Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro
Abstract
1. Introduction
2. Results
2.1. Optimization Analysis Using the BBD Method
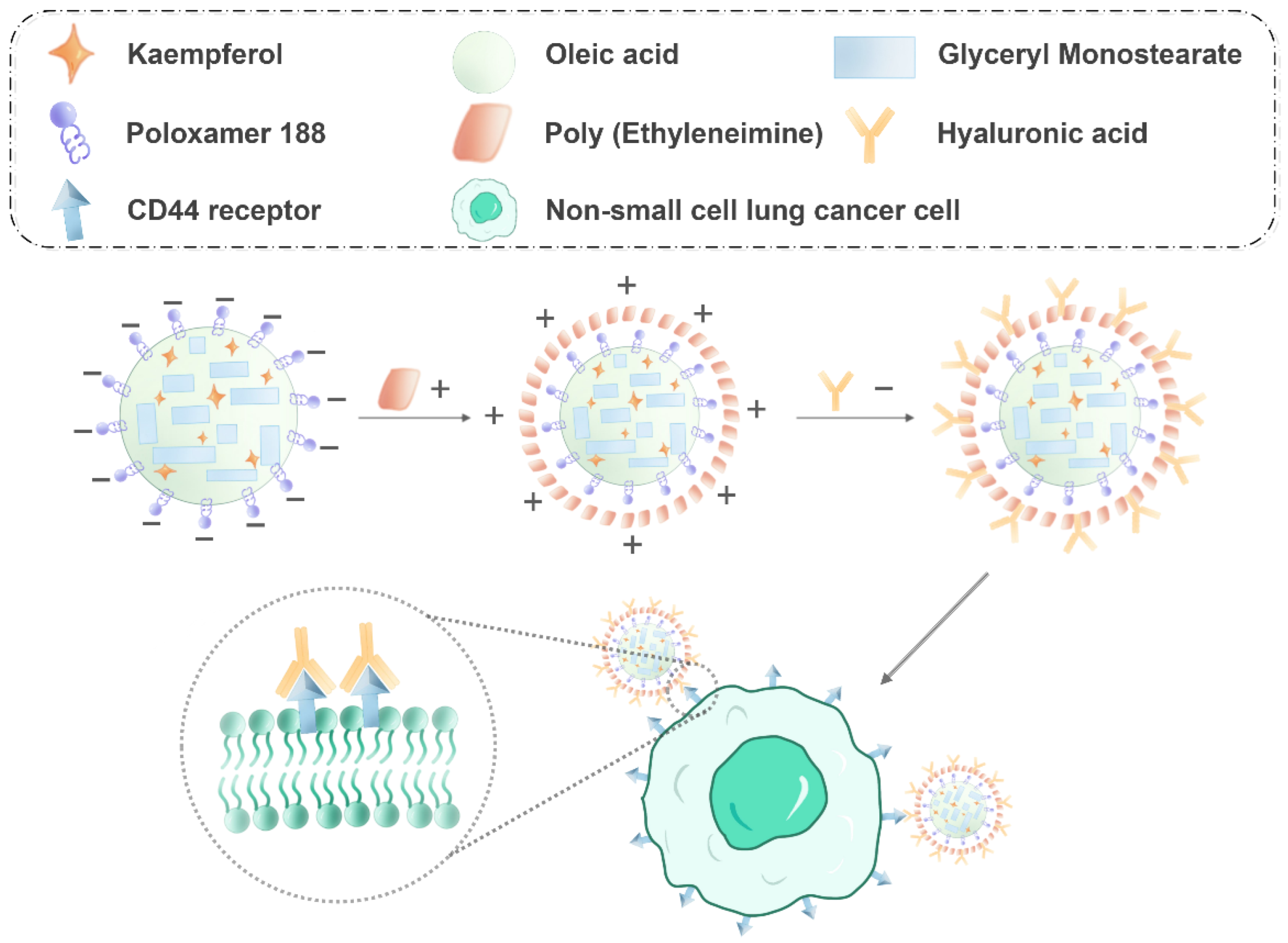
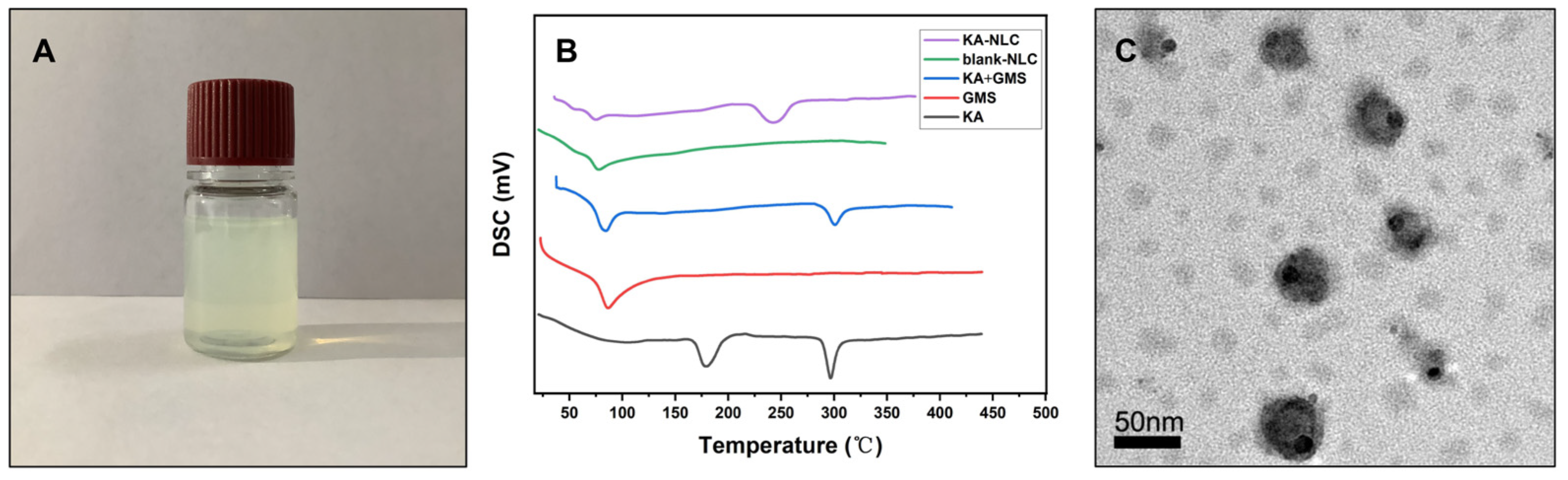
2.2. Characterization of KA-NLC
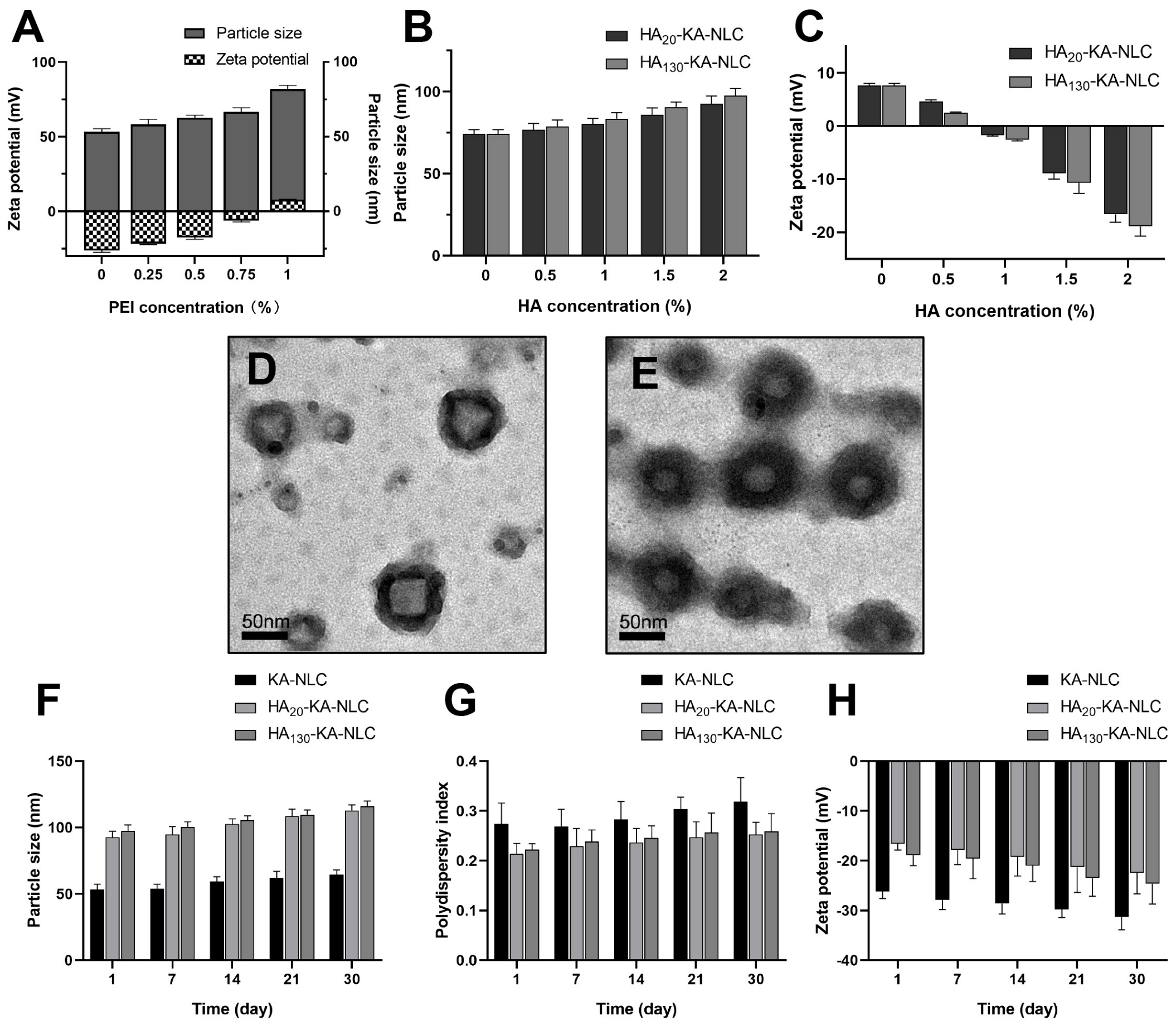
2.3. Characterization of HA-KA-NLC
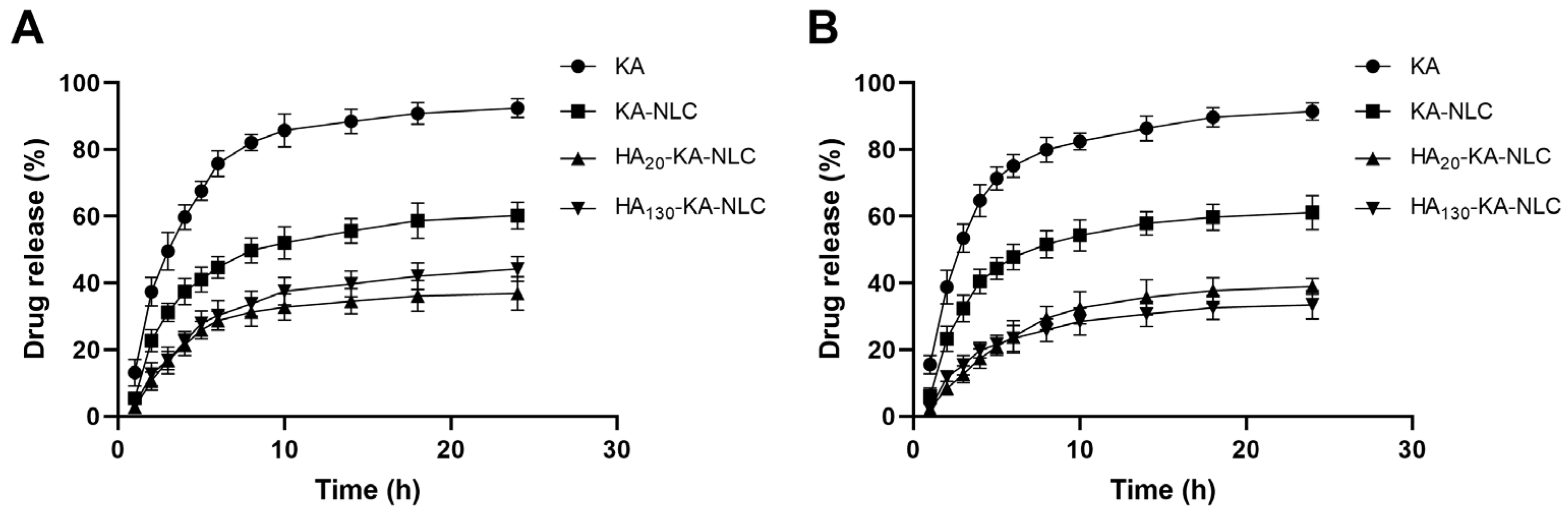
2.4. In Vitro Drug Release Study
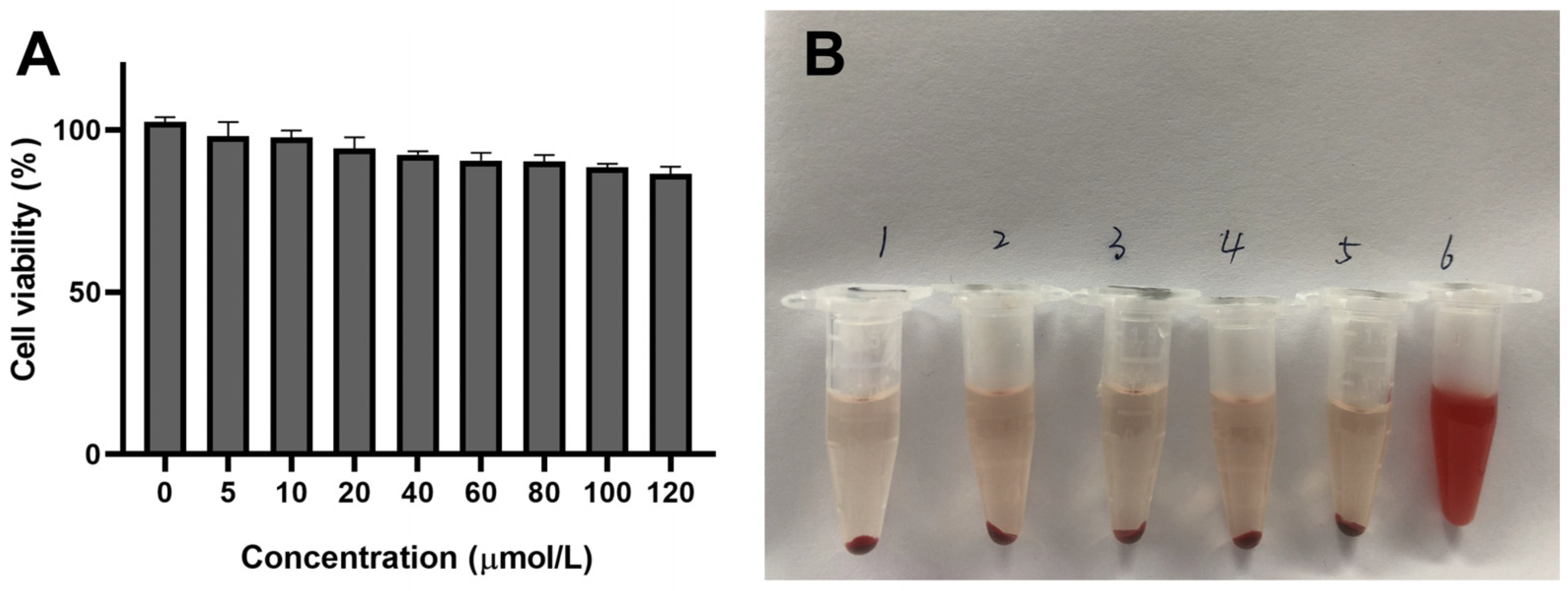
2.5. Safety Evaluation of Preparations In Vitro
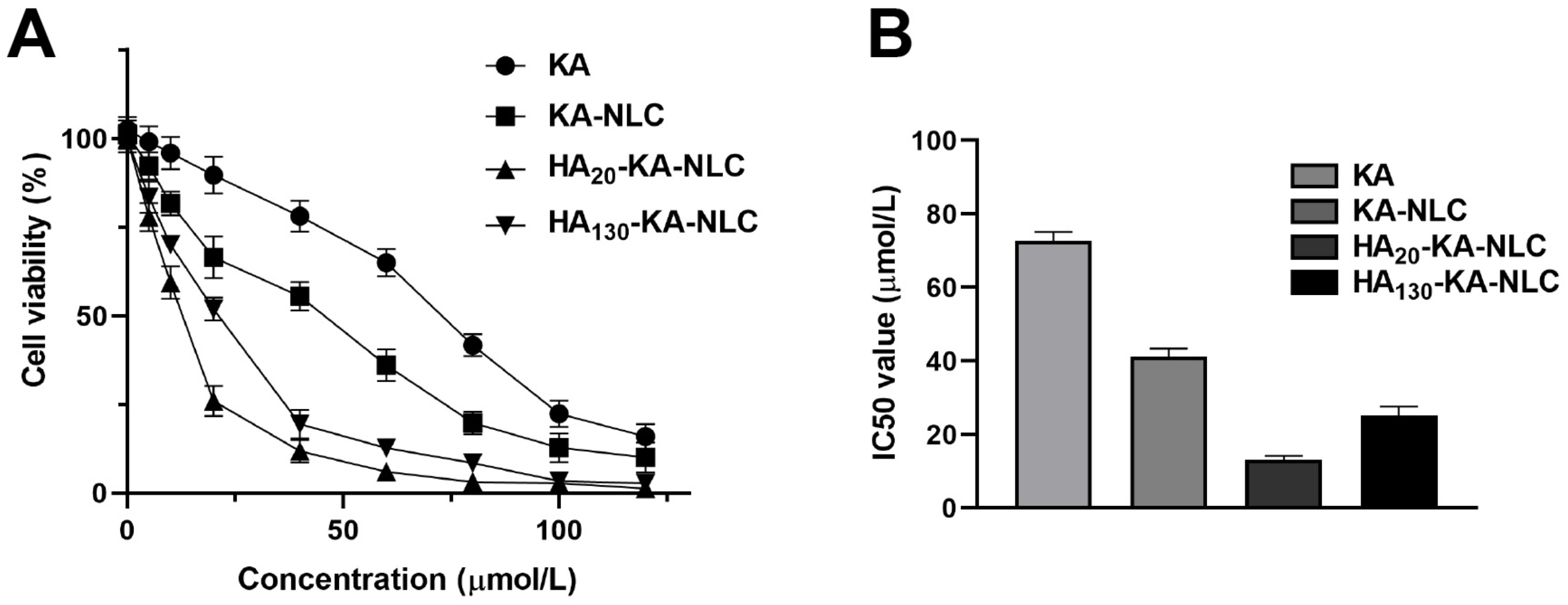
2.6. Evaluation of Cell Viability by CCK-8 Assay
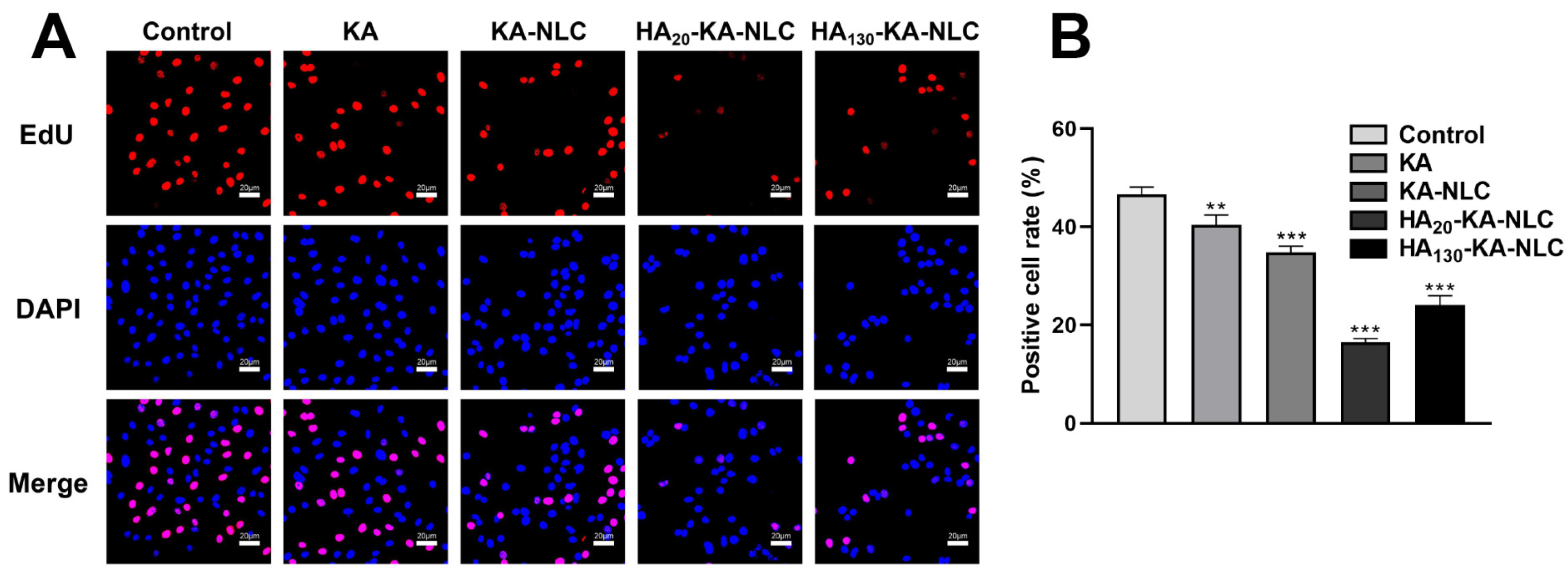
2.7. EdU Staining Assay for the Evaluation of Cell Proliferation
2.8. Clone Formation Assay for the Evaluation of Cell Proliferation
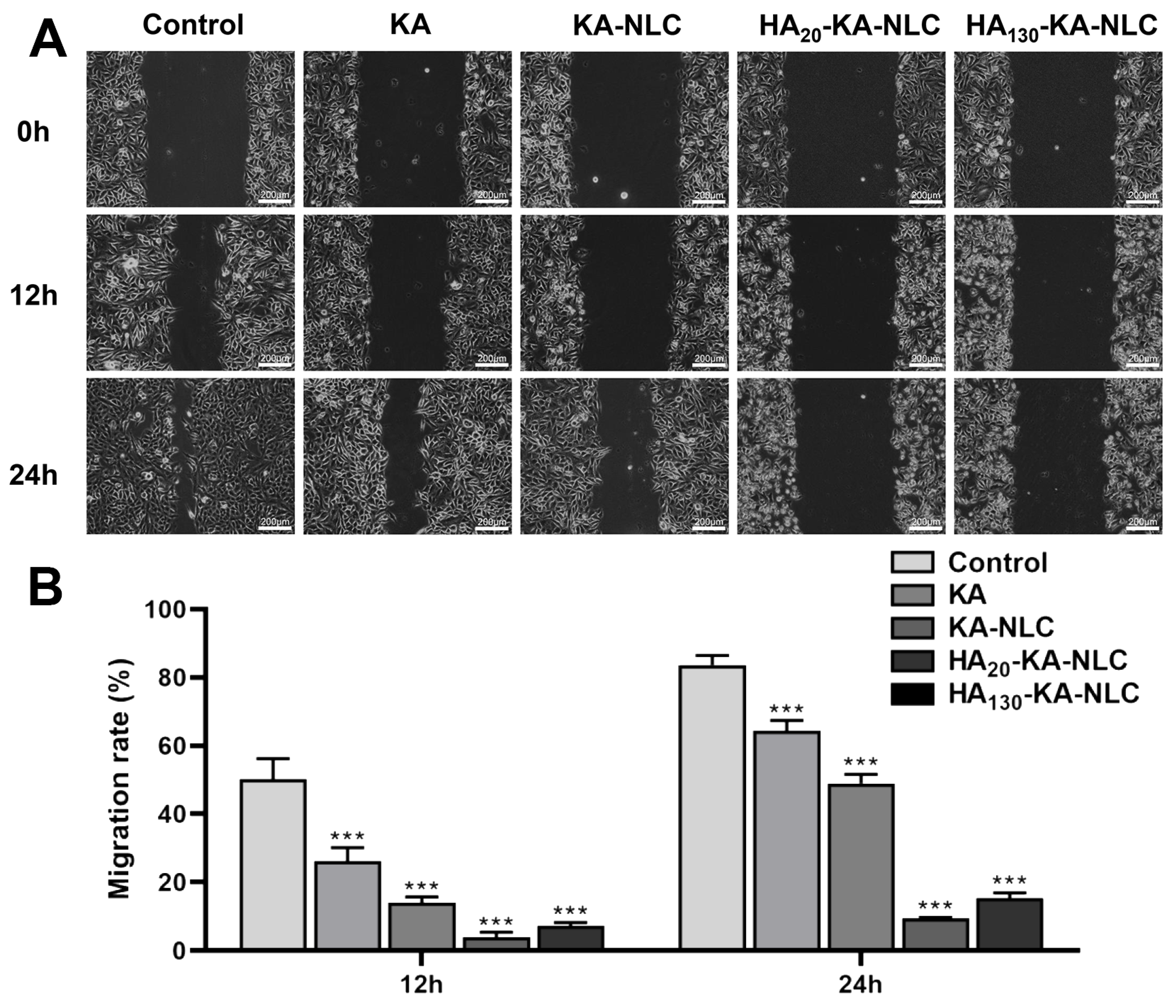
2.9. Evaluation of Cell Migration by Wound Healing Assay
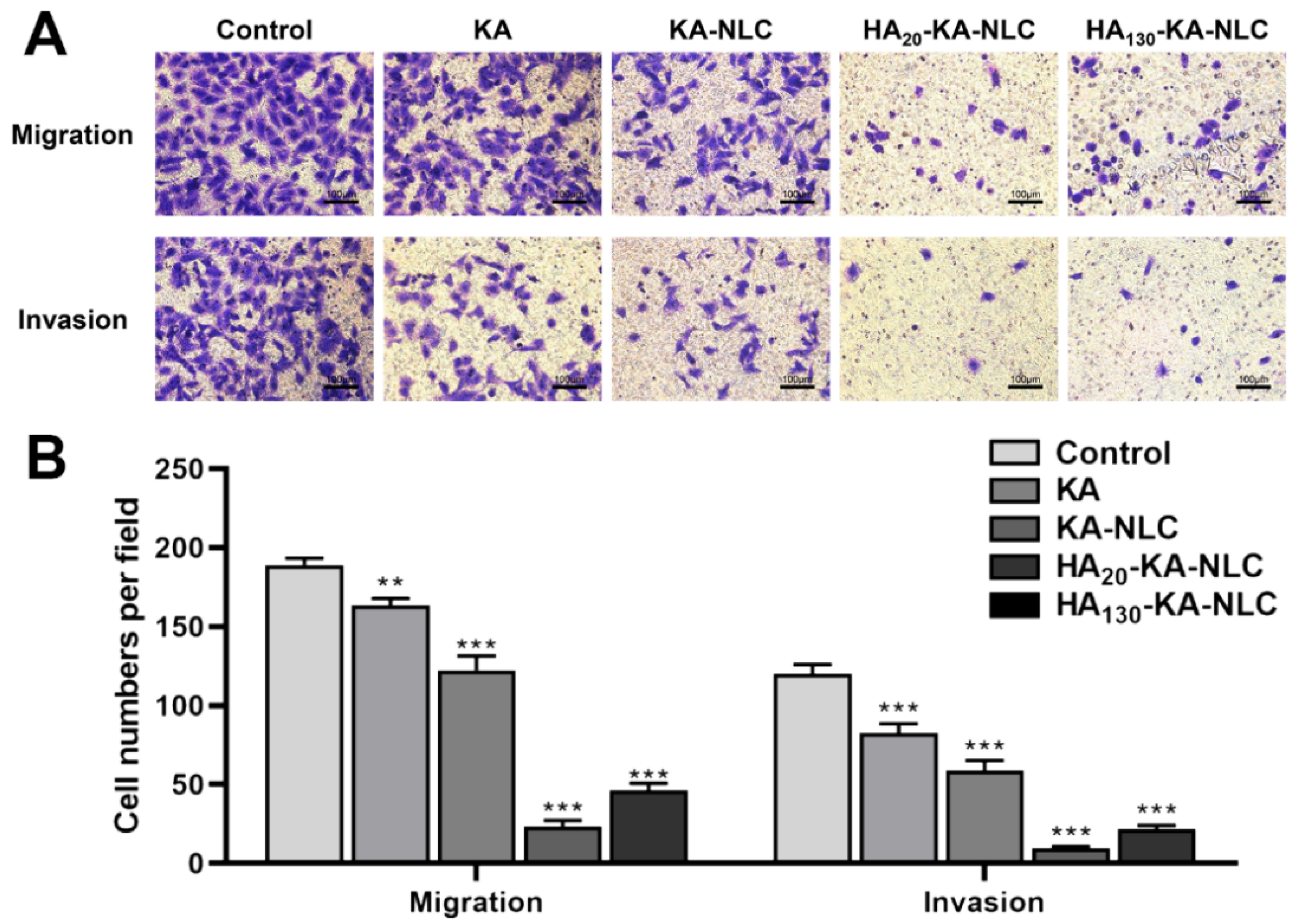
2.10. Evaluation of Cell Migration and Invasion by Transwell Assay
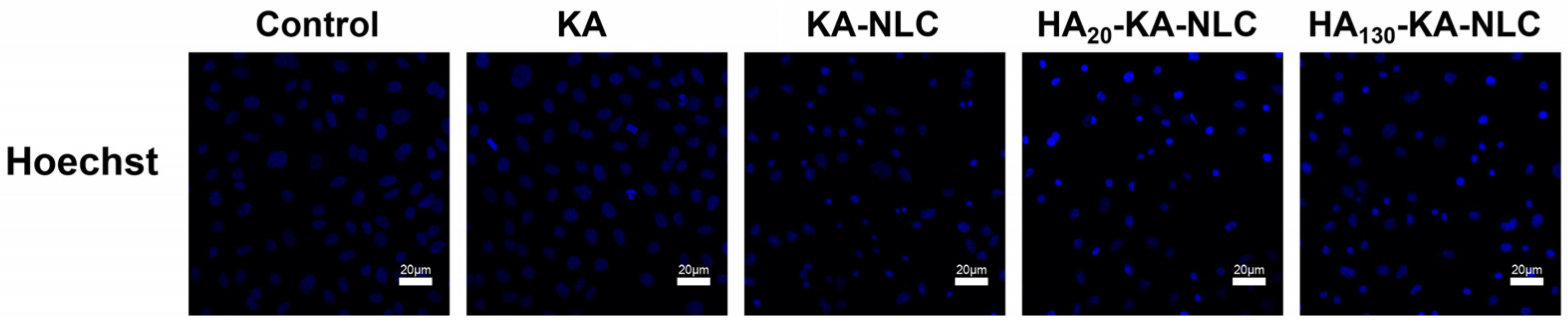
2.11. Analysis of Apoptosis Activity by Hoechst Staining Assay
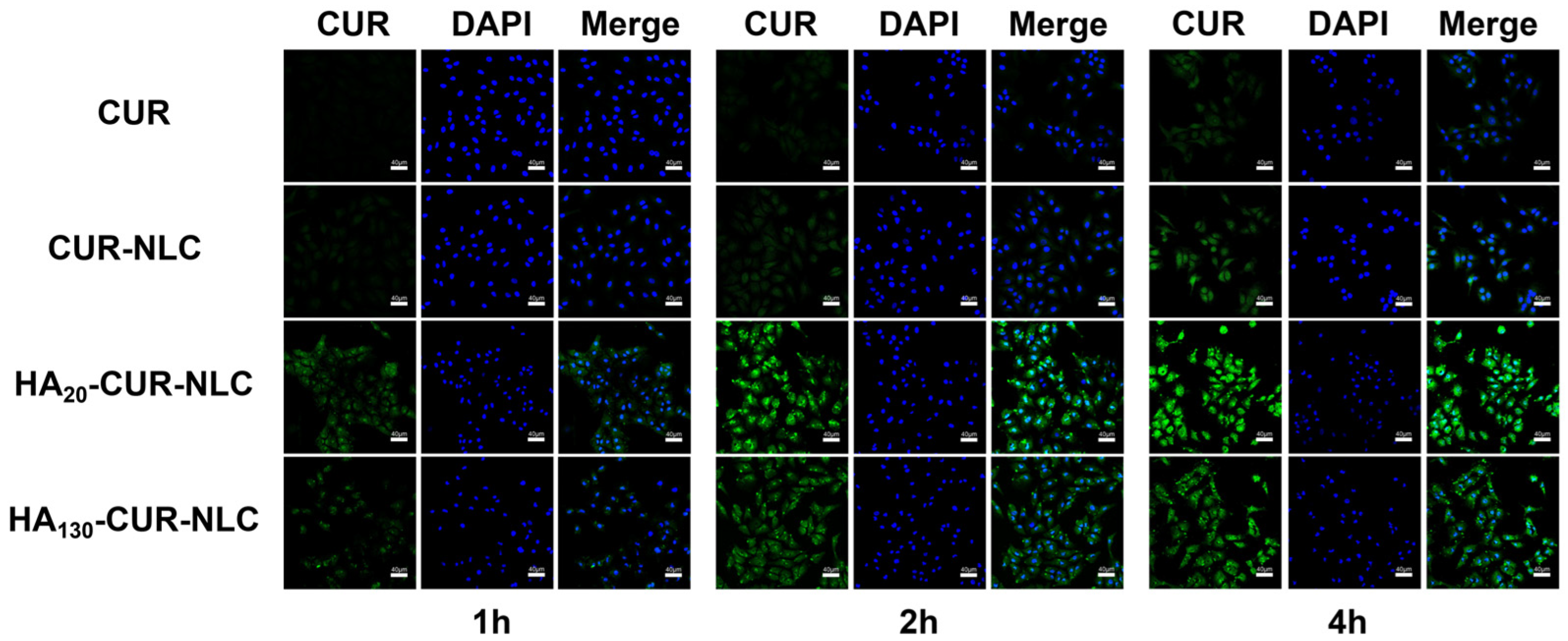
2.12. Cellular Uptake of Different Preparations
2.13. The Influence of Different Preparations on EMT
3. Materials and Methods
3.1. Materials
3.2. Preparation of KA-Loaded NLC
3.3. Optimization of KA-NLC
3.4. Characterization of NLC
3.4.1. Particle Size, Polydispersity Index, and Zeta Potential Analysis
3.4.2. DSC Analysis
3.4.3. Transmission Electron Microscopy
3.5. Preparation of HA-KA-NLC
3.6. Entrapment Efficiency and Drug Loading of Preparations
3.7. Stability of Preparations
3.8. In Vitro Drug Release Study
3.9. Cell Culture
3.10. Safety Evaluation of Preparations
3.10.1. Cytotoxicity Study
3.10.2. Hemolysis Activity
3.11. Cell Viability Assay
3.12. EdU Staining Assay
3.13. Clone Formation Assay
3.14. Wound Healing Assay
3.15. Transwell Assay
3.16. Apoptosis Assay
3.17. Cellular Uptake Assay
3.18. Western Blot Assay
