Atomic-Scale Tracking of Dynamic Nucleation and Growth of an Interfacial Lead Nanodroplet
Abstract
:1. Introduction
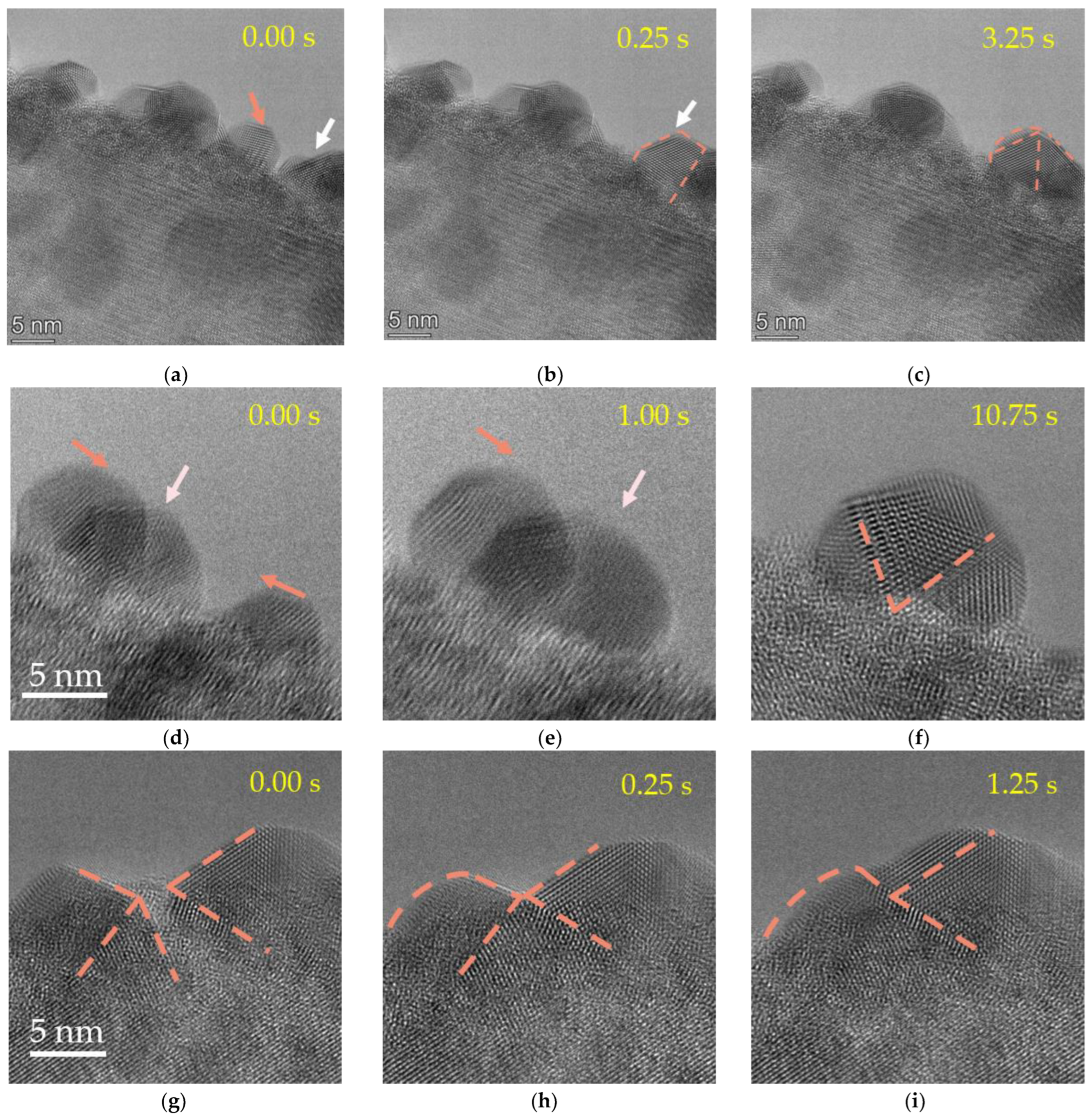
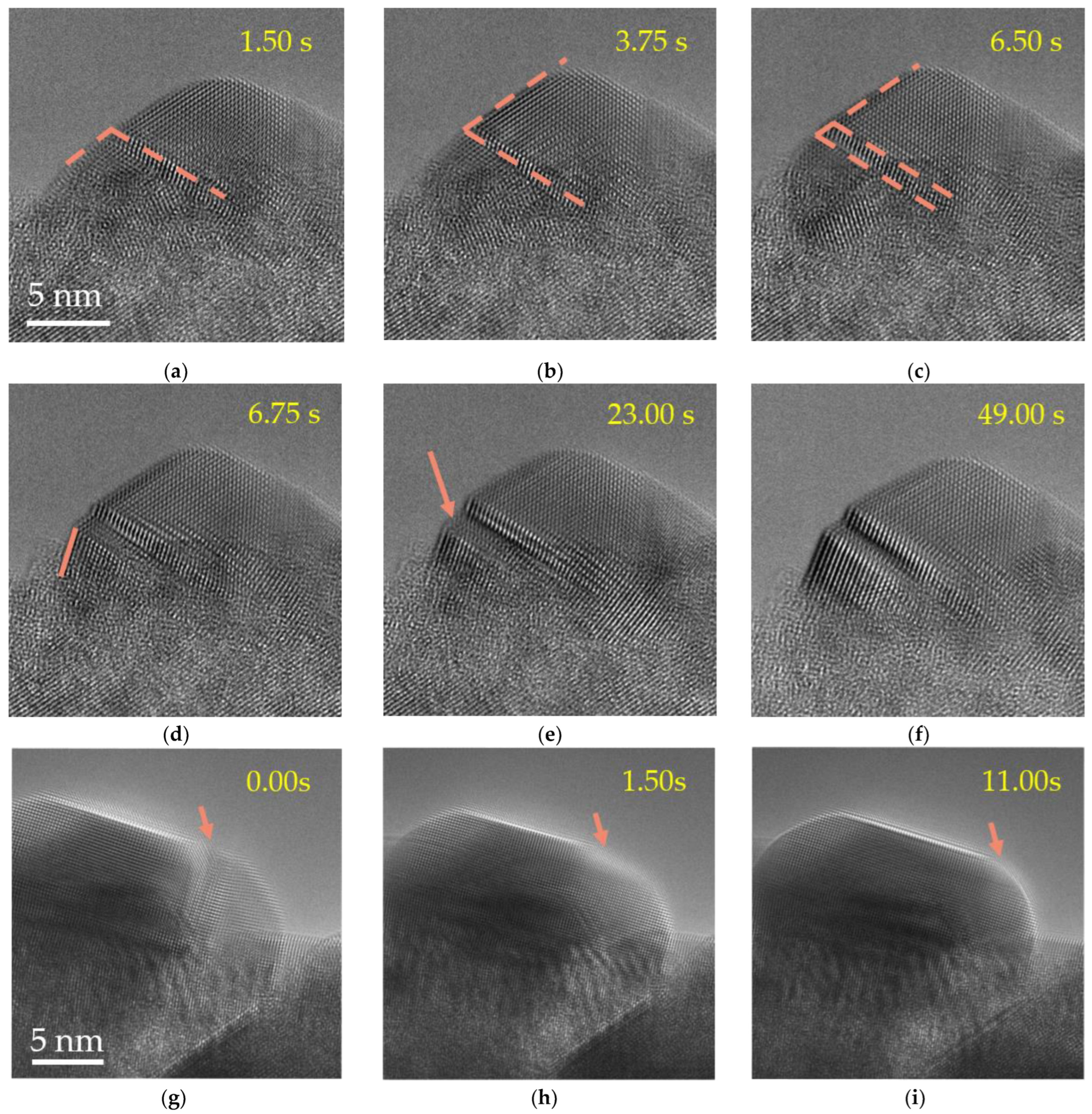
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Microscopic evidence for liquid-liquid separation in supersaturated CaCO3 solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Driessche, A.; Benning, L.G.; Rodriguez-Blanco, J.; Ossorio, M.; Bots, P.; García-Ruiz, J. The role and implications of bassanite as a stable precursor phase to gypsum precipitation. Science 2012, 336, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, D.; Lee, A.Y.; Myerson, A.S. Nucleation of crystals from solution: Classical and two-step models. Acc. Chem. Res. 2009, 42, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Li, Q.; Wang, Z.; Deepak, F.L. Atomic-scale dynamic observation reveals temperature-dependent multistep nucleation pathways in crystallization. Nanoscale Horiz. 2019, 4, 1302–1309. [Google Scholar] [CrossRef]
- Jacobson, L.C.; Molinero, V. Can amorphous nuclei grow crystalline clathrates? The size and crystallinity of critical clathrate nuclei. J. Am. Chem. Soc. 2011, 133, 6458–6463. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Deepak, F.L. In situ atomic-scale observation of droplet coalescence driven nucleation and growth at liquid/solid interfaces. ACS Nano 2017, 11, 5590–5597. