Abstract
The separation of chemical components from wild plants to develop new pesticides is a hot topic in current research. To evaluate the antimicrobial effects of metabolites of Ligusticum chuanxiong (CX), we systematically studied the antimicrobial activity of extracts of CX, and the active compounds were isolated, purified and structurally identified. The results of toxicity measurement showed that the extracts of CX had good biological activities against Botrytis cinerea, Sclerotinia sclerotiorum, Alternaria alternata and Pythium aphanidermatum, and the value of EC50 were 130.95, 242.36, 332.73 and 307.29 mg/L, respectively. The results of in vivo determination showed that under the concentration of 1000 mg/L, the control effect of CX extract on Blumeria graminis was more than 40%, and the control effect on Botrytis cinerea was 100%. The antifungal active components of CX were identified as Senkyunolide A and Ligustilide by mass spectrometry and nuclear magnetic resonance. The MIC (minimum inhibitory concentration) value of Senkyunolide A and Ligustilide against Fusarium graminearum were 7.81 and 62.25 mg/L, respectively. As a new botanical fungicide with a brightly exploitative prospect, CX extract has potential research value in the prevention and control of plant diseases.
1. Introduction
Since the 1980s, the research on plant resources with antimicrobial activity has been carried out at home and abroad. Scholars have conducted a lot of research on the effective components of plant antifungal and sterilization [1]. The functional components of many medicinal plants, such as volatile oils, alkaloids, flavonoids, organic acids, terpenoids, glycosides, quinones, esters, phenols and so on, have good antimicrobial effects [2]. It was found that 75% ethanol extract of Elsholtzia splendens had the most significant effect on Botryodiplodia theobromae, with an inhibition rate of 54.13% [3]. Cumin acid had good antimicrobial activity against a variety of pathogens, among which the virulence to Valsa mali was the strongest [4]. Carvanol had strong antimicrobial activity against Colletotrichum gloeosporioides and Alternaria alternata, with the values of IC50 being 40.89 μg/mL and 18.19 μg/mL, respectively [5]. Therefore, it is a valuable work to screen antimicrobial plants and identify their active components.
CX (named as Rhizoma chuanxiong before 2010 in the Chinese Pharmacopoeia), the dried rhizome of CX Hort., known as Chuan-Xiong (CX) in folk medicine, and belonging to the Umbelliferae family, is one of the oldest and most popular herbal medicines in the World. CX is warm in property and pungent in flavor, with the functions of activating qi, promoting blood circulation, expelling wind and alleviating pain, which has high medicinal value. It grows in a mild climate environment [6], and it is mainly produced in Guanxian Sichuan, Yunnan, Guizhou, Guangxi and other places. Its chemical composition includes phthalide and its dimer, alkaloids, organic acid phenols, polysaccharides, brain glycosides and ceramides. The active components of CX have various pharmacological activities on cardio-cerebrovascular system, nervous system, respiratory system and so on [7]. The acetone extract of CX had a good inhibitory effect on Fusarium oxysporum, Botrytis cinerea, poplar canker, Sclerotinia sclerotiorum and Fusarium graminearum [8].
In preliminary research, it was determined that CX had broad-spectrum antifungal activity, but its antifungal activity components were not clear. Therefore, the purpose of this study is to clarify the fungicide active components and structure of CX. Then, extraction, silica gel column chromatography and high performance liquid chromatography (HPLC), and the data were analyzed by modern spectral techniques (EI-MS, 1H-NMR, 13C-NMR) to clarify the structure of the active components, which laid a foundation for further development and utilization of CX extract as a fungicide.
2. Results
2.1. Toxicity Determination of Extracts of CX
The extract of CX showed different degrees of inhibition on the mycelial growth of 10 different plant-pathogenic fungi (Table 1). Among them, the inhibitory effects of the extract on Botrytis cinerea, Sclerotinia sclerotiorum, Pythium aphanidermatum, Alternaria alternata and Didymella glomerata were significant, and the EC50 values were 130.95, 242.36, 307.29, 332.73 and 470.53 mg/L, respectively. The extract had a poor inhibitory effect on Fusarium graminearum, Colletotrichum gloeosporioides, Fusarium oxysporum, Fusarium lateritium and Phytophthora infestans, and their EC50 values were in the range of 500–1000 mg/L.

Table 1.
Toxicity test results of CX plant extracts against 10 kinds of plant pathogens.
2.2. Bioassay of Extracts of CX In Vivo
2.2.1. Control Effect on Blumeria graminis
Different concentrations of extracts of CX had different effects on the control of Blumeria graminis (Table 2). The higher the concentration, the better the prevention and control effects were. Among them, the protective effect is better than the curative effect. Under the protective effect, the extract concentration of 4000 mg/L had the best control effect on Blumeria graminis.