3.19. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Santini, D.; Barni, S.; Intagliata, S.; Falcone, A.; Ferraù, F.; Galetta, D.; Moscetti, L.; La Verde, N.; Ibrahim, T.; Petrelli, F.; et al. Erratum: Corrigendum: Natural History of Non-Small-Cell Lung Cancer with Bone Metastases. Sci. Rep. 2016, 6, 22205. [Google Scholar] [CrossRef] [PubMed]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Ricciuti, B.; Brambilla, M.; Cortellini, A.; De Giglio, A.; Ficorella, C.; Sidoni, A.; Bellezza, G.; Crino, L.; Ludovini, V.; Baglivo, S.; et al. Clinical outcomes to pemetrexed-based versus non-pemetrexed-based platinum doublets in patients with KRAS-mutant advanced non-squamous non-small cell lung cancer. Clin. Transl. Oncol. 2020, 22, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Dickens, E.; Ahmed, S. Principles of cancer treatment by chemotherapy. Surgery 2021, 39, 215–220. [Google Scholar] [CrossRef]
- Jablonska, P.A.; Bosch-Barrera, J.; Serrano, D.; Valiente, M.; Calvo, A.; Aristu, J. Challenges and Novel Opportunities of Radiation Therapy for Brain Metastases in Non-Small Cell Lung Cancer. Cancers 2021, 13, 2141. [Google Scholar] [CrossRef]
- Rahnasto-Rilla, M.; Jarvenpaa, J.; Huovinen, M.; Schroderus, A.M.; Ihantola, E.L.; Kublbeck, J.; Khadeer, M.; Moaddel, R.; Lahtela-Kakkonen, M. Effects of galloflavin and ellagic acid on sirtuin 6 and its anti-tumorigenic activities. Biomed. Pharm. 2020, 131, 110701. [Google Scholar] [CrossRef]
- Masadah, R.; Ikram, D.; Rauf, S. Effects of propolis and its bioactive components on breast cancer cell pathways and the molecular mechanisms involved. Breast Dis. 2021, 40, S15–S25. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–25. [Google Scholar] [CrossRef]
- Kannanoor, M.; Lakshmi, B.A.; Kim, S. Synthesis of silver nanoparticles conjugated with kaempferol and hydrocortisone and an evaluation of their antibacterial effects. 3 Biotech 2021, 11, 317. [Google Scholar] [CrossRef]
