Formation of Carcinogens in Processed Meat and Its Measurement with the Usage of Artificial Digestion—A Review
Abstract
:1. Introduction
2. Thermally Processed Meat Is a Source of Carcinogenic Compounds
3. Parameters Affecting HAAs Content in Cooked Meat
3.1. Cooking Parameters Affect Content of Carcinogenic Compounds in Meat
3.2. Bioactive Compounds Are Effective Inhibitors of Carcinogen Formation in Heat-Treated Meat
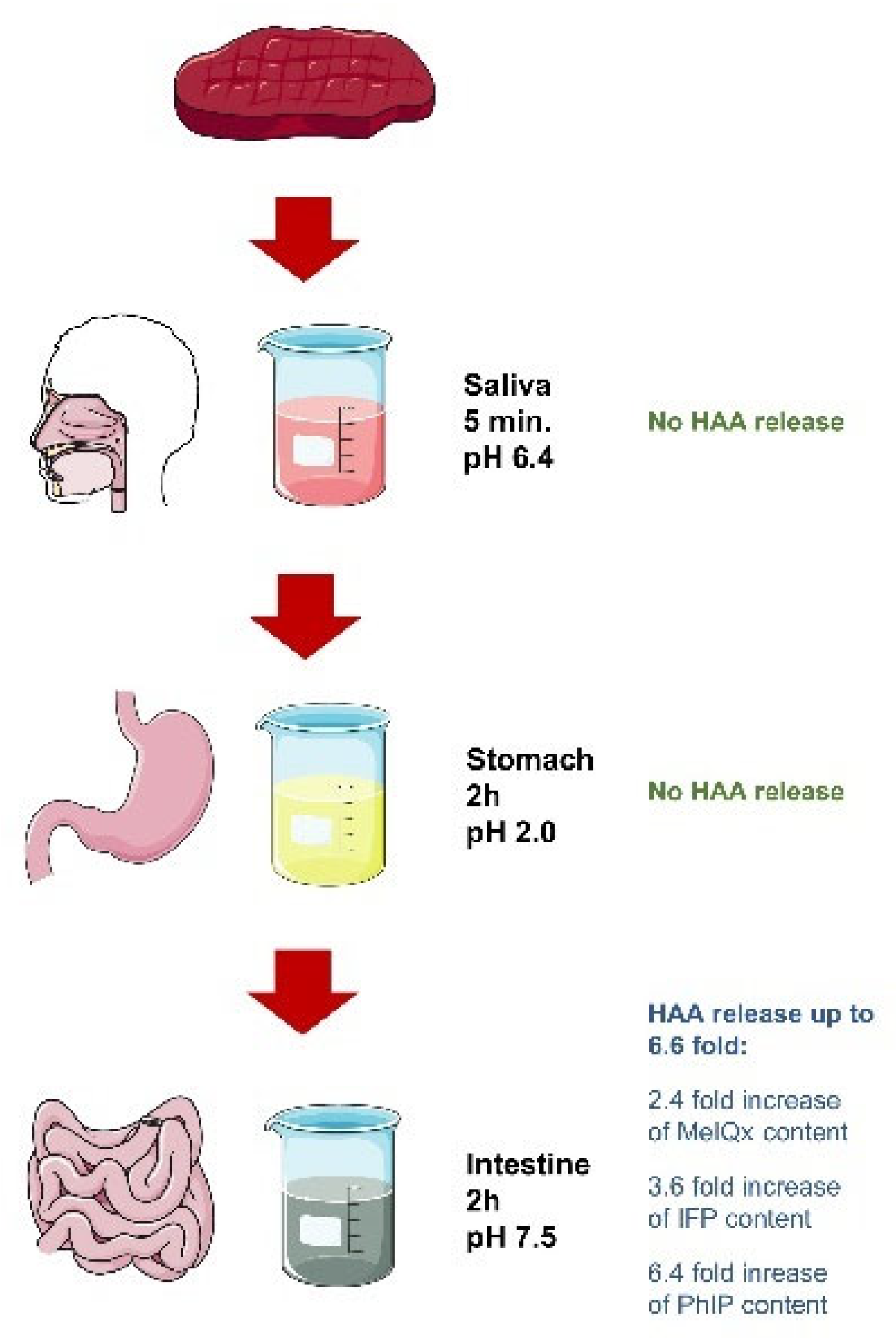
4. Digestion Significantly Increases the Number of Total Carcinogens Detected in Heat-Treated Meat
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA. Food Labeling Guide. Available online: www.fda.gov/FoodLabelingGuide (accessed on 2 May 2022).
- Cashman, K.D.; Hayes, A. Red meat’s role in addressing ‘nutrients of public health concern’. Meat Sci. 2017, 132, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fu, J.; Moore, J.B.; Stoner, L.; Li, R. Processed and Unprocessed Red Meat Consumption and Risk for Type 2 Diabetes Mellitus: An Updated Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2021, 18, 10788. [Google Scholar] [CrossRef]
- USDA. Department of Health and Human Services. 2015–2020 Dietary Guidlines for Americans. Washington; 2015. Available online: https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 31 May 2022).
- Cosgrove, M.; Flynn, A.; Kiely, M. Consumption of red meat, white meat and processed meat in Irish adults in relation to dietary quality. Br. J. Nutr. 2005, 93, 933–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer. Red Meat and Processed Meat. Lyon; 2015. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Red-Meat-And-Processed-Meat-2018 (accessed on 31 May 2022).
