Effect of Erosive Agents on Surface Characteristics of Nano-Fluorapatite Ceramic: An In-Vitro Study
Abstract
:1. Introduction
2. Results
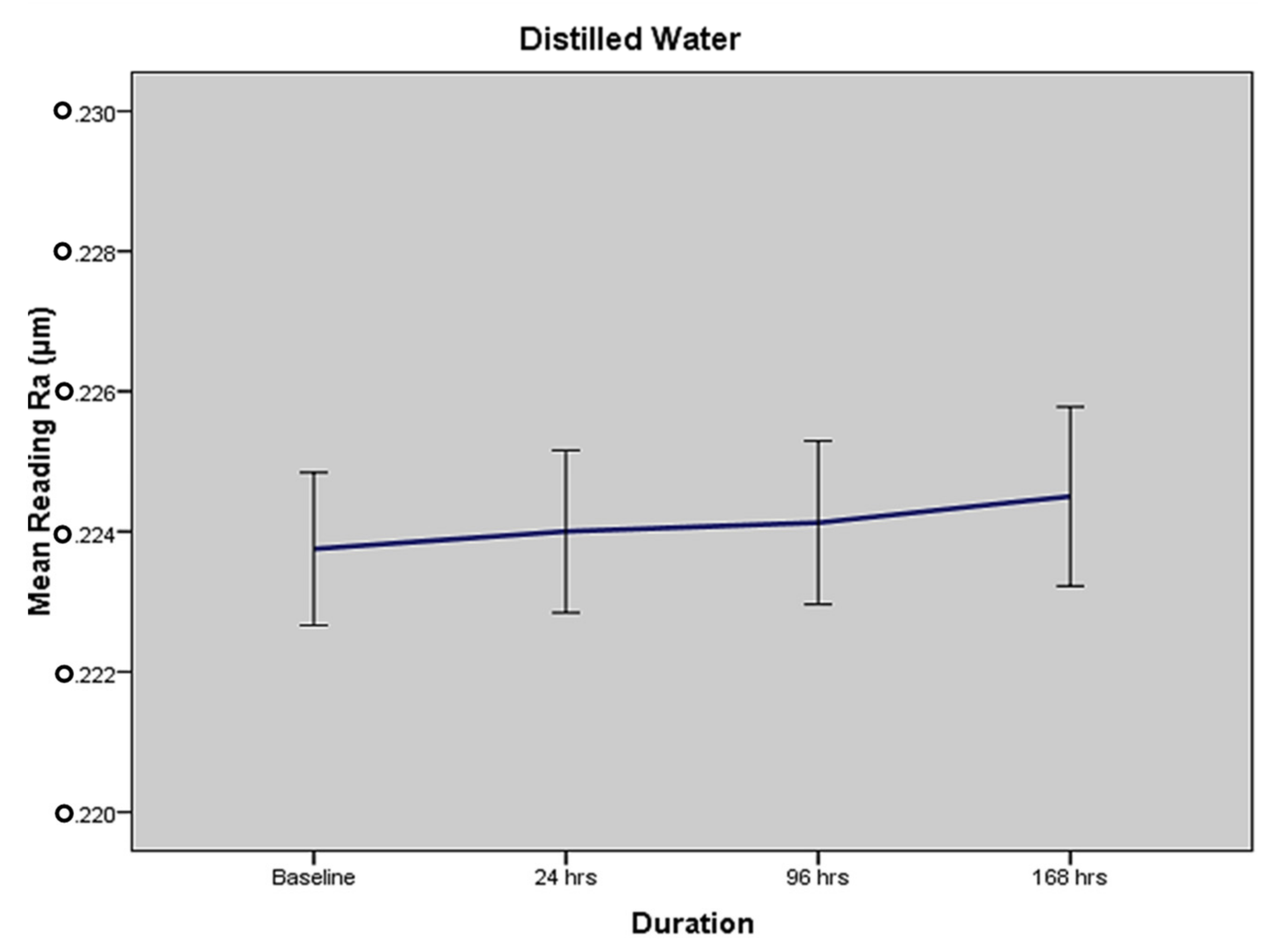
2.1. Mean Surface Roughness in Terms of Medium I—Distilled Water
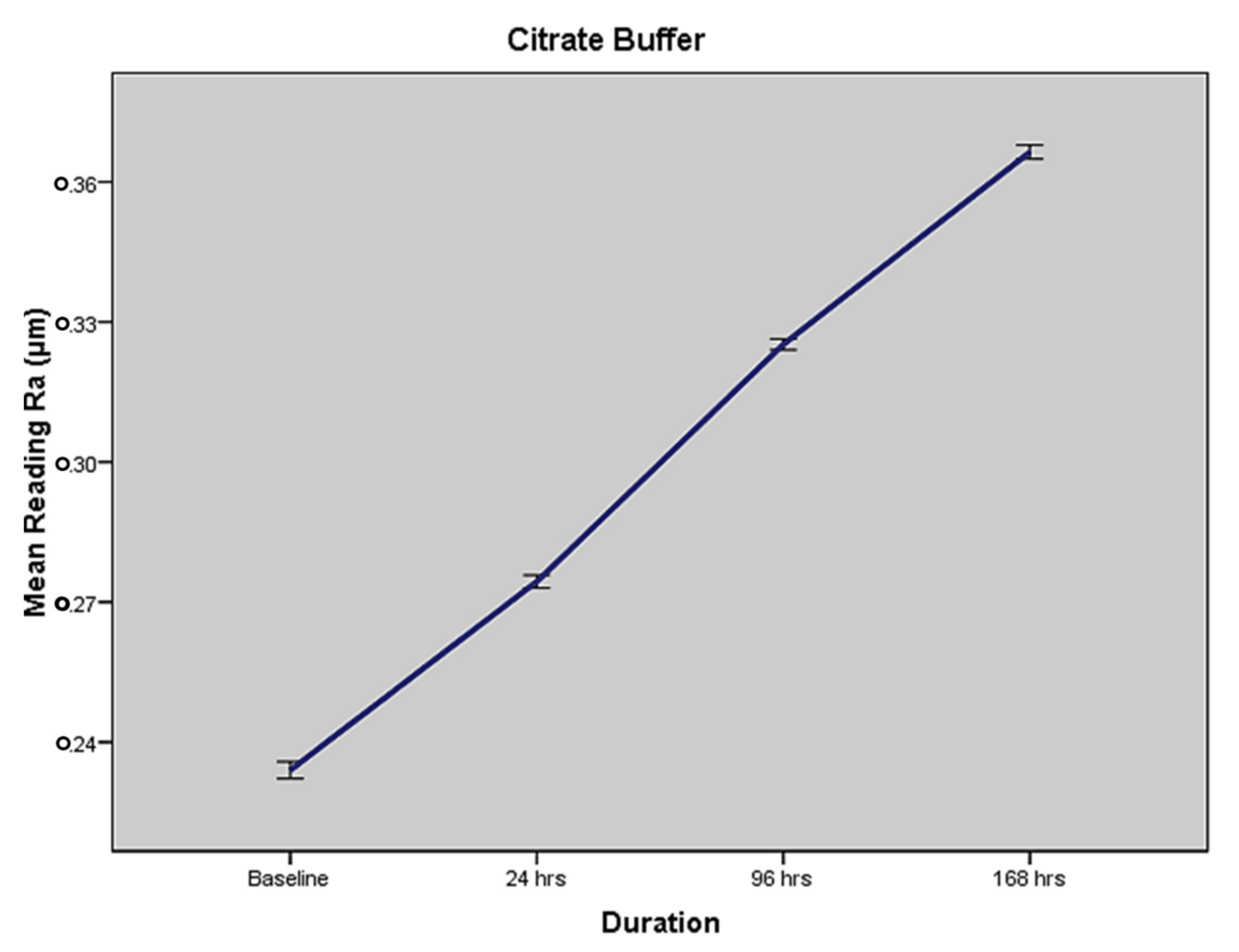
2.2. Mean Surface Roughness in Terms of MEDIUM III—Citrate Buffer
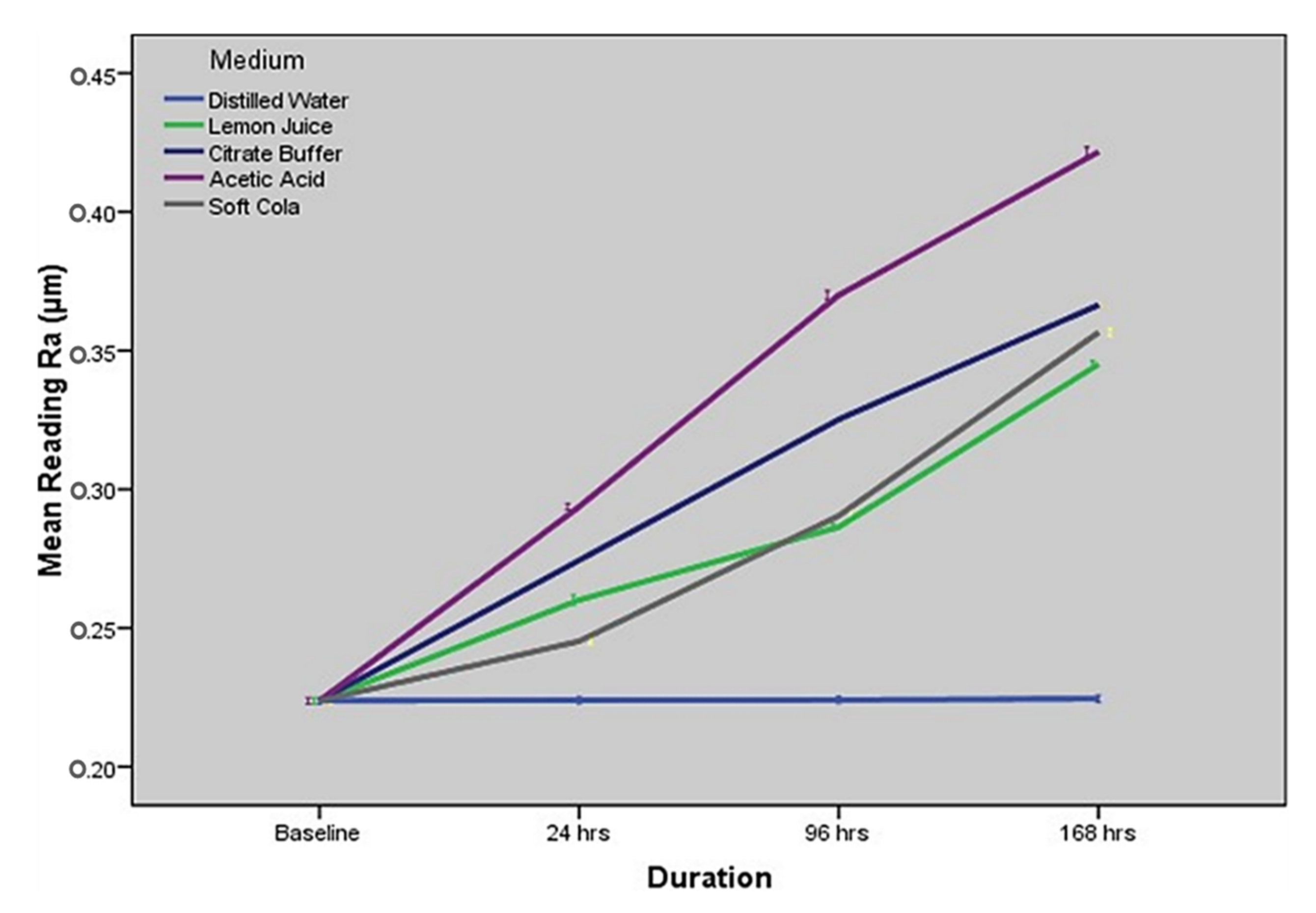
2.3. Comparison of Surface Roughness Assessment at Different Time Intervals for Specified Immersion Solutions
2.4. Comparison of Surface Roughness amongst Immersion Solutions at Specified Time Intervals
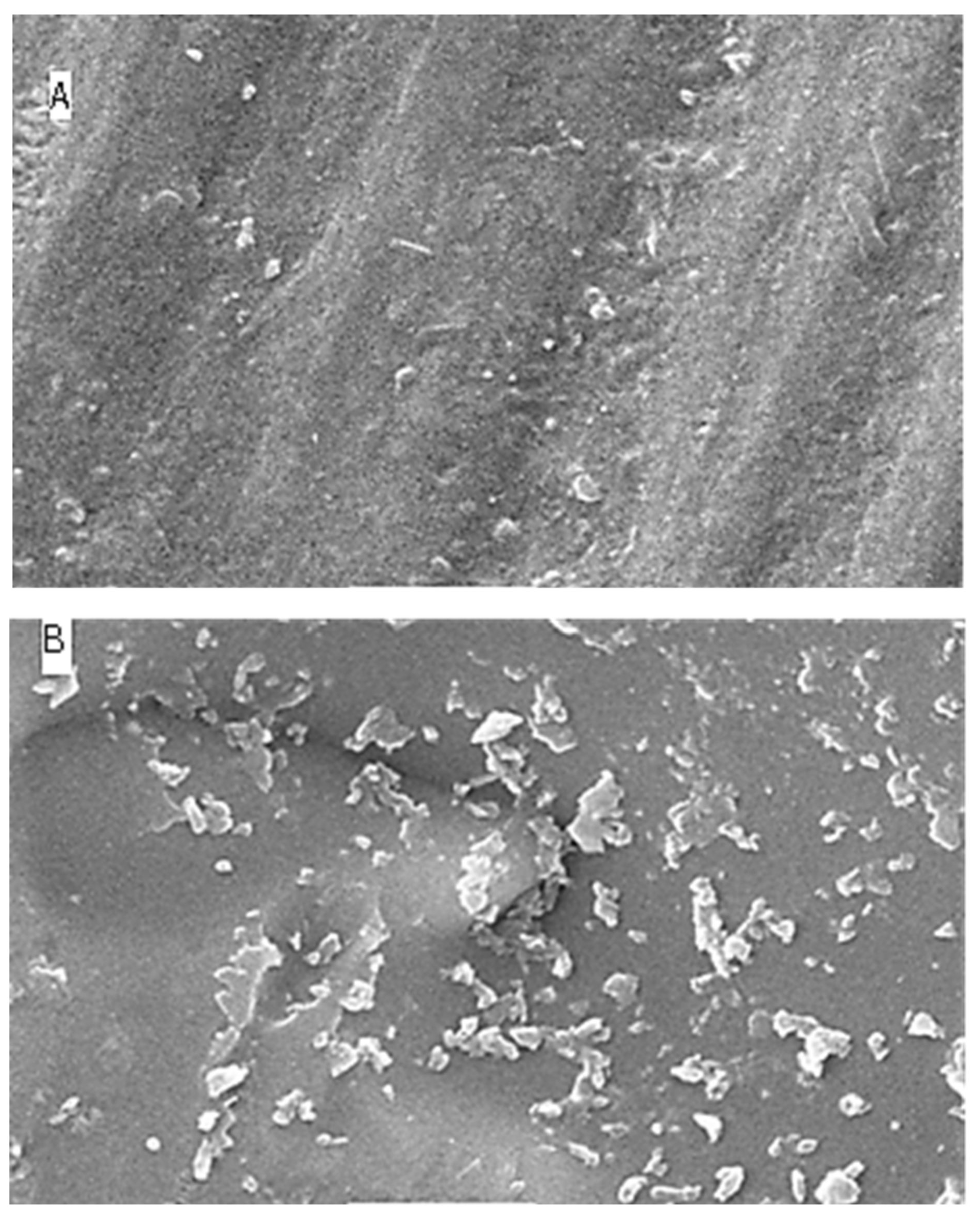
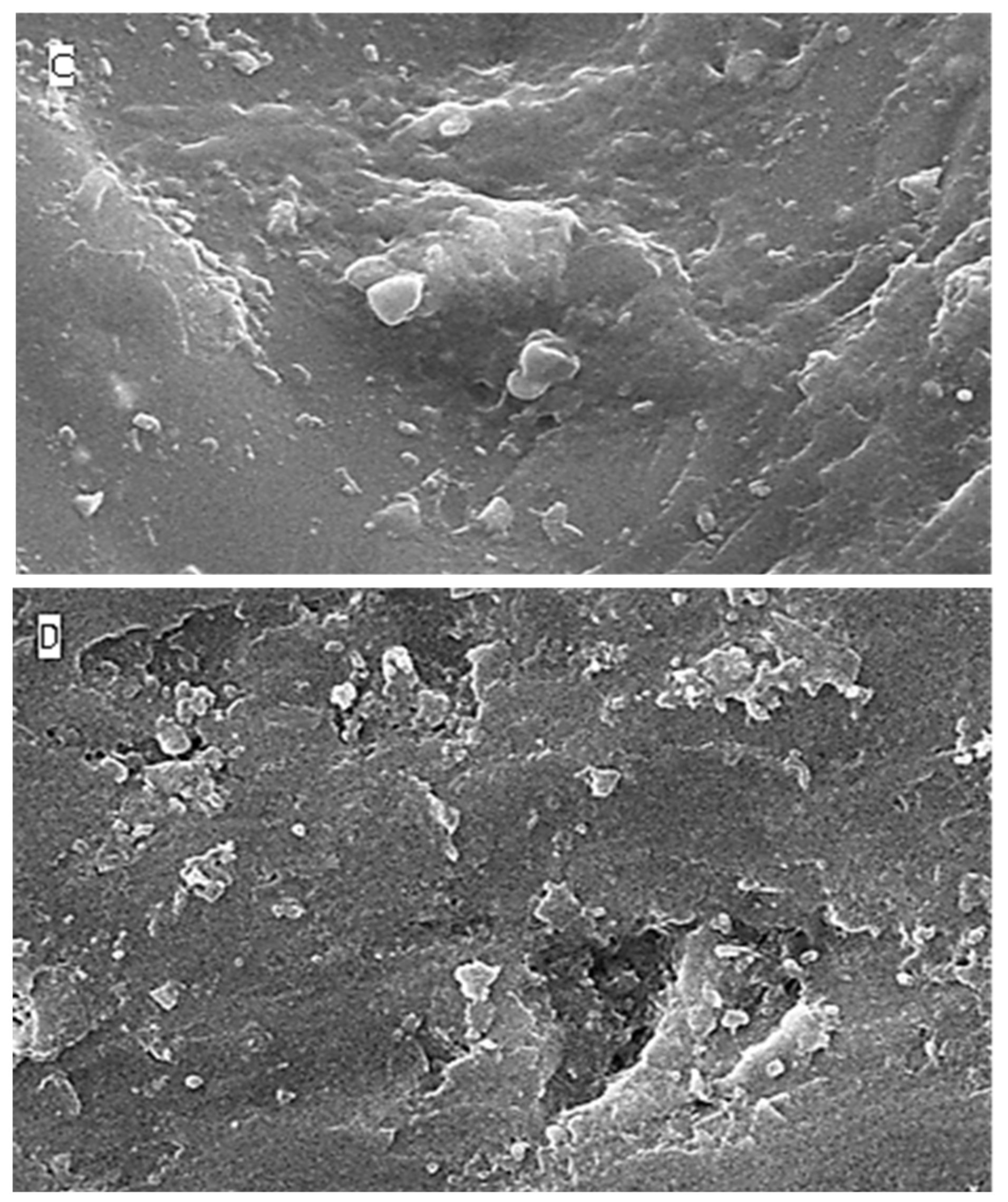
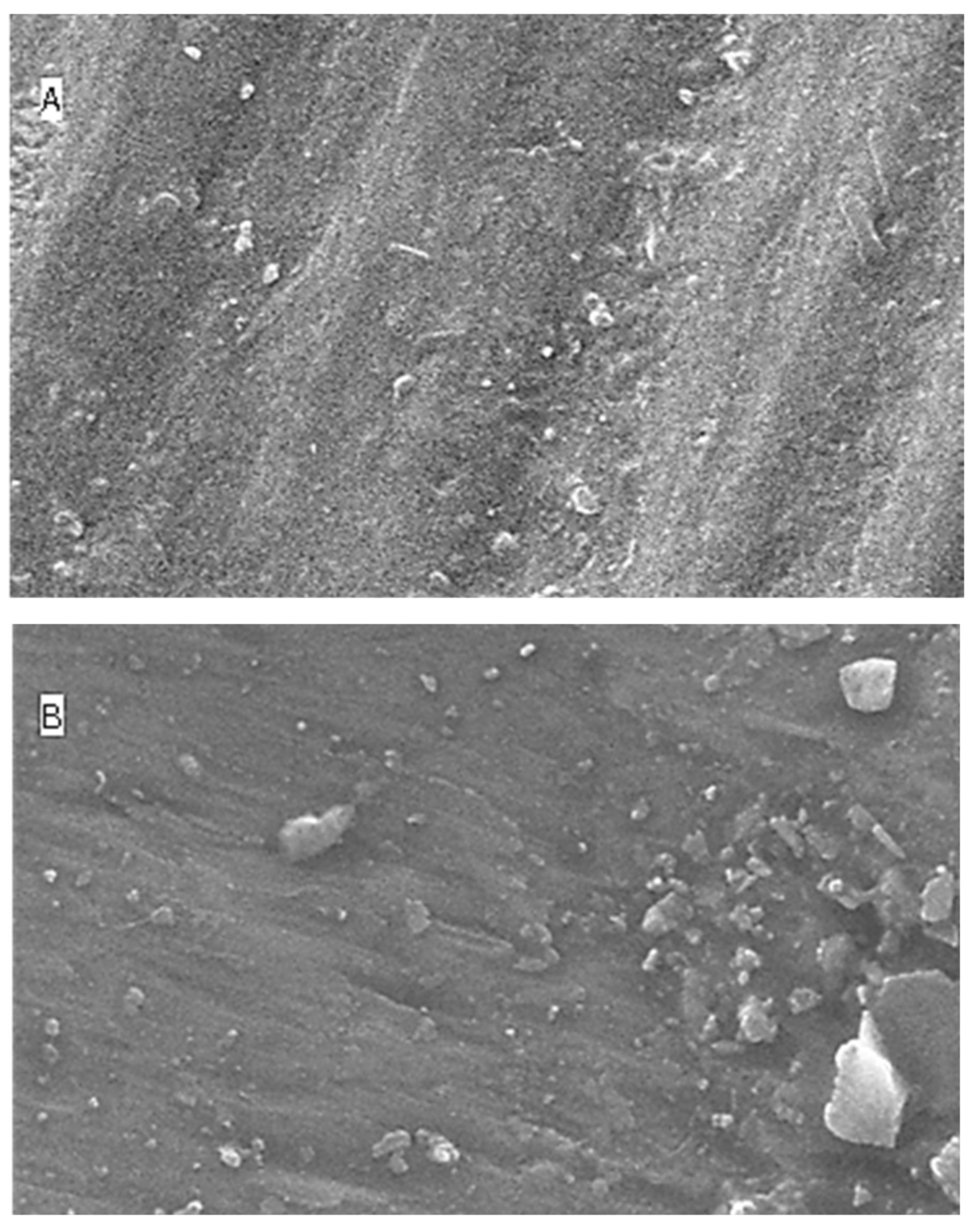
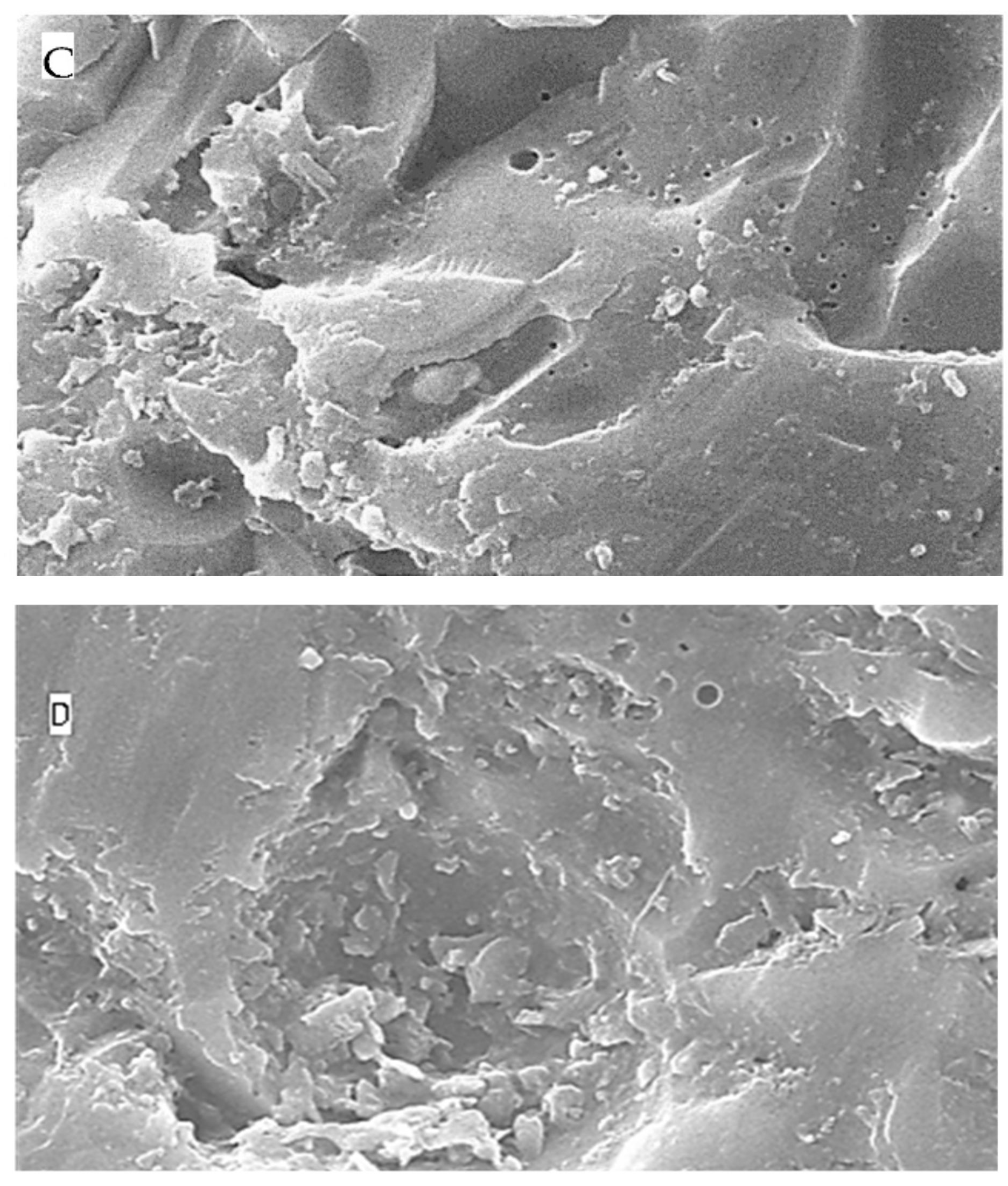
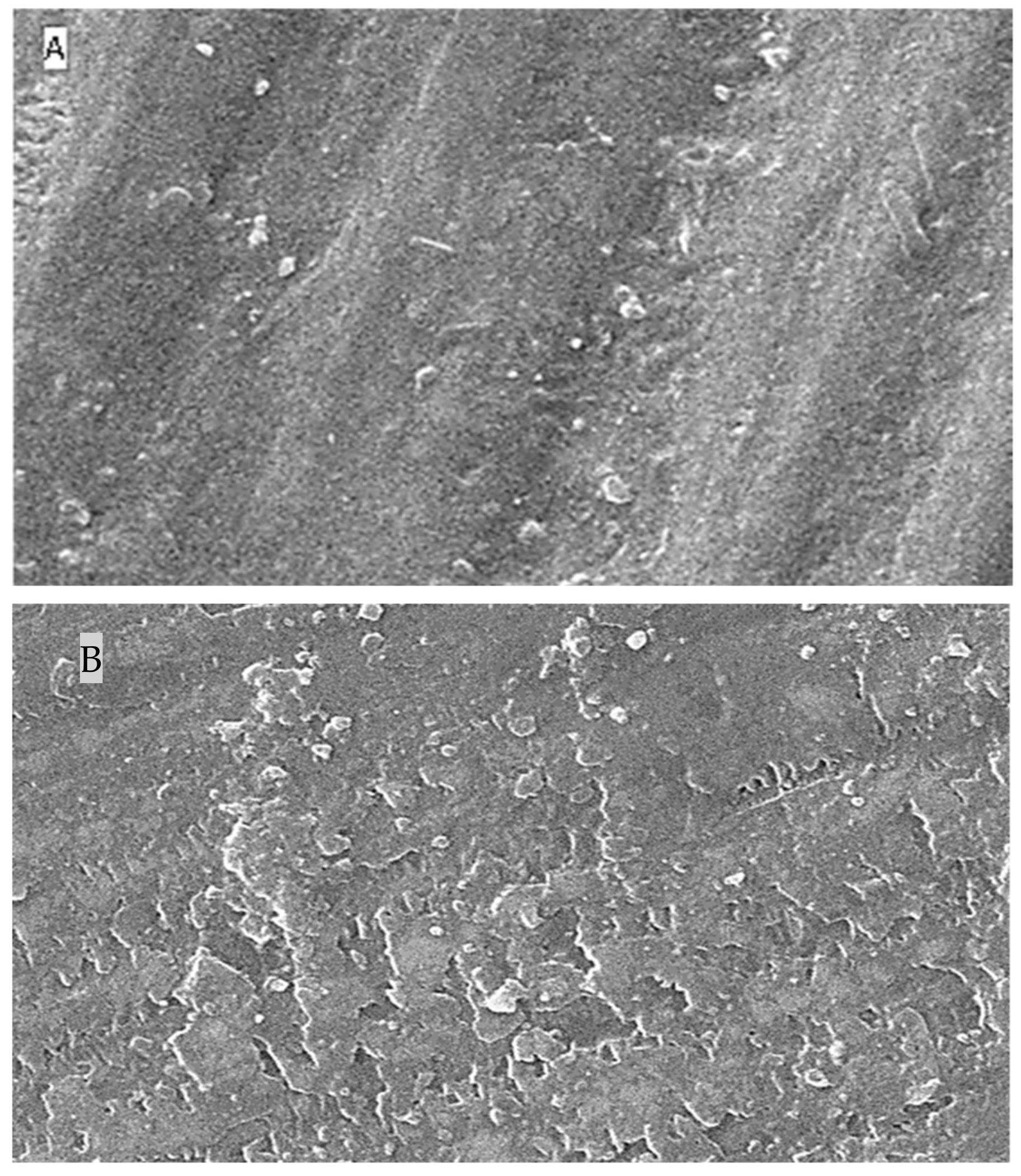
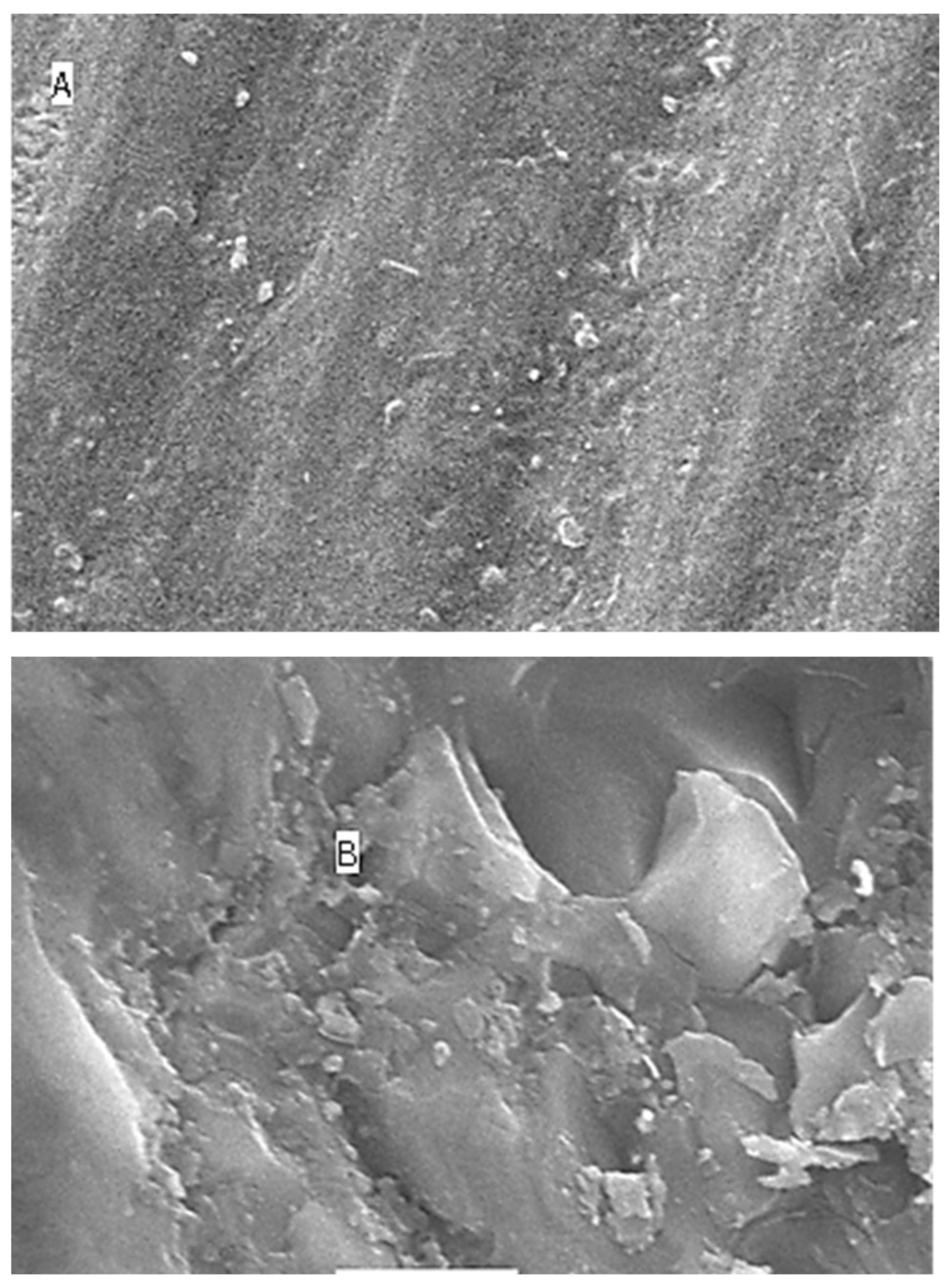
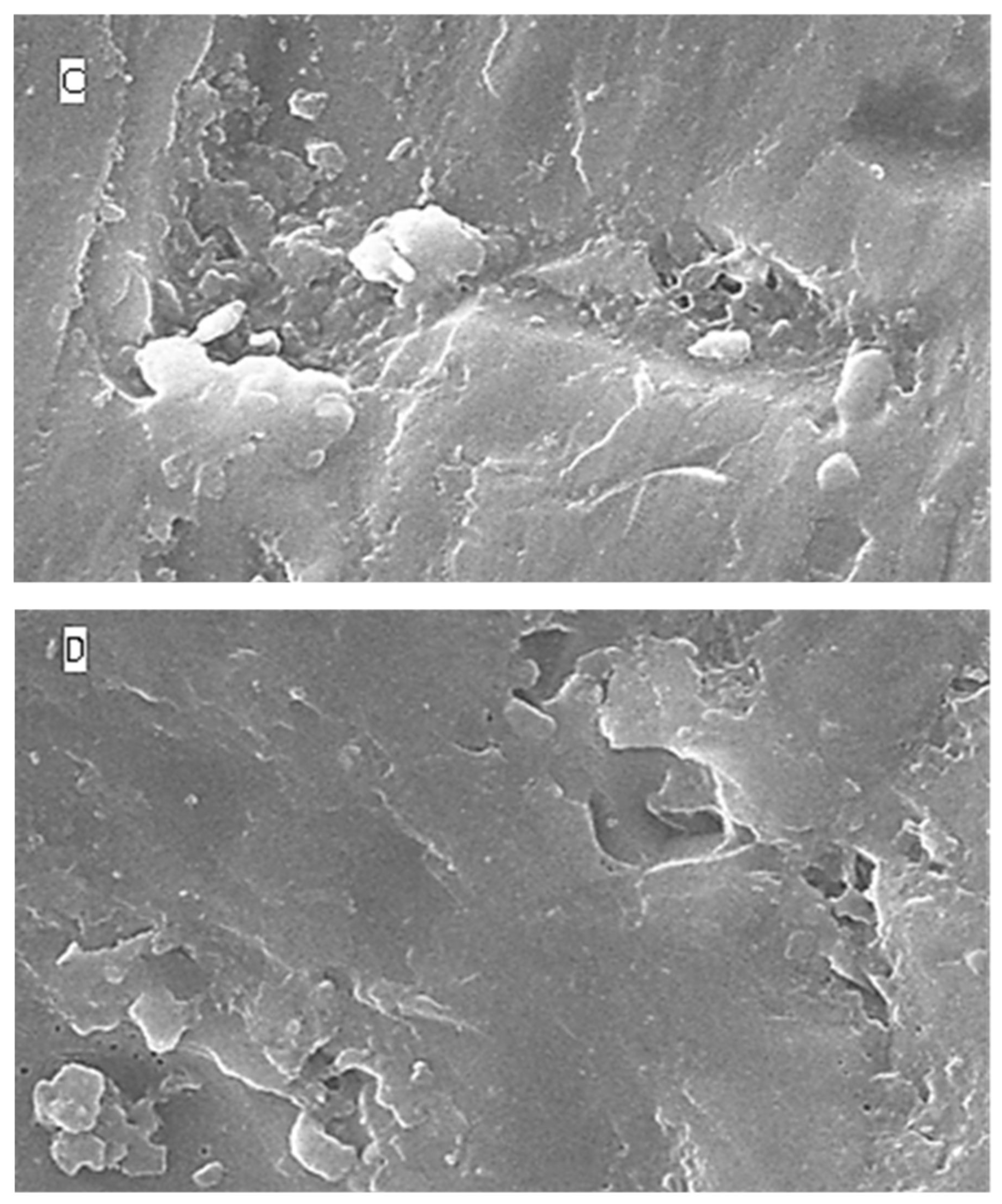
2.5. Surface Topography Analysis Using SEM (Non-Contact Method)
3. Discussion
3.1. Study Strengths
- This is a study related to the performance of ceramics in clinical dentistry in local settings as it involves the utilization of a profilometer and scanning electron microscope (SEM) to record the changes which occur on the surface of ceramics when exposed to acidic beverages available on the local market.
- The present research focuses on IPS e.max Ceram, a nano-fluorapatite type ceramic, which is considered to be a superior ceramic in terms of its properties, therefore any surface effect on this high-quality type of ceramic gives an insight about the changes which can occur on other ceramic types.
- The research findings will provide clinicians and students an understanding of detrimental effects produced by routinely consumed acidic beverages and juices on ceramic restorations which are usually considered as inert restorative materials.
- The study findings will assist dental practitioners in disseminating useful information to their patients about caution relating to consumption of acidic beverages, thereby enhancing the longevity of their intra-oral ceramic restorations and reducing the chances of clinical failure.