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Wang, L.; Dong, W.; Yao, L.; Chen, Y.; Dong, L.; Zheng, K.; Han, X. In situ TEM revealing pretreatment and interface effects in Ge2Sb2Te5. Appl. Phys. Lett. 2020, 116, 222105. [Google Scholar] [CrossRef]
- Shpyrko, O.G.; Streitel, R.; Balagurusamy, V.S.; Grigoriev, A.Y.; Deutsch, M.; Ocko, B.M.; Meron, M.; Lin, B.; Pershan, P.S. Surface crystallization in a liquid AuSi alloy. Science 2006, 313, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Sleutel, M.; Van Driessche, A.E. Role of clusters in nonclassical nucleation and growth of protein crystals. Proc. Natl. Acad. Sci. 2014, 111, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, Z.; Li, Y.; Deepak, F.L. In situ atomic-scale observation of kinetic pathways of sublimation in silver nanoparticles. Adv. Sci. 2019, 6, 1802131. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Wang, Z.; Deepak, F.L. Real-Time Dynamical Observation of Lattice Induced Nucleation and Growth in Interfacial Solid–Solid Phase Transitions. Cryst. Growth Des. 2016, 16, 7256–7262. [Google Scholar] [CrossRef]
- Kádárová, J.; Janeková, J.; Suhányiová, A. Possibilities to Increase Assembly Line Productivity Using Different Management Approaches. Processes 2022, 10, 553. [Google Scholar] [CrossRef]
