Novel Phosphonium-Based Ionic Liquid Electrolytes for Battery Applications
Abstract
:1. Introduction
2. Results
2.1. Synthesis of the Ionic Liquids, Alkali Metal Compatibility and Thermal Properties
2.1.1. Synthesis of the Phosphonium-Based Ionic Liquids and Electrolyte Mixtures
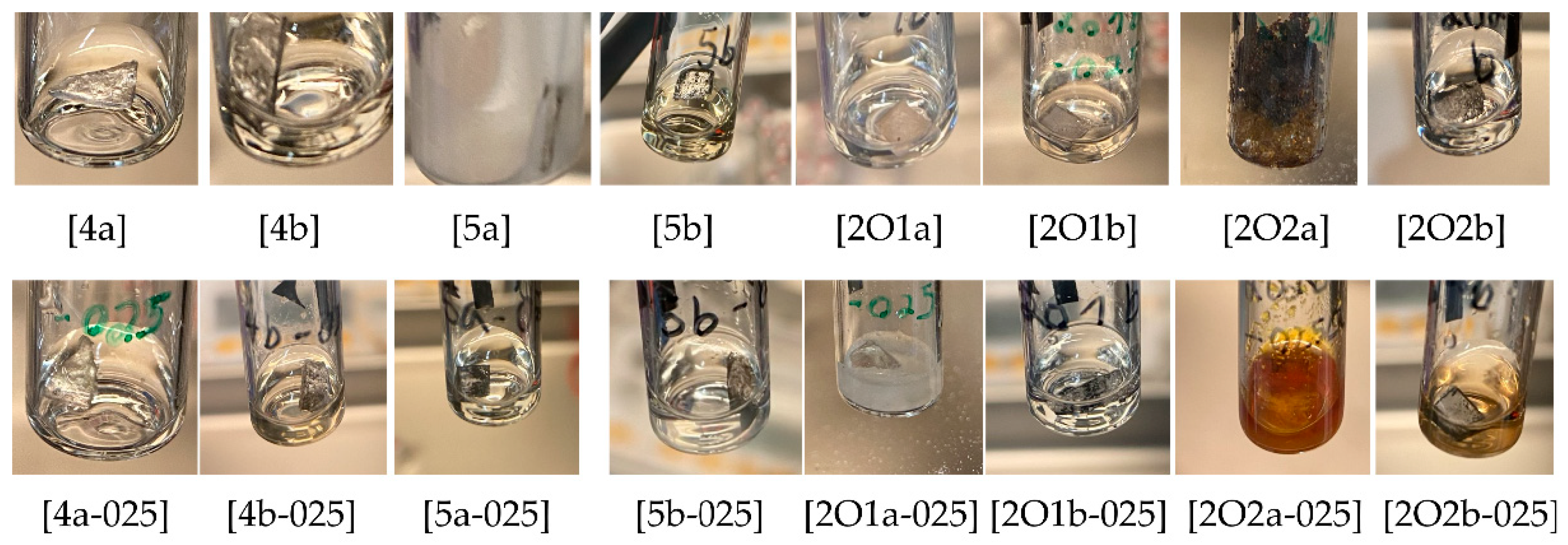
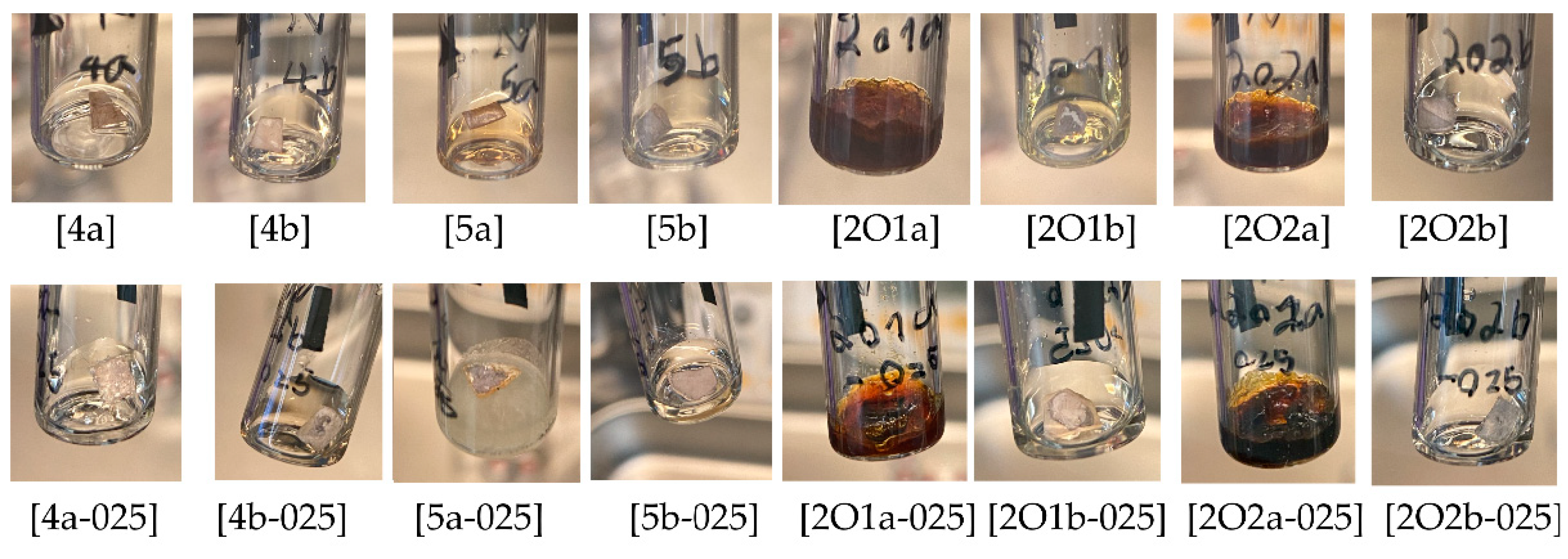
2.1.2. Compatibility with Alkali Metals (Li, Na)
2.1.3. Thermal Properties of the Ionic Liquids and Salt Mixtures
2.1.4. Density Values of Pure Ionic Liquids and Binary Salt Mixtures
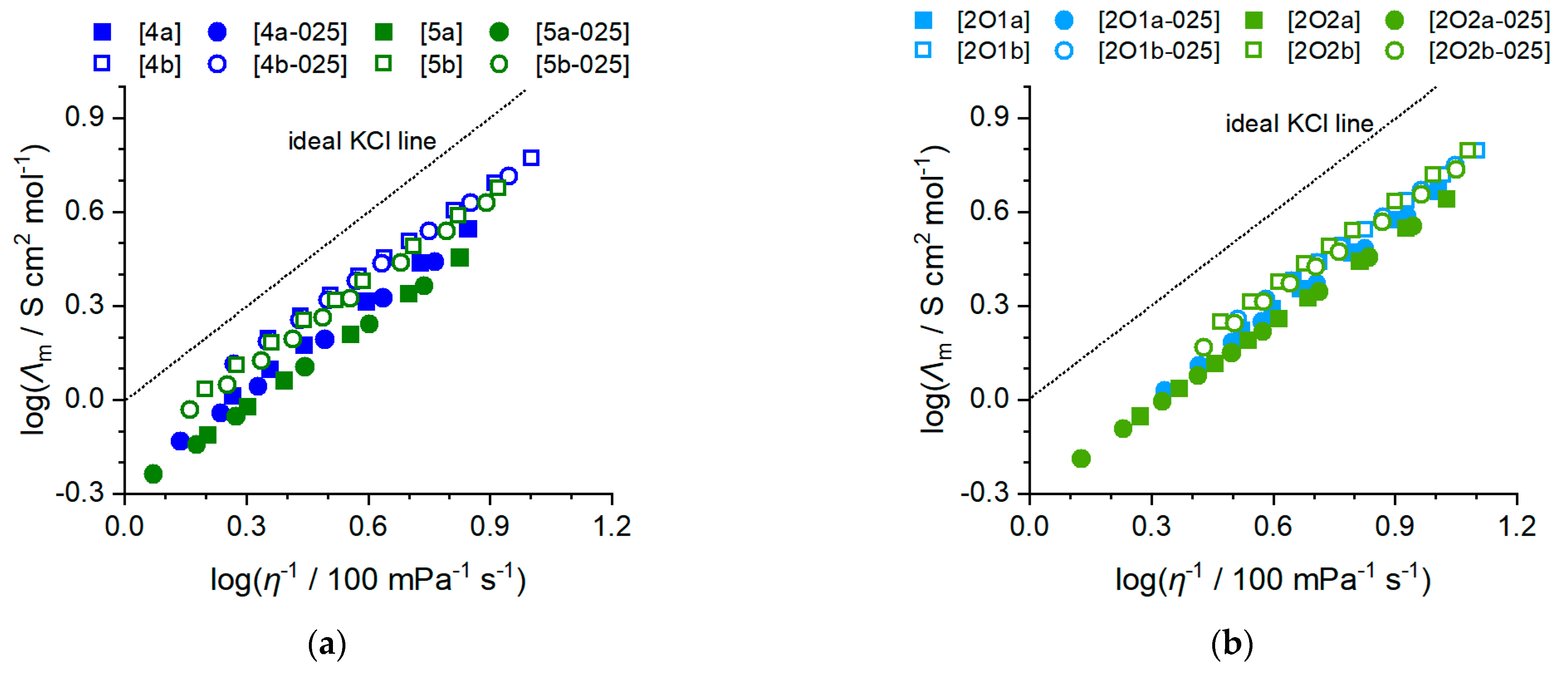
2.2. Transport Properties
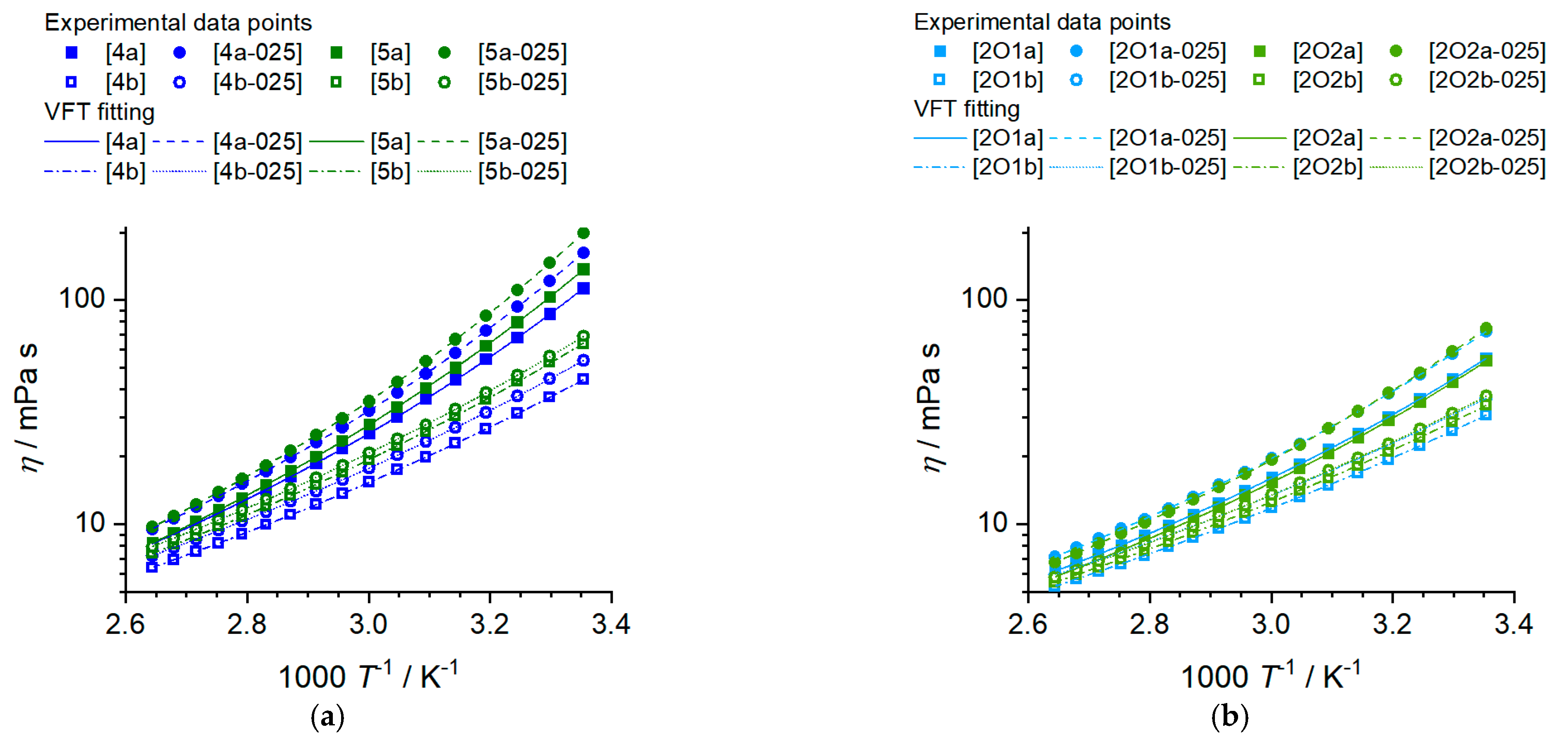
2.2.1. Viscosity of the Ionic Liquids and Ionic Liquid–Based Mixtures
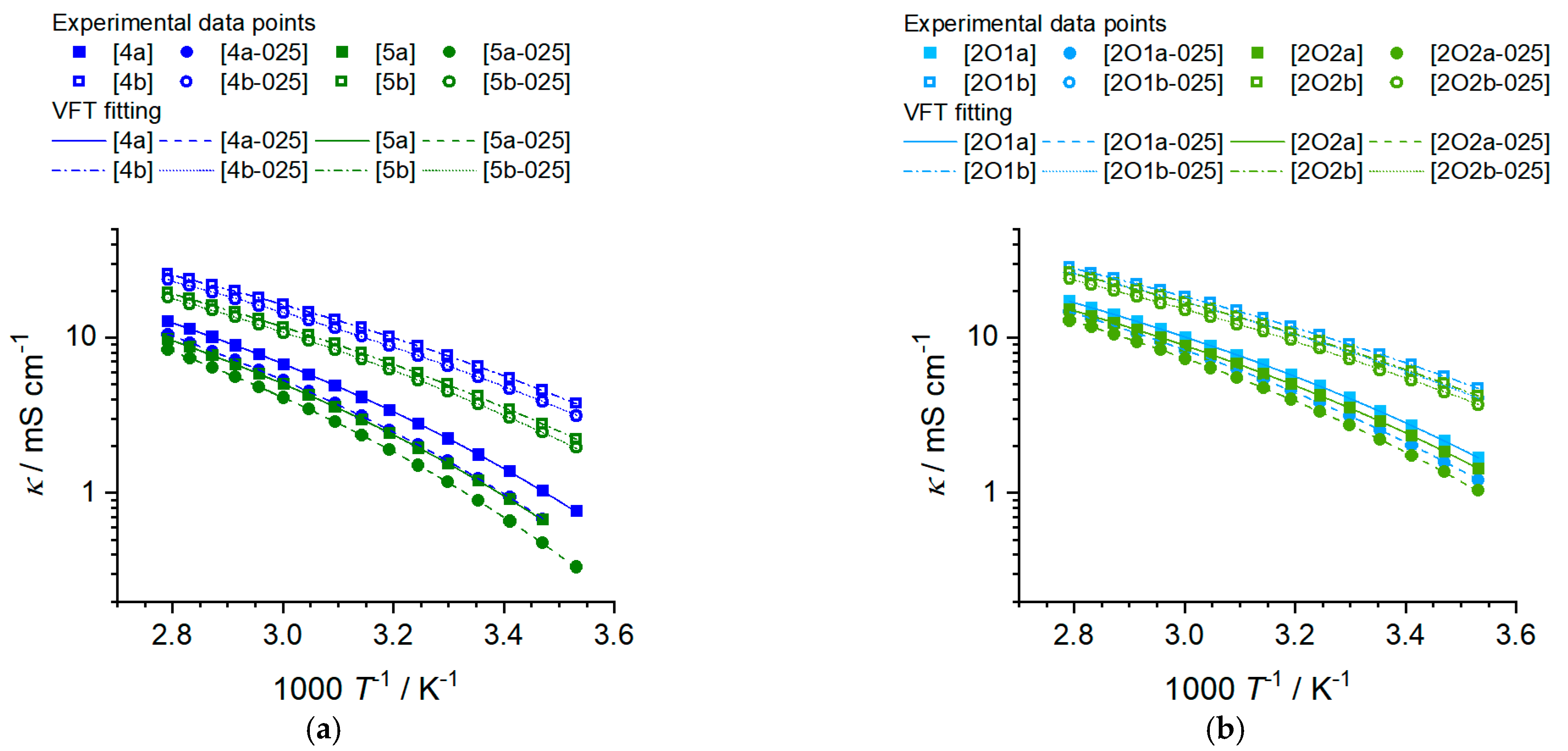
2.2.2. Ionic Conductivity
2.2.3. Li Self-Diffusion Coefficients
2.3. Electrochemical Window and Aluminum Resistivity
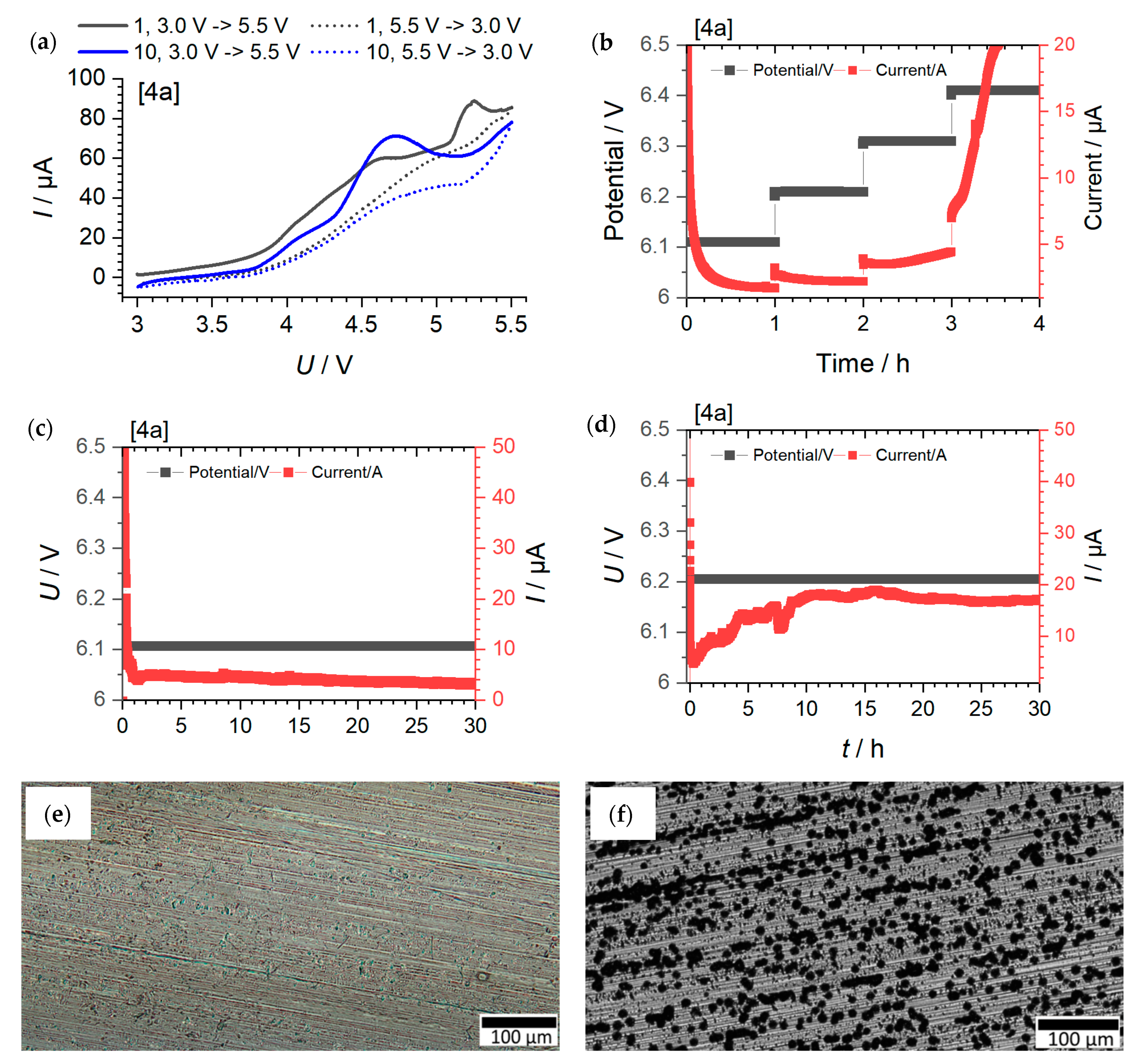
Cyclic Voltammetry Measurements, Potential Limit and Long-Term Potential Measurements
2.4. Cell Test Results
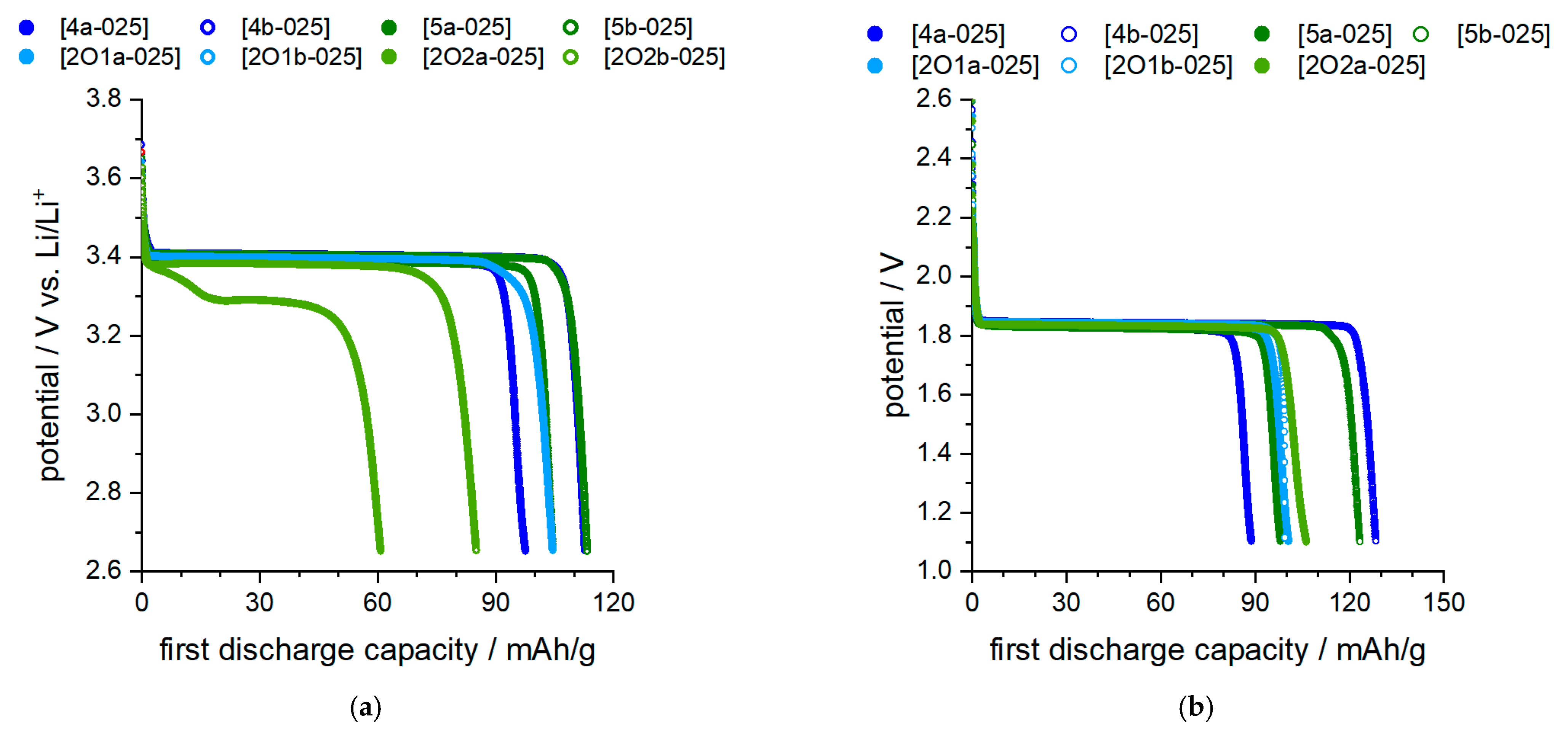
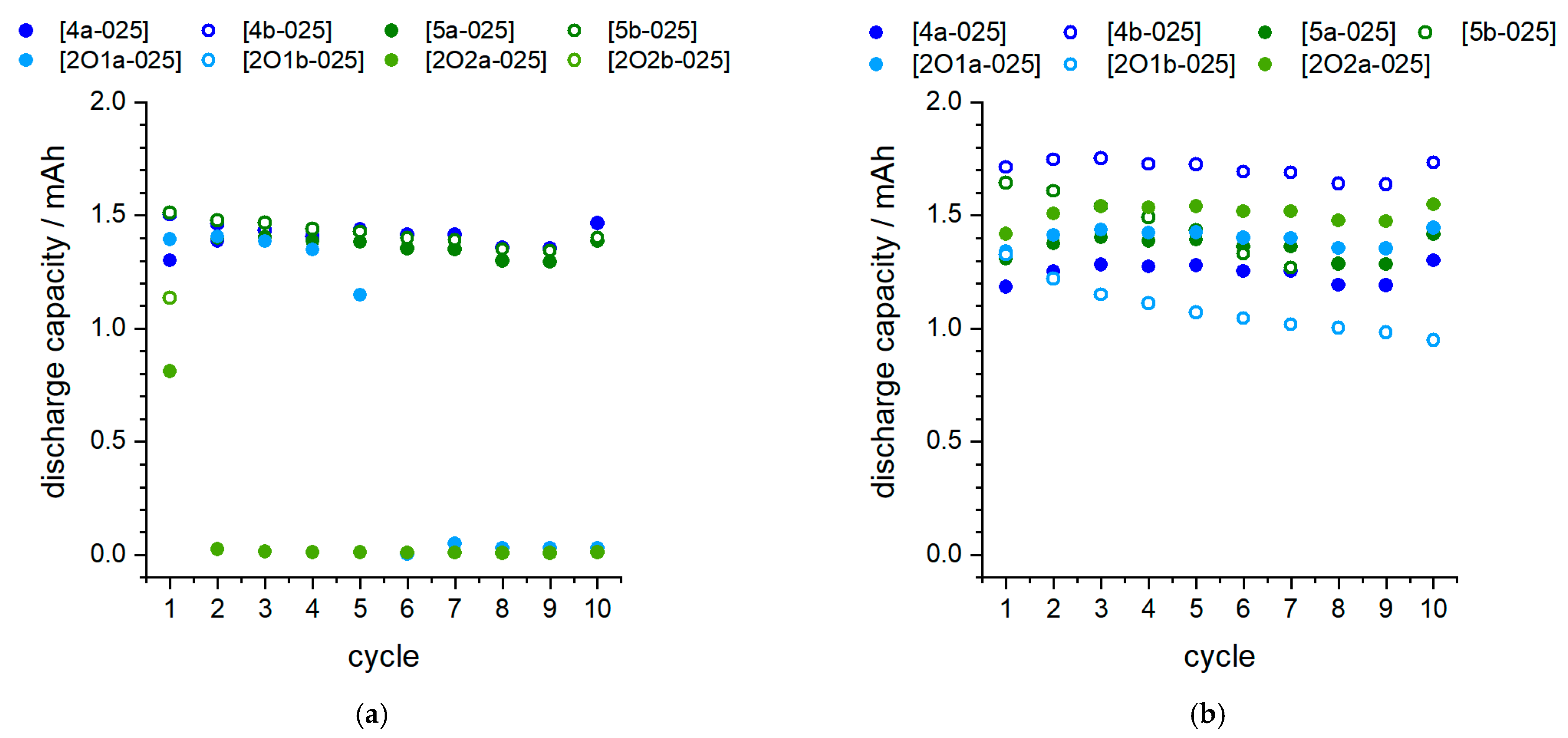
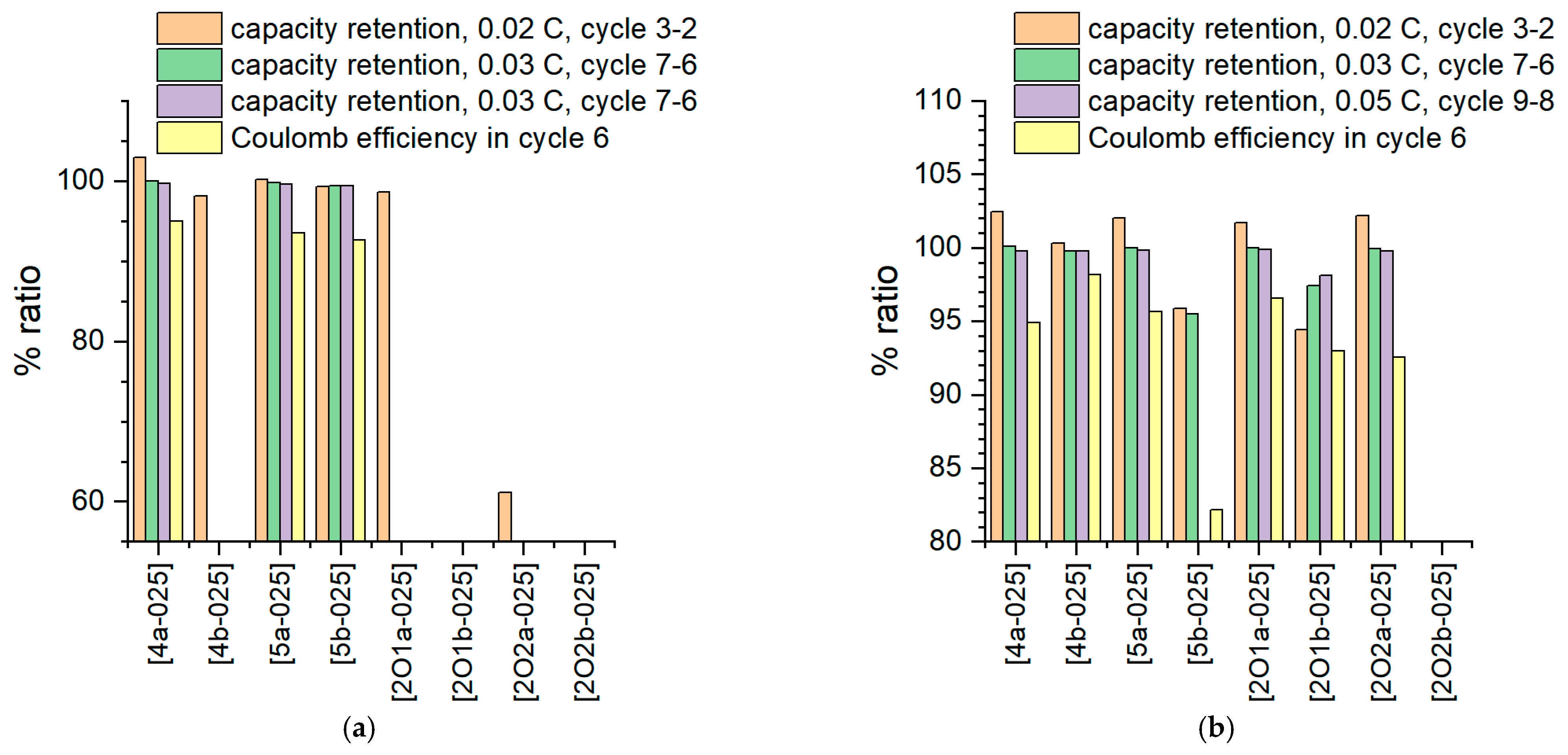
2.4.1. Proof of Principle in Coin Cell Tests
2.4.2. Overpotential Measurements in Half Cells and Full Cells
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Ionic Liquids
4.2. Thermal Transitions
4.3. Density
4.4. Viscosity
4.5. Conductivity
4.6. Self-Diffusion Coefficients
4.7. Fitting of the Transport Properties
4.8. Metal Compatibility
4.9. Electrochemistry
4.10. Cyclic Voltammetry
4.11. Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tsuzuki, S. Factors Controlling the Diffusion of Ions in Ionic Liquids. ChemPhysChem 2012, 13, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Nordness, O.; Brennecke, J.F. Ion Dissociation in Ionic Liquids and Ionic Liquid Solutions. Chem. Rev. 2020, 120, 12873–12902. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Hussey, C.L. Electrochemical Applications of Room-Temperature Ionic Liquids. Electrochem. Soc. Int. 2007, 2007, 42–49. [Google Scholar] [CrossRef]
- Esperanca, J.M.S.S.; Lopes, J.N.C.; Tariq, M.; Santos, L.M.N.B.F.; Magee, J.W.; Rebelo, L.P.N. Volatility of Aprotic Ionic Liquids—A Review. J. Chem. Eng. Data 2010, 55, 3–12. [Google Scholar] [CrossRef]
- Khazalpour, S.; Yarie, M.; Kianpour, E.; Amani, A.; Asadabadi, S.; Seyf, J.Y.; Rezaeivala, M.; Azizian, S.; Zolfigol, M.A. Applications of phosphonium-based ionic liquids in chemical processes. J. Iran. Chem. Soc. 2020, 17, 1775–1917. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are Ionic Liquids Chemically Stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef]
- Sakaebe, H.; Matsumoto, H.; Tatsumi, K. Application of room temperature ionic liquids to Li batteries. Electrochim. Acta 2007, 53, 1048–1054. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Martins, V.L.; Sanchez-Ramirez, N.; Ribeiro, M.C.; Torresi, R.M. Two phosphonium ionic liquids with high Li(+) transport number. Phys. Chem. Chem. Phys. 2015, 17, 23041–23051. [Google Scholar] [CrossRef]
