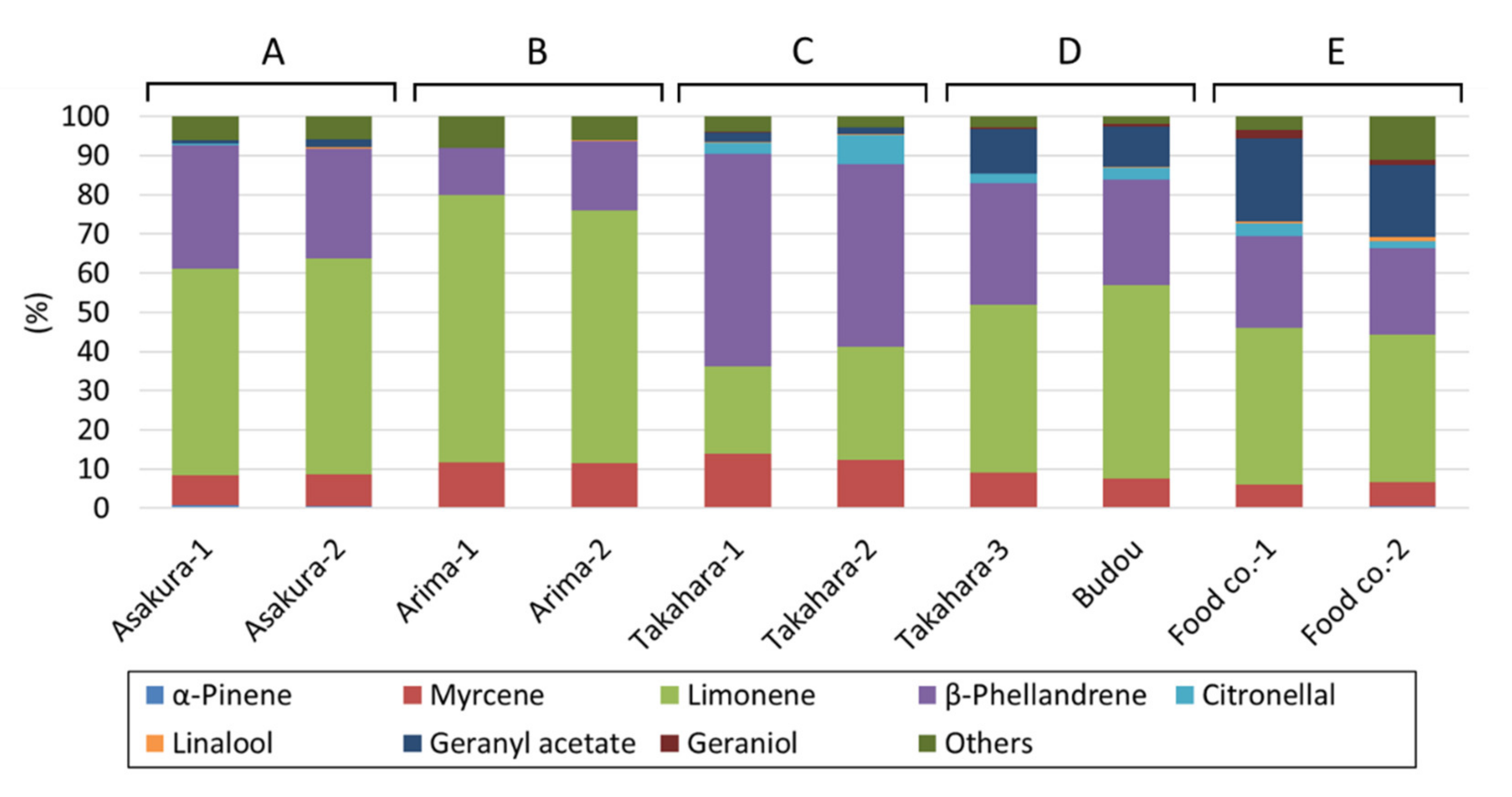
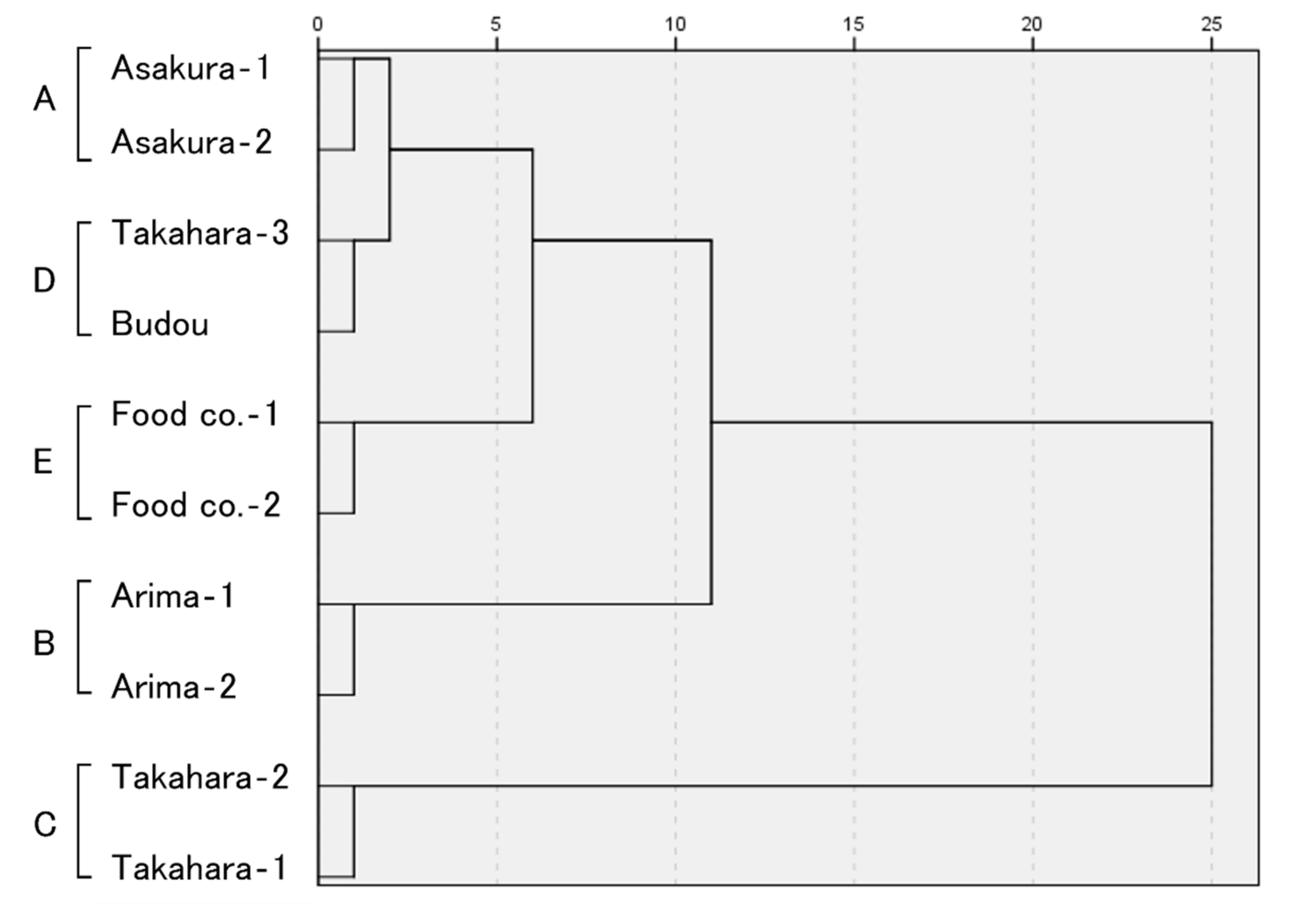

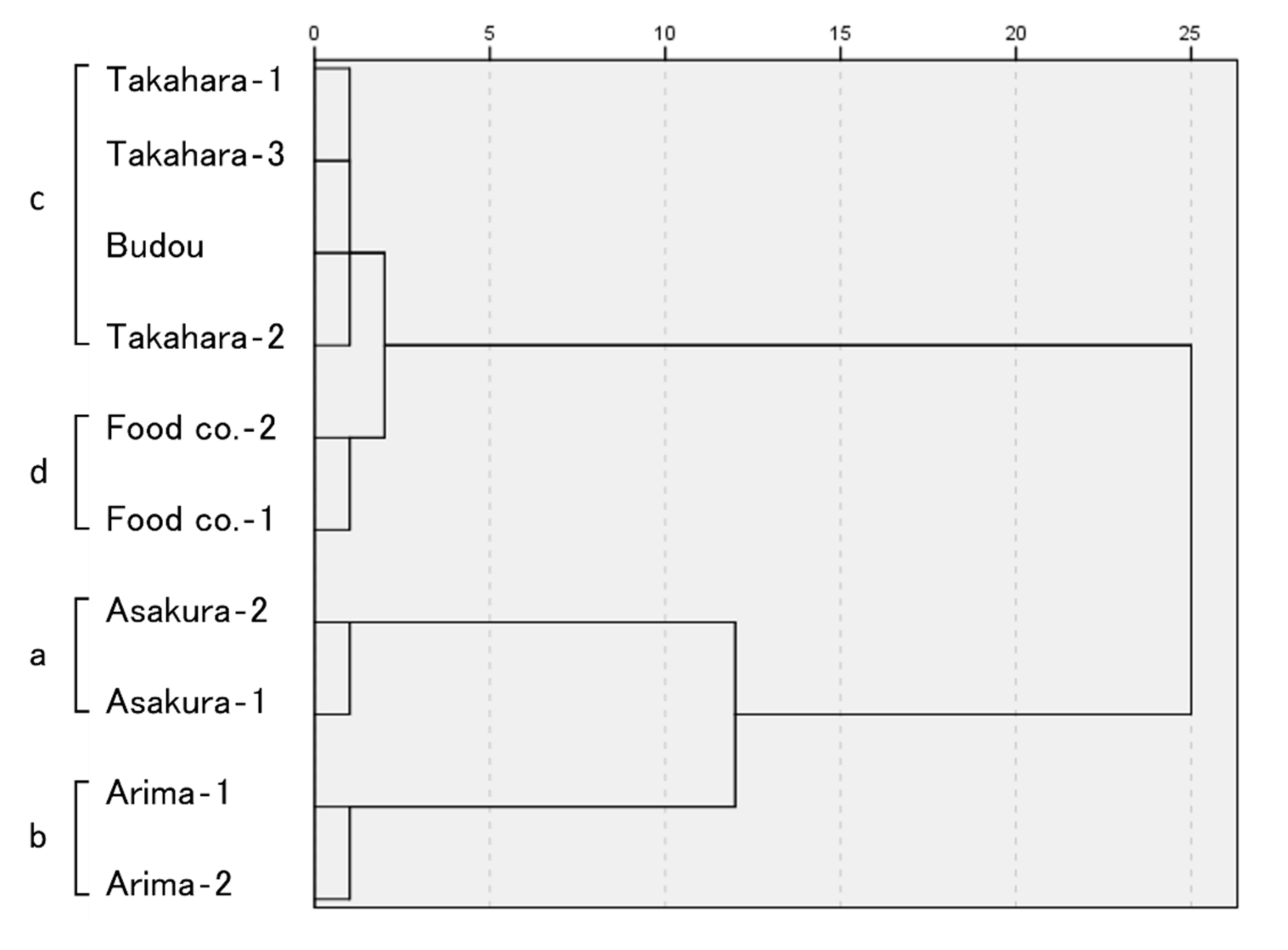
| No. | Product | GC and GC/MS Analysis | Sensory Evaluation |
|---|---|---|---|
| 1 | Asakura-1 | A | a |
| 2 | Asakura-2 | A | a |
| 3 | Arima-1 | B | b |
| 4 | Arima-2 | B | b |
| 5 | Takahara-1 | C | c |
| 6 | Takahara-2 | C | c |
| 7 | Takahara-3 | D | c |
| 8 | Budou | D | c |
| 9 | Major food manufacturing company in Japan-1 (Food co.-1) | E | d |
| 10 | Major food manufacturing company in Japan-2 (Food co.