Table 2.
Control effect of CX extract on Blumeria graminis by pot trial.
2.2.2. Control Effect on Fusarium graminearum
Different concentrations of extracts of CX had different degrees of control effects on Fusarium graminearum (Table 3). The higher the concentration, the better the control effect was, and the protective activity was better than the curative activity, but the control effect of the extract was lower than that of the control agent (86 mg/L Tebuconazole). When the concentration of extracts was 4000 mg/L, the protective and curative activities of Fusarium graminearum were the best. The RDCEs of the protective activities were 50.01%, 39.98% and 11.90%, respectively.

Table 3.
Control effect of CX extract on Fusarium graminearum by pot trial.
2.2.3. Control Effect on Botrytis cinerea
Different concentrations of extracts of CX had different degrees of control effects on Botrytis cinerea (Table 4). When the concentration of the extract was 1000 mg/L, it had obvious protective and curative activities on Botrytis cinerea. The RDCEs of the protective activities were 100.00%, and those of the curative activities were 100.00%, 75.00%, 45.47% at 3, 5 and 7 days after treatment, respectively. When the concentration of extract was 2000 mg/L, the RDCE of protective and curative activities reached 100%, which can achieve the control effect of the control agent (100 mg/L pyraclostrobin).

Table 4.
In vivo control effect of CX plant extracts on Botrytis cinerea.
2.2.4. Control Effect on Colletotrichum gloeosporioides
Different concentrations of CX extract have different degree of control effect on Colletotrichum gloeosporioides (Table 5). The higher the concentration, the better the control effect was, and among which the protective effect is better than the curative effect. Under the protective effect, the control effect was the best when the concentration of CX extract was 4000 mg/L, and the control effect was 48.04% after being treated for 7 days.

Table 5.
In vivo control effect of CX plant extracts on Colletotrichum gloeosporioides.
2.3. Tracking Results of Antifungal Activity of Different Fractions of CX Extract by Silica Gel Column Chromatography
Seven fractions of CX extracts were obtained, and their antifungal activities are shown in Table 6. Results showed that the fractions of FrD2 and FrD5 had antifungal activity. At the concentration of 400 mg/L, spore germination inhibition rates of FrD2 and FrD5 on Botrytis cinerea were 0.00%.

Table 6.
Spore germination inhibition rate of 7 fractions of CX extract obtained by silica gel column chromatography and 5 fractions obtained by HPLC on Botrytis cinerea.
2.4. Separation and Purification and Tracking Results of Active Compounds by HPLC in CX Extract
The compounds CQ1-CQ5 were isolated and purified. The antifungal activity of the compounds were traced (Table 6). The results showed that CQ2 and CQ4 had obvious inhibitory effect on Botrytis cinerea. At the concentration of 62.25 mg/L, spore germination inhibition rates of CQ2 and CQ4 on Botrytis cinerea were 0.00%.
2.5. Chemical Structure Identification of Active Compounds from CX
2.5.1. Compound CQ2
Compound CQ2 was identified as Yellowish oil. Its molecular formula is determined as C12H16O2 (M/Z 193.2[M+H]+) based on ESIMS data (M/Z 193.2[M+H]+), showing 5 degrees of unsaturated. 1H-NMR spectra (Table 7) showed two olefinic signals (1H, DT, J = 3.4, 8.9 Hz), 6.20 (1H, DT, J = 2.0, 9.7 Hz) and one mesine signal at X4.91 (1H, DD, J = 3.7,7.6 Hz). Amethyl group is in (3H, t, J = 7.1). 13C-NMR spectra (Table 8) show 12 carbon signals assigned to a Lactone carbonyl carbon (UNK C 171.4), four olefin atoms (UNK C 161.6, 128.5, 124.6, 116.9), One oxy-methine carbon (UNK C 82.6), five methyl groups (UNK C 32.0, 26.8, 22.5, 20.9, 22.4), and one methyl carbon (UNK C 14.0). These spectral data are the same as those released by Senkyunolide A [9,10]. Thus, the compound was identified as Senkyunolide A and its molecular formula was obtained (Figure 1).

Table 7.
1H and 13C NMR data of CQ2 and CQ4 (CDCl3, δ in ppm, J in Hz).

Table 8.
Determination results of the MIC of compounds CQ2 and CQ4 against the four pathogenic fungi.
