Electrospun Silk Fibroin/Polylactic-co-glycolic Acid/Black Phosphorus Nanosheets Nanofibrous Membrane with Photothermal Therapy Potential for Cancer
Abstract
:1. Introduction
2. Results and Discussions
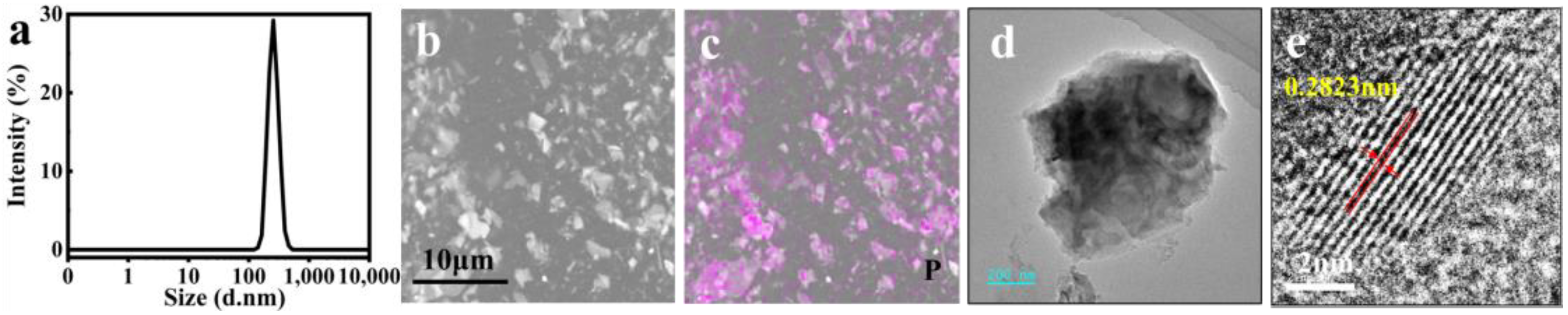
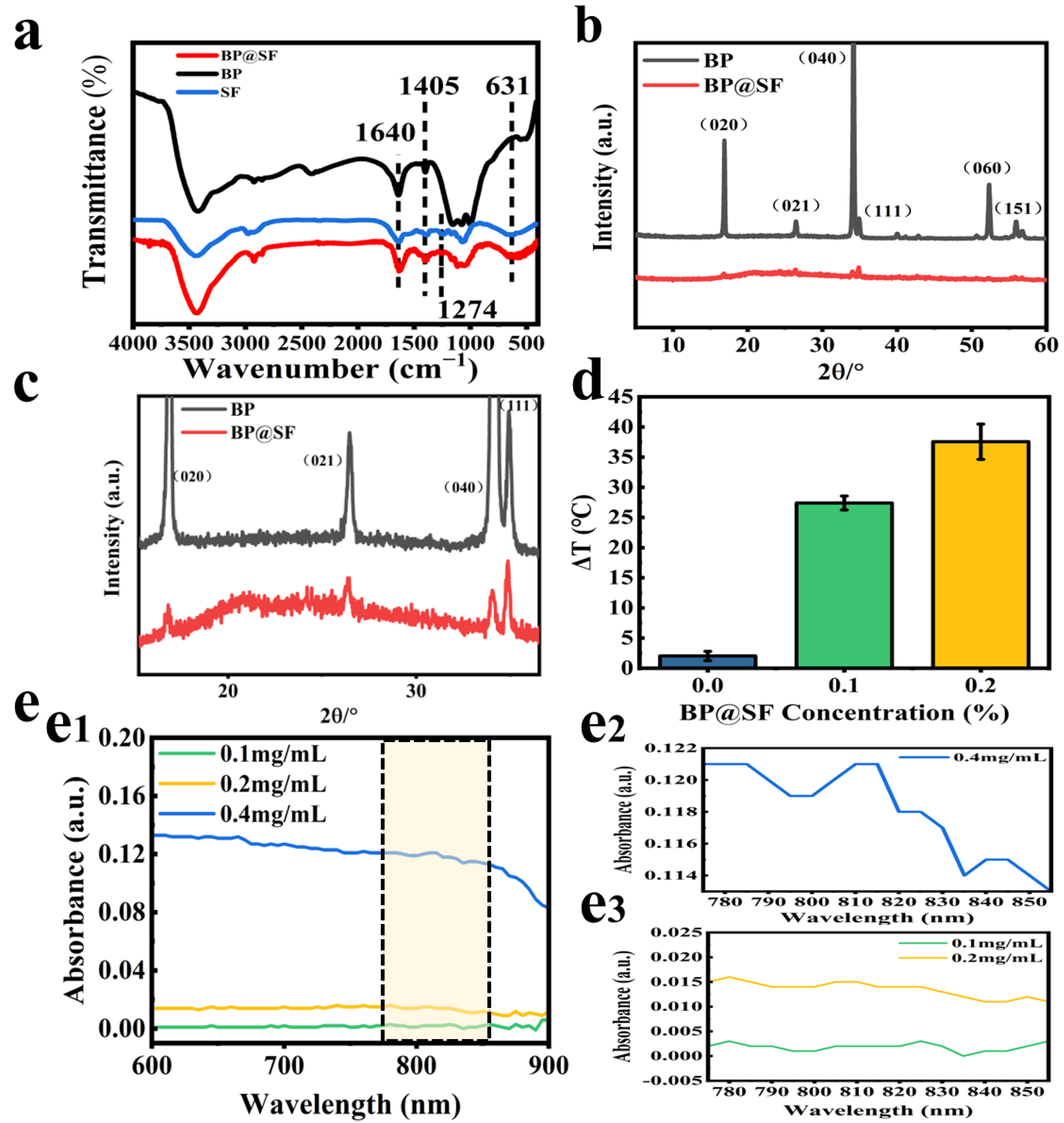
2.1. Preparation and Characterization of BP@SF Sheets
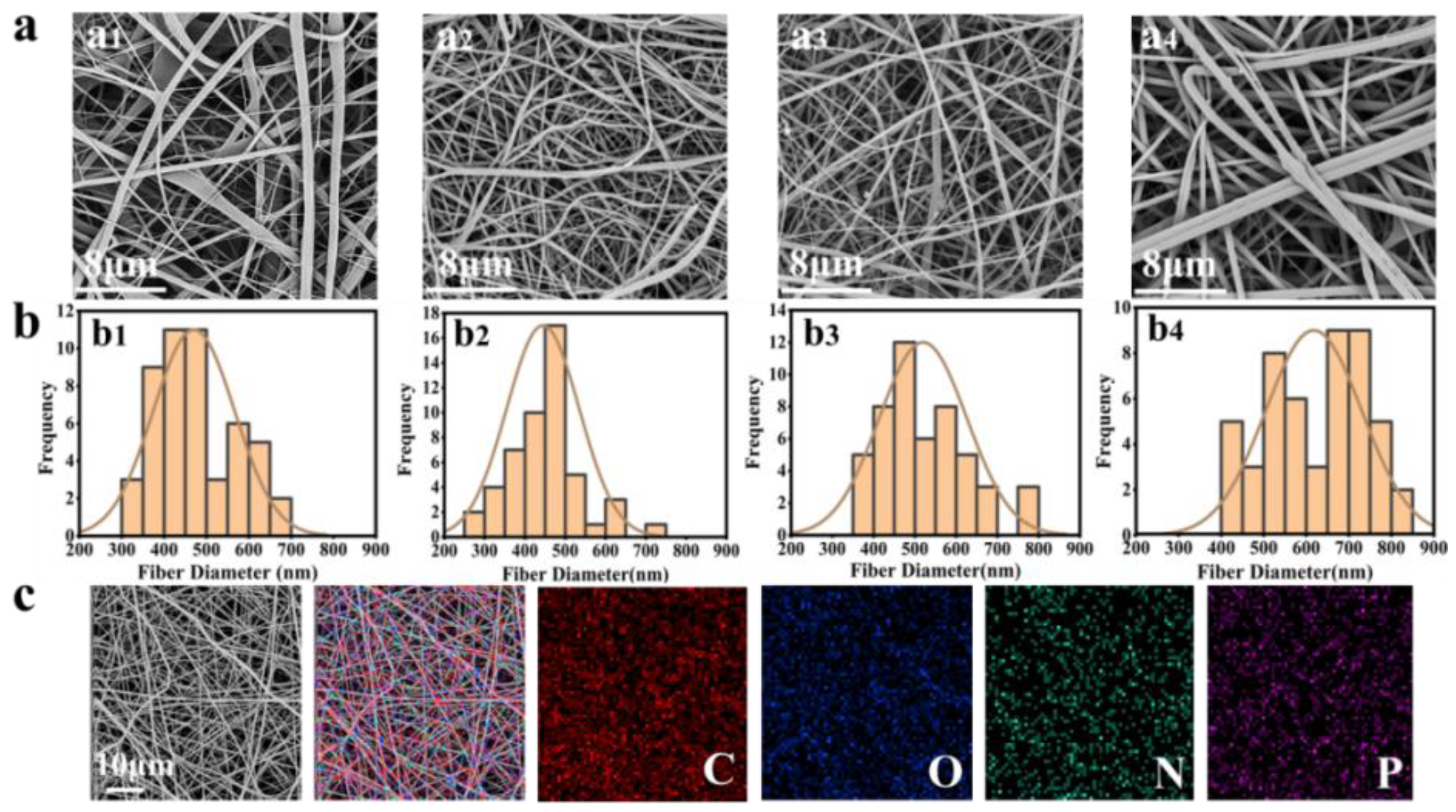
2.2. Preparation and Characterization of BP@SF Electrospun Membranes
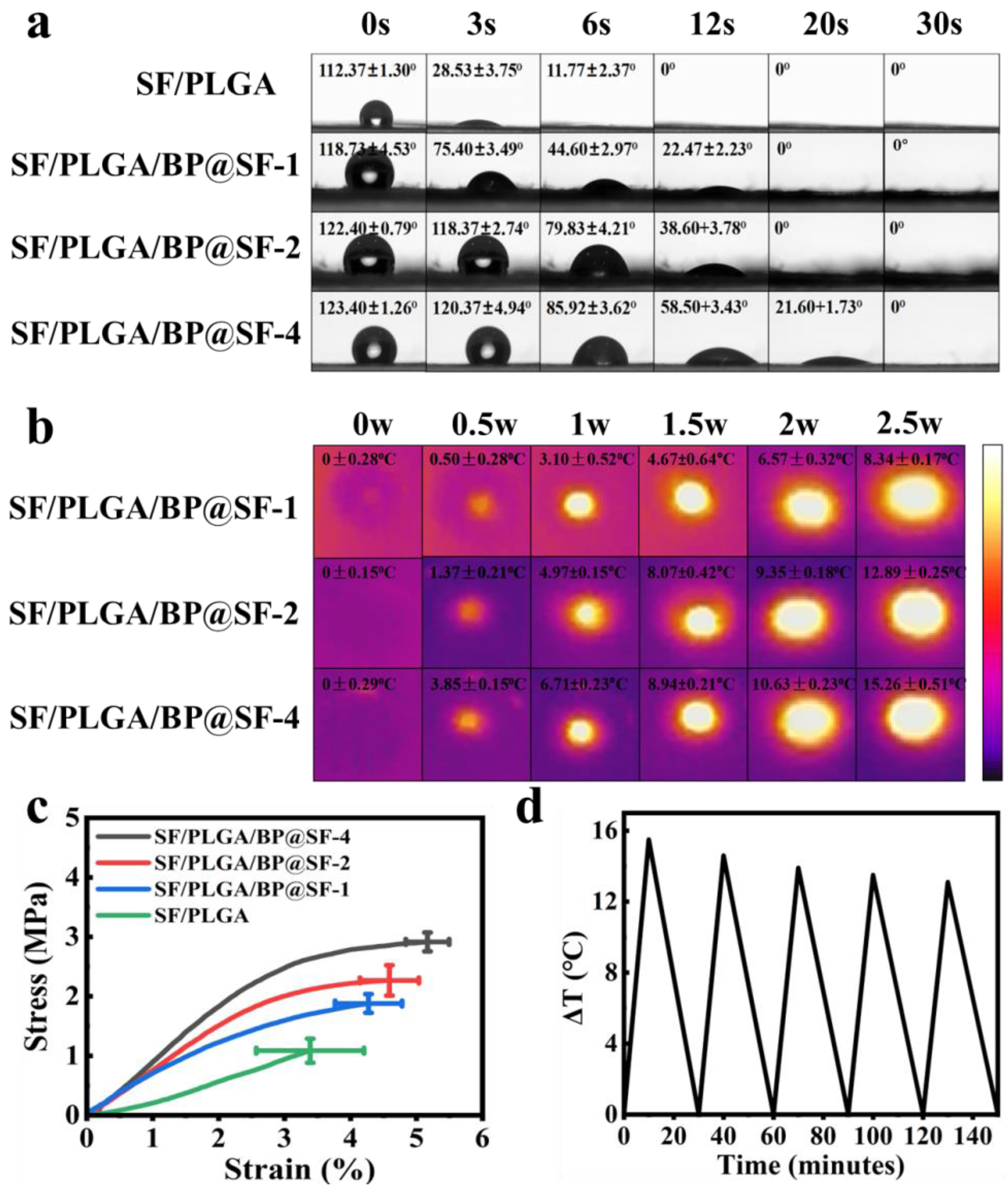
2.3. Hydrophilic and Hydrophobic Properties
2.4. Tensile Properties
2.5. Photothermal Effects and Stability Tests
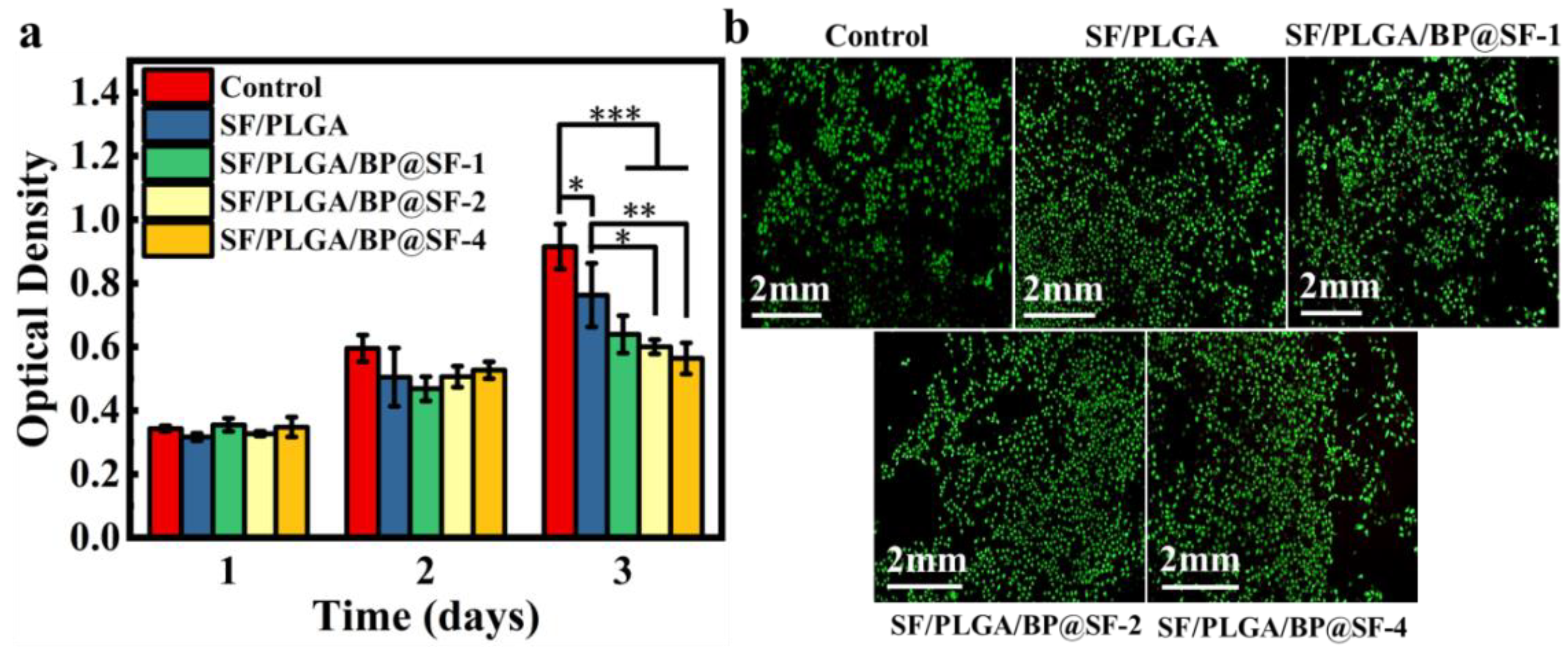
2.6. Biocompatibility
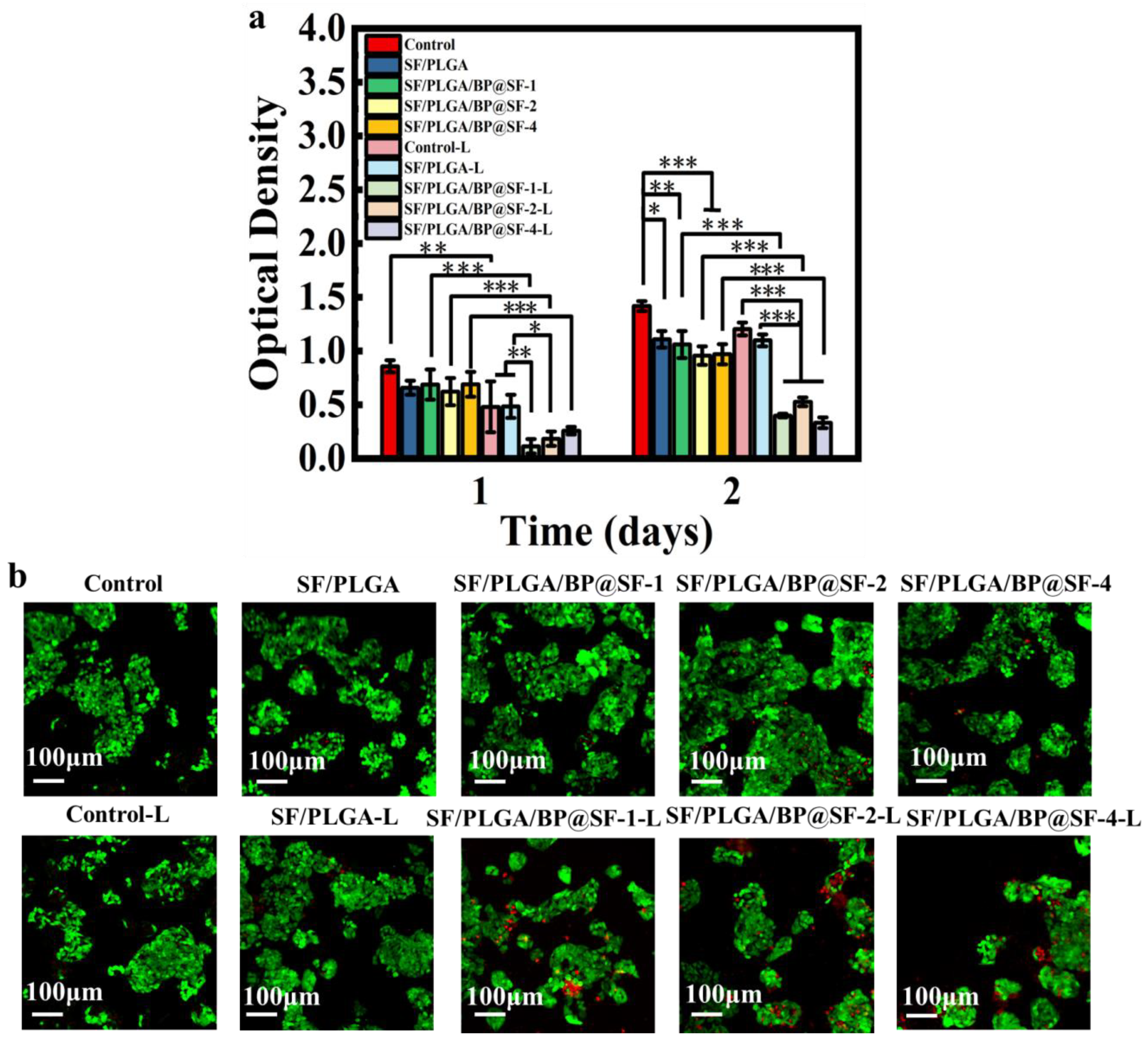
2.7. In Vitro Anti-Tumor Properties
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of BP Nanosheets
4.2.1. Preparation of SF Aqueous Solutions
4.2.2. Preparation of BP@SF
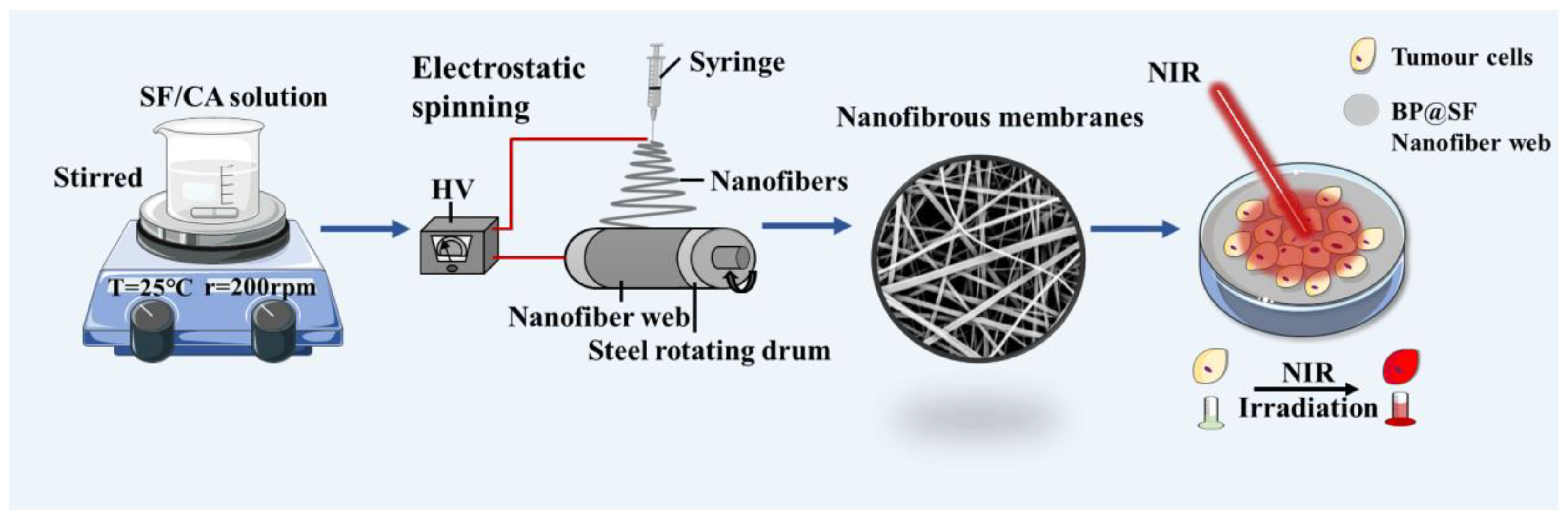
4.3. Preparation of SF/PLGA/BP@SF Electrospun Composite Membranes
4.4. Characterizations of BP@SF
4.5. Characterizations of SF/PLGA/BP@SF Electrospun Composite Membranes
4.6. Photothermal Effect Measurement
4.7. Cell Culture
4.8. Biocompatibility Test
4.9. In Vitro Anti-Tumor Performance Testing
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Ramadas, K.; Qiao, Y. Managing the changing burden of cancer in Asia. BMC Med. 2014, 12, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C. Cancer screening in the United States 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA A Cancer J. Clin. 2019, 69, 184–210. [Google Scholar] [CrossRef] [PubMed]
