Correlation between Irradiation Treatment and Metabolite Changes in Bactrocera dorsalis (Diptera: Tephritidae) Larvae Using Solid-Phase Microextraction (SPME) Coupled with Gas Chromatography-Mass Spectrometry (GC-MS)
Abstract
:1. Introduction
2. Results and Discussion
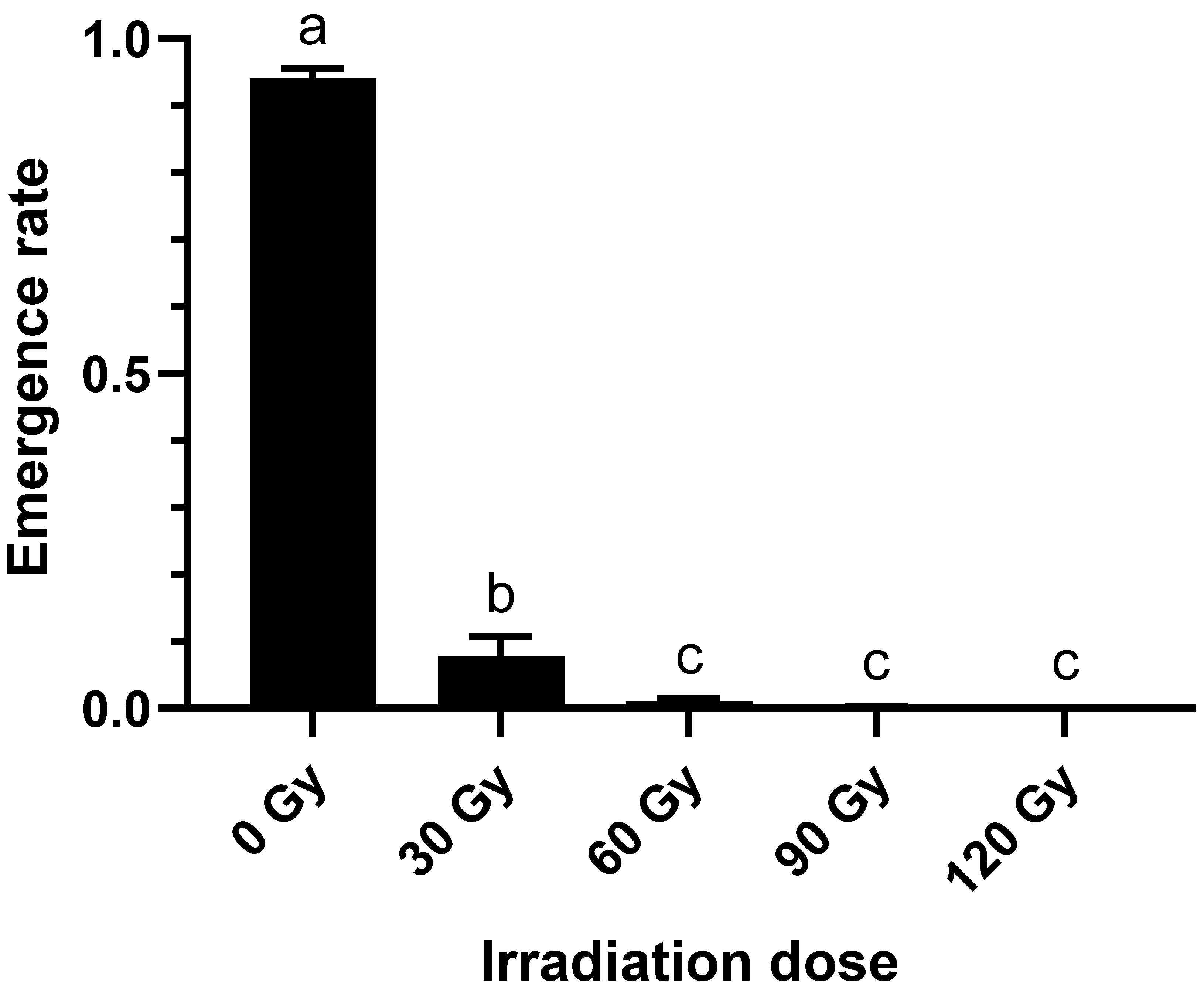
2.1. Effect of Irradiation Doses on Emergence Rate of B. dorsalis Larvae
2.2. Metabolite Expression in Response to Irradiation Dose
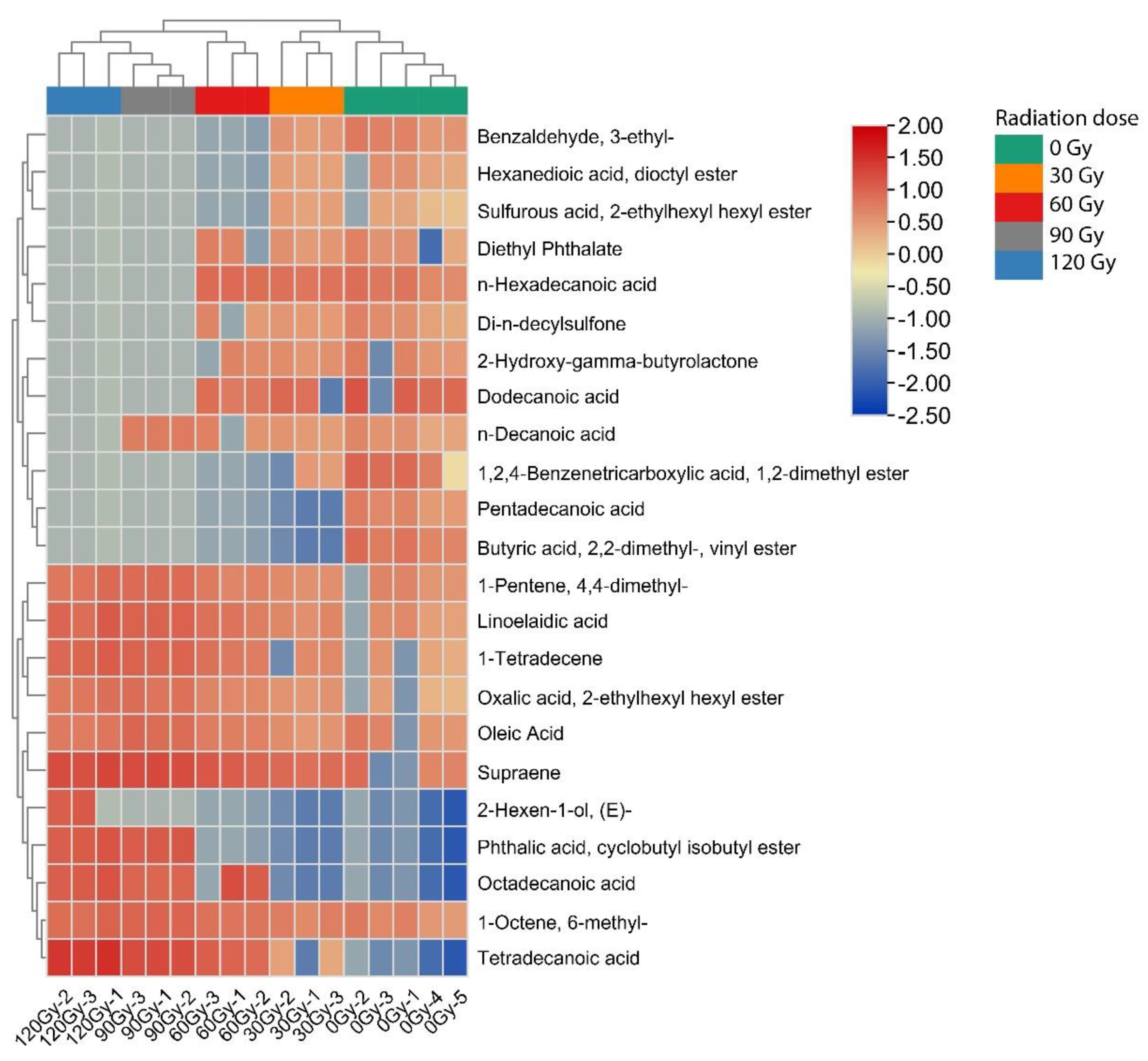
2.3. Hierarchical Cluster Analysis
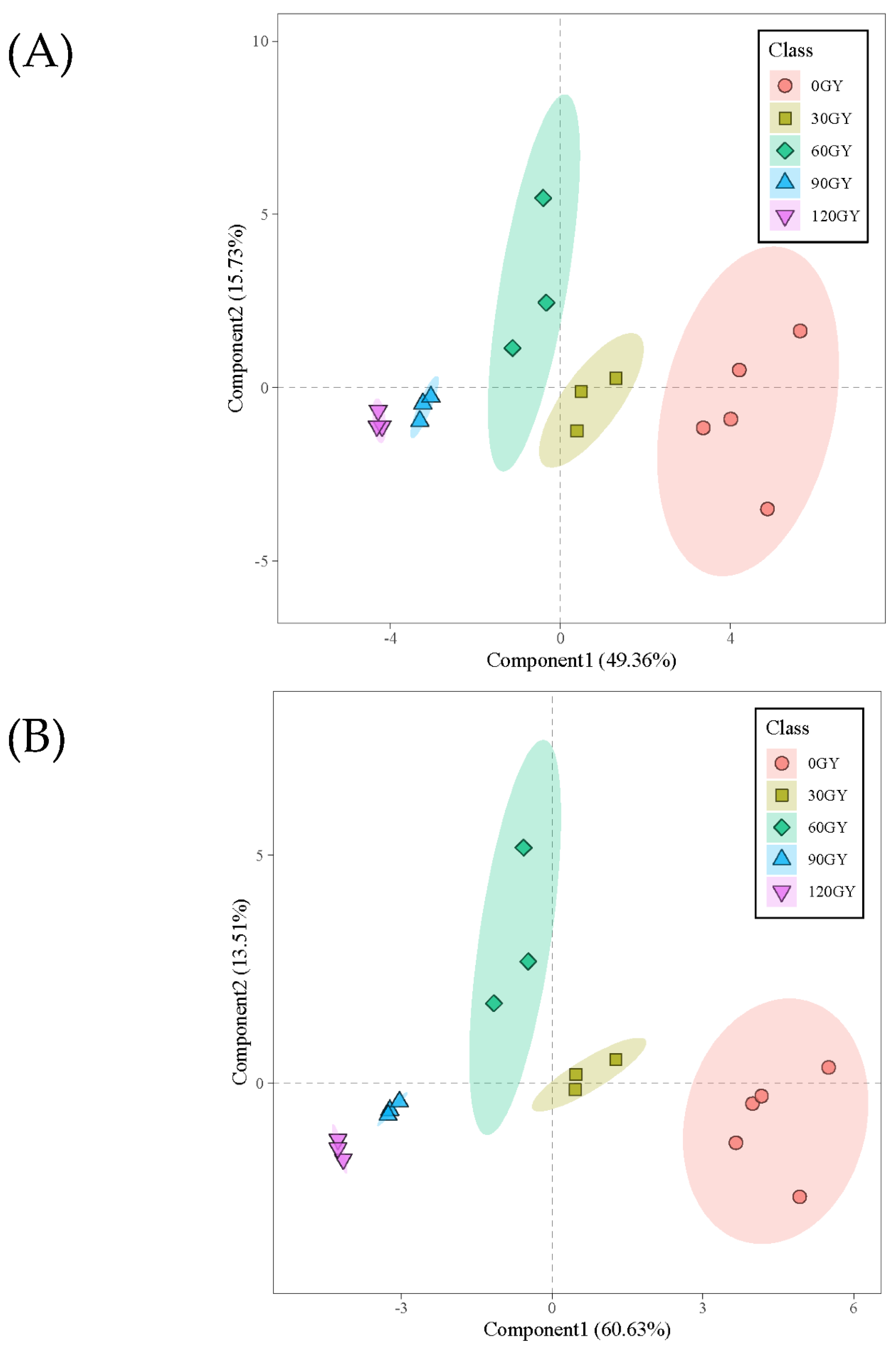
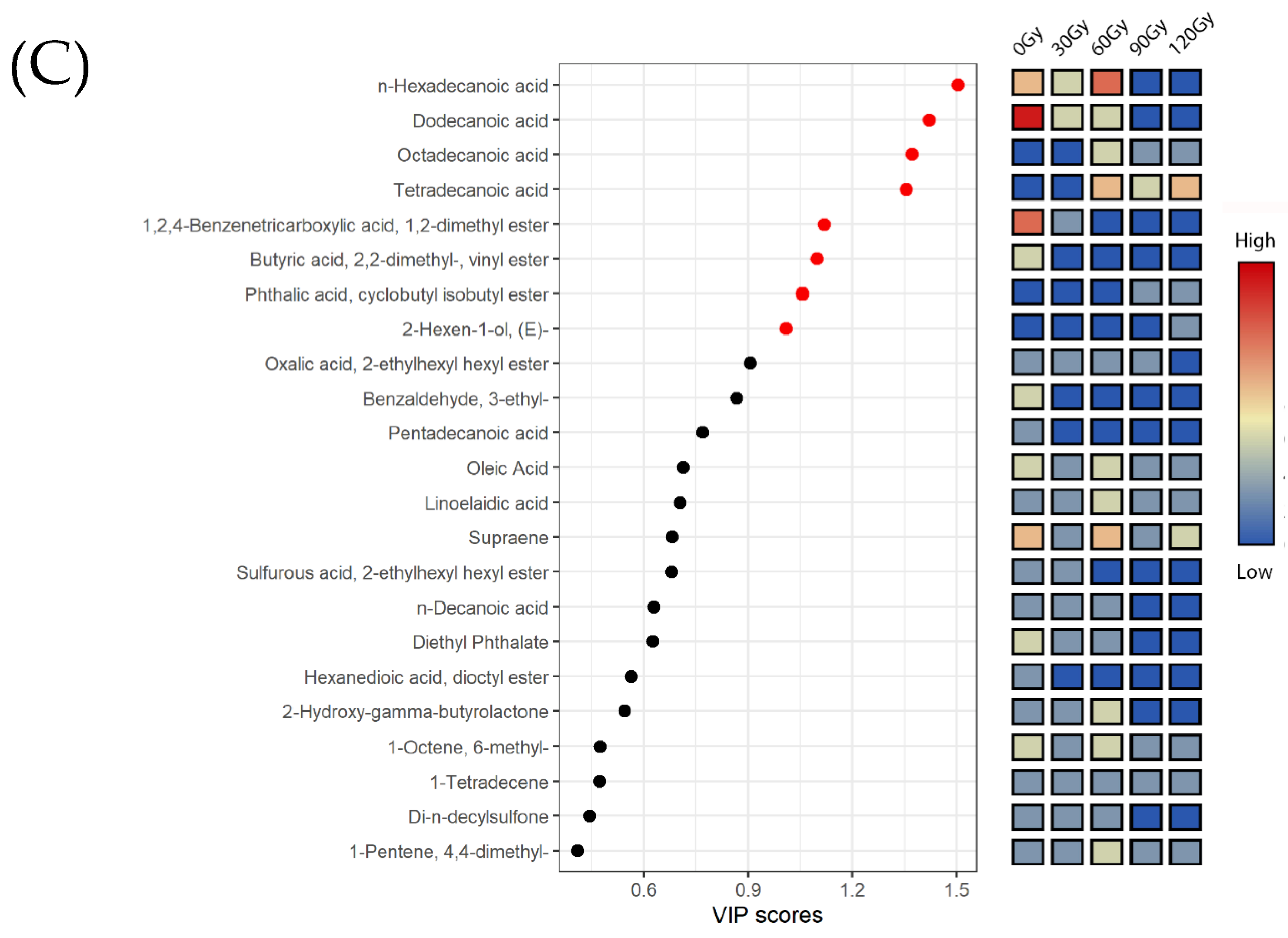
2.4. Multivariate Analysis of Metabolites in B. dorsalis Larvae Exposed to Different Irradiation Doses
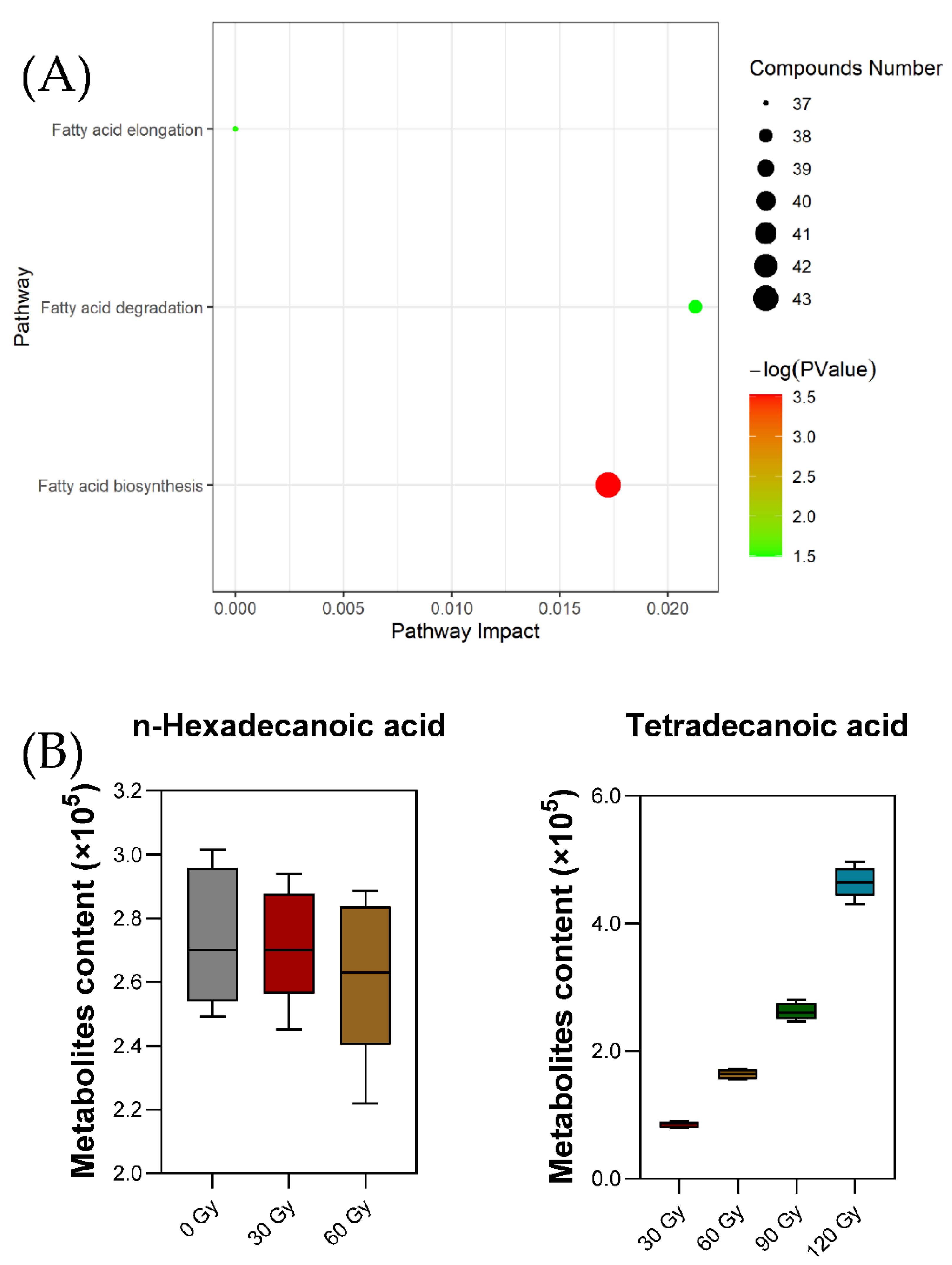
2.5. Preliminary Pathway Analysis of Differential Metabolites
3. Materials and Methods
3.1. Insect Culture
3.2. Irradiation Treatment
3.3. Solid Phase Microextraction (SPME) Procedure and Sampling Setup
3.4. Gas Chromatography-Mass Spectrometry (GC-MS) Conditions
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, X.; Liu, Y.; Zhang, B. Invasion history of the oriental fruit fly, Bactrocera dorsalis, in the Pacific-Asia region: Two main invasion routes. PLoS ONE 2012, 7, e36176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heve, W.K.; Adjadeh, T.A.; Billah, M.K. Overview and future research needs for development of effective biocontrol strategies for management of Bactrocera dorsalis Hendel (Diptera: Tephritidae) in sub-Saharan Africa. Pest Manag. Sci. 2021, 77, 4224–4237. [Google Scholar] [CrossRef] [PubMed]
- Dohino, T.; Hallman, G.J.; Grout, T.G.; Clarke, A.R.; Follett, P.A.; Cugala, D.R.; Minh Tu, D.; Murdita, W.; Hernandez, E.; Pereira, R.; et al. Phytosanitary Treatments Against Bactrocera dorsalis (Diptera: Tephritidae): Current Situation and Future Prospects. J. Econ. Entomol. 2017, 110, 67–79. [Google Scholar] [PubMed] [Green Version]
- Hallman, G.J. Phytosanitary Applications of Irradiation. Compr. Rev. Food Sci. Food Saf. 2011, 10, 143–151. [Google Scholar] [CrossRef]
- McDonald, H.; Arpaia, M.L.; Caporaso, F.; Obenland, D.; Were, L.; Rakovski, C.; Prakash, A. Effect of gamma irradiation treatment at phytosanitary dose levels on the quality of ‘Lane Late’ navel oranges. Postharvest Biol. Technol. 2013, 86, 91–99. [Google Scholar] [CrossRef]