- de Jonge, R. Predictable and unpredictable survival of foodborne pathogens during non-isothermal heating. Int. J. Food Microbiol. 2019, 291, 151–160. [Google Scholar] [CrossRef]
- Ježek, F.; Kameník, J.; Macharáčková, B.; Bogdanovičová, K.; Bednář, J. Cooking of meat: Effect on texture, cooking loss and microbiological quality—A review. Acta Vet. Brno 2019, 88, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Turesky, R.J. Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol. Lett. 2007, 168, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Szterk, A. Chemical state of heterocyclic aromatic amines in grilled beef: Evaluation by in vitro digestion model and comparison of alkaline hydrolysis and organic solvent for extraction. Food Chem. Toxicol. 2013, 62, 653–660. [Google Scholar] [CrossRef]
- Alaejos, M.S.; Afonso, A.M. Factors That Affect the Content of Heterocyclic Aromatic Amines in Foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 52–108. [Google Scholar] [CrossRef]
- Oz, F.; Kaya, M. Heterocyclic Aromatic Amines in meat. J. Food Process. Preserv. 2011, 35, 739–753. [Google Scholar] [CrossRef]
- Haskaraca, G.; Demirok, E.; Kolsarıcı, N.; Öz, F.; Özsaraç, N. Effect of green tea extract and microwave pre-cooking on the formation of heterocyclic aromatic amines in fried chicken meat products. Food Res. Int. 2014, 63, 373–381. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, G.; Wang, S.; Yu, J.; Xie, F.; Wang, H.; Xie, J. Simultaneous determination of fifteen heterocyclic aromatic amines in the urine of smokers and nonsmokers using ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1333, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Trafialek, J.; Kolanowski, W. Dietary exposure to meat-related carcinogenic substances: Is there a way to estimate the risk? Int. J. Food Sci. Nutr. 2014, 65, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Reinik, M.; Tamme, T.; Roasto, M.; Juhkam, K.; Tenno, T.; Kiis, A. Polycyclic aromatic hydrocarbons (PAHs) in meat products and estimated PAH intake by children and the general population in Estonia. Food Addit. Contam. 2007, 24, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Ciemniak, A. A comparison of N-nitrosodimethylamine contents in selected meat products. Rocz. Panstw. Zakl. Hig. 2006, 57, 341–346. [Google Scholar] [PubMed]
- Park, J.; Seo, J.; Lee, J.; Kwon, H. Erratum to ‘Distribution of Seven N-Nitrosamines in Food’ [Toxicol. Res. 31 (2015) 279–298]. Toxicol. Res. 2018, 34, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingo, J.L.; Nadal, M. Carcinogenicity of consumption of red and processed meat: What about environmental contaminants? Environ. Res. 2016, 145, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Truswell, A. Meat consumption and cancer of the large bowel. Eur. J. Clin. Nutr. 2002, 56, S19–S24. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, Y.; Satija, A.; Pan, A.; Sotos-Prieto, M.; Rimm, E.; Willett, W.C.; Hu, F.B. Association of changes in red meat consumption with total and cause specific mortality among US women and men: Two prospective cohort studies. BMJ 2019, 365, 12110. [Google Scholar] [CrossRef] [Green Version]
- Nagle, C.M.; Wilson, L.F.; Hughes, M.C.B.; Ibiebele, T.I.; Miura, K.; Bain, C.J.; Whiteman, D.C.; Webb, P.M. Cancers in Australia in 2010 attributable to the consumption of red and processed meat. Aust. N. Z. J. Public Health 2015, 39, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; He, Z.; Gao, D.; Qin, F.; Deng, S.; Wang, P.; Xu, X.; Chen, J.; Zeng, M. Effects of smoking or baking procedures during sausage processing on the formation of heterocyclic amines measured using UPLC-MS/MS. Food Chem. 2019, 276, 195–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Mei, J.; Wang, S. Formation and mitigation of heterocyclic aromatic amines in fried pork. Food Addit. Contam. Part A 2013, 30, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.A.; Layton, D.W.; Felton, J.S. Factors determining dietary intakes of heterocyclic amines in cooked foods. Mutat. Res./Genet. Toxicol. Environ. Mutagenes. 1999, 443, 149–156. [Google Scholar] [CrossRef]
- Solyakov, A.; Skog, K. Screening for heterocyclic amines in chicken cooked in various ways. Food Chem. Toxicol. 2002, 40, 1205–1211. [Google Scholar] [CrossRef]
- Chiu, C.P.; Yang, D.Y.; Chen, B.H. Formation of Heterocyclic Amines in Cooked Chicken Legs. J. Food Prot. 1998, 61, 712–719. [Google Scholar] [CrossRef]