- Wu, F.; Schür, A.R.; Kim, G.-T.; Dong, X.; Kuenzel, M.; Diemant, T.; D’Orsi, G.; Simonetti, E.; De Francesco, M.; Bellusci, M.; et al. A novel phosphonium ionic liquid electrolyte enabling high-voltage and high-energy positive electrode materials in lithium-metal batteries. Energy Storage Mater. 2021, 42, 826–835. [Google Scholar] [CrossRef]
- Larhrib, B.; Nikiforidis, G.; Anouti, M. Safe and efficient phosphonium ionic liquid based electrolyte for high-potential LiMn2O4 and LiNi0.8Co0.15Al0.05O2 cathodes for Li-ion batteries. Electrochim. Acta 2021, 371, 137841–137852. [Google Scholar] [CrossRef]
- Lin, X.; Kavian, R.; Lu, Y.; Hu, Q.; Shao-Horn, Y.; Grinstaff, M.W. Thermally-responsive, nonflammable phosphonium ionic liquid electrolytes for lithium metal batteries: Operating at 100 degrees celsius. Chem. Sci. 2015, 6, 6601–6606. [Google Scholar] [CrossRef] [Green Version]
- Tsunashima, K.; Sugiya, M. Electrochemical Behavior of Lithium in Room-Temperature Phosphonium Ionic Liquids as Lithium Battery Electrolytes. Electrochem. Solid-State Lett. 2008, 11, A17–A19. [Google Scholar] [CrossRef]
- Rogstad, D.T.; Einarsrud, M.-A.; Svensson, A.M. Evaluation of Selected Ionic Liquids as Electrolytes for Silicon Anodes in Li-Ion Batteries. J. Electrochem. Soc. 2021, 168, 110506–110521. [Google Scholar] [CrossRef]
- Hofmann, A.; Merklein, L.; Schulz, M.; Hanemann, T. Anodic Aluminum Dissolution of LiTFSA Containing Electrolytes for Li-Ion-Batteries. Electrochim. Acta 2014, 116, 388–395. [Google Scholar] [CrossRef]
- Hofmann, A.; Schulz, M.; Winkler, V.; Hanemann, T. Anodic Aluminum Dissolution in Conducting Salt Containing Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A431–A438. [Google Scholar] [CrossRef]
- Cha, E.-H.; Mun, J.-Y.; Cho, E.R.; Yim, T.-E.; Kim, Y.-G.; Oh, S.-M.; Lim, S.-A.; Lim, J.-W. The Corrosion Study of Al Current Collector in Phosphonium Ionic Liquid as Solvent for Lithium Ion Battery. J. Korean Electrochem. Soc. 2011, 14, 152–156. [Google Scholar] [CrossRef]
- Lee, J.S.; Bae, J.Y.; Lee, H.; Quan, N.D.; Kim, H.S.; Kim, H. Ionic liquids as electrolytes for Li ion batteries. J. Ind. Eng. Chem. 2004, 10, 1086–1089. [Google Scholar]
- Lewandowski, A.; Świderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries—An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Scarbath-Evers, L.K.; Hunt, P.A.; Kirchner, B.; MacFarlane, D.R.; Zahn, S. Molecular features contributing to the lower viscosity of phosphonium ionic liquids compared to their ammonium analogues. Phys. Chem. Chem. Phys. 2015, 17, 20205–20216. [Google Scholar] [CrossRef]