- Glazer, S.E.; Curley, S.A. The Ongoing History of Thermal Therapy for Cancer. Surg. Oncol. Clin. North Am. 2011, 20, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolba, M.F.; Santa-Maria, C.A.; Albini, A.; Chimusa, E.R.; Al-Ramadi, B.K.; Tolaney, S.M. Editorial: Immunotherapy as an Evolving Approach for the Treatment of Breast Cancer. Front. Oncol. 2021, 11, 752689. [Google Scholar] [CrossRef]
- Shahid, K.; Khalife, M.; Dabney, R.; Phan, A.T. Immunotherapy and targeted therapy—The new roadmap in cancer treatment. Ann. Transl. Med. 2019, 7, 595. [Google Scholar] [CrossRef]
- Gondhowiardjo, S.A.; Handoko; Jayalie, V.F.; Apriantoni, R.; Barata, A.R.; Senoaji, F.; Utami, I.J.; Maubere, F.; Nuryadi, E.; Giselvania, A. Tackling Resistance to Cancer Immunotherapy: What Do We Know? Molecules 2020, 25, 4096. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Yang, W.; Lyu, Q.; Zhao, J.; Cao, L.; Hao, Y.; Zhang, H. Recent advance in near-infrared/ultrasound-sensitive 2D-nanomaterials for cancer therapeutics. Sci. China Mater. 2020, 63, 2397–2428. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.; Zhong, M.; Xie, X. Coordination-Driven Hierarchical Assembly of Hybrid Nanostructures Based on 2D Materials. Small 2020, 16, 1902779. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Li, Z.; Zhu, S.; Zheng, Y.; Yeung, K.W.K.; Wu, S. Repeatable Photodynamic Therapy with Triggered Signaling Pathways of Fibroblast Cell Proliferation and Differentiation to Promote Bacteria-Accompanied Wound Healing. ACS Nano 2018, 12, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.; Zhao, Y.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef]
- Qin, L.; Ling, G.; Peng, F.; Zhang, F.; Jiang, S.; He, H.; Yang, D.; Zhang, P. Black phosphorus nanosheets and gemcitabine encapsulated thermo-sensitive hydrogel for synergistic photothermal-chemotherapy. J. Colloid Interface Sci. 2019, 556, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Z.; Zhu, C.; Cai, X.; Qu, L.; Du, D.; Lin, Y. Graphene-like Metal-Free 2D Nanosheets for Cancer Imaging and Theranostics. Trends Biotechnol. 