- Jägerstad, M.; Skog, K.; Arvidsson, P.; Solyakov, A. Chemistry, formation and occurrence of genotoxic heterocyclic amines identified in model systems and cooked foods. Z. Für Lebensm. Und-Forsch. A 1998, 207, 419–427. [Google Scholar] [CrossRef]
- Kondjoyan, A.; Chevolleau, S.; Grève, E.; Gatellier, P.; Santé-Lhoutellier, V.; Bruel, S.; Touzet, C.; Portanguen, S.; Debrauwer, L. Formation of heterocyclic amines in slices of Longissimus thoracis beef muscle subjected to jets of superheated steam. Food Chem. 2010, 119, 19–26. [Google Scholar] [CrossRef]
- Oz, F.; Kotan, G. Effects of different cooking methods and fat levels on the formation of heterocyclic aromatic amines in various fishes. Food Control 2016, 67, 216–224. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Z.; Pan, H.; Li, X.; Chen, L.; Rao, W.; Gao, Y.; Zhang, D. Effects of traditional chinese cooking methods on formation of heterocyclic aromatic amines in lamb patties. Food Sci. Biotechnol. 2014, 23, 747–753. [Google Scholar] [CrossRef]
- Omojola, A.B.; Ahmed, S.A.; Attoh-Kotoku, V.; Wogar, G.I. Effect of cooking methods on cholesterol, mineral composition and formation of total heterocyclic aromatic amines in Muscovy drake meat. J. Sci. Food Agric. 2015, 95, 98–102. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Abas, F.; Sakar, Z.I. Determination of polycyclic aromatic hydrocarbons in grilled meat. Food Control 2010, 21, 606–610. [Google Scholar] [CrossRef]
- Zorrilla, S.E.; Singh, R.P. Heat transfer in double-sided cooking of meat patties considering two-dimensional geometry and radial shrinkage. J. Food Eng. 2003, 57, 57–65. [Google Scholar] [CrossRef]
- Persson, E.; Sjöholm, I.; Skog, K. Heat and mass transfer in chicken breasts—Effect on PhIP formation. Eur. Food Res. Technol. 2002, 214, 455–459. [Google Scholar]
- Jinap, S.; Mohd-Mokhtar, M.S.; Farhadian, A.; Hasnol, N.D.S.; Jaafar, S.N.; Hajeb, P. Effects of varying degrees of doneness on the formation of Heterocyclic Aromatic Amines in chicken and beef satay. Meat Sci. 2013, 94, 202–207. [Google Scholar] [CrossRef]
- EFSA. Polycyclic Aromatic Hydrocarbons in Food—Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 724. [Google Scholar] [CrossRef]
- Oz, F.; Kızıl, M.; Çelık, T. Effects of Different Cooking Methods on the Formation of Heterocyclic Aromatic Amines in Goose Meat. J. Food Process. Preserv. 2016, 40, 1047–1053. [Google Scholar] [CrossRef]
- Oz, F.; Zikirov, E. The effects of sous-vide cooking method on the formation of heterocyclic aromatic amines in beef chops. LWT—Food Sci. Technol. 2015, 64, 120–125. [Google Scholar] [CrossRef]
- Liao, G.Z.; Wang, G.Y.; Xu, X.L.; Zhou, G.H. Effect of cooking methods on the formation of heterocyclic aromatic amines in chicken and duck breast. Meat Sci. 2010, 85, 149–154. [Google Scholar] [CrossRef]
- Han-Seung, S. Influence of Food Ingredients on the Formation of Heterocyclic Aromatic Amine in Cooked pork patties. Food Sci. Biotechnol. 2005, 14, 572–575. Available online: https://www.koreascience.or.kr/article/JAKO200509905833740.page (accessed on 23 June 2022).
- Yang, Z.; Lu, R.; Song, H.; Zhang, Y.; Tang, J.; Zhou, N. Effect of Different Cooking Methods on the Formation of Aroma Components and Heterocyclic Amines in Pork Loin. J. Food Process. Preserv. 2017, 41, e12981. [Google Scholar] [CrossRef]
- Raza, A.; Shabbir, M.A.; Khan, M.I.; Suleria, H.A.R.; Sultan, S. Effect of Thermal Treatments on the Formation of Heterocyclic Aromatic Amines in Various Meats. J. Food Process. Preserv. 2015, 39, 376–383. [Google Scholar] [CrossRef]
- Liao, G.Z.; Wang, G.Y.; Zhang, Y.J.; Xu, X.L.; Zhou, G.H. Formation of heterocyclic amines during cooking of duck meat. Food Addit. Contam. Part A 2012, 29, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Keskekoglu, H.; Uren, A. Inhibitory effects of grape seed extract on the formation of heterocyclic aromatic amines in beef and chicken meatballs cooked by different techniques. Int. J. Food Prop. 2017, 20, S722–S734. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Dong, L.; Zhang, Y.; Yu, H.; Wang, S. Reduction of the Heterocyclic Amines in Grilled Beef Patties through the Combination of Thermal Food Processing Techniques without Destroying the Grilling Quality Characteristics. Foods 2021, 10, 1490. [Google Scholar] [CrossRef] [PubMed]
- Suleman, R.; Hui, T.; Wang, Z.; Liu, H.; Zhang, D. Comparative analysis of charcoal grilling, infrared grilling and superheated steam roasting on the colour, textural quality and heterocyclic aromatic amines of lamb patties. Int. J. Food Sci. Technol. 2020, 55, 1057–1068. [Google Scholar] [CrossRef]