- The current research provides dental practitioners with valuable information to plan dental restorations, keeping under consideration any underlying erosive conditions, such as GERD, bulimic conditions, alcoholism, and occupation-related exposures.
3.2. Study Limitations
- Since the present study was an in-vitro investigation, the influence of natural oral conditions such as the presence of saliva, water, intra-oral temperature changes and pH level could not be taken into consideration, since they may affect the study outcome. Henceforth, in vivo experiments should be undertaken to evaluate the true effect of erosive agents on dental ceramics.
- Due to limited financial resources, only one ceramic type i.e., fluorapatite (IPS e.max Ceram), was evaluated in the current study. Therefore, more studies are required to determine the impact of acidic beverages/agents on other types of dental ceramics.
- Artificial saliva could have been used as an additional test group, since it offers a better medium to reproduce natural oral conditions.
- A contact-type profilometer was used in the present study. A disadvantage of this type is that the stylus tip, upon contact with disc specimen, causes irreversible damage to the discs. Hence, such specimens cannot be re-used for further study purposes.
3.3. Recommendations
- The effect of natural saliva on the ceramic surface should be explored to determine its behavior in in vivo conditions.
- Additional potential erosive juices and beverages should be included to explore their effect on ceramics.
- More ceramic types should be added in future research endeavors.
- Additional testing methods, including atomic force microscopy (AFM) should be employed to give a three-dimensional image of the ceramic surface.
- The effect of temperature changes should be included in future studies.
- Titrability of acidic drinks, along with their respective pH values, should be considered in future endeavors.