- Drake, S.R.; Neven, L.G. Irradiation as an Alternative to Methyl Bromide for Quarantine Treatment of Stone Fruits. J. Food Qual. 1998, 21, 529–538. [Google Scholar] [CrossRef]
- Zahran, N.F.; Hamza, A.F.; Ramadan, M.H. Gamma Irradiation as an Alternative Treatment for Controlling of Lyctus africanus Lesne (Coleoptera: Bostrichidae) in Dry Wood. Egypt. J. Biol. Pest Control 2016, 26, 97–101. [Google Scholar]
- ISPM 28; International Plant Protection Convention (IPPC). Irradiation treatment for fruit flies of the family Tephritidae (generic). Food and Agricultural Organization: Rome, Italy, 2009. [Google Scholar]
- Hallman, G.J. Expanding radiation quarantine treatments beyond fruit flies. Agric. For. Entomol. 2000, 2, 85–95. [Google Scholar] [CrossRef]
- Hallman, G.J.; Levang-Brilz, N.M.; Zettler, J.L.; Winborne, I.C. Factors affecting ionizing radiation phytosanitary treatments, and implications for research and generic treatments. J. Econ. Entomol. 2010, 103, 1950–1963. [Google Scholar] [CrossRef]
- von Sonntag, C. The Chemical Basis of Radiation Biology; Taylor & Francis: London, UK, 1987. [Google Scholar]
- Hallman, G.J.; Loaharanu, P. Phytosanitary irradiation—Development and application. Radiat. Phys. Chem. 2016, 129, 39–45. [Google Scholar] [CrossRef]
- Woruba, D.N.; Morrow, J.L.; Reynolds, O.L.; Chapman, T.A.; Collins, D.P.; Riegler, M. Diet and irradiation effects on the bacterial community composition and structure in the gut of domesticated teneral and mature Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). BMC Microbiol. 2019, 19, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Furuta, M. Biological effects of low-dose γ-ray irradiation on chromosomes and DNA of Drosophila melanogaster. J. Radiat. Res. 2021, 62, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oinata, K.; Chambers, D.L.; Fujimoto, M.; Kashiwai, S.; Miyabara, R. Sterilization of the Mediterranean fruit fly by irradiation: Comparative mating effectiveness of treated pupae and adults. J. Econ. Entomol. 1971, 64, 781–787. [Google Scholar] [CrossRef]
- Baye, Z.K. Determine the Gamma Radiation Technique to Sterilization of Insects from Risk Factor. World J. Appl. Phys. 2021, 6, 35–40. [Google Scholar]