2018, 36, 1145–1156. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Ma, L.; Mao, F.; Jiang, A.; Liu, D.; Wang, L.; Jia, Q.; Zhou, J. Artemisinin-Loaded Mesoporous Nanoplatform for pH-Responsive Radical Generation Synergistic Tumor Theranostics. ACS Appl. Mater. Interfaces 2018, 10, 6155–6167. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Jiang, D.; Duan, H.; Sun, Z.; Zhang, M.; Jin, H.; Guan, R.; Liu, Y.; Chen, M.; et al. Stabilizing black phosphorus nanosheets via edge-selective bonding of sacrificial C60 molecules. Nat. Commun. 2018, 9, 4177. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhang, H.; Lu, S.; Wang, Z.; Tang, S.; Shao, J.; Sun, Z.; Xie, H.; Wang, H.; Xue-Feng, Y.; et al. From Black Phosphorus to Phosphorene: Basic Solvent Exfoliation, Evolution of Raman Scattering, and Applications to Ultrafast Photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Ge, X.; Xia, Z.; Guo, S. Recent Advances on Black Phosphorus for Biomedicine and Biosensing. Adv. Funct. Mater. 2019, 29, 1900318. [Google Scholar] [CrossRef]
- Huang, X.; Wei, J.; Zhang, M.; Zhang, X.; Yin, X.; Lu, C.; Song, J.; Bai, S.; Yang, H. Water-Based Black Phosphorus Hybrid Nanosheets as a Moldable Platform for Wound Healing Applications. ACS Appl. Mater. Interfaces 2018, 10, 35495–35502. [Google Scholar] [CrossRef]
- Sutrisno, L.; Chen, H.; Chen, Y.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Composite scaffolds of black phosphorus nanosheets and gelatin with controlled pore structures for photothermal cancer therapy and adipose tissue engineering. Biomaterials 2021, 275, 120923. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wan, X.; Gu, Z.; Zeng, X.; Tang, J. Near infrared photothermal-responsive poly(vinyl alcohol)/black phosphorus composite hydrogels with excellent on-demand drug release capacity. J. Mater. Chem. B 2018, 6, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Dai, C.; Li, Y.; Yin, Y.; Gong, L.; Machuki, J.O.; Yang, Y.; Qiu, S.; Guo, K.; Gao, F. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials 2020, 239, 119851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kuang, G.; Zong, S.; Liu, S.; Xiao, H.; Chen, X.; Zhou, D.; Huang, Y. Sandwich-Like Fibers/Sponge Composite Combining Chemotherapy and Hemostasis for Efficient Postoperative Prevention of Tumor Recurrence and Metastasis. Adv. Mater. 2018, 30, 1803217. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, L.; Fan, Y.; Tian, L.; Luan, S.; Niu, S.; Ren, L.; Ming, W.; Zhao, J. Synergistic Photodynamic and Photothermal Antibacterial Nanocomposite Membrane Triggered by Single NIR Light Source. ACS Appl. Mater. Interfaces 2019, 11, 26581–26589. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Fiorica, C.; Giuffrè, M.; Calà, C.; Maida, C.M.; Giammona, G. A self-sterilizing fluorescent nanocomposite as versatile material with broad-spectrum antibiofilm features. Mater. Sci. Eng. C 2020, 117, 111308. [Google Scholar] [CrossRef]
- Wang, X.; Lv, F.; Li, T.; Han, Y.; Yi, Z.; Liu, M.; Chang, J.; Wu, C. Electrospun Micropatterned Nanocomposites Incorporated with Cu2S Nanoflowers for Skin Tumor Therapy and Wound Healing. ACS Nano 2017, 11, 11337–11349. [Google Scholar] [CrossRef]
- Lee, Y.B.; Song, S.; Shin, Y.; Jung, Y.; Kim, B.; Kang, M.; Kwon, I.K.; Hyon, S.; Lee, H.; Jung, S. Ternary nanofiber matrices composed of PCL/black phosphorus/collagen to enhance osteodifferentiation. J. Ind. Eng. Chem. 2019, 80, 802–810. [Google Scholar] [CrossRef]