- The synergistic effect of abrasion/attrition on wear with chemical erosion should be assessed in further studies.
4. Materials and Methods
4.1. Specimen Fabrication
4.2. Solution Preparation and pH Testing
4.3. Specimen Immersion and Surface Characteristic Changes Using Profilometer and SEM
4.4. Data Management and Analysis
5. Conclusions
- The tested erosive agents had a negative effect on the surface roughness of tested ceramic.
- A significant deterioration in the ceramic surfaces was observed in relation to all of the immersion media except distilled water.
- The surface roughness increased with increased storage time interval.
- The low pH found in beverages and fruit juices was observed to be a causative factor in increasing the surface roughness of the material.
- This study outcome should be considered during advertising of and in consumers’ habits of consuming erosive beverages.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kukiattrakoon, B.; Hengtrakool, C.; Kedjarune-Leggat, U. Effect of acidic agents on surface roughness of dental ceramics. Dent. Res. J. 2011, 8, 6–15. [Google Scholar]
- Wongkhantee, S.; Patanapiradej, V.; Maneenut, C.; Tantbirojn, D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J. Dent. 2006, 34, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Milleding, P.; Haraldsson, C.; Karlsson, S. Ion leaching from dental ceramics during static in vitro corrosion testing. J. Biomed. Mater. Res. 2002, 61, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Raptis, N.V.; Michalakis, K.X.; Hirayama, H. Optical behavior of current ceramic systems. Int. J. Periodontics Restorative Dent. 2006, 26, 31–41. [Google Scholar]
- Kukiattrakoon, B.; Hengtrakool, C.; Kedjarune-Leggat, U. Chemical durability and microhardness of dental ceramics immersed in acidic agents. Acta Odontol. Scand. 2010, 68, 1–10. [Google Scholar] [CrossRef]
- Sınmazışık, G.; Öveçoğlu, M. Physical properties and microstructural characterization of dental porcelains mixed with distilled water and modeling liquid. Dent. Mater. 2006, 22, 735–745. [Google Scholar] [CrossRef]