- Bordas, M.; Moyano, E.; Puignou, L.; Galceran, M.T. Formation and stability of heterocyclic amines in a meat flavour model system. J. Chromatogr. B 2004, 802, 11–17. [Google Scholar] [CrossRef]
- Turesky, R.J.; Taylor, J.; Schnackenberg, L.; Freeman, J.P.; Holland, R.D. Quantitation of Carcinogenic Heterocyclic Aromatic Amines and Detection of Novel Heterocyclic Aromatic Amines in Cooked Meats and Grill Scrapings by HPLC/ESI-MS. J. Agric. Food Chem. 2005, 53, 3248–3258. [Google Scholar] [CrossRef]
- Lee, J.-G.; Kim, S.-Y.; Moon, J.-S.; Kim, S.-H.; Kang, D.-H.; Yoon, H.-J. Effects of grilling procedures on levels of polycyclic aromatic hydrocarbons in grilled meats. Food Chem. 2016, 199, 632–638. [Google Scholar] [CrossRef]
- Skog, K.; Jägerstad, M. Incorporation of carbon atoms from glucose into the food mutagens MeIQx and 4,8-DiMeIQx using 14C-labelled glucose in a model system. Carcinogenesis 1993, 14, 2027–2031. [Google Scholar] [CrossRef]
- Cao, H.; Chen, B.H.; Inbaraj, B.S.; Chen, L.; Alvarez-Rivera, G.; Cifuentes, A.; Zhang, N.; Yang, D.J.; Simal-Gandara, J.; Wang, M.; et al. Preventive potential and mechanism of dietary polyphenols on the formation of heterocyclic aromatic amines. Food Front. 2020, 1, 134–151. [Google Scholar] [CrossRef]
- Jackson, L.S.; Hargraves, W.A.; Stroup, W.H.; Diachenko, G.W. Heterocyclic aromatic amine content of selected beef flavors. Mutat. Res./Genet. Toxicol. 1994, 320, 113–124. [Google Scholar] [CrossRef]
- Jägerstad, M.; Skog, K. Formation of Meat Mutagens. Adv Exp Med Biol. 1991, 289, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Skog, K.; Jagerstad, M. Effects of monosaccharides and disaccharides on the formation of food mutagens in model systems. Mutat. Res./Fundam. Mol. Mech. Mutagenes. 1990, 230, 263–272. [Google Scholar] [CrossRef]
- Olsson, V.; Solyakov, A.; Skog, K.; Lundström, K.; Jägerstad, M. Natural Variations of Precursors in Pig Meat Affect the Yield of Heterocyclic Amines—Effects of RN Genotype, Feeding Regime, and Sex. J. Agric. Food Chem. 2002, 50, 2962–2969. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Wang, J.; Zhang, M.; Chen, J.; He, Z.; Qin, F.; Xu, Z.; Cao, D.; Chen, J. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chem. 2018, 239, 111–118. [Google Scholar] [CrossRef]
- Sinha, R.; Rothman, N.; Brown, E.D.; Salmon, C.P.; Knize, M.G.; Swanson, C.A.; Rossi, S.C.; Mark, S.D.; Levander, O.A.; Felton, J.S. High concentrations of the carcinogen 2-amino-1-methyl-6-phenylimidazo-[4,5-b]pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res. 1995, 55, 4516–4519. [Google Scholar]
- Polak, M.L.; Demšar, L.; Zahija, I.; Polak, T. Influence of temperature on the formation of heterocyclic aromatic amines in pork steaks. Czech J. Food Sci. 2020, 38, 248–254. [Google Scholar] [CrossRef]
- Persson, E.; Oroszvári, B.K.; Tornberg, E.; Sjöholm, I.; Skog, K. Heterocyclic amine formation during frying of frozen beefburgers. Int. J. Food Sci. Technol. 2008, 43, 62–68. [Google Scholar] [CrossRef]
- Knize, M.G.; Andresen, B.D.; Healy, S.K.; Shen, N.H.; Lewis, P.R.; Bjeldanes, L.F.; Hatch, F.T.; Felton, J.S. Effects of temperature, patty thickness and fat content on the production of mutagens in fried ground beef. Food Chem. Toxicol. 1985, 23, 1035–1040. [Google Scholar] [CrossRef]
- Zamora, R.; Alcón, E.; Hidalgo, F.J. Effect of lipid oxidation products on the formation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in model systems. Food Chem. 2012, 135, 2569–2574. [Google Scholar] [CrossRef]
- Stumpe-Vīksna, I.; Bartkevičs, V.; Kukāre, A.; Morozovs, A. Polycyclic aromatic hydrocarbons in meat smoked with different types of wood. Food Chem. 2008, 110, 794–797. [Google Scholar] [CrossRef]
- Balogh, Z.; Gray, J.I.; Gomaa, E.A.; Booren, A.M. Formation and inhibition of heterocyclic aromatic amines in fried ground beef patties. Food Chem. Toxicol. 2000, 38, 395–401. [Google Scholar] [CrossRef]
- Ruan, E.D.; Juárez, M.; Thacker, R.; Yang, X.; Dugan, M.E.R.; Aalhus, J.L. Dietary vitamin E effects on the formation of heterocyclic amines in grilled lean beef. Meat Sci. 2014, 96, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Monti, S.; Ambrosino, P.; Skog, K.; Fogliano, V. Carotenoids from tomatoes inhibit heterocyclic amine formation. Eur. Food Res. Technol. 2002, 215, 108–113. [Google Scholar] [CrossRef]