- Depeigne, L.; Zdraveva, E. Electrospun Biomaterials’ Applications and Processing. J. Biomim. Biomater. Biomed. Eng. 2021, 49, 91–100. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Hou, Z.; Huang, S.; Liu, B.; He, F.; Luo, L.; Lin, J. Polyaniline electrospinning composite fibers for orthotopic photothermal treatment of tumors in vivo. New J. Chem. 2015, 39, 4987–4993. [Google Scholar] [CrossRef]
- Huang, X.; Wei, J.; Liu, T.; Zhang, X.; Bai, S.; Yang, H. Silk fibroin-assisted exfoliation and functionalization of transition metal dichalcogenide nanosheets for antibacterial wound dressings. Nanoscale 2017, 9, 17193–17198. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.M.; Kim, H.S.; Dupnock, T.L.; Hu, K.; Yingling, Y.G.; Tsukruk, V.V. Silk Fibroin—Substrate Interactions at Heterogeneous Nanocomposite Interfaces. Adv. Funct. Mater. 2016, 26, 6380–6392. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C. Multifunctional surface modification of silk fabric via graphene oxide repeatedly coating and chemical reduction method. Appl. Surf. Sci. 2017, 405, 380–388. [Google Scholar] [CrossRef]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Y.; Zhou, Y.; Wu, J.; Wang, X.; Qu, Y.; Wang, Y.; Zhang, Y.; Yu, Q. Photothermally Activated Electrospun Nanofiber Mats for High-Efficiency Surface-Mediated Gene Transfection. ACS Appl. Mater. Interfaces 2020, 12, 7905–7914. [Google Scholar] [CrossRef]
- Ma, K.; Liao, C.; Huang, L.; Liang, R.; Zhao, J.; Zheng, L.; Su, W. Electrospun PCL/MoS2 Nanofiber Membranes Combined with NIR-Triggered Photothermal Therapy to Accelerate Bone Regeneration. Small 2021, 17, 2104747. [Google Scholar] [CrossRef]