- Yang, L.; Bovet, P.; Liu, Y.; Zhao, M.; Ma, C.; Liang, Y.; Xi, B. Consumption of carbonated soft drinks among young adolescents aged 12 to 15 years in 53 low-and middle-income countries. Am. J. Public Health 2017, 107, 1095–1100. [Google Scholar] [CrossRef]
- Enan, E.T. ErosivE and abrasivE PotEntials of soft drinks and air Polishing on nano-filled ComPositE rEsin. Egypt. Dent. J. 2016, 62, 1221–1229. [Google Scholar] [CrossRef]
- Yu, P.; Xu, Z.; Arola, D.D.; Min, J.; Zhao, P.; Gao, S. Effect of acidic agents on the wear behavior of a polymer infiltrated ceramic network (PICN) material. J. Mech. Behav. Biomed. Mater. 2017, 74, 154–163. [Google Scholar] [CrossRef]
- Milleding, P.; Wennerberg, A.; Alaeddin, S.; Karlsson, S.; Simon, E. Surface corrosion of dental ceramics in vitro. Biomaterials 1999, 20, 733–746. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert Rev. Med. Devices 2016, 13, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Kukiattrakoon, B.; Junpoom, P.; Hengtrakool, C. Vicker’s microhardness and energy dispersive x-ray analysis of fluorapatite-leucite and fluorapatite ceramics cyclically immersed in acidic agents. J. Oral Sci. 2009, 51, 443–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeggi, T.; Lussi, A. Prevalence, Incidence and Distribution of Erosion; Dental erosion. 20; Karger Publishers: Basel, Switzerland, 2006; pp. 44–65. [Google Scholar]
- Jakovac, M.; Živko-Babić, J.; Ćurković, L.; Carek, A. Chemical durability of dental ceramic material in acid medium. Acta Stomatol. Croat. 2006, 40, 65–71. [Google Scholar]
- Kukiattrakoon, B.; Hengtrakool, C.; Kedjarune-Leggat, U. Elemental release and surface changes of fluorapatite–leucite porcelain upon immersion in acidic agents. J. Dent. Sci. 2010, 5, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Kukiattrakoon, B.; Hengtrakool, C.; Kedjarune-Leggat, U. The effect of acidic agents on surface ion leaching and surface characteristics of dental porcelains. J. Prosthet. Dent. 2010, 103, 148–162. [Google Scholar] [CrossRef]