- Wong, D.; Cheng, K.-W.; Wang, M. Inhibition of heterocyclic amine formation by water-soluble vitamins in Maillard reaction model systems and beef patties. Food Chem. 2012, 133, 760–766. [Google Scholar] [CrossRef]
- Sabally, K.; Sleno, L.; Jauffrit, J.-A.; Iskandar, M.M.; Kubow, S. Inhibitory effects of apple peel polyphenol extract on the formation of heterocyclic amines in pan fried beef patties. Meat Sci. 2016, 117, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Li, K.; Li, C. Effects of turmeric on reducing heterocyclic aromatic amines in Chinese tradition braised meat products and the underlying mechanism. Food Sci. Nutr. 2021, 9, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Kaya, M. The inhibitory effect of Red Pepper on Heterocyclic Aromatic Amines in fried beef Longissimus dorsi muscle. J. Food Process. Preserv. 2011, 35, 806–812. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, S.; Wang, M.; Chen, J.; Zheng, Z.-P. Inhibitory effects of selected dietary flavonoids on the formation of total heterocyclic amines and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in roast beef patties and in chemical models. Food Funct. 2016, 7, 1057–1066. [Google Scholar] [CrossRef]
- Viegas, O.; Amaro, L.F.; Ferreira, I.M.P.L.V.O.; Pinho, O. Inhibitory Effect of Antioxidant-Rich Marinades on the Formation of Heterocyclic Aromatic Amines in Pan-Fried Beef. J. Agric. Food Chem. 2012, 60, 6235–6240. [Google Scholar] [CrossRef]
- Kikugawa, K. Involvement of free radicals in the formation of heterocyclic amines and prevention by antioxidants. Cancer Lett. 1999, 143, 123–126. [Google Scholar] [CrossRef]
- Lan, C.M.; Kao, T.H.; Chen, B.H. Effects of heating time and antioxidants on the formation of heterocyclic amines in marinated foods. J. Chromatogr. B 2004, 802, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, R.; Edenharder, R.; Platt, K.L. In vitro antimutagenic and in vivo anticlastogenic effects of carotenoids and solvent extracts from fruits and vegetables rich in carotenoids. Mutat. Res./Genet. Toxicol. Environ. Mutagenes. 1998, 413, 129–142. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Khatib, A.; Manap, M.Y.A.; Razis, A.F.A.; Hajeb, P. Inhibitory effect of mixture herbs/spices on formation of heterocyclic amines and mutagenic activity of grilled beef. Food Addit. Contam. Part A 2018, 35, 1911–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogorzelska-Nowicka, E.D.; Brodowska, M.; Górska-Horczyczak, E.; Godziszewska, J.; Sakowska, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A. Physicochemical and Biochemical Properties of Ground Pork Formulated with Addition of Prunus cerasus (cv Montmorency) Extract and Subjected to Freezing Storage. Acta Univ. Cibiniensis. Ser. E Food Technol. 2019, 23, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Yeung, A.W.K.; Tzvetkov, N.T.; El-Demerdash, A.; Horbanczuk, O.K.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; Santini, A.; et al. Apple polyphenols in human and animal health. Anim. Sci. Pap. Rep. 2021, 39, 105–118. [Google Scholar]
- Jamali, M.; Zhang, Y.; Teng, H.; Li, S.; Wang, F.; Peng, Z. Inhibitory Effect of Rosa rugosa Tea Extract on the Formation of Heterocyclic Amines in Meat Patties at Different Temperatures. Molecules 2016, 21, 173. [Google Scholar] [CrossRef]
- Gibis, M.; Weiss, J. Antioxidant capacity and inhibitory effect of grape seed and rosemary extract in marinades on the formation of heterocyclic amines in fried beef patties. Food Chem. 2012, 134, 766–774. [Google Scholar] [CrossRef]
- Cheng, K.W.; Wong, C.C.; Chao, J.; Lo, C.; Chen, F.; Chu, I.K.; Che, C.M.; Ho, C.T.; Wang, M. Inhibition of mutagenic PhIP formation by epigallocatechin gallate via scavenging of phenylacetaldehyde. Mol. Nutr. Food Res. 2009, 53, 716–725. [Google Scholar] [CrossRef]
- Tengilimoglu-Metin, M.M.; Kizil, M. Reducing effect of artichoke extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Meat Sci. 2017, 134, 68–75. [Google Scholar] [CrossRef]
- Keşkekoğlu, H.; Üren, A. Inhibitory effects of pomegranate seed extract on the formation of heterocyclic aromatic amines in beef and chicken meatballs after cooking by four different methods. Meat Sci. 2014, 96, 1446–1451. [Google Scholar] [CrossRef]
- Tengilimoglu-Metin, M.M.; Hamzalioglu, A.; Gokmen, V.; Kizil, M. Inhibitory effect of hawthorn extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Food Res. Int. 2017, 99, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Quelhas, I.; Petisca, C.; Viegas, O.; Melo, A.; Pinho, O.; Ferreira, I.M.P.L.V.O. Effect of green tea marinades on the formation of heterocyclic aromatic amines and sensory quality of pan-fried beef. Food Chem. 2010, 122, 98–104. [Google Scholar] [CrossRef]
