Surfactant-Assisted Assembly of Dipeptide Forming a Broom-like Structure
Abstract
:1. Introduction
2. Results and Discussion
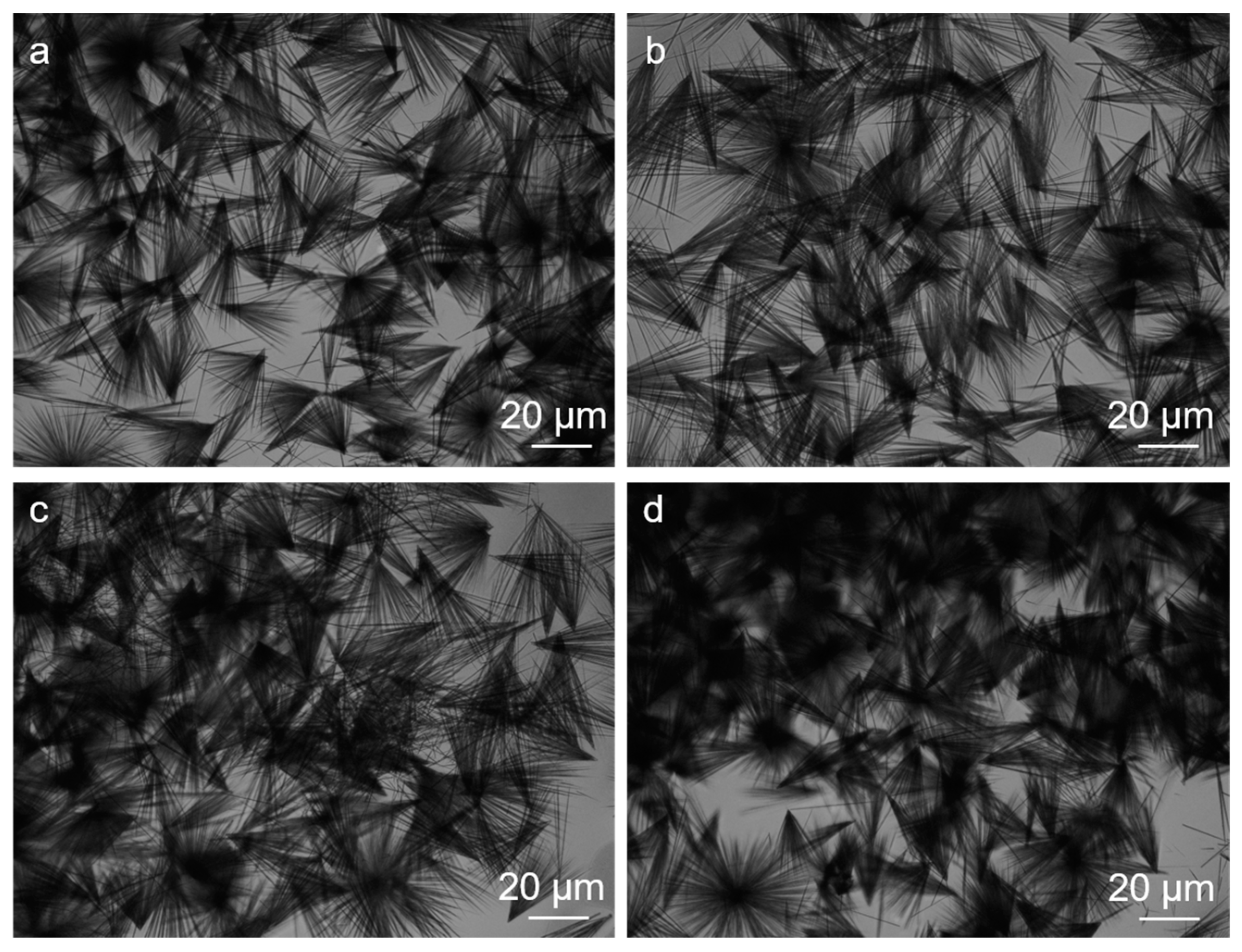
2.1. Assembly Induced by NaC (Phase Behavior)
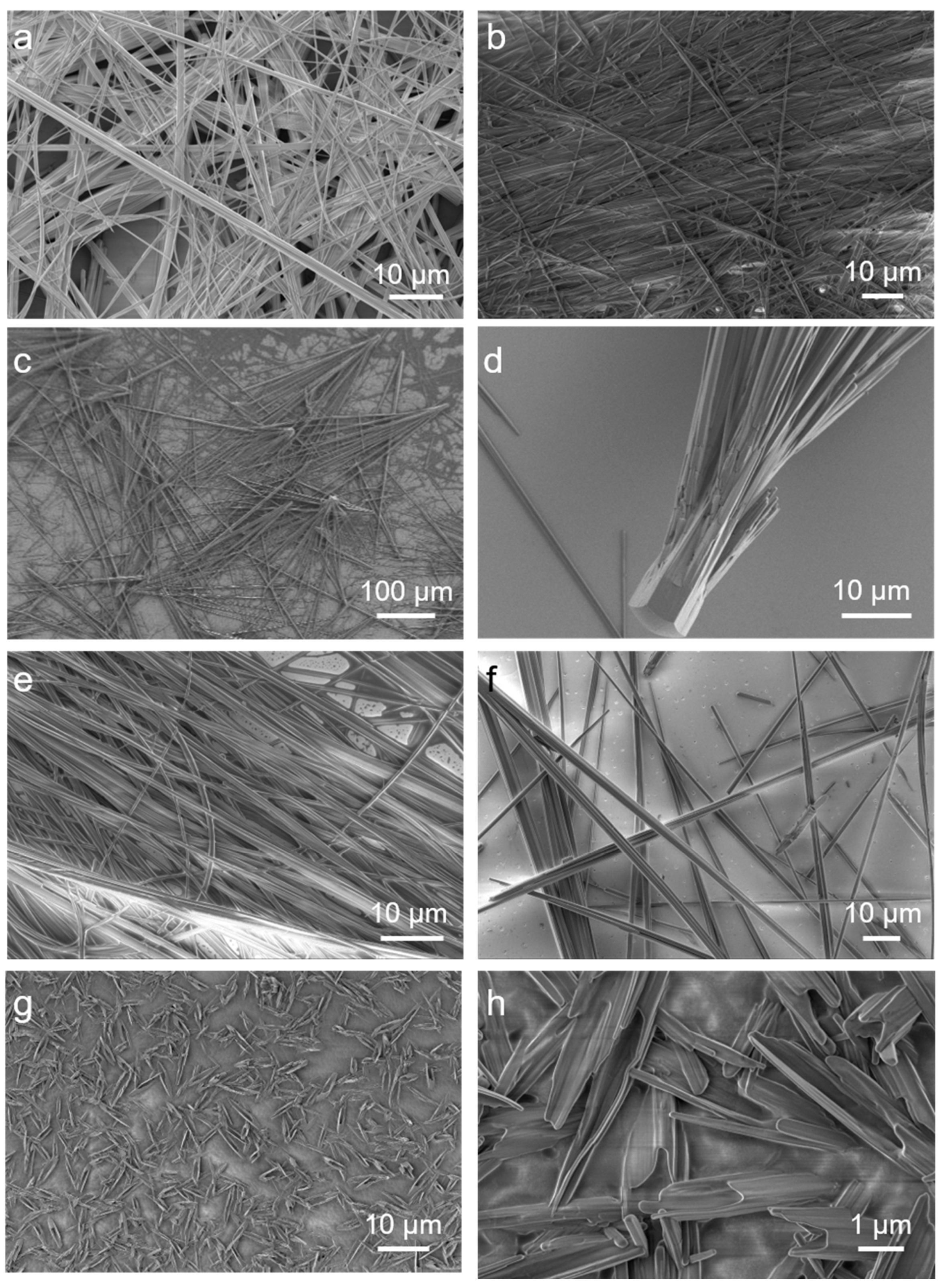
2.2. The Secondary Structures of the FF/NaC Mixture
2.3. The General Observation of Different Surfactants
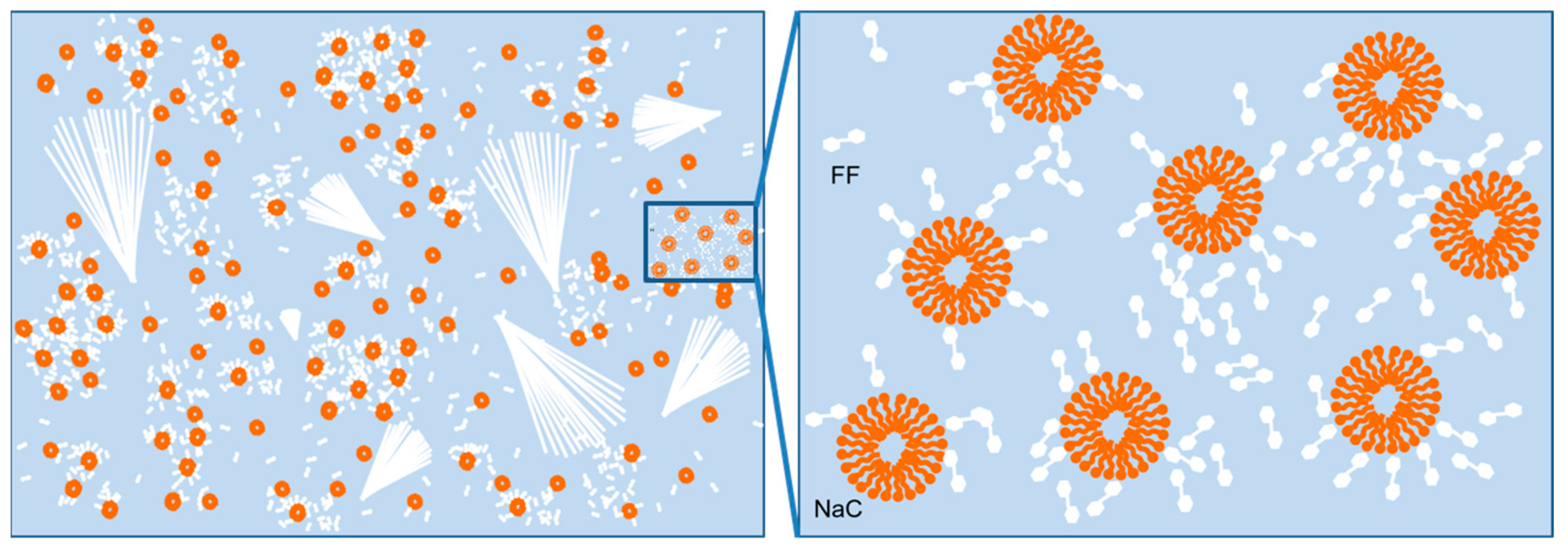
2.4. Mechanism of the Formation of the Assemblies
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Samples
3.3. Measurements and Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bera, S.; Mondal, S.; Xue, B.; Shimon, L.J.W.; Cao, Y.; Gazit, E. Rigid helical-like assemblies from a self-aggregating tripeptide. Nat. Mater. 2019, 18, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Gazit, E. Minimalistic peptide supramolecular co-assembly: Expanding the conformational space for nanotechnology. Chem. Soc. Rev. 2018, 47, 3406–3420. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, M.; Ulijn, R.V. Next-generation peptide nanomaterials: Molecular networks, interfaces and supramolecular functionality. Chem. Soc. Rev. 2010, 39, 3351–3357. [Google Scholar] [CrossRef] [PubMed]
- Omosun, T.O.; Hsieh, M.-C.; Childers, W.S.; Das, D.; Mehta, A.K.; Anthony, N.R.; Pan, T.; Grover, M.A.; Berland, K.M.; Lynn, D.G. Catalytic diversity in self-propagating peptide assemblies. Nat. Chem. 2017, 9, 805–809. [Google Scholar] [CrossRef]
- Jiang, Q.C.; Liu, X.Y.; Liang, G.L.; Sun, X.B. Self-assembly of peptide nanofibers for imaging applications. Nanoscale 2021, 13, 15142–15150. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.B.; Song, N.; Cao, Y.W.; Li, M.M.; Liu, X.; Zhou, Z.F.; Shi, L.Q.L.; Yu, Z.L. Noncanonical amino acids for hypoxia-responsive peptide self-assembly and fluorescence. J. Am. Chem. Soc. 2021, 143, 13854–13864. [Google Scholar] [CrossRef]