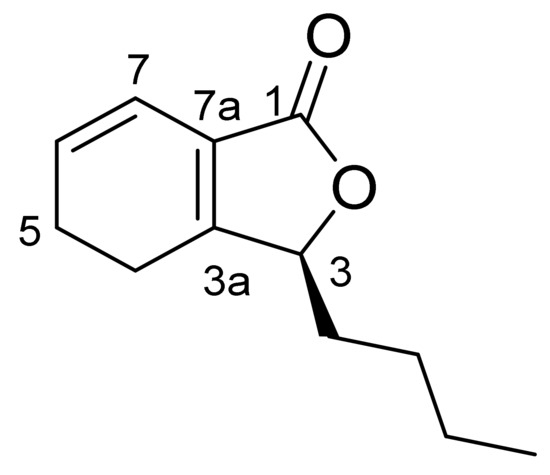
Figure 1.
Structural formula of Senkyunolide A.
2.5.2. Compound CQ4
Compound CQ4 was identified as colorless oil. According to the HRESIMS data (M/Z 191.1051 [M+H]+), its molecular formula is determined as C12H14O2, indicating six degrees of unsaturated. 1H-NMR data (Table 6) show that δH is 6.24 (1H, D, J = 9.5Hz) 5.97 (1H, m) and 5.20 (1H, T, J = 8.0 Hz) for three olefin protons and one methyl proton (δH 0.93 (3H, T, J = 7.4 Hz)). 13C-NMR spectrum (Table 7) contains twelve carbon signals, one lactone carbonyl carbon (δC 167.8), six olefin carbons (δC 148.7, 147.2, 130.0, 124.1, 117.2, 113.1), four methylene carbons (δC 28.2, 22.5, 18.6, 18.6) and one methyl carbon (δC 13.9), respectively. These spectral data are consistent with the published data of Ligustilide [11,12]. Thus, compound CQ4 was identified as Ligustilide, and its molecular formula was obtained (Figure 2).
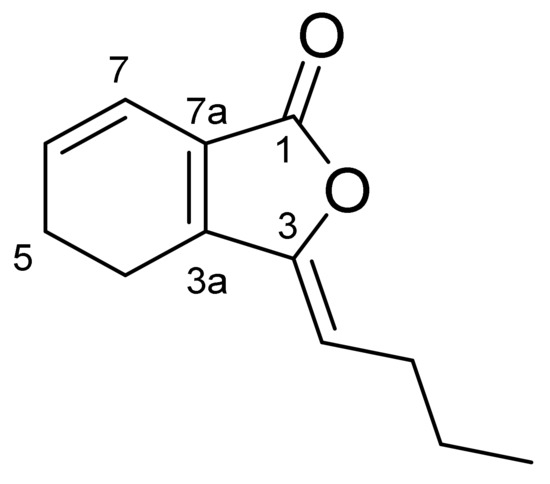
Figure 2.
Structural formula of Ligustilide.
2.6. Antifungal Activity of Active Compounds Ingredients
Senkyunolide A and Ligustilide isolated from extracts of CX had inhibitory effects on the spore germination of Fusarium graminearum, Botryis cinerea, Colletotrichum gloeosporioides and Fusarium oxysporum (Table 8). The MIC values of Senkyunolide A against Fusarium graminearum, Botryis cinerea, Colletotrichum gloeosporioides and Fusarium oxysporum were 7.81 mg/L, 250 mg/L, 250 mg/L and 250 mg/L, respectively; the MIC values of Ligustilide effective against Fusarium graminearum, Botryis cinerea, Colletotrichum gloeosporioides and Fusarium oxysporum were 62.25 mg/L, 125 mg/L, 500 mg/L, and 250 mg/L, respectively.
3. Discussion
Plants can produce rich secondary metabolites. Statistics shows that more than 400,000 kinds of secondary metabolites that have significant biological activities against phytopathogenic fungi and bacteria have been found thus far. The phytochemical components of CX is complex, and more than 100 compounds such as haplophthalides, polyploides, alkaloids, terpenes, organic acids, lipids and polysaccharides have been isolated from it [13]. The use of these active natural products to develop new botanical fungicides has become an important research field.