- Griffin, P.J.; Holt, A.P.; Tsunashima, K.; Sangoro, J.R.; Kremer, F.; Sokolov, A.P. Ion transport and structural dynamics in homologous ammonium and phosphonium-based room temperature ionic liquids. J. Chem. Phys. 2015, 142, 084501. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Hayamizu, K.; Tsuzuki, S.; Fujii, K.; Umebayashi, Y.; Mitsugi, T.; Kobayashi, T.; Ohno, Y.; Kobayashi, Y.; Mita, Y.; et al. Relationships between center atom species (N, P) and ionic conductivity, viscosity, density, self-diffusion coefficient of quaternary cation room-temperature ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Bencherifi, Y.; Larhrib, B.; Sayegh, A.; Nikiforidis, G.; Anouti, M. Phosphonium ionic liquid-based electrolyte for high voltage Li-ion batteries: Effect of ionic liquid ratio. J. Appl. Electrochem. 2021, 51, 1651–1664. [Google Scholar] [CrossRef]
- Leclere, M.; Bernard, L.; Livi, S.; Bardet, M.; Guillermo, A.; Picard, L.; Duchet-Rumeau, J. Gelled Electrolyte Containing Phosphonium Ionic Liquids for Lithium-Ion Batteries. Nanomaterials 2018, 8, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsunashima, K.; Taguchi, H.; Yonekawa, F. Influence of Nonflammable Diluents on Properties of Phosphonium Ionic Liquids as Lithium Battery Electrolytes. ECS Trans. 2012, 50, 419–424. [Google Scholar] [CrossRef]
- Tsunashima, K.; Yonekawa, F.; Sugiya, M. A Lithium Battery Electrolyte Based on a Room-temperature Phosphonium Ionic Liquid. Chem. Lett. 2008, 37, 314–315. [Google Scholar] [CrossRef]
- Rauber, D.; Hofmann, A.; Philippi, F.; Kay, C.W.M.; Zinkevich, T.; Hanemann, T.; Hempelmann, R. Structure-Property Relation of Trimethyl Ammonium Ionic Liquids for Battery Applications. Appl. Sci. 2021, 11, 5679. [Google Scholar] [CrossRef]
- Salem, N.; Zavorine, S.; Nucciarone, D.; Whitbread, K.; Moser, M.; Abu-Lebdeh, Y. Physical and Electrochemical Properties of Some Phosphonium-Based Ionic Liquids and the Performance of Their Electrolytes in Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, H5202–H5209. [Google Scholar] [CrossRef] [Green Version]
- Philippi, F.; Rauber, D.; Zapp, J.; Präsang, C.; Scheschkewitz, D.; Hempelmann, R. Multiple Ether-Functionalized Phosphonium Ionic Liquids as Highly Fluid Electrolytes. ChemPhysChem 2019, 20, 443–455. [Google Scholar] [CrossRef]
- Rüther, T.; Bhatt, A.I.; Best, A.S.; Harris, K.R.; Hollenkamp, A.F. Electrolytes for Lithium (Sodium) Batteries Based on Ionic Liquids: Highlighting the Key Role Played by the Anion. Batter. Supercaps 2020, 3, 793–827. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Z.; Shi, L.; Jungsuttiwong, S.; Yuan, S. Ionic liquids for high performance lithium metal batteries. J. Energy Chem. 2021, 59, 320–333. [Google Scholar] [CrossRef]
- Kim, H.T.; Kang, J.; Mun, J.; Oh, S.M.; Yim, T.; Kim, Y.G. Pyrrolinium-based Ionic Liquid as a Flame Retardant for Binary Electrolytes of Lithium Ion Batteries. ACS Sus. Chem. Eng. 2016, 4, 497–505. [Google Scholar] [CrossRef]
- Passerini, S.; Henderson, W.A. Secondary batteries—Lithium rechargeable systems. Electrolytes: Ionic Liquids. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 85–91. [Google Scholar]