-2) | E | d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamasaki, K.; Fukutome, N.; Hayakawa, F.; Ibaragi, N.; Nagano, Y. Classification of Japanese Pepper (Zanthoxylum piperitum DC.) from Different Growing Regions Based on Analysis of Volatile Compounds and Sensory Evaluation. Molecules 2022, 27, 4946. https://doi.org/10.3390/molecules27154946
Yamasaki K, Fukutome N, Hayakawa F, Ibaragi N, Nagano Y. Classification of Japanese Pepper (Zanthoxylum piperitum DC.) from Different Growing Regions Based on Analysis of Volatile Compounds and Sensory Evaluation. Molecules. 2022; 27(15):4946. https://doi.org/10.3390/molecules27154946
Chicago/Turabian StyleYamasaki, Kazuaki, Nami Fukutome, Fumiyo Hayakawa, Nobuo Ibaragi, and Yukio Nagano. 2022. "Classification of Japanese Pepper (Zanthoxylum piperitum DC.) from Different Growing Regions Based on Analysis of Volatile Compounds and Sensory Evaluation" Molecules 27, no. 15: 4946. https://doi.org/10.3390/molecules27154946
APA StyleYamasaki, K., Fukutome, N., Hayakawa, F., Ibaragi, N., & Nagano, Y. (2022). Classification of Japanese Pepper (Zanthoxylum piperitum DC.) from Different Growing Regions Based on Analysis of Volatile Compounds and Sensory Evaluation. Molecules, 27(15), 4946. https://doi.org/10.3390/molecules27154946