In this study, the extract of CX was found that it has significant inhibitory effect on many plant pathogens, such as Botryis cinerea, Sclerotinia sclerotiorum and Fusarium graminearum. Among them, CX extract has the best control effect of on Blumeria graminis and Botrytis cinerea, and the inhibition rates (1000 mg/L) were more than 40% and 100%, respectively. Currently, studies of the medical and agricultural antimicrobial activity of CX extract have drawn more and more attention. In medicine, studies have reported that CX extract has significant antimicrobial effect on Escherichia coli, Salmonella, solanacearum, Staphylococcus albicans, Staphylococcus aureus and double-streptococcus pneumoniae [14]. In agriculture, studies have reported that CX extract has good antimicrobial effect on Penicillium, Sclerotinia sclerotiorum and Fusarium graminearum. For example, Xie et al. found that CX extract had a significant inhibitory effect on white mold and brown rot phytophthora and finger penicillium, which could reach 100% at concentrations of 1.0 × 10−3 g/mL [8,15]. Chen et al. found that the ethanol extract of CX had a good inhibitory effect on Penicillium, and the inhibiting rate was over 92.79% [16]. Tang et al., found that CX extract has antifungal effect on Penicillium citrus and the antifungal effect decreased with the decrease in the concentration of the extract. When the concentration was 500 mg/mL, the inhibition rate to Penicillium citrus was 100% [17]. Zhang et al. found that the inhibition rates of CX extract on the mycelial growth of Sclerotinia sclerotiorum and Fusarium graminearum were more than 60% [8].
Two active components were isolated and purified from the alcohol extract of CX Hort in this study, and they were identified as Senkyunolide A and Ligustilide, respectively. Senkyunolide A and Ligustilide are both phthalide compounds. The bioassay results showed that Senkyunolide A and Ligustilide had different inhibitory effects on Fusarium graminearum, Botryis cinerea, Colletotrichum gloeosporioides and Fusarium oxysporum. Among them, the MIC amounts of Senkyunolide A and Ligustilide against Fusarium graminearum were 7.81 mg/L and 62.25 mg/L. At present, the biological activities of these two compounds are mainly in medicine, and to a lesser extent in agriculture. For example, Senkyunolide A has good pharmacological effects such as anti-oxidative injury, anti-inflammation, anti-tumor and vasodilation [18]. Ligustilide has good pharmacological effects such as anti-atherosclerosis, anti-inflammation and analgesia, anti-senile dementia and anti-tumor [14]. In agriculture, Ligustilide has significant insecticidal activity against Spodoptera litura [19]. However, it has not been reported that these two compounds can inhibit plant pathogens such as Fusarium graminearum, Botryis cinerea. Therefore, the extract of CX as a plant fungicide will have potential applications.
The extract of CX had broad-spectrum antifungal activity in vitro and in vivo. The active components were isolated and purified by extraction, silica gel column chromatography and HPLC, and Senkyunolide A and Ligustilide further were identified by modern spectrum technology. The results of microporous plate method showed that Senkyunolide A and Ligustilide had an obvious inhibitory effect on Fusarium graminearum. However, the mechanism of Senkyunolide and Ligustilide inhibiting the growth and toxin production of wheat scab is not clear. Because Fusarium asiaticum is one of the main pathogens of wheat scab, it accumulates a large amount of single-ended mycotoxins such as nivalenol (NIV) in wheat grains [20]. Further, we will continue analyzing the effects of Senkyunolide and Ligustilide on the accumulation of NIV and the related indexes of mycelial cell membrane, cell wall and respiratory metabolism, and illustrate the mechanism of Senkyunolide and Ligustilide inhibiting the growth and NIV production of Fusarium asiaticum at the molecular level.
4. Materials and Methods
4.1. Materials
CX was provided by Sichuan Shushan Daogen Chinese Herbal Medicine Co., Ltd. Pathogens including Fusarium graminearum, Blumeria graminis (Bgt), Botryis cinerea, Colletotrichum gloeosporioides, Sclerotinia sclerotiorum, Fusarium oxysporum, Fusarium lateritium, Alternaria alternata, Pythium aphanidermatum, Didymella glomerata and Phytophthora infestans were provided by the College of Agronomy of Sichuan Agricultural University.
4.2. Preparation of Extracts of CX
Solvent extraction method was used [21,22]. The plant samples were dried in an electrothermal constant temperature blast drying box at 55 °C, and the dried plant powder was crushed by the grinder and sealed away from light in the fresh-keeping bag. The dried plant powder (2 kg) was soaked and extracted with 5 times volume of ethanol in dark. The extract was filtered, and then the same amount of ethanol was added after 2 days. The extract was filtered out after that and the two parts of the filtrate was combined. The extract was concentrated to the paste extract by rotary evaporator at 50–60 °C, scraped off, and stored in the refrigerator at 4 °C.