- Molitorisova, M.; Sutovska, M.; Kazimierova, I.; Barborikova, J.; Joskova, M.; Novakova, E.; Franova, S. The anti-asthmatic potential of flavonol kaempferol in an experimental model of allergic airway inflammation. Eur. J. Pharmacol. 2021, 891, 173698. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, J.G.; Sung, G.H.; Yang, S.; Yang, W.S.; Kim, E.; Kim, J.H.; Ha, V.T.; Kim, H.G.; Yi, Y.S.; et al. Kaempferol, a dietary flavonoid, ameliorates acute inflammatory and nociceptive symptoms in gastritis, pancreatitis, and abdominal pain. Mol. Nutr. Food Res. 2015, 59, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front. Pharmacol. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Avakumova, N.H.; Hadjimitova, V.; Traykov, T.; Petrov, P. Evaluation of physicochemical and antioxidant properties of nanosized copolymeric micelles loaded with kaempferol. Pharmacia 2020, 67, 49–54. [Google Scholar] [CrossRef]
- Alshehri, A.S.; El-Kott, A.F.; Eleawa, S.M.; El-Gerbed, M.S.A.; Khalifa, H.S.; El-Kenawy, A.E.; Albadrani, G.M.; Abdel-Daim, M.M. Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1. J. Physiol. Pharmacol. 2021, 72, 339–355. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, C.; Yue, J.; Meng, Q.; Wu, J.; Sun, H. Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm. Biol. 2021, 59, 1106–1116. [Google Scholar] [CrossRef]
- Fouzder, C.; Mukhuty, A.; Kundu, R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch. Biochem. Biophys. 2021, 697, 108700. [Google Scholar] [CrossRef]
- Jeong, H.; Phan, A.N.H.; Choi, J.W. Anti-cancer Effects of Polyphenolic Compounds in Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-resistant Non-small Cell Lung Cancer. Pharmacogn. Mag. 2017, 13, 595–599. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.F.; Gao, N.; Zhao, J.; Xu, J. RETRACTED: Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. Biomed. Pharmacother. 2018, 108, 809–816. [Google Scholar] [CrossRef]
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. [Google Scholar] [CrossRef]
- Huang, M.; Su, E.; Zheng, F.; Tan, C. Encapsulation of flavonoids in liposomal delivery systems: The case of quercetin, kaempferol and luteolin. Food Funct. 