- Rezk, A.I.; Rajan Unnithan, A.; Hee Park, C.; Sang Kim, C. Rational design of bone extracellular matrix mimicking tri-layered composite nanofibers for bone tissue regeneration. Chem. Eng. J. 2018, 350, 812–823. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, X.; He, C.; Wang, H. Intermolecular interactions in electrospun collagen-chitosan complex nanofibers. Carbohydr. Polym. 2008, 72, 410–418. [Google Scholar] [CrossRef]
- Shin, Y.; Yang, R.; Shi, Y.; Li, X.; Fu, Q.; Lu, J.; Ye, J.; Wang, K.; Ma, S.; Zheng, X. Light-sensitive Albino Tea Plants and Their Characterization. HortScience 2018, 53, 144–147. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 79, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, R.S.; Demarquette, N.R. Surface modification to control the water wettability of electrospun mats. Int. Mater. Rev. 2019, 64, 249–287. [Google Scholar] [CrossRef]
- Huang, Y.; Qiao, J.; He, K.; Bliznakov, S.; Sutter, E.; Chen, X.; Luo, D.; Meng, F.; Su, D.; Decker, J. Interaction of Black Phosphorus with Oxygen and Water. Chem. Mater. 2016, 28, 8330–8339. [Google Scholar] [CrossRef] [Green Version]
- Nauman, S.; Lubineau, G.; Alharbi, H.F. Post Processing Strategies for the Enhancement of Mechanical Properties of ENMs (Electrospun Nanofibrous Membranes): A Review. Membranes 2021, 11, 39. [Google Scholar] [CrossRef]
- Tian, D.; He, J. Control of Macromolecule Chains Structure in a Nanofiber. Polymers 2020, 12, 2305. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Wang, J.; Sun, M. Two-dimensional black phosphorus: Physical properties and applications. Mater. Today Phys. 2019, 8, 92–111. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, D.; Liu, C.; Zhang, Q.; Tang, L.; Lu, Y.; Xiao, H. Biodegradable Polymer with Effective Near-Infrared-II Absorption as a Photothermal Agent for Deep Tumor Therapy. Adv. Mater. 2022, 34, 2105976. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, 1902333. [Google Scholar] [CrossRef]