- Siddiqui, M.S.; Filomeni, E.; François, M.; Collins, S.R.; Cooper, T.; Glatz, R.V.; Taylor, P.W.; Fenech, M.; Leifert, W.R. Exposure of insect cells to ionising radiation in vivo induces persistent phosphorylation of a H2AX homologue (H2AvB). Mutagenesis 2013, 28, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Arthur, C.L.; Pawliszyn, J.B. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Kataoka, H.; Heather, L.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2020, 880, 35–62. [Google Scholar] [CrossRef]
- Laopongsit, W.; Srzednicki, G.; Craske, J.D. Preliminary study of solid phase micro-extraction (SPME) as a method for detecting insect infestation in wheat grain. J. Stored Prod. Res. 2014, 59, 88–95. [Google Scholar] [CrossRef]
- Cai, L.; Macfadyen, S.; Hua, B.; Zhang, H.; Xu, W.; Ren, Y. Identification of Biomarker Volatile Organic Compounds Released by Three Stored-Grain Insect Pests in Wheat. Molecules 2022, 27, 1963. [Google Scholar] [CrossRef]
- Alnajim, I.; Du, X.; Lee, B.; Agarwal, M.; Liu, T.; Ren, Y. New Method of Analysis of Lipids in Tribolium castaneum (Herbst) and Rhyzopertha dominica (Fabricius) Insects by Direct Immersion Solid-Phase Microextraction (DI-SPME) Coupled with GC–MS. Insects 2019, 10, 363. [Google Scholar] [CrossRef] [Green Version]
- Al-Khshemawee, H.; Du, X.; Agarwal, M.; Yang, J.; Ren, Y. Application of Direct Immersion Solid-Phase Microextraction (DI-SPME) for Understanding Biological Changes of Mediterranean Fruit Fly (Ceratitis capitata) During Mating Procedures. Molecules 2018, 23, 2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, F.; Magariyama, Y.; Miyanoshita, A. Volatile biomarkers for early-stage detection of insect-infested brown rice: Isopentenols and polysulfides. Food Chem. 2019, 303, 125381. [Google Scholar] [CrossRef] [PubMed]
- Fulano, A.M.; Lengai, G.M.; Muthomi, J.W. Phytosanitary and Technical Quality Challenges in Export Fresh Vegetables and Strategies to Compliance with Market Requirements: Case of Smallholder Snap Beans in Kenya. Sustainability 2021, 13, 1546. [Google Scholar] [CrossRef]
- Nawaz, A.; Sufyan, M.; Gogi, M.D.; Javed, M.W. Sustainable Management of Insect-Pests. Innov. Sustain. Agric. 2019, 10, 287–335. [Google Scholar]
- Heather, N.W.; Hallman, G.J. Pest Management and Phytosanitary Trade Barriers; CABI: Wallingford, UK, 2008. [Google Scholar]
- Hallman, G.J. Process control in phytosanitary irradiation of fresh fruits and vegetables as a model for other phytosanitary treatment processes. Food Control 2017, 72, 372–377. [Google Scholar] [CrossRef]