2017, 8, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Teo, S.Y.; Yew, M.Y.; Lee, S.Y.; Rathbone, M.J.; Gan, S.N.; Coombes, A.G.A. In Vitro Evaluation of Novel Phenytoin-Loaded Alkyd Nanoemulsions Designed for Application in Topical Wound Healing. J. Pharm. Sci. 2017, 106, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Premarathne, E.P.; Karunaratne, D.N.; Perera, A.D. Controlled release of diclofenac sodium in glycolipid incorporated micro emulsions. Int. J. Pharm. 2016, 511, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Venugopalarao, G.; Lakshmipathy, R.; Sarada, N.C. Preparation and characterization of cefditoren pivoxil-loaded liposomes for controlled in vitro and in vivo drug release. Int. J. Nanomed. 2015, 10 (Suppl. S1), 149–157. [Google Scholar] [CrossRef]
- Arantes, V.T.; Faraco, A.A.G.; Ferreira, F.B.; Oliveira, C.A.; Martins-Santos, E.; Cassini-Vieira, P.; Barcelos, L.S.; Ferreira, L.A.M.; Goulart, G.A.C. Retinoic acid-loaded solid lipid nanoparticles surrounded by chitosan film support diabetic wound healing in in vivo study. Colloids Surf. B Biointerfaces 2020, 188, 110749. [Google Scholar] [CrossRef]
- Narang, J.; Khan, S.; Baboota, S.; Ali, J.; Khan, S.; Narang, R. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. Int. J. Pharm. Investig. 2015, 5, 182–191. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Muller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Gonzalez-Mira, E.; Nikolic, S.; Calpena, A.C.; Egea, M.A.; Souto, E.B.; Garcia, M.L. Improved and safe transcorneal delivery of flurbiprofen by NLC and NLC-based hydrogels. J. Pharm. Sci. 2012, 101, 707–725. [Google Scholar] [CrossRef]
- Mussi, S.V.; Parekh, G.; Pattekari, P.; Levchenko, T.; Lvov, Y.; Ferreira, L.A.M.; Torchilin, V.P. Improved pharmacokinetics and enhanced tumor growth inhibition using a nanostructured lipid carrier loaded with doxorubicin and modified with a layer-by-layer polyelectrolyte coating. Int. J. Pharm. 2015, 495, 186–193. [Google Scholar] [CrossRef][Green Version]
- Sudha, P.N.; Rose, M.H. Beneficial effects of hyaluronic acid. Adv. Food Nutr. Res. 2014, 72, 137–176. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf. B Biointerfaces 2021, 197, 111404. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Parkkinen, J.; Tammi, M.; Ågren, U.; Johansson, R.; Hirvikoski, P.; Eskelinen, M.; Kosma, V.-M. Hyaluronan in Peritumoral Stroma and Malignant Cells Associates with Breast Cancer Spreading and Predicts Survival. Am. J. Pathol. 2000, 156, 529–536. [Google Scholar] [CrossRef]