4.3. Toxicity Determination of Extracts of CX
Mycelial growth inhibition assay was used to evaluate the inhibitory effect of extracts of CX on fungi [23]. The extract of CX was dissolved with dimethyl sulfoxide and then diluted with sterile water to 2000, 1000, 500, 250, 125 and 62.5 mg/L. The solution (1 mL) was mixed with 9 mL Potato Dextrose Agar (PDA) medium (containing 1% streptomycin sulfate) and then poured into a sterile Petri dish (9 cm in diameter) to make a medium-filled plate. After the medium was solidified, an agar block (with a diameter of 0.5 cm) containing pathogenic fungi to be tested was placed in each medium plane, and the fungi-carrying side of the agar block containing fungi was placed onto the surface of the medium. Every concentration repeated three times, and double-distilled water (containing 0.1% Tween 80 and 2% DMSO) was used as the negative control. Then, pathogenic fungi were cultured at 25 °C. The diameter of colony growth was measured by the cross method after 2–7 days, and the inhibition rate was calculated. Then, the toxicity regression equation and EC50 value of the extract were obtained according to the probit analysis method for toxicity.
CK: Control colony growth diameter; PT: Colony growth diameter of chemical treatment.
4.4. Bioassay of Extracts of CX In Vivo
4.4.1. Control Effect on Blumeria graminis
The paste extract was dissolved in DMSO, and then prepared into 250 mg/L, 500 mg/L, 1000 mg/L, 2000 mg/L and 4000 mg/L solutions with double-distilled water (contain 0.1% Tween 80). Wheat seeds were planted in a glass tube (4 cm in diameter), and the tube was sealed with parafilm. Seeds were cultured to the three-leaf stage at 20 ± 1 °C and 60–70% humidity with a 16:8 h light/dark photoperiod [24]. Then, wheat leaves were sprayed with 250 mg/L, 500 mg/L and 1000 mg/L extract solutions with three replications at each concentration. Leaves were also sprayed with 100 mg/L prothioconazole as a positive control and with double-distilled water (contain 2% DMSO and 0.1% Tween 80) as a negative control. Each treatment was repeated three times. Fresh spores of Bgt were inoculated onto the plants by shaking over the foliage of the wheat seedlings [25]. The protective activity was determined by spray application of the extract solution first followed by inoculation with the pathogenic fungi 24 h later, and the curative activity was determined by inoculation with the pathogenic fungi first, followed by spray application of the extract solution 24 h later. The methods for detecting the protective and curative activities of the subsequent experiment were the same. Wheat was further cultured after treatment, and the disease incidence was investigated 7, 9 and 10 days after treatment. A disease classification grading scale was established, and the disease index and the relative disease control efficiency (RDCE) were calculated according to Standards of China “GB/T 17980-22-2000” [26].
4.4.2. Control Effect on Fusarium graminearum
Wheat seeds were planted in pots (15 cm in diameter) and cultured to the blooming stage at 26 ± 2 °C and 65–75% humidity in the greenhouse. Then, wheat leaves were sprayed with 1000 mg/L and 4000 mg/L extract solutions with three replications at each concentration. Leaves were also sprayed with 100 mg/L tebuconazole as a positive control and with double-distilled water (contain 2% DMSO and 0.1% Tween 80) as a negative control. A spore suspension of Fusarium graminearum (10 μL) was injected wheat panicles with a microinjector. Spores were observed and counted on a hemocytometer under an optical microscope (4 × 10). The method for adjusting the spore suspension in the later experiment was the same. It is advisable to adjust the spore concentration to 80–100 spores per field. Wheat was further cultured after treatment, and the disease incidence was investigated 7, 9 and 10 days after treatment. A disease classification grading scale was established, and the disease index and the RDCE were calculated referring to Standards of China “NY/T 1464.15_2007” [27].
4.4.3. Control Effect on Botrytis cinerea
Fresh strawberry fruits of uniform size were selected, soaked in 75% ethanol for 2 min, washed with double-distilled water and then dried. Then, fruits were sprayed with 500 mg/L, 1000 mg/L and 2000 mg/L extract solutions with three replications at each concentration. Leaves were also sprayed with 100 mg/L pyraclostrobin as a positive control and with double-distilled water (contain 2% DMSO and 0.1% Tween 80) as a negative control. The equator of the strawberry was pricked with a sterile needle of a 1 ml injector, a 2 mm wound was formed, and then a suspension of Botrytis cinerea was evenly spread on the fruit surface. The fruit was placed in a culture plate and stored at 25 °C and 95% humidity after treatment. The disease incidence was investigated 3, 5 and 7 days after treatment. The disease was divided into five grades according to the percentage of Botrytis cinerea diseased area to fruit surface area, and then the disease index and the RDCE were calculated [28].