- Demirel, F.; Yüksel, G.; Muhtarogulları, M.; Çekiç, C. Effect of topical fluorides and citric acid on heat-pressed all-ceramic material. Int. J. Periodontics Restor. Dent. 2005, 25, 277–281. [Google Scholar]
- Zakir, T.; Dandekeri, S.; Suhaim, K.S.; Shetty, N.H.; Ragher, M.; Shetty, S.K. Influence of aerated drink, mouthwash, and simulated gastric acid on the surface roughness of dental ceramics: A comparative In Vitro study. J. Pharm. Bioallied Sci. 2020, 12, 480. [Google Scholar]
- Junpoom, P.; Kukiattrakoon, B.; Hengtrakool, C. Flexural strength of fluorapatite-leucite and fuorapatite porcelains exposed to erosive agents in cyclic immersion. J. Appl. Oral Sci. 2011, 19, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.-M.; Ren, F.; Chen, Z.; Kim, M.; Fares, C.; Clark, A.E.; Neal, D.; Esquivel-Upshaw, J.F. Novel coating to minimize corrosion of glass-ceramics for dental applications. Materials 2020, 13, 1215. [Google Scholar] [CrossRef] [Green Version]
- Zaninovich, M. The Effect of Clinical Polishing Protocols on Ceramic Surface Texture and Wear Rate of Opposing Enamel: A Laboratory Study. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2014. [Google Scholar]
- Alnasser, M.; Finkelman, M.; Papathanasiou, A.; Suzuki, M.; Ghaffari, R.; Ali, A. Effect of acidic pH on surface roughness of esthetic dental materials. J. Prosthet. Dent. 2019, 122, 567.e1–e8. [Google Scholar] [CrossRef]
- Rashid, H. The effect of surface roughness on ceramics used in dentistry: A review of literature. Eur. J. Dent. 2014, 8, 571. [Google Scholar] [CrossRef]
- Anusavice, K.J. Standardizing failure, success, and survival decisions in clinical studies of ceramic and metal–ceramic fixed dental prostheses. Dent. Mater. 2012, 28, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Näpänkangas, R.; Raustia, A. Twenty-year follow-up of metal-ceramic single crowns: A retrospective study. Int. J. Prosthodont. 2008, 21, 307–311. [Google Scholar] [PubMed]