- Hallman, G.J.; Blackburn, C.M. Phytosanitary Irradiation. Foods 2016, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Barry, J.D.; McInnis, D.O.; Gates, D.; Morse, J.G. Effects of irradiation on Mediterranean fruit flies (Diptera: Tephritidae): Emergence, survivorship, lure attraction, and mating competition. J. Econ. Entomol. 2003, 96, 615–622. [Google Scholar] [CrossRef]
- Benelli, M.; Ponton, F.; Lallu, U.; Mitchell, K.A.; Taylor, P.W. Cool storage of Queensland fruit fly pupae for improved management of mass production schedules. Pest Manag. Sci. 2019, 75, 3184–3192. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, G.; Mishra, H.N. Identification and differentiation of insect infested rice grains varieties with FTNIR spectroscopy and hierarchical cluster analysis. Food Chem. 2018, 268, 402–410. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Antti, H.; Holmes, E. Multi- and Megavariate Data Analysis: Finding and Using Regularities in Metabonomics Data; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Sharin, S.N.; Sani, M.S.A.; Jaafar, M.A.; Yuswan, M.H.; Kassim, N.K.; Manaf, Y.N. Discrimination of Malaysian stingless bee honey from different entomological origins based on physicochemical properties and volatile compound profiles using chemometrics and machine learning. Food Chem. 2020, 346, 128654. [Google Scholar] [CrossRef]
- Feng, Y.; Fu, T.; Zhang, L.; Wang, C.; Zhang, D. Research on Differential Metabolites in Distinction of Rice (Oryza sativa L.) Origin Based on GC-MS. J. Chem. 2019, 2019, 1614504. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Feng, X.; Lyu, C.; Zhou, S.; Liu, Z. Effects of different processing methods on the lipid composition of hazelnut oil: A lipidomics analysis. Food Sci. Hum. Wellness 2022, 11, 427–435. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Li, X.; Zhang, H.; Chen, Q.; Kong, B. Application of lactic acid bacteria for improving the quality of reduced-salt dry fermented sausage: Texture, color, and flavor profiles. LWT 2021, 154, 112723. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Sun, L.; Fan, K.; Wang, L.; Ma, D.; Wang, Y.; Kong, X.; Li, H.; Ren, Y.; Ding, Z. Correlation among Metabolic Changes in Tea Plant Camellia sinensis (L.) Shoots, Green Tea Quality and the Application of Cow Manure to Tea Plantation Soils. Molecules 2021, 26, 6180. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Ji, H.; Wang, W.; Liu, J.; Wang, F.; Xie, F.; Yu, Y.; Qin, Y.; Wang, X. Metabolic profiling of tobacco leaves at different growth stages or different stalk positions by gas chromatography–mass spectrometry. Ind. Crops Prod. 2018, 116, 46–55. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Jurenka, R.; Cripps, C.; Blomquist, G.J.; Renobales, M.D. Fatty acids in insects: Composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 1988, 9, 1–33. [Google Scholar] [CrossRef]
- Beenakkers, A.M.; Van der Horst, D.J.; Van Marrewijk, W. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 1985, 24, 19–67. [Google Scholar] [CrossRef]
- Parvy, J.P.; Napal, L.; Rubin, T.; Poidevin, M.; Perrin, L.; Wicker, T.C.; Montagne, J. Drosophila melanogaster Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System. PLoS Genet. 2012, 8, e1002925. [Google Scholar] [CrossRef] [Green Version]
- Urbanski, J.; Benoit, J.; Michaud, M.; Denlinger, D.; Armbruster, P. The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proc. Biol. Sci. 2010, 277, 2683–2692. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jiang, Y.; Liu, Z.; You, L.; Wu, Y.; Xu, B.; Ge, L.; Stanley, D.; Song, Q.; Wu, J. Jinggangmycin increases fecundity of the brown planthopper, Nilaparvata lugens (Stål) via fatty acid synthase gene expression. J. Proteom. 2016, 130, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.; Denlinger, D.L. Transcription profiling and regulation of fat metabolism genes in diapausing adults of the mosquito Culex pipiens. Physiol. Genom. 2009, 39, 202–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, S.N. A review and comparative characterization of the fatty acid compositions of seven insect orders. Comp. Biochem. Physiol. Part B Comp. Biochem. 1973, 45, 467–482. [Google Scholar] [CrossRef]
- Abdullah, R. Insecticidal Activity of Secondary Metabolites of Locally Isolated Fungal Strains against some Cotton Insect Pests. J. Plant Prot. Pathol. 2019, 10, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Alagarmalai, J.; Chinnamani, T.C. Chemical composition and growth inhibitory activities of Solonum pseudocapsicum against Spodoptera litura and Helicoverpa armigera (Lepidoptera: Noctuidae). Int. J. Entomol. Res. 2017, 2, 2455–4758. [Google Scholar]