- Shen, S.; Lu, H.; Liu, L.; Wang, Y.; Zhang, C.; Yang, W.; Xu, W. Role of CD44 in tumor-initiating cells of salivary gland pleomorphic adenoma: More than a surface biomarker. Oral Dis. 2020, 26, 547–557. [Google Scholar] [CrossRef]
- Hu, B.; Ma, Y.; Yang, Y.; Zhang, L.; Han, H.; Chen, J. CD44 promotes cell proliferation in non-small cell lung cancer. Oncol. Lett. 2018, 15, 5627–5633. [Google Scholar] [CrossRef] [PubMed]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid Decorated Naringenin Nanoparticles: Appraisal of Chemopreventive and Curative Potential for Lung Cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Choi, Y.J.; Jeong, M.S.; Park, Y.I.; Motoyama, K.; Kim, M.W.; Kwon, S.H.; Choi, J.H. Hyaluronic Acid-Decorated Glycol Chitosan Nanoparticles for pH-Sensitive Controlled Release of Doxorubicin and Celecoxib in Nonsmall Cell Lung Cancer. Bioconjugate Chem. 2020, 31, 923–932. [Google Scholar] [CrossRef]
- Chiesa, E.; Greco, A.; Riva, F.; Dorati, R.; Conti, B.; Modena, T.; Genta, I. CD44-Targeted Carriers: The Role of Molecular Weight of Hyaluronic Acid in the Uptake of Hyaluronic Acid-Based Nanoparticles. Pharmaceuticals 2022, 15, 103. [Google Scholar] [CrossRef]
- Maharjan, S.; Singh, B.; Bok, J.D.; Kim, J.I.; Jiang, T.; Cho, C.S.; Kang, S.K.; Choi, Y.J. Exploring codon optimization and response surface methodology to express biologically active transmembrane RANKL in E. coli. PLoS ONE 2014, 9, e96259. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, Y.J. Improved Antitumor Efficacy of Hyaluronic Acid-Complexed Paclitaxel Nanoemulsions in Treating Non-Small Cell Lung Cancer. Biomol. Ther. 2017, 25, 411–416. [Google Scholar] [CrossRef]