- De Backer, H.; Van Maele, G.; De Moor, N.; Van den Berghe, L.; De Boever, J. A 20-Year Retrospective Survival Study of Fixed Partial Dentures. Int. J. Prosthodont. 2006, 19, 143–153. [Google Scholar] [PubMed]
- Sunnegårdh-Grönberg, K.; van Dijken, J.W. Surface roughness of a novel “ceramic restorative cement” after treatment with different polishing techniques in vitro. Clin. Oral Investig. 2003, 7, 27–31. [Google Scholar] [CrossRef]
- Han, J.-M.; Zhang, H.; Choe, H.-S.; Lin, H.; Zheng, G.; Hong, G. Abrasive wear and surface roughness of contemporary dental composite resin. Dent. Mater. J. 2014, 33, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Chaiyabutr, Y.; McGowan, S.; Phillips, K.M.; Kois, J.C.; Giordano, R.A. The effect of hydrofluoric acid surface treatment and bond strength of a zirconia veneering ceramic. J. Prosthet. Dent. 2008, 100, 194–202. [Google Scholar] [CrossRef]
- Syed, S.; Yassin, S.M.; Almalki, A.Y.; Ali, S.A.A.; Alqarni, A.M.M.; Moadi, Y.M.; Alkhaldi, A.M.; Alqahtani, N.M.; Hosmani, J. Structural Changes in Primary Teeth of Diabetic Children: Composition and Ultrastructure Analysis. Children 2022, 9, 317. [Google Scholar] [CrossRef]
- Heboyan, A.; Marya, A.; Syed, A.U.Y.; Khurshid, Z.; Zafar, M.S.; Rokaya, D.; Anastasyan, M. In vitro microscopic evaluation of metal- and zirconium-oxide-based crowns’ marginal fit. Pesqui Bras Odontopediatria Clín Integr. 2022, 22, e210144. [Google Scholar] [CrossRef]
- Ramakrishnaiah, R.; Alkheraif, A.A.; Divakar, D.D.; Matinlinna, J.P.; Vallittu, P.K. The effect of hydrofluoric acid etching duration on the surface micromorphology, roughness, and wettability of dental ceramics. Int. J. Mol. Sci. 2016, 17, 822. [Google Scholar] [CrossRef] [Green Version]
- Kamala, K.; Annapurni, H. Evaluation of surface roughness of glazed and polished ceramic surface on exposure to fluoride gel, bleaching agent and aerated drink: An in vitro study. J. Indian Prosthodont. Soc. 2006, 6, 128. [Google Scholar] [CrossRef]