- Sivakumar, R.; Jebanesan, A.; Govindarajan, M.; Rajasekar, P. Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera:Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 706–710. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Ma, J.; Mutao, W.; Jiao, X.; Wang, Z.; Liang, F.; Zhan, G. Gamma radiation as a phytosanitary treatment against larvae and pupae of Bactrocera dorsalis (Diptera: Tephritidae) in guava fruits. Food Control 2017, 72, 360–366. [Google Scholar] [CrossRef]
- Vargas, R.I.; Miyashita, D.H.; Nishida, T. Life History and Demographic Parameters of Three Laboratory-reared Tephritids (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1984, 77, 651–656. [Google Scholar] [CrossRef]
- Zhan, G.; Zhao, J.; Ma, F.; Liu, B.; Zhong, Y.; Song, Z.; Zhao, Q.; Chen, N.; Ma, C. Radioprotective Effects on Late Third-Instar Bactrocera dorsalis (Diptera: Tephritidae) Larvae in Low-Oxygen Atmospheres. Insects 2020, 11, 526. [Google Scholar] [CrossRef]
- Geiselhardt, S.F.; Geiselhardt, S.; Peschke, K. Comparison of tarsal and cuticular chemistry in the leaf beetle Gastrophysa viridula (Coleoptera: Chrysomelidae) and an evaluation of solid-phase microextraction and solvent extraction techniques. Chemoecology 2009, 19, 185–193. [Google Scholar] [CrossRef]
- Ismarti, I.; Handoko, D.D.; Triyana, K.; Salleh, H.M.; Fadzillah, N.A.; Nordin, N.F. Study on Volatile Compounds of Gelatine and The Maillard Reaction Products from Different Species Using SPME-GCMS. Sci. Technol. Indones. 2022, 7, 132–139. [Google Scholar] [CrossRef]
- Du, W.; Liu, X.; Zhao, L.; Xu, Y.; Yin, Y.; Wu, J.; Ji, R.; Sun, Y.; Guo, H. Response of cucumber (Cucumis sativus) to perfluorooctanoic acid in photosynthesis and metabolomics. Sci. Total Environ. 2020, 724, 138257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.D.; Wiemann, S. KEGG graph: A graph approach to KEGG PATHWAY in R and bioconductor. Bioinformatics 2009, 25, 1470–1471. [Google Scholar] [CrossRef] [PubMed]
| Metabolites | Retention Time | Retention Index | GC Response (105) ± SD | CAS Number | ||||
|---|---|---|---|---|---|---|---|---|
| 0 Gy | 30 Gy | 60 Gy | 90 Gy | 120 Gy | ||||
| 2-Hydroxy-gamma-butyrolactone | 8.404 | 997.26 | 1.2 ± 0.06 | 1.12 ± 0.13 | 1.06 ± 0.14 | N.D. | N.D. | 19444-84-9 |
| 1-Pentene, 4,4-dimethyl- | 9.825 | 1037.86 | 1.41 ± 0.12 | 1.25 ± 0.15 | 1.14 ± 0.13 | 1.26 ± 0.16 | 1.01 ± 0.10 | 762-62-9 |
| Benzaldehyde, 3-ethyl- | 14.305 | 1061.02 | 1.36 ± 0.17 | 0.91 ± 0.11 | N.D. | N.D. | N.D. | 34246-54-3 |