- Oz, E. Inhibitory effects of black cumin on the formation of heterocyclic aromatic amines in meatball. PLoS ONE 2019, 14, e0221680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puangsombat, K.; Jirapakkul, W.; Smith, J.S. Inhibitory Activity of Asian Spices on Heterocyclic Amines Formation in Cooked Beef Patties. J. Food Sci. 2011, 76, T174–T180. [Google Scholar] [CrossRef]
- Damašius, J.; Venskutonis, P.R.; Ferracane, R.; Fogliano, V. Assessment of the influence of some spice extracts on the formation of heterocyclic amines in meat. Food Chem. 2011, 126, 149–156. [Google Scholar] [CrossRef]
- Zeng, M.; He, Z.; Zheng, Z.; Qin, F.; Tao, G.; Zhang, S.; Gao, Y.; Chen, J. Effect of Six Chinese Spices on Heterocyclic Amine Profiles in Roast Beef Patties by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry and Principal Component Analysis. J. Agric. Food Chem. 2014, 62, 9908–9915. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, M.; Chen, J.; He, Z.; Qin, F.; Hu, C.; Xu, H.; Tao, G.; Zhang, S.; Chen, J. UPLC-MS/MS and multivariate analysis of inhibition of heterocyclic amine profiles by black pepper and piperine in roast beef patties. Chemom. Intell. Lab. Syst. 2017, 168, 96–106. [Google Scholar] [CrossRef]
- Xue, C.; He, Z.; Qin, F.; Chen, J.; Zeng, M. Effects of amides from pungent spices on the free and protein-bound heterocyclic amine profiles of roast beef patties by UPLC–MS/MS and multivariate statistical analysis. Food Res. Int. 2020, 135, 109299. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; He, Z.; Qin, F.; Chen, J. Effect of phenolic compounds from spices consumed in China on heterocyclic amine profiles in roast beef patties by UPLC–MS/MS and multivariate analysis. Meat Sci. 2016, 116, 50–57. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; He, Z.; Qin, F.; Tao, G.; Zhang, S.; Gao, Y.; Chen, J. Discrimination and investigation of inhibitory patterns of flavonoids and phenolic acids on heterocyclic amine formation in chemical model systems by UPLC-MS profiling and chemometrics. Eur. Food Res. Technol. 2016, 242, 313–319. [Google Scholar] [CrossRef]
- Cheng, K.-W.; Chen, F.; Wang, M. Inhibitory activities of dietary phenolic compounds on heterocyclic amine formation in both chemical model system and beef patties. Mol. Nutr. Food Res. 2007, 51, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Arámbula-Villa, G.; Hidalgo, F.J.; Zamora, R. Structural characteristics that determine the inhibitory role of phenolic compounds on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) formation. Food Chem. 2014, 151, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibis, M. Heterocyclic Aromatic Amines in Cooked Meat Products: Causes, Formation, Occurrence, and Risk Assessment. Compr. Rev. Food Sci. Food Saf. 2016, 15, 269–302. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Miyake, M.; Nishioka, S.; Matsumoto, T.; Saito, K.; Mitani, K. Formation of protein adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in cooked foods. Mol. Nutr. Food Res. 2010, 54, 1039–1048. [Google Scholar] [CrossRef]
- Chen, J.; He, Z.; Qin, F.; Chen, J.; Zeng, M. Formation of Free and Protein-Bound Heterocyclic Amines in Roast Beef Patties Assessed by UPLC-MS/MS. J. Agric. Food Chem. 2017, 65, 4493–4499. [Google Scholar] [CrossRef]
- Kulp, K.S.; Fortson, S.L.; Knize, M.G.; Felton, J.S. An in vitro model system to predict the bioaccessibility of heterocyclic amines from a cooked meat matrix. Food Chem. Toxicol. 2003, 41, 1701–1710. [Google Scholar] [CrossRef]
- Kim, H.S.; Hur, S.J. Changes in the mutagenicity of heterocyclic amines, nitrite, and N-nitroso compound in pork patties during in vitro human digestion. LWT 2018, 92, 47–53. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Corradini, C.; Elviri, L.; Mangia, A.; Zagnoni, I. Investigation of the separation of heterocyclic aromatic amines by reversed phase ion-pair liquid chromatography coupled with tandem mass spectrometry: The role of ion pair reagents on LC–MS/MS sensitivity. J. Chromatogr. B 2005, 825, 193–200. [Google Scholar] [CrossRef]
- Xue, C.; Chen, Q.; He, Z.; Qin, F.; Wang, Z.; Chen, J.; Zeng, M. Release mechanism between sarcoplasmic protein–bound and free heterocyclic amines and the effects of dietary additives using an in-vitro digestion model. Food Chem. 2022, 377, 131993. [Google Scholar] [CrossRef]