- Song, N.; Zhou, Z.F.; Song, Y.Q.; Li, M.M.; Yu, X.N.; Hu, B.B.; Yu, Z.L. In situ oxidation-regulated self-assembly of peptides into transformable scaffolds for cascade therapy. Nano Today 2021, 38, 101198. [Google Scholar] [CrossRef]
- Song, Y.Q.; Li, M.M.; Song, N.; Liu, X.; Wu, G.Y.; Zhou, H.; Long, Z.F.; Shi, L.Q.; Yu, Z.L. Self-amplifying assembly of peptides in macrophages for enhanced inflammatory treatment. J. Am. Chem. Soc. 2022, 144, 6907–6917. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.M.; Liu, J.Z.; Song, Y.Q.; Hu, B.B.; Wu, C.X.; Liu, A.A.; Zhou, H.; Long, J.F.; Shi, L.Q.; et al. In situ self-sorting peptide assemblies in living cells for simultaneous organelle targeting. J. Am. Chem. Soc. 2022, 144, 9312–9323. [Google Scholar] [CrossRef]
- Jiang, L.H.; Zuo, X.B.; Li, J.P.; Traaseth, N.J.; Kirshenbaum, K. Programmed supramolecular assemblies using orthogonal pairs of heterodimeric coiled coil peptides. Angew. Chem. Int. Ed. 2022, 134, e202201895. [Google Scholar] [CrossRef]
- Hamsici, S.; White, A.D.; Acar, H. Peptide framework for screening the effects of amino acids on assembly. Sci. Adv. 2022, 8, eabj0305. [Google Scholar] [CrossRef]
- Heinz-Kunert, S.L.; Pandya, A.; Dang, V.T.; Tran, P.N.; Ghosh, S.; McElheny, D.; Santarsiero, B.D.; Ren, Z.; Nguyen, A.I. Assembly of π-stacking helical peptides into a porous and multivariable proteomimetic framework. J. Am. Chem. Soc. 2022, 144, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.C.; Zhang, Y.; Han, L.X.; Liu, H.L.; Sun, B.G. Quantum confined peptide assemblies in a visual photoluminescent hydrogel platform and smartphone-assisted sample-to-answer analyzer for detecting trace pyrethroids. Biosens. Bioelectron. 2022, 210, 114265. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Lee, J.S.; Park, C.B. Beta-sheet-forming, self-assembled peptide nanomaterials towards optical, energy and healthcare applications. Small 2015, 11, 3623–3640. [Google Scholar] [CrossRef] [PubMed]