4.4.4. Control Effect on Colletotrichum gloeosporioides
Fruits of Jincheng orange with consistent appearance and no mechanical damage were selected, soaked in 75% ethanol for 2 min, washed with double-distilled water and then dried. Then, the fruits were sprayed with 1000 mg/L, 2000 mg/L, and 4000 mg/L extract solutions. Three biological replicates were performed at each concentration with 10 fruits per treatment. Leaves were also sprayed with 86 mg/L pyraclostrobin as a positive control and with double-distilled water (containing 2% DMSO and 0.1% Tween 80) as a negative control. Colletotrichum gloeosporioides was inoculated by needle puncture. The depth of the pinhole was 2 mm, and a spore suspension (10 μL) was dropped at the pinhole [29,30]. The fruits were cultured at 28 °C and 95% relative humidity after treatment. The diameters of the lesions were measured by the cross method, and the inhibition rate was calculated 7 days after treatment using the following formula:
CK: Control colony growth diameter; PT: Colony growth diameter of chemical treatment.
4.4.5. Data Processing and Analysis
SPSS.23 software was used for regression analysis, variance analysis and multiple comparison of the test data.
4.5. Isolation, Purification and Structure Identification of Active Compounds from CX
4.5.1. Macroporous Resin Separation
The D101 macroporous resin was soaked in absolute ethanol for 24 h. After the resin was fully swollen, it was loaded into a chromatography column and rinsed repeatedly with absolute ethanol until the supernatant was free of white turbidity. Then, the resin was rinsed with distilled water until there was no ethanol. After 10 L of the treated CX extract was statically adsorbed with 2 kg D101 macroporous resin for 48 h, the D101 macroporous resin was loaded into the chromatographic column, first eluted with 3 times the column volume of distilled water, and the eluent was discarded; then, 5 Elution with 70% ethanol was used, and the filtrate was collected and concentrated under reduced pressure to obtain the CX extract.
4.5.2. Petroleum Ether Extraction and Silica Gel Column Chromatography
The CX extract was evenly dispersed with water, and the aqueous solution of the CX extract was extracted four times with an equal volume of petroleum ether, and the organic phase was concentrated under reduced pressure at 45 °C to obtain the petroleum ether extract. The petroleum ether extract was separated by column chromatography using normal phase silica gel, and the elution solvent was successively eluted with different mixtures of chloroform and methanol (1:0, 3:1 and 0:1, v/v), and the fractions were collected. The extract was separated into 7 fractions (Fr. D1-D7) by silica gel column chromatography. Further separation is performed by silica gel column chromatography: using petroleum ether:ethyl acetate (5:1-2:1) as eluent, elute Fr. D2 to obtain fraction Fr. D2-1; using petroleum ether:acetone (5:1-2:1) as the eluent, elute Fr. D5 to obtain fraction Fr. D5-1. The antimicrobial active fractions were traced and selected by the spore germination method using Botrytis cinerea as an indicator pathogen.
4.5.3. HPLC Preparation
The components (FR. D2-1, FR. D5-1) are fitted with Phenomenex C18 columns (250 × 10 mm, Phenomenex; Aschaffenburg, Germany) and UV detector. The fr. D2-1 component was eluted with 30% (v/v) acetonitrile containing 0.1% formic acid to obtain compound numbers CQC1 (20 mg), CQC2 (20 mg) and CQC3 (5 mg). The fr. D5-1 component was eluted with 35% (v/v) acetonitrile containing 0.1% formic acid to obtain compound codes CQC4 (20 mg) and CQC5 (5 mg). The antimicrobial active compound was traced and selected by spore germination method with Botrytis cinerea as indicator pathogen.
4.5.4. Compound Structure Identification
The purity was determined by HPLC. The mass spectrum, 1H-NMR (nuclear magnetic resonance) and 13C-NMR spectra of the compounds with higher purity were determined (the 1H-NMR spectra were 400 MHz, the 13C-NMR spectra were 100 MHz and the solvent was CDCl3). The chemical structure of the compound was identified according to spectroscopic data.
4.5.5. Determination of Compound Activity
The MIC against the pathogen spores was determined by the microtiter method [26]. Dimethyl sulphoxide was used to prepare the pure compound into 20.00 mg/L mother liquor, and the mother liquor was diluted into 1000 mg/L, 500 mg/L, 250 mg/L, 125 mg/L, 62.25 mg/L, 31.13 mg/L, 15.63 mg/L, 7.813 mg/L, 3.91 mg/L, and 1.95 mg/L aqueous solution with sterile water by double-dilution method. Add 5μL of compound solution of different concentrations to each single well of 96-well plate, add 45 μL of medium at 50 °C to each well, mix thoroughly, prepare the medium containing drug with the desired concentration, stand for 10 min, add 10 μL of spore suspension to each well, and add the same amount of dimethyl sulphoxide solution to the single well of 96-well plate as control. Each treatment was repeated 3 times. After incubation at 28 °C, for 48 h, the growth of mycelium in all pore plates was observed under microscope, and the concentration of no mycelium growth was the minimum inhibitory concentration.