- Habibi, A.; Sadat Shandiz, S.A.; Salehzadeh, A.; Moradi-Shoeili, Z. Novel pyridinecarboxaldehyde thiosemicarbazone conjugated magnetite nanoparticulates (MNPs) promote apoptosis in human lung cancer A549 cells. J. Biol. Inorg. Chem. 2020, 25, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Liu, J.; Zhao, Z.; Dong, L.; Cai, H.; Yin, L.; Zhou, J.; Huo, M. Smart nanoparticles with a detachable outer shell for maximized synergistic antitumor efficacy of therapeutics with varying physicochemical properties. J. Control. Release 2016, 243, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Karimi Roshan, M.; Soltani, A.; Soleimani, A.; Rezaie Kahkhaie, K.; Afshari, A.R.; Soukhtanloo, M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie 2019, 165, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Shehata, E.M.M.; Gowayed, M.A.; El-Ganainy, S.O.; Sheta, E.; Elnaggar, Y.S.R.; Abdallah, O.Y. Pectin coated nanostructured lipid carriers for targeted piperine delivery to hepatocellular carcinoma. Int. J. Pharm. 2022, 619, 121712. [Google Scholar] [CrossRef]
- Elsayed, I.; El-Dahmy, R.M.; El-Emam, S.Z.; Elshafeey, A.H.; El Gawad, N.A.A.; El-Gazayerly, O.N. Response surface optimization of biocompatible elastic nanovesicles loaded with rosuvastatin calcium: Enhanced bioavailability and anticancer efficacy. Drug Deliv. Transl. Res. 2020, 10, 1459–1475. [Google Scholar] [CrossRef]
- Nnamani, P.O.; Hansen, S.; Windbergs, M.; Lehr, C.M. Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application. Int. J. Pharm. 2014, 477, 208–217. [Google Scholar] [CrossRef]
- Divya, G.; Panonnummal, R.; Gupta, S.; Jayakumar, R.; Sabitha, M. Acitretin and aloe-emodin loaded chitin nanogel for the treatment of psoriasis. Eur. J. Pharm. Biopharm. 2016, 107, 97–109. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Shen, J. The development and characterization of a glutathione-sensitive cross-linked polyethylenimine gene vector. Biomaterials 2006, 27, 5292–5298. [Google Scholar] [CrossRef]
- Pereira, R.L.; Leites, F.I.; Paese, K.; Sponchiado, R.M.; Michalowski, C.B.; Guterres, S.S.; Schapoval, E.E. Hydrogel containing adapalene- and dapsone-loaded lipid-core nanocapsules for cutaneous application: Development, characterization, in vitro irritation and permeation studies. Drug Dev. Ind. Pharm. 2016, 42, 2001–2008. [Google Scholar] [CrossRef]