- Retout, M.; Mantri, Y.; Jin, Z.C.; Zhou, J.J.; Noël, G.; Donovan, B.; Yim, W.J.; Jokerst, J.V. Peptide-induced fractal assembly of silver nanoparticles for visual detection of disease biomarkers. ACS Nano 2022, 16, 6165–6175. [Google Scholar] [CrossRef]
- Yan, X.H.; Mohwald, H. Organized peptidic nanostructures as functional materials. Biomacromolecules 2017, 18, 3469–3470. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Yan, K.; Xiong, S.J.; Wei, T.T.; Wu, X.L.; Chu, P.K. Controlled fiberization of dipeptide in merging phases leads to collagen-level strength and opto/electric mechanofunctionalities. Biomaterials 2019, 208, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zelenovskiy, P.S.; Domingues, E.M.; Slabov, V.; Kopyl, Z.; Ugolkov, V.L.; Figueiredo, F.M.L.; Kholkin, A.L. Efficient water self-diffusion in diphenylalanine peptide nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 27485–27492. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Zhu, R.; Jenkins, K.; Yang, R. Self-assembly of diphenylalanine peptide with controlled polarization for power generation. Nat. Commun. 2016, 7, 13566. [Google Scholar] [CrossRef] [Green Version]
- Ben-Nun, Y.; Fichman, G.; Adler-Abramovich, L.; Turk, B.; Gazit, E.; Blum, G. Cathepsin nanofiber substrates as potential agents for targeted drug delivery. J. Control Release 2017, 257, 60–67. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, S.W.; Kang, K.; Park, C.B. Synthesis of diphenylalanine/cobalt oxide hybrid nanowires and their application to energy storage. ACS Nano 2010, 4, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Qin, S.Y.; Wu, X.L.; Chu, P.K. Morphology and pattern control of diphenylalanine self-assembly via evaporative dewetting. ACS Nano 2016, 10, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Arnon, Z.A.; Sui, X.M.; Azuri, I.; Cohen, H.; Hod, O.; Kronik, L.; Shimon, L.J.W.; Wagner, H.D.; Gazit, E. Bioinspired flexible and tough layered peptide crystals. Adv. Mater. 2018, 30, 1704551. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Tang, Y.M.; Guterman, T.; Arnon, Z.A.; Yao, Y.F.; Wei, G.H.; Gazit, E. Co-assembly between Fmoc diphenylalanineand diphenylalanine within a 3D fibrous viscous network confers atypical curvature and branching. Angew. Chem. Int. Ed. 2020, 59, 23731–23739. [Google Scholar] [CrossRef] [PubMed]