- Shao, R.; Gao, P.; Zheng, K. The effect of tensile and bending strain on the electrical properties of p-type<110> silicon nanowires. Nanotechnology 2015, 26, 265703. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.; Zaziski, D.; Scher, E.C.; Herhold, A.B.; Paul Alivisatos, A. Activation volumes for solid-solid transformations in nanocrystals. Science 2001, 293, 1803–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asta, M.; Hoyt, J.; Karma, A. Calculation of alloy solid-liquid interfacial free energies from atomic-scale simulations. Phys. Rev. B 2002, 66, 100101. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Purans, J.; Jia, R. Comparative Hybrid Hartree-Fock-DFT Calculations of WO2-Terminated Cubic WO3 as Well as SrTiO3, BaTiO3, PbTiO3 and CaTiO3 (001) Surfaces. Crystals 2021, 11, 455. [Google Scholar] [CrossRef]
- Li, Y.; Huang, M.; Zang, L.; Jacobs, D.L.; Zhao, J.; Zhu, Y.; Wang, C. Real-Time Atomic Scale Observation of Surface-Induced Crystallization of a Bismuth Nanodroplet by Stepwise Ordering Mechanism. Cryst. Growth Des. 2018, 18, 5808–5815. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Smith, R.K.; Jun, Y.-W.; Kisielowski, C.; Dahmen, U.; Alivisatos, A.P. Observation of single colloidal platinum nanocrystal growth trajectories. Science 2009, 324, 1309–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, M.; Yuk, J.M.; Lee, J.Y. Observation of surface atoms during platinum nanocrystal growth by monomer attachment. Chem. Mater. 2015, 27, 3200–3202. [Google Scholar] [CrossRef]