Author Contributions
Conceptualization, G.Q. and H.C.; methodology, H.C. and G.Q.; software, G.Q.; formal analysis, G.Q.; data curation, H.C., G.Q. and Y.Z.; writing—original draft preparation, Y.Z. and Y.B.; writing—review and editing, Y.Z. and C.Y.; supervision, G.Y., M.Z., X.C., X.Q. and L.L.; project administration, C.Y. and H.C.; funding acquisition, C.Y. and H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Biotechnology and Medicine of the Major Scientific and Technological Project of Sichuan Province under Grant No. 2017NZDZX0003 and Sichuan Tianfu New District Rural Revitalization Research Institute "the open competition mechanism to select the best candidates" project (The first batch) No.XZY1-13.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The data generated and analyzed during the study are available in this article.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the assistance of Yuqing Ma with spraying drugs in farmlands and field investigation at Sichuan Agriculture University (SICAU).
Conflicts of Interest
All authors declare that there are no conflict of interest.
References
- Li, X.F.; Xu, Z. The research progress on botanical fungicides. J. South. Agric. 2018, 12, 40–42,45. [Google Scholar]
- Gao, Y.C. Study on the Antibacterial Effect of Polygonum orientale Essential Oils against Three Plant Pathogens. Ph.D. Thesis, Shanxi University, Taiyuan, China, 2020. [Google Scholar]
- Ni, L.; Shi, W.Y.; Yan, J.; Yan, J.; Wu, L.H. Content of Total Flavonoids and its antifungal effect Elsholtzia splendens. Bull. Sci. Technol. 2010, 26, 546–549. [Google Scholar]
- Wang, Y.; Sun, Y.; Zhou, M.X.; Wang, M.M.; Han, L.R.; Feng, J.T.; Zhang, X. Antiungal activities screening of 14 small molecular organic acids. J. Hebei Agric. Univ. 2018, 200, 26–32. [Google Scholar]
- Yang, T.; Shi, H.; Li, C.L.; Li, J.H.; Wang, L.H.; Li, G.Y.; Zhang, Z.H. Antifungal activitiy of 13 terpenoids against Colletotrichum gloeosporioides and Alternaria sp. Plant Prot. 2017, 43, 192–195. [Google Scholar]
- Zhang, X.J.; Zhang, Y.L.; Zuo, D.D. Research Progress on chemical constituents and pharmacological effects of Ligusticum chuanxiong. Hort. Inf. Tradit. Chin. Med. 2020, 37, 128–133. [Google Scholar]
- Han, W. Research Progress on chemical constituents and pharmacological effects of Ligusticum chuanxiong. Chin. Mod. Tradit. Med. 2017, 19, 1341–1349. [Google Scholar]
- Zhang, Y.L.; Yin, C.P. Fungistasis of extracts from 18 kinds of Chinese Herbs against plant pathogens. J. Jiangxi Plant Prot. 2010, 33, 75–78. [Google Scholar]
- Lee, I.S.; Huong, D.T.L.; Lee, M.S.; Kim, J.W.; Na, D.S.; Kim, Y.H. NFAT transcription Factor Inhibitory Cnostituents from Cnidium officinale. Nat. Prod. Sci. 2002, 8, 94–96. [Google Scholar]
- Naito, T.; Katsuhara, T.; Niitsu, K.; Ikeya, Y.; Okada, M.; Mitsuhashi, H. Two Phthalides from Ligusticum. chuangxiong. Phytochemistry 1992, 31, 639–642. [Google Scholar] [CrossRef]
- Zhang, J.L.; Yu, D.Q.; Zhou, Z.H. Studies on the Chemical Constituents of the Root and Rhizoma of Ligusticum jeholense. J. Pharm. 1996, 31, 33–37. [Google Scholar]
- Awat, W.; Florian, T.; Schevenels, Y.; Ratsami, L. Chemical Constituents and Their Biolocal Activities from The Roots of Diospyros filipendula. Nat. Prod. Res. 2019, 3, 46–47. [Google Scholar]
- Li, Q.; Wu, X.K. New Progress in Research on Chemical constituents and Pharmacological Action of Ligusticum. chuanxiong Hort. Chem. Eng. 2020, 34, 62–64, +44. [Google Scholar]