- Azuri, I.; Adler-Abramovich, L.; Gazit, E.; Hod, O.; Kronik, L. Why are diphenylalanine-based peptide nanostructures so rigid? Insights from first principles calculations. J. Am. Chem. Soc. 2014, 136, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Chen, P.L.; Dong, H.L.; Zhen, Y.G.; Liu, M.H.; Hu, W.P. Porphyrin supramolecular 1D structures via surfactant-assisted self-assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Fei, J.B.; Wang, A.H.; Cui, W.; Zhu, P.L.; Li, J.B. Transformation of dipeptide-based organogels into chiral crystals by cryogenic treatment. Angew. Chem. Int. Ed. 2017, 56, 2660–2663. [Google Scholar] [CrossRef]
- Liu, X.C.; Riegler, H.; Ma, L.; Li, Q.; Hao, J.C. Vapor-stimuli shape transformation cycles of assembled dipeptide film. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129483. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Y.; Dai, L.R.; Yang, Y.; Li, J.B. Controlled rod nanostructured assembly of diphenylalanine and their optical waveguide properties. ACS Nano 2015, 9, 2689–2695. [Google Scholar] [CrossRef]
- Panja, S.; Bharti, R.; Dey, G.; Lynd, N.A.; Chattopadhyay, S. Coordination-assisted self-assembled polypeptide nanogels to selectively combat bacterial infection. ACS Appl. Mater. Interfaces 2019, 11, 33599–33611. [Google Scholar] [CrossRef]
- Shi, L.; Kuang, D.Q.; Ma, X.M.; Jalalah, M.; Alsareii, S.A.; Gao, T.; Harraz, F.A.; Yang, J.; Li, G.X. Peptide assembled in a nano-confined space as a molecular rectifier for the availability of ionic current modulation. Nano Lett. 2022, 22, 1083–1090. [Google Scholar] [CrossRef]