| 2-Hexen-1-ol, (E)- | 15.58 | 1102.61 | N.D. | N.D. | N.D. | N.D. | 2.11 ± 0.09 | 928-95-0 |
| n-Decanoic acid | 20.686 | 1274.26 | 1.14 ± 0.16 | 1.09 ± 0.13 | 0.89 ± 0.10 | 0.67 ± 0.02 | N.D. | 334-48-5 |
| 1-Octene, 6-methyl- | 21.288 | 1295.34 | 1.55 ± 0.16 | 1.54 ± 0.12 | 1.29 ± 0.26 | 1.16 ± 0.12 | 1.05 ± 0.14 | 13151-10-5 |
| 1-Tetradecene | 23.28 | 1369.70 | 1.7 ± 0.10 | 1.62 ± 0.01 | 1.27 ± 0.12 | 1.23 ± 0.08 | 1.39 ± 0.16 | 1120-36-1 |
| Dodecanoic acid | 24.966 | 1435.12 | 3.72 ± 0.25 | 3.31 ± 0.17 | 1.13 ± 0.13 | N.D. | N.D. | 143-07-7 |
| Diethyl Phthalate | 25.772 | 1467.36 | 1.32 ± 0.12 | 1.06 ± 0.08 | 1.04 ± 0.06 | N.D. | N.D. | 84-66-2 |
| Tetradecanoic acid | 29.365 | 1739.12 | N.D. | 0.85 ± 0.10 | 1.64 ± 0.10 | 2.62 ± 0.23 | 4.6 ± 0.43 | 544-63-8 |
| Pentadecanoic acid | 31.94 | 1817.30 | 1.45 ± 0.13 | N.D. | N.D. | N.D. | N.D. | 1002-84-2 |
| Phthalic acid, cyclobutyl isobutyl ester | 35.306 | 1947.82 | N.D. | N.D. | N.D. | 1.66 ± 0.17 | 1.61 ± 0.22 | 1000314-91-1 |
| n-Hexadecanoic acid | 35.23 | 2058.07 | 2.73 ± 0.19 | 2.72 ± 0.12 | 2.63 ± 0.28 | N.D. | N.D. | 57-10-3 |
| Linoelaidic acid | 35.969 | 2135.05 | 1.54 ± 0.21 | 1.53 ± 0.19 | 1.14 ± 0.09 | 1.28 ± 0.11 | 1.19 ± 0.03 | 506-21-8 |
| Oleic acid | 38.664 | 2181.34 | 1.68 ± 0.23 | 1.35 ± 0.18 | 1.09 ± 0.1 | 0.98 ± 0.09 | 0.89 ± 0.03 | 112-80-1 |
| Octadecanoic acid | 39.014 | 2187.36 | N.D. | N.D. | 1.08 ± 0.04 | 1.23 ± 0.01 | 1.56 ± 0.21 | 57-11-4 |
| Oxalic acid, 2-ethylhexyl hexyl ester | 40.585 | 2221.02 | 1.22 ± 0.16 | 1.08 ± 0.10 | 0.85 ± 0.21 | 0.83 ± 0.07 | 0.91 ± 0.08 | 1000309-38-9 |
| Hexanedioic acid, dioctyl ester | 41.759 | 2350.94 | 1.27 ± 0.13 | 0.79 ± 0.11 | N.D. | N.D. | N.D. | 123-79-5 |
| Di-n-decylsulfone | 42.046 | 2439.44 | 1.57 ± 0.17 | 1.16 ± 0.14 | 0.96 ± 0.07 | N.D. | N.D. | 111530-37-1 |
| Sulfurous acid, 2-ethylhexyl hexyl ester | 43.309 | 2561.14 | 1.32 ± 0.17 | 0.9 ± 0.04 | N.D. | N.D. | N.D. | 1000309-20-2 |
| 1,2,4-Benzenetricarboxylic acid, 1,2-dimethyl ester | 44.34 | 2678.85 | 3.26 ± 0.21 | 1.04 ± 0.11 | N.D. | N.D. | N.D. | 54699-35-3 |
| Butyric acid, 2,2-dimethyl-, vinyl ester | 45.219 | 2793.95 | 2.13 ± 0.14 | N.D. | N.D. | N.D. | N.D. | 13170-00-8 |
| Supraene | 45.568 | 2867.19 | 2.69 ± 0.29 | 2.82 ± 0.01 | 2.04 ± 0.25 | 2.65 ± 0.26 | 2.39 ± 0.31 | 7683-64-9 |
| Metabolites | VIP Scores | p Value | FDR | Class |