- Fu, W.; Zhou, W.; Chu, P.K.; Yu, X. Inherent Chemotherapeutic Anti-Cancer Effects of Low-Dimensional Nanomaterials. Chem. Eur. J. 2019, 25, 10995–11006. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L.; Cai, Z.; Chen, L.; Zhu, H. Recent progress of black phosphorus and its emerging multifunction applications in biomedicine. J. Phys. Mater. 2021, 4, 042004. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Li, Y.; Hou, Y.; Fei, X.; Su, C.; Wang, S.; Zhuang, Z.; Guo, Z. Facile synthesis of black phosphorus-Au nanocomposites for enhanced photothermal cancer therapy and surface-enhanced Raman scattering analysis. Biomater. Sci. 2017, 5, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, Z.; Xie, H.; Sun, Z.; Wang, B.; Huang, H.; Han, G.; Wang, H.; Chu, P.K.; Yu, X. Different-sized black phosphorus nanosheets with good cytocompatibility and high photothermal performance. RSC Adv. 2017, 7, 14618–14624. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, K.; Li, X.; Xu, Q.; Weng, J.; Xu, J. Electron Matters: Recent Advances in Passivation and Applications of Black Phosphorus. Adv. Mater. 2021, 33, 2005924. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Tang, R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787. [Google Scholar] [CrossRef]
- Liu, H.; Mei, Y.; Zhao, Q.; Zhang, A.; Tang, L.; Gao, H.; Wang, W. Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy. Pharmaceutics 2021, 13, 1344. [Google Scholar] [CrossRef]
- Liu, P.; Dai, M.; L i, M.; Song, H.; Gong, J.; Yin, Y.; Wang, J. Research progress of sericin and its composite materials in cartilage and bone tissue engineering. J. Silk 2021, 58, 1–7. [Google Scholar]