- Li, J.F.; Li, X.D.; Xu, J.; Wang, Y.; Wu, L.X.; Wang, Y.Q.; Wang, L.Y.; Lee, M.; Li, W. Engineering the ionic self-assembly of polyoxometalates and facial-like peptides. Chem. Eur. J. 2016, 22, 15751–15759. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Llamas, S.; Maestro, A.; Fernández-Peña, L.; Akanno, A.; Miller, R.; Ortega, F.; Rubio, R.G. Polymer-surfactant systems in bulk and at fluid interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. [Google Scholar] [CrossRef] [PubMed]
- Roussel, G.; Caudano, Y.; Matagne, A.; Sansom, M.S.; Perpète, E.A.; Michaux, C. Peptide-surfactant interactions: A combined spectroscopic and molecular dynamics simulation approach. Spectrochim. Acta Part A 2018, 190, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Winogradoff, D.; John, S.; Aksimentiev, A. Protein unfolding by SDS: The microscopic mechanisms and the properties of the SDS-protein assembly. Nanoscale 2020, 12, 5422–5434. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, N.; Kulkarni, Y.S.; Misiaszek, A.D.; Wallin, C.; Kruger, D.M.; Liao, Q.H.; Rad, F.M.; Jarvet, J.; Strodel, B.; Warmlander, S.K.T.S. Amyloid-beta peptide interactions with amphiphilic surfactants: Electrostatic and hydrophobic effects. ACS Chem. Neurosci. 2018, 9, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Walther, F.J.; Sharma, S.; Gordon, L.M. Structural and functional stability of the sulfur-free surfactant protein B peptide mimic B-YL in synthetic surfactant lipids. BMC Pulm Med. 2021, 21, 330. [Google Scholar] [CrossRef]
- Wang, D.; Cao, Y.Y.; Sun, Y.W.; Wang, J.Q. Co-assembly behaviors and rheological properties of a salt-free catanionic tetrapeptide/ surfactant system in water. J. Mol. Liq. 2017, 243, 406–413. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.B.; Möhwald, H. Self-assembly of hexagonal peptide microtubes and their optical waveguiding. Adv. Mater. 2011, 23, 2796–2801. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, C.Q.; Han, Y.C.; Wang, Y.L.; Liu, X.M.; Zhang, S.J.; Yan, X.H. Trace water as prominent factor to induce peptide self-assembly: Dynamic evolution and governing interactions in ionic liquids. Small 2017, 13, 1702175. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, Y.; Yang, X.K.; Dai, L.R.; Das, B.; Acharya, S.; Sun, B.B.; Yang, Y.; Liu, X.C.; Ariga, K.; et al. Unidirectional branching growth of dipeptide single crystals for remote light multiplication and collection. ACS Appl. Mater. Interfaces 2019, 11, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, H.; Moroi, Y. Micelle formation of sodium cholate and solubilization into the micelle. Biochim. Biophys. Acta 1998, 1394, 99–110. [Google Scholar] [CrossRef]
- Santhanalakshmi, J.; Lakshmi, G.S.; Aswal, V.K.; Goyal, P.S. Small-angle neutron scattering study of sodium cholate and sodium deoxycholate interacting micelles in aqueous medium. Proc. Indian Acad. Sci. (Chem. Sci.) 2001, 113, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Sugioka, H.; Matsuoka, K.; Moroi, Y. Temperature effect on formation of sodium cholate micelles. J. Colloid Interface Sci. 2003, 259, 156–162. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Jones, W. Formation of tubular crystals of pharmaceutical compounds. Cryst. Growth Des. 2010, 10, 365–370. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhang, J.; Liu, X. Surfactant-Assisted Assembly of Dipeptide Forming a Broom-like Structure. Molecules 2022, 27, 4876. https://doi.org/10.3390/molecules27154876
Wei Y, Zhang J, Liu X. Surfactant-Assisted Assembly of Dipeptide Forming a Broom-like Structure. Molecules. 2022; 27(15):4876. https://doi.org/10.3390/molecules27154876
Chicago/Turabian StyleWei, Yunping, Jie Zhang, and Xingcen Liu. 2022. "Surfactant-Assisted Assembly of Dipeptide Forming a Broom-like Structure" Molecules 27, no. 15: 4876. https://doi.org/10.3390/molecules27154876
APA StyleWei, Y., Zhang, J., & Liu, X. (2022). Surfactant-Assisted Assembly of Dipeptide Forming a Broom-like Structure. Molecules, 27(15), 4876. https://doi.org/10.3390/molecules27154876