|---|---|---|---|---|
| n-Hexadecanoic acid | 1.505 | 0.049 | 0.069 | Acid |
| Dodecanoic acid | 1.421 | 0.003 | 0.008 | |
| Octadecanoic acid | 1.371 | 0.001 | 0.006 | |
| Tetradecanoic acid | 1.355 | 0.005 | 0.012 | |
| 1,2,4-Benzenetricarboxylic acid, 1,2-dimethyl ester | 1.120 | 0.003 | 0.008 | Ester |
| Butyric acid, 2,2-dimethyl-, vinyl ester | 1.098 | 0.003 | 0.008 | |
| Phthalic acid, cyclobutyl isobutyl ester | 1.056 | 0.000 | 0.000 | |
| 2-Hexen-1-ol, (E)- | 1.009 | 0.017 | 0.029 | Alcohol |
| Pathway Name | Total | Expected | Hits | Raw p | Impact | Hit Metabolites |
|---|---|---|---|---|---|---|
| Fatty acid biosynthesis | 43 | 0.28289 | 2 | 0.029454 | 0.01724 | C00249: n-Hexadecanoic acidC06424: Tetradecanoic acid |
| Fatty acid elongation | 37 | 0.24342 | 1 | 0.22001 | 0 | C00249: n-Hexadecanoic acid |
| Fatty acid degradation | 38 | 0.25 | 1 | 0.22532 | 0.02128 | C00249: n-Hexadecanoic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, C.; Li, B.; Li, L.; Li, B.; Ren, Y.; Liu, T. Correlation between Irradiation Treatment and Metabolite Changes in Bactrocera dorsalis (Diptera: Tephritidae) Larvae Using Solid-Phase Microextraction (SPME) Coupled with Gas Chromatography-Mass Spectrometry (GC-MS). Molecules 2022, 27, 4641. https://doi.org/10.3390/molecules27144641
Shan C, Li B, Li L, Li B, Ren Y, Liu T. Correlation between Irradiation Treatment and Metabolite Changes in Bactrocera dorsalis (Diptera: Tephritidae) Larvae Using Solid-Phase Microextraction (SPME) Coupled with Gas Chromatography-Mass Spectrometry (GC-MS). Molecules. 2022; 27(14):4641. https://doi.org/10.3390/molecules27144641
Chicago/Turabian StyleShan, Changyao, Baishu Li, Li Li, Beibei Li, YongLin Ren, and Tao Liu. 2022. "Correlation between Irradiation Treatment and Metabolite Changes in Bactrocera dorsalis (Diptera: Tephritidae) Larvae Using Solid-Phase Microextraction (SPME) Coupled with Gas Chromatography-Mass Spectrometry (GC-MS)" Molecules 27, no. 14: 4641. https://doi.org/10.3390/molecules27144641
APA StyleShan, C., Li, B., Li, L., Li, B., Ren, Y., & Liu, T. (2022). Correlation between Irradiation Treatment and Metabolite Changes in Bactrocera dorsalis (Diptera: Tephritidae) Larvae Using Solid-Phase Microextraction (SPME) Coupled with Gas Chromatography-Mass Spectrometry (GC-MS). Molecules, 27(14), 4641. https://doi.org/10.3390/molecules27144641