- Hofmann, A.; Migeot, M.; Arens, L.; Hanemann, T. Investigation of Ternary Mixtures Containing 1-Ethyl-3-methylimidazolium Bis(trifluoromethanesulfonyl)azanide, Ethylene Carbonate and Lithium Bis(trifluoromethanesulfonyl)azanide. Int. J. Mol. Sci. 2016, 17, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, B.; Lavallée, S.; Perron, G.; Michot, C.; Armand, M. Room temperature molten salts as lithium battery electrolyte. Electrochim. Acta 2004, 49, 4583–4588. [Google Scholar] [CrossRef]
- Seki, S.; Ohno, Y.; Kobayashi, Y.; Miyashiro, H.; Usami, A.; Mita, Y.; Tokuda, H.; Watanabe, M.; Hayamizu, K.; Tsuzuki, S.; et al. Imidazolium-based room-temperature ionic liquid for lithium secondary batteries—Effects of lithium salt concentration. J. Electrochem. Soc. 2007, 154, A173–A177. [Google Scholar] [CrossRef]
- Hofmann, A.; Schulz, M.; Hanemann, T. Effect of Conducting Salts in Ionic Liquid based Electrolytes: Viscosity, Conductivity, and Li-Ion Cell Testing. Int. J. Electrochem. Sci. 2013, 8, 10170–10189. [Google Scholar]
- Hofmann, A.; Migeot, M.; Hanemann, T. Investigation of Binary Mixtures Containing 1-Ethyl-3-methylimidazolium Bis(trifluoromethane-sulfonyl)azanide and Ethylene Carbonate J. Chem. Eng. Data 2016, 61, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Indris, S.; Heinzmann, R.; Schulz, M.; Hofmann, A. Ionic Liquid Based Electrolytes: Correlating Li Diffusion Coefficients and Battery Performance. J. Electrochem. Soc. 2014, 161, A2036–A2041. [Google Scholar] [CrossRef]
- Philippi, F.; Rauber, D.; Zapp, J.; Hempelmann, R. Transport properties and ionicity of phosphonium ionic liquids. Phys. Chem. Chem. Phys. 2017, 19, 23015–23023. [Google Scholar] [CrossRef]
- Zheng, Q.-G.; Liu, H.; Xia, Q.; Liu, Q.-S.; Mou, L. Density, Dynamic Viscosity and Electrical Conductivity of Two Hydrophobic Phosphonium Ionic Liquids. Acta Phys.-Chim. Sin. 2017, 33, 736–744. [Google Scholar] [CrossRef]
- Tantray, A.A.; Rode, N.R.; Shinde, S.S.; Nandre, V.; Kodam, K.M.; Terdale, S.S. Influence of the alkyl chain length on the physicochemical properties and microbial biocompatibility of phosphonium based fatty acid ionic liquids. Can. J. Chem. 2022, 100, 35–45. [Google Scholar] [CrossRef]
- Ferreira, A.G.M.; Simões, P.N.; Ferreira, A.F.; Fonseca, M.A.; Oliveira, M.S.A.; Trino, A.S.M. Transport and thermal properties of quaternary phosphonium ionic liquids and IoNanofluids. J. Chem. Thermodyn. 2013, 64, 80–92. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Marin, T.W.; Zhu, Y.; Abraham, D.P. Why Bis(fluorosulfonyl)imide Is a “Magic Anion” for Electrochemistry. J. Phys. Chem. C 2014, 118, 19661–19671. [Google Scholar] [CrossRef]
- Kerner, M.; Plylahan, N.; Scheers, J.; Johansson, P. Ionic liquid based lithium battery electrolytes: Fundamental benefits of utilising both TFSI and FSI anions? Phys. Chem. Chem. Phys. 2015, 17, 19569–19581. [Google Scholar] [CrossRef] [PubMed]
- Philippi, F.; Rauber, D.; Kuttich, B.; Kraus, T.; Kay, C.W.M.; Hempelmann, R.; Hunt, P.A.; Welton, T. Ether functionalisation, ion conformation and the optimisation of macroscopic properties in ionic liquids. Phys. Chem. Chem. Phys. 2020, 22, 23038–23056. [Google Scholar] [CrossRef]