- Esquivel-Upshaw, J.F.; Dieng, F.Y.; Clark, A.E.; Neal, D.; Anusavice, K.J. Surface degradation of dental ceramics as a function of environmental pH. J. Dent. Res. 2013, 92, 467–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abhay, S.S.; Ganapathy, D.; Veeraiyan, D.N.; Ariga, P. Wear Resistance, Color Stability and Displacement Resistance of Milled PEEK Crowns Compared to Zirconia Crowns under Stimulated Chewing and High-Performance Aging. Polymers 2021, 13, 3761. [Google Scholar] [CrossRef] [PubMed]
- Srimaneepong, V. Fixed Prosthetic Restorations and Periodontal Health: A Narrative Review. J. Funct. Biomater. 2022, 13, 15. [Google Scholar] [CrossRef]
- Heboyan, A.; Syed, A.U.Y.; Rokaya, D.; Cooper, P.R.; Manrikyan, M.; Markaryan, M. Cytomorphometric Analysis of Inflammation Dynamics in the Periodontium Following the Use of Fixed Dental Prostheses. Molecules 2020, 25, 4650. [Google Scholar] [CrossRef] [PubMed]
- De Jager, N.; Feilzer, A.; Davidson, C. The influence of surface roughness on porcelain strength. Dent. Mater. 2000, 16, 381–388. [Google Scholar] [CrossRef]
- Ersu, B.; Yuzugullu, B.; Yazici, A.R.; Canay, S. Surface roughness and bond strengths of glass-infiltrated alumina-ceramics prepared using various surface treatments. J. Dent. 2009, 37, 848–856. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markaryan, M.; Rokaya, D.; Tovani-Palone, M.R.; Zafar, M.S.; Khurshid, Z.; Vardanyan, A. Characteristics of Periodontal Tissues in Prosthetic Treatment with Fixed Dental Prostheses. Molecules 2021, 26, 1331. [Google Scholar] [CrossRef]
- Heboyan, A.; Manrikyan, M.; Zafar, M.S.; Rokaya, D.; Nushikyan, R.; Vardanyan, I.; Vardanyan, A.; Khurshid, Z. Bacteriological Evaluation of Gingival Crevicular Fluid in Teeth Restored Using Fixed Dental Prostheses: An In Vivo Study. Int. J. Mol. Sci. 2021, 22, 5463. [Google Scholar] [CrossRef]
- No IS. 6872; Dental Ceramics. 2nd ed. International Organization for Standardization: Geneva, Switzerland, 1995; pp. 1–9.