- Yao, S.Y.; Zhang, K.X.; Ma, Q.; Jin, Y.S. Preclinical research progress of Ligustilide. Pharm. Care Res. 2019, 19, 106–110. [Google Scholar] [CrossRef]
- Xie, J.; Huang, G.; Jin, C.Z. Application of chuanxiong and other Chinese herbal medicine extracts in Restraining Citrus pathogens and Citrus fresh-keeping. Chin. Agron. Bull. 2014, 30, 226–231. [Google Scholar]
- Cheng, X.M.; Peng, Y.J.; Yang, Y.; Xu, H.; Bu, F.W. Identification of the pathogen causing fruit penicilliosis on kiwifruit and the inhibition effect of Chinese herb extracts on the pathogen. Plant Prot. 2018, 44, 186–189. [Google Scholar]
- Tang, J.; Feng, S.; Hou, R.T.; Yang, Z.L. Study on the bacteriostatic and insecticidal effect of Ligusticum chuanxiong extract. Li Shizhen Med. Mater. Med. Res. 2008, 2437–2439. [Google Scholar]
- Zhang, L.J.; Liu, J.Y.; Yao, K.; Wang, X.Y.; Gao, S.; Liu, J.Y.; Sun, L.P. Research progress on pharmacological effects of lactone compounds of Ligusticum. chuanxiong. Chin. J. Pharm. 2015, 50, 1081–1084. [Google Scholar]
- Yang, Y.; Dou, G.J.; Yu, Z.Y.; He, H.; Wang, C.Q.; Li, L.; Zhou, J.; Liu, D.J.; Shi, J.Y.; Li, G.R. Z-Ligustilide Exerted Hormetic Effect on Growth and Detoxification Enzymes of Spodoptera Litura Larvae. Evid.-Based Complementary Altern. Med. 2018, 2018, 7104513. [Google Scholar]
- Chen, H.W.; Wu, J.W.; Zhuang, Y.Q.; Yang, H.F.; Wu, Q.Y.; Xu, C.; Miao, K.; Yao, K.B. Control efficacy of different fungicides on Fusarium head blight and deoxynivaleno in wheat grain L. Plant Prot. 2021, 47, 307–317. [Google Scholar]
- Zhang, T.; Wang, G.Q. Research advances in extraction of antimicrobial activity components from the plant materials. Guangdong Agric. Sci. 2011, 38, 59–62. [Google Scholar]
- Chen, X.L.; Pan, R.Q.; Gai, Y.P.; Yi, S.; Xiao, X.; Jiang, Z.D.; Xu, H.H. Antifungal activities in methanol extracts of 31 plant species against Phytophthora litchii and Colletotrichum gloeosporioides. Guangdong Agric. Sci. 2012, 39, 1–3. [Google Scholar]
- Wang, M.H.; Shen, H.M.; Zhou, X.M. Plant Chemical Protection Experiment; Peking University Press: Beijing, China, 2014; pp. 113–115. [Google Scholar]
- Wang, J.F.; Yan, X.J.; Yang, D.B.; Yan, H.Z. Toxcity Analysis of cyclopropanol against wheat powdery mildew as Alternative Triadimefon. Crop Mag. 2011, 28–31. [Google Scholar]
- Xie, D.; Cai, X.; Yang, C.; Xie, L.; Qin, G.; Zhang, M.; Huang, Y.; Gong, G.; Chang, X.; Chen, H. Studies on the control effect of Bacillus subtilis on wheat powdery mildew. Pest Manag. Sci. 2021, 77, 4375–4382. [Google Scholar] [CrossRef]
- GB/T 17980-22-2000; Pesticide-Guidelines for the Field Eficacy Trials (1)-Fungicides Against Cereal Powdery Mildew. Standards Press of China: Beijing, China, 2000; pp. 90–93.
- NY/T 1464.15_2007; Guidelines on Efficacy of Pesticides Part 15: Fungicides Against Fusarium Head Blight of Wheat. Standards Press of China: Beijing, China, 2007; pp. 1–4.
- Yang, X.N.; Wang, M.; Shen, R.P.; Liu, F. Microtiter Method to Test the Sensitivity of Botrytis cinerea to Fungicides. Chin. Agric. Sci. 2012, 45, 3075–3082. [Google Scholar]
- Zhang, S.; Liu, Y.C.; Yang, T.X. Toxicity Determination and Field Control Effects of Different Fungicides to Yam Anthracnose. Pesticides 2013, 52, 142–144. [Google Scholar]
- Gai, Z.X. Studies on the Infection Process and Main Physiological and Biochemical Changes of Colletotrichum gloeosporioides in Citrus; Chongqing Southwest University: Chongqing, China, 2016; pp. 12–13. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).