- Guo, A.; Xiong, Y.L. Myoprotein–phytophenol interaction: Implications for muscle food structure-forming properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2801–2824. [Google Scholar] [CrossRef]
- Platel, K.; Srinivasan, K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung/Food 2000, 44, 42–46. [Google Scholar] [CrossRef]
| Meat Type | Parameters | Effect | Reference |
|---|---|---|---|
| Leg and breast of goose | Boiling at 100 °C; grilling, pan frying without fat and oil, pan frying with oil, deep fat frying at 180 °C; oven at 200 °C, microwave (automatic). | The highest HAAs content was measured in breast heated with a microwave (2.20 ng/g) and in boiled leg meat (2.42 ng/g). | [38] |
| Beef chops | Sous-vide cooking at 75, 85, and 95 °C; pan frying at 75, 85, and 95 °C; boiling in pressure cooker. | The highest total content of HAAs was measured in pan-fried beef chops at the temperature of 95 °C. | [39] |
| Chicken breast and duck breast | Pan frying with no oil at 180 °C; deep fat frying at 180 °C; charcoal grilling at 200 °C and roasting (oven) at 200 °C. | Charcoal-grilled chicken breast had the highest total amount of HAAs followed by pan-fried and charcoal-grilled duck breast. | [40] |
| Pork patties | Boiling to internal temperature of 71 and 77 °C; oven-broiling at 177 and 225 °C; pan frying at 177 and 225 °C. | Greatest HAAs formation was observed in pan-fried pork patties. HAAs concentration increased in meat samples with the increase in internal temperature. | [41] |
| Pork loin | Electric oven cooking at 180 °C; hot air frying at 180 °C and deep oil frying at maximum power (household electric oven). | Highest HAAs content was observed in meat samples subjected to cooking in an electric oven. | [42] |
| Lamb patties | Roasting at 200 °C; frying at 200 °C; pan frying at 200 °C and stewing in seasonings at 100 °C. | Higher content of HAAs was noted for patties stewed in seasonings in comparison to roasted, fried, or pan-fried. | [31] |
| Chicken, beef, mutton | Charcoal grilling (200 °C on the meat surface); deep frying at 180 °C; pan frying at 180 °C; roasting at 200 °C. | Charcoal grilling and deep frying generated higher HAAs formation in comparison to other methods. | [43] |
| Duck breast | Boiling at 100 °C; roasting at 160, 180, and 200 °C; electric oven at 200 °C; deep frying at 100, 150, and 200 °C; charcoal grilling and microwave cooking (2450 MHz, 700 W). | Pan-fried samples were characterized by the highest final amount of HAAs followed by charcoal-grilled, deep-fried, roasted, microwave-cooked, and boiled. | [44] |
| Beef and chicken meatballs | Deep fat frying at 150 °C; pan-cooking at 180 °C; charcoal grilling (temperature of 280 °C on meatball surface); oven roasting at 180 °C. | Charcoal grilling was responsible for the highest HAAs generation in beef meatballs while pan frying for chicken meatballs. | [45] |
| Beef patties | Steam roasting at 100 °C; infrared grilling at 180, 200, and 220 °C; charcoal grilling and microwave cooking using powers of 1000 and 500 W. | The highest content of HAAs was measured in charcoal-grilled beef samples. | [46] |
| Lamb patties | Charcoal grilling at 450–500 °C; infrared grilling at 240 °C and superheated steam roasting at 240 °C. | Charcoal grilling generated the highest rate of HAAs formation in lamb patties followed by infrared grilling and superheated steam roasting. | [47] |
| Factor | Parameters | Effect | Reference |
|---|---|---|---|
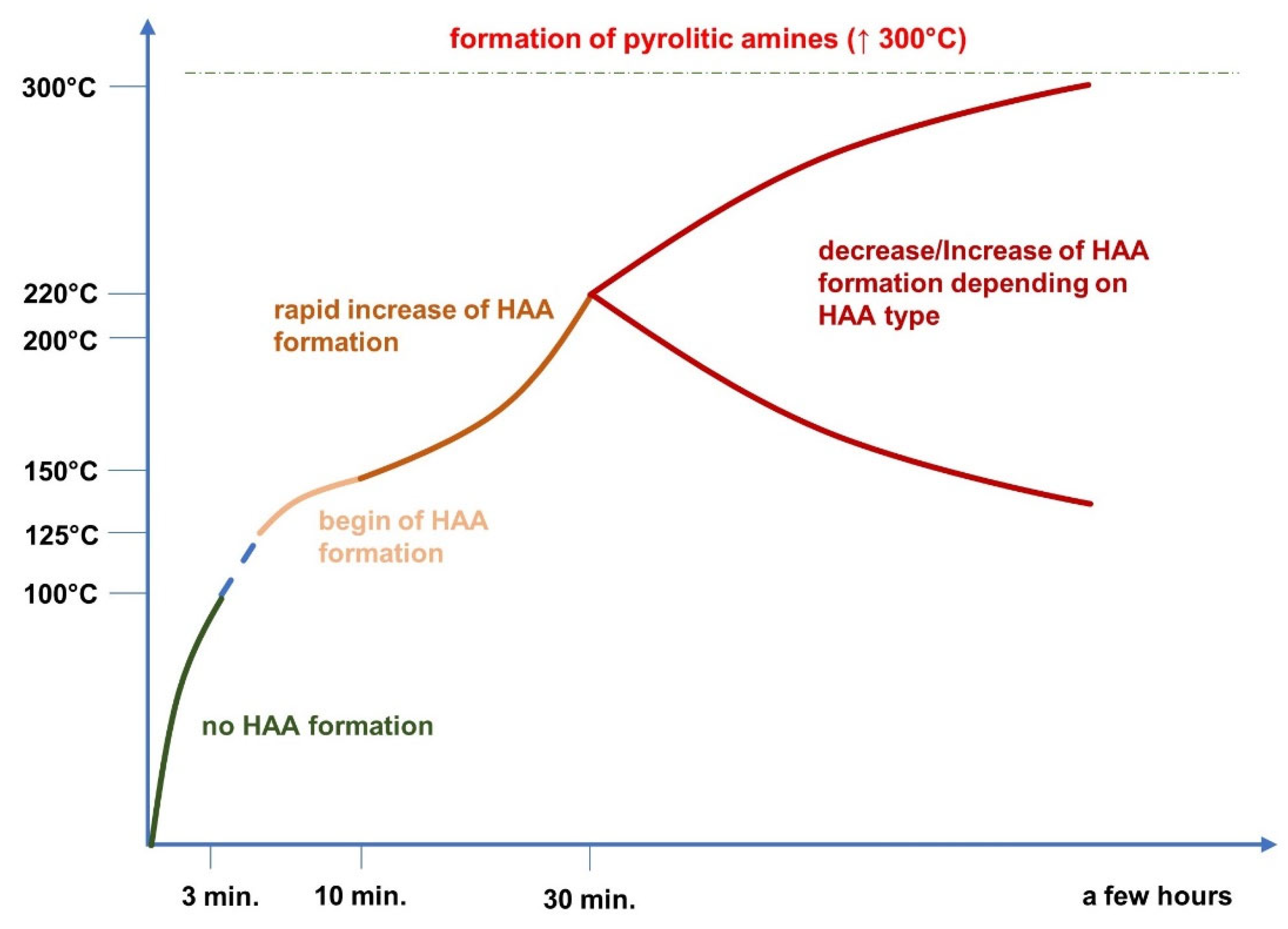
| Temperature | 100 °C | HAAs not formed for most compounds. | [27] |
| Temperature | 150 °C | HAAs formation at relatively low level. | [29] |
| Temperature | 200 °C | Rapid increase of HAAs formation. | [29] |
| Time | Time increase from 1 to 2 h | Threefold increase in 4,8-DiMeIQx content. | [48] |
| Time | 3 min and longer | The concentration of HAAs increased during the time of cooking. | [9] |
| Cooking method | Deep frying, roasting, pan frying, grilling | Highest content of HAAs for deep frying followed by roasting, pan frying, and grilling. | [32] |
| Cooking method | Broiling, deep frying, pan frying | PhIP was formed in broiled meat in a quantity of 0.07 ng/g, in pan-fried of 0.04 ng/g, and in deep-fried of 0.02 ng/g. | [35] |
| Cooking method | Grilling, microwave heating, deep frying | Grilled samples had higher content of HAAs in comparison to microwaved and pan-fried. | [36] |