- Graham, P.J.; Farhangi, M.M.; Dolatabadi, A. Dynamics of droplet coalescence in response to increasing hydrophobicity. Phys. Fluids 2012, 24, 112105. [Google Scholar] [CrossRef]
- Klopp, C.; Trittel, T.; Stannarius, R. Self similarity of liquid droplet coalescence in a quasi-2D free-standing liquid-crystal film. Soft Matter 2020, 16, 4607–4614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Bunes, B.R.; Zang, L.; Zhao, J.; Li, Y.; Zhu, Y.; Wang, C. Atomic scale imaging of nucleation and growth trajectories of an interfacial bismuth nanodroplet. ACS Nano 2016, 10, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Cajigas, S.; Orozco, J. Nanobioconjugates for Signal Amplification in Electrochemical Biosensing. Molecules 2020, 25, 3542. [Google Scholar] [CrossRef] [PubMed]
- Oprisan, A.; Garrabos, Y.; Lecoutre, C.; Beysens, D. Pattern Evolution during Double Liquid-Vapor Phase Transitions under Weightlessness. Molecules 2017, 22, 947. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Hu, J.; Yang, Z.; Li, X.; Zhang, H.; Zhang, G. Dielectric Properties Investigation of Metal–Insulator–Metal (MIM) Capacitors. Molecules 2022, 27, 3951. [Google Scholar] [CrossRef]
- Nanda, K.; Sahu, S.; Behera, S. Liquid-drop model for the size-dependent melting of low-dimensional systems. Phys. Rev. A 2002, 66, 013208. [Google Scholar] [CrossRef]
- Nanda, K.K. Liquid-drop model for the surface energy of nanoparticles. Phys. Rev. A 2012, 376, 1647–1649. [Google Scholar] [CrossRef]