- Rauber, D.; Philippi, F.; Kuttich, B.; Becker, J.; Kraus, T.; Hunt, P.; Welton, T.; Hempelmann, R.; Kay, C.W.M. Curled cation structures accelerate the dynamics of ionic liquids. Phys. Chem. Chem. Phys. 2021, 23, 21042–21064. [Google Scholar] [CrossRef]
- Schreiner, C.; Zugmann, S.; Hartl, R.; Gores, H.J. Fractional Walden Rule for Ionic Liquids: Examples from Recent Measurementsand a Critique of the So-Called Ideal KCl Line for the Walden Plot. J. Chem. Eng. Data 2010, 55, 1784–1788. [Google Scholar] [CrossRef]
- Harris, K.R.; Kanakubo, M. Self-Diffusion Coefficients and Related Transport Properties for a Number of Fragile Ionic Liquids. J. Chem. Eng. Data 2016, 61, 2399–2411. [Google Scholar] [CrossRef]
- Gouverneur, M.; Schmidt, F.; Schonhoff, M. Negative effective Li transference numbers in Li salt/ionic liquid mixtures: Does Li drift in the “Wrong” direction? Phys. Chem. Chem. Phys. 2018, 20, 7470–7478. [Google Scholar] [CrossRef]
- Gouverneur, M.; Kopp, J.; van Wullen, L.; Schonhoff, M. Direct determination of ionic transference numbers in ionic liquids by electrophoretic NMR. Phys. Chem. Chem. Phys. 2015, 17, 30680–30686. [Google Scholar] [CrossRef] [Green Version]
- Tsuzuki, S.; Hayamizu, K.; Seki, S. Origin of the Low-Viscosity of [emim][(FSO2)2N] Ionic Liquid and Its Lithium Salt Mixture: Experimental and Theoretical Study of Self-Diffusion Coefficients, Conductivities, and Intermolecular Interactions. J. Phys. Chem. B 2010, 114, 16329–16336. [Google Scholar] [CrossRef] [PubMed]
- Lassègues, J.-C.; Grondin, J.; Aupetit, C.; Johansson, P. Spectroscopic Identification of the Lithium Ion Transporting Species in LiTFSI-Doped IonicLiquids. J. Phys. Chem. A 2009, 113, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Russina, O.; Triolo, A.; Gontrani, L.; Caminiti, R.; Xiao, D.; Hines, L.G., Jr.; Bartsch, R.A.; Quitevis, E.L.; Pleckhova, N.; Seddon, K.R. Morphology and intermolecular dynamics of 1-alkyl-3-methylimidazolium bis{(trifluoromethane)sulfonyl}amide ionic liquids: Structural and dynamic evidence of nanoscale segregation. J. Phys. Condens. Matter 2009, 21, 424121–424130. [Google Scholar] [CrossRef]
- Amith, W.D.; Araque, J.C.; Margulis, C.J. A Pictorial View of Viscosity in Ionic Liquids and the Link to Nanostructural Heterogeneity. J. Phys. Chem. Lett. 2020, 11, 2062–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, J.N.A.C.; Pádua, A.A.H. Nanostructural Organization in Ionic Liquids. J. Phys. Chem. B 2006, 110, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Bernardes, C.E.; Triolo, A.; Canongia Lopes, J.N. Nano-segregation in ionic liquids: Scorpions and vanishing chains. Phys. Chem. Chem. Phys. 2013, 15, 16256–16262. [Google Scholar] [CrossRef]
- Schreiner, C.; Zugmann, S.; Hartl, R.; Gores, H.J. Temperature Dependence of Viscosity and Specific Conductivity ofFluoroborate-Based Ionic Liquids in Light of the Fractional Walden Rule andAngell’s Fragility Concept. J. Chem. Eng. Data 2010, 55, 4372–4377. [Google Scholar] [CrossRef]