- Feng, P.; Dong, Z.; Zhang, Y.; Cheng, L.; Tang, L.; Gui, Y. Study on extraction of silk fibroin fiber. J. Silk 2022, 59, 9–15. [Google Scholar]
| Sample | Atomic Conc. | Weight Conc. |
|---|---|---|
| SF/PLGA | 0.06% | 0.13% |
| SF/PLGA/BP@SF-1 | 0.06% | 0.14% |
| SF/PLGA/BP@SF-2 | 0.08% | 0.18% |
| SF/PLGA/BP@SF-4 | 0.11% | 0.25% |
| Element Number | Element Symbol | Element Name | Atomic Conc. | Weight Conc. |
|---|---|---|---|---|
| 6 | C | Carbon | 43.45% | 37.82% |
| 8 | O | Oxygen | 32.02% | 37.13% |
| 7 | N | Nitrogen | 24.42% | 24.79% |
| 15 | P | Phosphorus | 0.11% | 0.25% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhou, J.; Wu, H.; Dai, F.; Li, J.; Li, Z. Electrospun Silk Fibroin/Polylactic-co-glycolic Acid/Black Phosphorus Nanosheets Nanofibrous Membrane with Photothermal Therapy Potential for Cancer. Molecules 2022, 27, 4563. https://doi.org/10.3390/molecules27144563
Li X, Zhou J, Wu H, Dai F, Li J, Li Z. Electrospun Silk Fibroin/Polylactic-co-glycolic Acid/Black Phosphorus Nanosheets Nanofibrous Membrane with Photothermal Therapy Potential for Cancer. Molecules. 2022; 27(14):4563. https://doi.org/10.3390/molecules27144563
Chicago/Turabian StyleLi, Xia, Jiale Zhou, Haiyan Wu, Fangyin Dai, Jiashen Li, and Zhi Li. 2022. "Electrospun Silk Fibroin/Polylactic-co-glycolic Acid/Black Phosphorus Nanosheets Nanofibrous Membrane with Photothermal Therapy Potential for Cancer" Molecules 27, no. 14: 4563. https://doi.org/10.3390/molecules27144563
APA StyleLi, X., Zhou, J., Wu, H., Dai, F., Li, J., & Li, Z. (2022). Electrospun Silk Fibroin/Polylactic-co-glycolic Acid/Black Phosphorus Nanosheets Nanofibrous Membrane with Photothermal Therapy Potential for Cancer. Molecules, 27(14), 4563. https://doi.org/10.3390/molecules27144563