- Sartaj, A.; Annu; Biswas, L.; Verma, A.K.; Sahoo, P.K.; Baboota, S.; Ali, J. Ribociclib Nanostructured Lipid Carrier Aimed for Breast Cancer: Formulation Optimization, Attenuating In Vitro Specification, and In Vivo Scrutinization. BioMed Res. Int. 2022, 2022, 6009309. [Google Scholar] [CrossRef]
- Keeratichamroen, S.; Lirdprapamongkol, K.; Svasti, J. Mechanism of ECM-induced dormancy and chemoresistance in A549 human lung carcinoma cells. Oncol. Rep. 2018, 39, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Zhang, W.J.; Li, X.D.; Yang, N.; Pan, W.S.; Kong, J.; Zhang, J.S. Sustained-release genistein from nanostructured lipid carrier suppresses human lens epithelial cell growth. Int. J. Ophthalmol. 2016, 9, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Hu, Y.; Zhu, Y.; Che, L.; Shen, Q.; Zhang, J.; Li, X. Oligoamines conjugated chitosan derivatives: Synthesis, characterization, in vitro and in vivo biocompatibility evaluations. Carbohydr. Polym. 2011, 83, 1153–1161. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Noacco, N.; Perez de Berti, I.; Stewart, S.J.; Cabrera, A.F.; Alvarez, V.A.; Garcia de Bravo, M.; Duran, N.; Castro, G.R.; Islan, G.A. Design of magnetic hybrid nanostructured lipid carriers containing 1,8-cineole as delivery systems for anticancer drugs: Physicochemical and cytotoxic studies. Colloids Surf. B Biointerfaces 2021, 202, 111710. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Pahuja, K.B.; Wickliffe, K.E.; Gorur, A.; Baumgartel, C.; Schekman, R.; Rape, M. Ubiquitin-dependent regulation of COPII coat size and function. Nature 2012, 482, 495–500. [Google Scholar] [CrossRef]
- Jun, S.; Kim, S.W.; Lim, J.Y.; Park, S.J. ABHD12 Knockdown Suppresses Breast Cancer Cell Proliferation, Migration and Invasion. Anticancer Res. 2020, 40, 2601–2611. [Google Scholar] [CrossRef]
- Dubey, A.K.; Podia, M.; Priyanka; Raut, S.; Singh, S.; Pinnaka, A.K.; Khatri, N. Insight Into the Beneficial Role of Lactiplantibacillus plantarum Supernatant Against Bacterial Infections, Oxidative Stress, and Wound Healing in A549 Cells and BALB/c Mice. Front. Pharmacol. 2021, 12, 728614. [Google Scholar] [CrossRef]