- Harris, K.R. Scaling the transport properties of molecular and ionic liquids. J. Mol. Liq. 2016, 222, 520–534. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yonezawa, T.; Koda, S. Study on the temperature-dependent coupling among viscosity, conductivity and structural relaxation of ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 19126–19133. [Google Scholar] [CrossRef]
- Harris, K.R. On the Use of the Angell-Walden Equation To Determine the “Ionicity” of Molten Salts and Ionic Liquids. J. Phys. Chem. B 2019, 123, 7014–7023. [Google Scholar] [CrossRef]
- Sippel, P.; Lunkenheimer, P.; Krohns, S.; Thoms, E.; Loidl, A. Importance of liquid fragility for energy applications of ionic liquids. Sci. Rep. 2015, 5, 13922–13930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, S.M.; Ries, M.E.; Moffat, J.; Budtova, T. NMR and Rheological Study of Anion Size Influence on the Properties of Two Imidazolium-based Ionic Liquids. Sci. Rep. 2017, 7, 8968–8979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
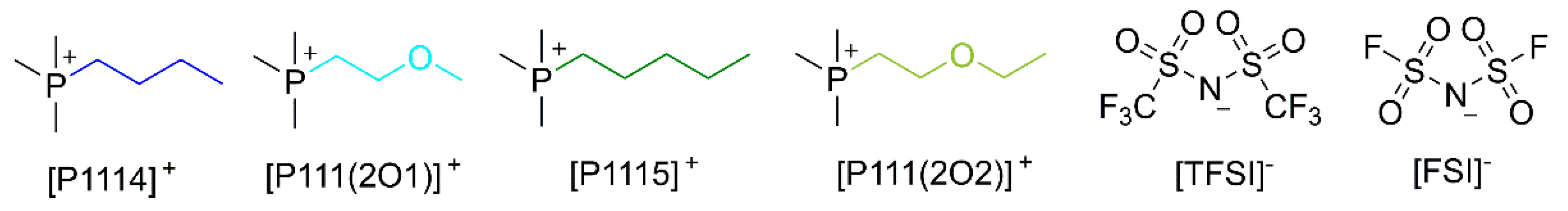

| Abbreviation | Ionic Liquid | Salt | Salt Concentration [mol L−1] | Molar Mass, Me [g mol−1] |
|---|---|---|---|---|
| [4a] | [P1114][TFSI] | --- | --- | 413.2 |
| [4a-025] | [P1114][TFSI] | [Li][TFSI] | 0.25 | 404.1 |
| [4b] | [P1114][FSI] | --- | --- | 313.2 |
| [4b-025] | [P1114][FSI] | [Li][FSI] | 0.25 | 305.8 |
| [5a] | [P1115][TFSI] | --- | --- | 427.2 |
| [5a-025] | [P1115][TFSI] | [Li][TFSI] | 0.25 | 416.5 |
| [5b] | [P1115][FSI] | --- | --- | 327.2 |
| [5b-025] | [P1115][FSI] | [Li][FSI] | 0.25 | 318.4 |
| [2O1a] | [P111(2O1)][TFSI] | --- | --- | 415.2 |
| [2O1a-025] | [P111(2O1)][TFSI] | [Li][TFSI] | 0.25 | 406.2 |
| [2O1b] | [P111(2O1)][FSI] | --- | --- | 315.2 |
| [2O1b-025] | [P111(2O1)][FSI] | [Li][FSI] | 0.25 | 308.0 |
| [2O2a] | [P111(2O2)][TFSI] | --- | --- | 429.2 |
| [2O2a-025] | [P111(2O2)][TFSI] | [Li][TFSI] | 0.25 | 418.6 |
| [2O2b] | [P111(2O2)][FSI] | --- | --- | 329.2 |
| [2O2b-025] | [P111(2O2)][FSI] | [Li][FSI] | 0.25 | 320.6 |
| Ionic Liquid | Li [25 °C] | Li [70 °C] | Na [25 °C] | Na [70 °C] | Electrolyte Mixture | Li [25 °C] | Li [70 °C] | Na [25 °C] | Na [70 °C] |
|---|---|---|---|---|---|---|---|---|---|
| [4a] | + | + | − | − | [4a-025] | + | + | + | + |
| [4b] | +, c | +, c | −, c | −, c | [4b-025] | −, c | −, c | −, c | −, c |
| [5a] | + | + | −, c | −, c | [5a-025] | −, c | −, c | −, c | −, c |
| [5b] | − | − | +, c | +, c | [5b-025] | −, c | −, c | −, c | −, c |
| [2O1a] | + | + | −, c | −, c | [2O1a-025] | + | + | −, c | −, c |
| [2O1b] | − | − | −, c | −, c | [2O1b-025] | + | +, c | − | − |
| [2O2a] | − | − | −, c | −, c | [2O2a-025] | −, c | −, c | −, c | −, c |
| [2O2b] | − | − | + | + | [2O2b-025] | −, c | − | − | − |
| Ionic Liquid | Tg/°C | Tc/°C | Tm/°C | Electrolyte Mixture | Tg/°C | Tc/°C | Tm/°C |
|---|---|---|---|---|---|---|---|
| [4a] | - | −12 | 42 | [4a-025] | - | −14 | 39 |