- Amer, R.; Kürklü, D.; Johnston, W. Effect of simulated mastication on the surface roughness of three ceramic systems. J. Prosthet. Dent. 2015, 114, 260–265. [Google Scholar] [CrossRef]
- Ferreiraa, M.C.; Ramos-Jorgea, M.L.; Carlos, A.; Delbemb, B.; Vieirac, R.D.S. Effect of Toothpastes with Different Abrasives on Eroded Human Enamel: An in Situ/Ex Vivo Study. Open Dent. J. 2013, 7, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Elbah, M.; Yallese, M.A.; Aouici, H.; Mabrouki, T.; Rigal, J.-F. Comparative assessment of wiper and conventional ceramic tools on surface roughness in hard turning AISI 4140 steel. Measurement 2013, 46, 3041–3056. [Google Scholar] [CrossRef]



| Specimen | Baseline Mean, SD | 24 h Mean, SD | 96 h Mean, SD | 168 h Mean, SD |
|---|---|---|---|---|
| Nano-fluorapatite ceramic | 0.223 ± 0.004 | 0.224 ± 0.005 | 0.224 ± 0.005 | 0.224 ± 0.005 |
| Specimen | Baseline Mean, SD | 24 h Mean, SD | 96 h Mean, SD | 168 h Mean, SD |
|---|---|---|---|---|
| Nano-fluorapatite ceramic | 0.223 ± 0.004 | 0.274 ± 0.005 | 0.325 ± 0.005 | 0.366 ± 0.006 |
| Storage Agent | Baseline Mean, SD | 24 h Mean, SD | 96 h Mean, SD | 168 h Mean, SD | p-Value |
|---|---|---|---|---|---|
| Distilled Water | 0.223 (±0.004) | 0.224 (±0.005) | 0.224 (±0.005) | 0.224 (±0.005) | 0.8289 |
| Lemon Juice | 0.223 (±0.004) | 0.260 (±0.008) | 0.286 (±0.004) | 0.349 (±0.005) | <0.0001 a |
| Citrate Buffer | 0.223 (±0.004) | 0.274 (±0.005) | 0.325 (±0.005) | 0.366 (±0.006) | <0.0001 a |
| Acetic Acid | 0.223 (±0.004) | 0.293 (±0.004) | 0.370 (±0.007) | 0.421 (±0.007) | <0.0001 a |
| Soft Cola | 0.223 (±0.004) | 0.245 (±0.007) | 0.290 (±0.007) | 0.355 (±0.007) | <0.0001 a |
| Time (Hours) | Distilled Water Mean, SD | Lemon Juice Mean, SD | Citrate Buffer Mean, SD | Acetic Acid Mean, SD | Soft Cola Mean, SD | p-Value |
|---|---|---|---|---|---|---|
| Baseline | 0.223 (±0.0048) | 0.223 (±0.004) | 0.223 (±0.004) | 0.223 (±0.004) | 0.223 (±0.004) | 1.00 |
| 24 h | 0.224 (±0.0051) | 0.260 (±0.008) | 0.274 (±0.005) | 0.293 (±0.009) | 0.245 (±0.001) | <0.001 a |
| 96 h | 0.221 (±0.0052) | 0.286 (±0.004) | 0.325 (±0.003) | 0.370 (±0.007) | 0.290 (±0.004) | <0.001 a |
| 168 h | 0.225 (±0.0057) | 0.344 (±0.005) | 0.366 (±0.006) | 0.421 (±0.005) | 0.355 (±0.007) | <0.001 a |
| Erosive Media | Prepared/Form | Composition | pH Mean and SD | Manufacturer |
|---|---|---|---|---|
| Lemon juice | Prepared from fresh lemons | Lemon juice vol% | 2.21 ± 0.03 | Laboratory prepared |
| Citrate buffer solution | Instant | Mixture of 5-chloro-2-methyl-4-isothaizolin-3-one and 2-methyl-4-isothaizolin-3-one (3:1) | 4.99 ± 0.01 | Laboratory prepared |
| Acetic acid | Diluted from 100% acetic acid | 4% acetic acid | 2.47 (0.01) | Laboratory prepared |
| Soft Cola drink | Instant | Mixture of sugar, water, lime juice, citrate of caffeine, citric acid, extract of vanilla, fluid extract of kola nut and fluid extract of coca | 2.41 (0.06) | Coca Cola™ Company |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanweer, N.; Qazi, F.-U.-R.; Das, G.; Bilgrami, A.; Basha, S.; Ahmed, N.; Bahammam, H.A.; Bahammam, S.A.; Basheer, S.N.; Assiry, A.A.; et al. Effect of Erosive Agents on Surface Characteristics of Nano-Fluorapatite Ceramic: An In-Vitro Study. Molecules 2022, 27, 4691. https://doi.org/10.3390/molecules27154691
Tanweer N, Qazi F-U-R, Das G, Bilgrami A, Basha S, Ahmed N, Bahammam HA, Bahammam SA, Basheer SN, Assiry AA, et al. Effect of Erosive Agents on Surface Characteristics of Nano-Fluorapatite Ceramic: An In-Vitro Study. Molecules. 2022; 27(15):4691. https://doi.org/10.3390/molecules27154691
Chicago/Turabian StyleTanweer, Navara, Fazal-Ur-Rehman Qazi, Gotam Das, Afreen Bilgrami, Sakeenabi Basha, Naseer Ahmed, Hammam Ahmed Bahammam, Sarah Ahmed Bahammam, Syed Nahid Basheer, Ali A. Assiry, and et al. 2022. "Effect of Erosive Agents on Surface Characteristics of Nano-Fluorapatite Ceramic: An In-Vitro Study" Molecules 27, no. 15: 4691. https://doi.org/10.3390/molecules27154691
APA StyleTanweer, N., Qazi, F.-U.-R., Das, G., Bilgrami, A., Basha, S., Ahmed, N., Bahammam, H. A., Bahammam, S. A., Basheer, S. N., Assiry, A. A., Karobari, M. I., Khan, A. S., & Heboyan, A. (2022). Effect of Erosive Agents on Surface Characteristics of Nano-Fluorapatite Ceramic: An In-Vitro Study. Molecules, 27(15), 4691. https://doi.org/10.3390/molecules27154691