- Lee, S.-T.; Lin, Z.; Jiang, X. CVD diamond films: Nucleation and growth. Mater. Sci. Eng. R 1999, 25, 123–154. [Google Scholar] [CrossRef]

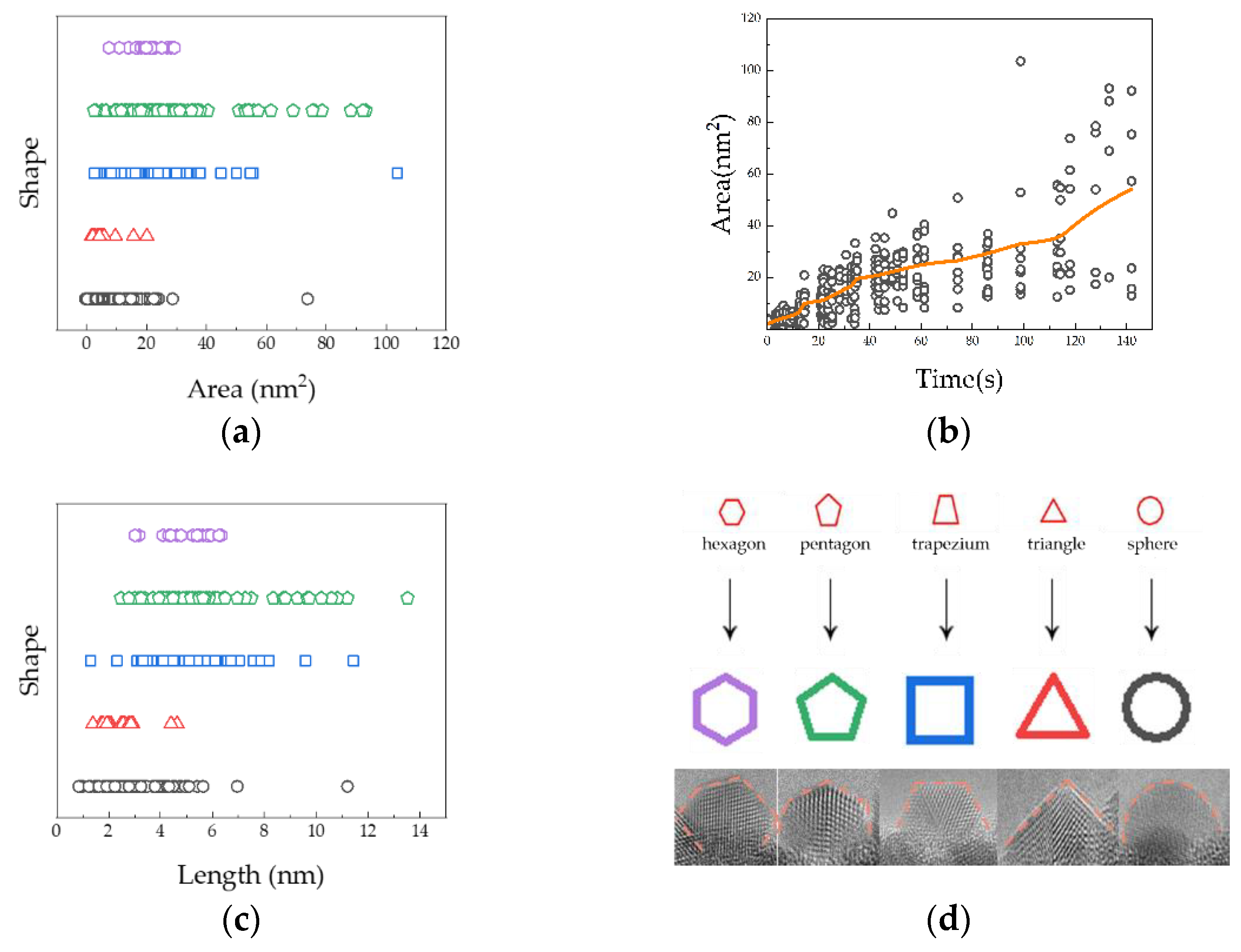
| Title 1 | Hemispherical | Triangular | Trapezoidal | Pentagon | Hexagon |
|---|---|---|---|---|---|
| Area mean (nm2) | 3.00 | 2.46 | 4.88 | 5.80 | 5.01 |
| Area standard deviation area | ±1.19 | ±0.95 | ±1.20 | ±1.39 | ±0.97 |
| Length mean (nm) | 8.47 | 5.63 | 19.80 | 28.73 | 20.45 |
| Length standard deviation area | ±2.92 | ±2.25 | ±3.65 | ±4.43 | ±2.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Sun, C.; Ran, L.; Cai, R.; Shao, R. Atomic-Scale Tracking of Dynamic Nucleation and Growth of an Interfacial Lead Nanodroplet. Molecules 2022, 27, 4877. https://doi.org/10.3390/molecules27154877
Chang X, Sun C, Ran L, Cai R, Shao R. Atomic-Scale Tracking of Dynamic Nucleation and Growth of an Interfacial Lead Nanodroplet. Molecules. 2022; 27(15):4877. https://doi.org/10.3390/molecules27154877
Chicago/Turabian StyleChang, Xiaoxue, Chunhao Sun, Leguan Ran, Ran Cai, and Ruiwen Shao. 2022. "Atomic-Scale Tracking of Dynamic Nucleation and Growth of an Interfacial Lead Nanodroplet" Molecules 27, no. 15: 4877. https://doi.org/10.3390/molecules27154877
APA StyleChang, X., Sun, C., Ran, L., Cai, R., & Shao, R. (2022). Atomic-Scale Tracking of Dynamic Nucleation and Growth of an Interfacial Lead Nanodroplet. Molecules, 27(15), 4877. https://doi.org/10.3390/molecules27154877