| [4b] | - | −36 | 0 | [4b-025] | - | −53 | −2 |
| [5a] | - | −9 | 41 | [5a-025] | −70 | - | 39 |
| [5b] | - | −5 | 28 | [5b-025] | - | −13 | 24 |
| [2O1a] | - | −20 | 42 | [2O1a-025] | - | −36 | 36 |
| [2O1b] | - | −3 | 36 | [2O1b-025] | - | −7 | 33 |
| [2O2a] | −82 | - | - | [2O2a-025] | −78 | - | - |
| [2O2b] | - | -38 | 8 | [2O2b-025] | - | −47 | 9 |
| Sample | η25 °C mPa∙s | η0/10−1 mPa∙s | Bη/K | T0,η/K | R2 | Ea,η /kJ∙mol−1 | |
|---|---|---|---|---|---|---|---|
| [4a] | 112.3 | 1.66 ± 0.05 | 772.8 ± 8.8 | 179.6 ± 0.8 | >0.99999 | 4.30 | 35.1 ± 0.6 |
| [4a-025] | 162.0 | 1.67 ± 0.05 | 783.2 ± 7.5 | 184.3 ± 0.6 | >0.99999 | 4.25 | 38.2 ± 0.7 |
| [4b] | 44.19 | 1.89 ± 0.05 | 796.4 ± 8.3 | 152.1 ± 0.9 | >0.99999 | 5.24 | 24.8 ± 0.3 |
| [4b-025] | 53.77 | 2.33 ± 0.04 | 747.6 ± 4.9 | 160.8 ± 0.5 | >0.99999 | 4.65 | 26.1 ± 0.4 |
| [5a] | 136.7 | 1.34 ± 0.01 | 812.2 ± 2.5 | 181.0 ± 6.9 | >0.99999 | 4.49 | 37.5 ± 0.7 |
| [5a-025] | 198.7 | 1.29 ± 0.02 | 837.2 ± 3.9 | 184.0 ± 0.9 | >0.99999 | 4.55 | 38.0 ± 2.1 |
| [5b] | 63.27 | 1.43 ± 0.32 | 887.3 ± 54.1 | 152.8 ± 6.9 | 0.99999 | 5.81 | 28.2 ± 0.3 |
| [5b-025] | 68.86 | 2.19 ± 0.06 | 765.9 ± 9.0 | 164.9 ± 0.9 | >0.99999 | 4.64 | 28.1 ± 0.4 |
| [2O1a] | 54.79 | 2.06 ± 0.03 | 694.0 ± 4.1 | 173.9 ± 0.4 | >0.99999 | 3.99 | 28.9 ± 0.5 |
| [2O1a-025] | 72.05 | 2.00 ± 0.06 | 727.1 ± 9.2 | 174.6 ± 0.9 | 0.99999 | 4.16 | 30.7 ± 0.5 |
| [2O1b] | 30.66 | 1.92 ± 0.04 | 765.1 ± 8.2 | 147.3 ± 0.9 | >0.99999 | 5.20 | 22.5 ± 0.3 |
| [2O1b-025] | 36.46 | 2.38 ± 0.05 | 714.5 ± 6.8 | 156.2 ± 0.8 | >0.99999 | 4.57 | 23.5 ± 0.3 |
| [2O2a] | 53.34 | 1.91 ± 0.03 | 694.4 ± 3.9 | 174.8 ± 0.4 | >0.99999 | 3.97 | 29.3 ± 0.5 |
| [2O2a-025] | 74.42 | 1.65 ±0.02 | 756.4 ± 2.9 | 174.4 ± 0.3 | >0.99999 | 4.34 | 31.7 ± 0.5 |
| [2O2b] | 33.83 | 1.96 ± 0.03 | 757.7 ± 4.8 | 151.0 ± 0.5 | >0.99999 | 5.02 | 23.3 ± 0.3 |
| [2O2b-025] | 37.26 | 2.15 ± 0.07 | 737.3 ± 11.0 | 155.1 ± 1.2 | 0.99999 | 4.75 | 23.9 ± 0.3 |
| Sample | κ25°C/ mS∙cm−1 | κ,0 / mS∙cm−1 | Bκ /K | T0,κ/K | R2 | Ea,κ /kJ∙mol−1 | |
|---|---|---|---|---|---|---|---|
| [4a] | 1.76 | 600.5 ± 13.1 | −684.6 ± 6.5 | 180.7 ± 0.7 | >0.99999 | 3.79 | 31.6 ± 0.6 |
| [4a-025] | 1.24 | 632.1 ± 26.2 | −719.2 ± 12.4 | 182.8 ± 1.3 | 0.99998 | 3.93 | 34.2 ± 0.6 |
| [4b] | 6.49 | 582.3 ± 8.0 | −615.3 ± 4.6 | 161.3 ± 0.6 | >0.99999 | 3.82 | 21.6 ± 0.3 |
| [4b-025] | 5.57 | 767.4 ± 36.8 | −717.3 ± 16.8 | 152.5 ± 2.0 | 0.99998 | 4.70 | 22.5 ± 0.3 |
| [5a] | 1.20 | 536.9 ±15.4 | −698.6 ± 8.5 | 183.7 ± 0.9 | >0.99999 | 3.80 | 33.7 ± 0.6 |
| [5a-025] | 0.89 | 528.7 ± 18.2 | −711.3 ± 10.0 | 186.8 ± 1.0 | 0.99999 | 3.81 | 36.0 ± 0.6 |
| [5b] | 4.16 | 607.9 ± 7.8 | −669.5 ± 4.2 | 163.9 ± 0.5 | >0.99999 | 4.09 | 24.3 ± 0.3 |
| [5b-025] | 3.73 | 537.2 ± 15.6 | −640.1 ± 9.2 | 169.4 ± 1.1 | 0.99999 | 3.78 | 25.0 ± 0.3 |
| [2O1a] | 3.35 | 506.2 ± 20.0 | −626.3 ± 12.2 | 173.4 ± 1.5 | 0.99998 | 3.61 | 25.7 ± 0.4 |
| [2O1a-025] | 2.53 | 559.5 ± 47.2 | −672.9 ± 26.3 | 173.4 ± 3.0 | 0.99994 | 3.88 | 27.8 ± 0.6 |