| Precursor content | Creatinine | In beef flavors with a low content of creatinine fewer HAAs were observed in contrast to beef flavors with a high content of creatinine characterized by the highest content of HAAs. | [53] |
| Precursor content | Sugar to amino acids ratio | Sugar content higher than in natural state leads to lower formation of HAAs in meat. | [55] |
| Precursor content | Glycogen content | Reduced content of HAAs in meat obtained from pigs with the RN allele (higher glycogen content) in comparison to normal pigs. | [56] |
| Meat type | Beef, pork, chicken | Highest content of PhIP was noted in broiled chicken fillet (480 ng/g), lower content for grilled ground beef patties (50 ng/g), and the lowest for grilled pork steaks (28.26 ng/g). | [57] [58] [59] |
| Fat content | Fat percentage | Higher content of fat decreased production of mutagenic compounds. In beef with 30% fat content 150,000 revertants/kg of fresh beef were detected while that containing 15% of fat 230,000 revertants/kg. | [61] |
| Fat content | Oxidized fat | The addition of oxidized soybean oil increased PhIP formation as well as addition of lipid oxidation products such as ω-6- and ω-3-derived lipid hydroperoxides, 4,5-epoxy-2-alkenals, 2,4-alkadienals, 2-alkenals, 4-oxo-2-alkenals, and 4-hydroxy-2-nonenal. | [62] |
| Vitamin E | Vitamin E addition (1% and 10%) | The addition of vitamin E at two concentrations 1% and 10% significantly decreased the formation of PhIP in cooked ground beef patties (of 69% and 72%, respectively). | [64] |
| Vitamin E | Animal supplementation with vitamin E | There was a trend observed that with increasing tissue levels of α-tocopherol meat mutagenicity was reduced. | [65] |
| Carotenoids | Carotenoid extracts | Tomato carotenoid extracts addition of 1000 mg/kg inhibited formation of MeIQx in 13% and 4,8-DiMeIQx in 5% in a meat juice system. | [66] |
| Pyridoxiamine | Pyridoxamine addition | Pyrydoxiamine (0.2 mmol power) lowered PhIP, 4,8-DiMeIQx, and MeIQx level in fried beef patties by about 40%. | [67] |
| Vitamin C | Vitamin C addition | Vitamin C (0.2 mmol power) lowered PhIP, 4,8-DiMeIQx, and MeIQx level in fried beef patties by about 20%. | [67] |
| Niacin | Niacin addition | Niacin (0.2 mmol power) lowered PhIP, 4,8-DiMeIQx, and MeIQx level in fried beef patties by about 20%. | [67] |
| Polyphenols | Apple peel extract addition | Addition of 0.3% of apple peel extract on the surface of beef patties reduced formation of MeIQx by 68%, 4,8-DiMeIQx by 56%, and PhIP by 83%. | [68] |
| Polyphenols | Turmeric | Addition of turmeric (5%) significantly inhibited norharman and harman formation (by 49.56% and 94.8%, respectively). | [69] |
| Polyphenols | Red pepper | Addition of 1% of red pepper reduced HAAs formation from 75% up to 100% depending on the compound. | [70] |
| Polyphenols | Pure phenolic compounds (apigenin, luteolin, kaempferol, quercetin, genistein, naringenin, phlorizin, EGCG) | Epigallocatechin gallate (EGCG), phlorizin and quercetin are the most effective in both reduction of total HAAs (55–70%) and PhIP (60–80%) content. | [71] |
| Polyphenols | Wine, beer | The addition of wine to marinades for beef sample marination prior to pan frying decreased HAAs formation of 72.5%. In the case of beer, a 25.9% reduction of HAAs content was observed. | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogorzelska-Nowicka, E.; Kurek, M.; Hanula, M.; Wierzbicka, A.; Półtorak, A. Formation of Carcinogens in Processed Meat and Its Measurement with the Usage of Artificial Digestion—A Review. Molecules 2022, 27, 4665. https://doi.org/10.3390/molecules27144665
Pogorzelska-Nowicka E, Kurek M, Hanula M, Wierzbicka A, Półtorak A. Formation of Carcinogens in Processed Meat and Its Measurement with the Usage of Artificial Digestion—A Review. Molecules. 2022; 27(14):4665. https://doi.org/10.3390/molecules27144665
Chicago/Turabian StylePogorzelska-Nowicka, Ewelina, Marcin Kurek, Monika Hanula, Agnieszka Wierzbicka, and Andrzej Półtorak. 2022. "Formation of Carcinogens in Processed Meat and Its Measurement with the Usage of Artificial Digestion—A Review" Molecules 27, no. 14: 4665. https://doi.org/10.3390/molecules27144665
APA StylePogorzelska-Nowicka, E., Kurek, M., Hanula, M., Wierzbicka, A., & Półtorak, A. (2022). Formation of Carcinogens in Processed Meat and Its Measurement with the Usage of Artificial Digestion—A Review. Molecules, 27(14), 4665. https://doi.org/10.3390/molecules27144665