| [2O1b] | 7.79 | 665.1 ± 32.8 | −656.4 ± 17.3 | 150.6 ± 2.3 | 0.99997 | 4.36 | 20.1 ± 0.3 |
| [2O1b-025] | 6.94 | 563.4 ± 8.2 | −609.7 ± 4.8 | 159.5 ± 0.7 | >0.99999 | 3.82 | 20.9 ± 0.3 |
| [2O2a] | 2.89 | 446.3 ± 5.9 | −618.2 ± 4.0 | 175.5 ± 0.5 | >0.99999 | 3.52 | 26.3 ± 0.4 |
| [2O2a-025] | 2.20 | 396.1 ± 23.2 | −603.7 ± 17.8 | 181.8 ± 2.1 | 0.99998 | 3.32 | 28.2 ± 0.5 |
| [2O2b] | 7.07 | 555.2 ± 12.1 | −615.0 ± 7.4 | 157.2 ± 1.0 | 0.99999 | 3.91 | 20.4 ± 0.3 |
| [2O2b-025] | 6.14 | 641.5 ± 76.1 | −686.3 ± 41.9 | 149.9 ± 5.3 | 0.99986 | 4.58 | 20.9 ± 0.5 |
| Electrolyte | DLi/10−12 m2 s−1 | Electrolyte | DLi/10−12 m2 s−1 | ||
|---|---|---|---|---|---|
| [4a-025] | 3.7 ± 0.1 | 3.64 ± 0.12 | [2O1a-025] | 6.9 ± 1.2 | 4.39 ± 0.77 |
| [4b-025] | 19.0 ± 1.0 | 2.14 ± 0.12 | [2O1b-025] | 26.0 ± 0.7 | 2.30 ± 0.08 |
| [5a-025] | 2.8 ± 0.2 | 3.93 ± 0.29 | [2O2a-025] | 8.2 ± 0.3 | 3.58 ± 0.15 |
| [5b-025] | 14.0 ± 0.3 | 2.27 ± 0.07 | [2O2b-025] | 24.0 ± 0.4 | 2.44 ± 0.06 |
| Ionic Liquid | Potential Limit from CV/V | Potential Limit via U-Ramping/V | Potential Limit Confirmed at 30 h Polarization | Electrolyte Mixture | Potential Limit from CV/V | Potential Limit via U-Ramping/V | Potential Limit Confirmed at 30 h Polarization |
|---|---|---|---|---|---|---|---|
| [4a] | 3.8 | 6.2 | 6.1 | [4a-025] | >5.5 | 5.3 | 5.2 |
| [4b] | 3.9 | 4.1 | 4.4 | [4b-025] | 4.0 | 4.4 | 4.4 |
| [5a] | 5.3 | 5.8 | 5.5 | [5a-025] | 5.3 | 5.3 | 5.3 |
| [5b] | 3.9 | 4.3 | 4.5 | [5b-025] | 4.2 | 3.7 | 3.7 |
| [2O1a] | 4.8 | 4.7 | 4.6 | [2O1a-025] | >5.5 | 5.0 | 4.8 |
| [2O1b] | 5.2 | 3.3 | 3.2 | [2O1b-025] | 4.2 | 3.4 | 3.4 |
| [2O2a] | 3.5 | 2.4 | 2.1 | [2O2a-025] | 3.5 | 5.0 | 4.8 |
| [2O2b] | 3.3 | 3.3 | 3.1 | [2O2b-025] | 3.8 | 3.3 | 3.1 |
| Electrolyte Mixture | ΔU/mV | Electrolyte Mixture | ΔU/mV |
|---|---|---|---|
| [4a-025] | 78 | [2O1a-025] | 60 |
| [4b-025] | 52 | [2O1b-025] | 50 |
| [5a-025] | 82 | [2O2a-025] | 63 |
| [5b-025] | 56 | [2O2b-025] | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, A.; Rauber, D.; Wang, T.-M.; Hempelmann, R.; Kay, C.W.M.; Hanemann, T. Novel Phosphonium-Based Ionic Liquid Electrolytes for Battery Applications. Molecules 2022, 27, 4729. https://doi.org/10.3390/molecules27154729
Hofmann A, Rauber D, Wang T-M, Hempelmann R, Kay CWM, Hanemann T. Novel Phosphonium-Based Ionic Liquid Electrolytes for Battery Applications. Molecules. 2022; 27(15):4729. https://doi.org/10.3390/molecules27154729
Chicago/Turabian StyleHofmann, Andreas, Daniel Rauber, Tzu-Ming Wang, Rolf Hempelmann, Christopher W. M. Kay, and Thomas Hanemann. 2022. "Novel Phosphonium-Based Ionic Liquid Electrolytes for Battery Applications" Molecules 27, no. 15: 4729. https://doi.org/10.3390/molecules27154729
APA StyleHofmann, A., Rauber, D., Wang, T.-M., Hempelmann, R., Kay, C. W. M., & Hanemann, T. (2022). Novel Phosphonium-Based Ionic Liquid Electrolytes for Battery Applications. Molecules, 27(15), 4729. https://doi.org/10.3390/molecules27154729