- Hong, W.G.; Jeong, G.W.; Nah, J.W. Evaluation of hyaluronic acid-combined ternary complexes for serum-resistant and targeted gene delivery system. Int. J. Biol. Macromol. 2018, 115, 459–468. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, J.; Ye, B.; Wu, Y.; Xu, Z.; Chen, J.; Chen, J. Dual-Responsive Core Crosslinking Glycopolymer-Drug Conjugates Nanoparticles for Precise Hepatocarcinoma Therapy. Front. Pharmacol. 2018, 9, 663. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef]
- Tiwari, N.; Nawale, L.; Sarkar, D.; Badiger, M.V. Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin. Gels 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, Y.; Cheng, Z.; Hu, Y.; Liu, T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-alpha/NF-kappaB and PI3K/AKT signaling pathway. Cell Death Discov. 2018, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Rodino-Janeiro, B.K.; Lobo, B.; Stanifer, M.L.; Klaus, B.; Granzow, M.; Gonzalez-Castro, A.M.; Salvo-Romero, E.; Alonso-Cotoner, C.; Pigrau, M.; et al. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut 2017, 66, 1537–1538. [Google Scholar] [CrossRef] [PubMed]



| Std Order | Run Order | Factors | Responses | |||
|---|---|---|---|---|---|---|
| A | B | C | Y1 | Y2 | ||
| 1 | 4 | 20 | 3 | 1.25 | 78.57 ± 2.22 | 2.90 ± 0.23 |
| 2 | 3 | 40 | 3 | 1.25 | 82.26 ± 2.01 | 2.01 ± 0.11 |
| 3 | 7 | 20 | 5 | 1.25 | 80.76 ± 1.58 | 3.24 ± 0.32 |
| 4 | 5 | 40 | 5 | 1.25 | 83.34 ± 2.64 | 2.01 ± 0.18 |
| 5 | 9 | 20 | 4 | 1 | 83.87 ± 1.32 | 4.32 ± 0.42 |
| 6 | 6 | 40 | 4 | 1 | 89.19 ± 1.65 | 2.23 ± 0.33 |
| 7 | 15 | 20 | 4 | 1.5 | 82.45 ± 2.62 | 3.77 ± 0.46 |
| 8 | 17 | 40 | 4 | 1.5 | 85.48 ± 2.91 | 2.06 ± 0.19 |
| 9 | 13 | 30 | 3 | 1 | 88.71 ± 3.01 | 2.96 ± 0.34 |
| 10 | 2 | 30 | 5 | 1 | 87.57 ± 2.87 | 2.98 ± 0.49 |
| 11 | 8 | 30 | 3 | 1.5 | 84.69 ± 1.94 | 2.89 ± 0.37 |
| 12 | 1 | 30 | 5 | 1.5 | 83.35 ± 1.68 | 2.10 ± 0.16 |
| 13 | 14 | 30 | 4 | 1.25 | 85.23 ± 1.54 | 2.86 ± 0.25 |
| 14 | 16 | 30 | 4 | 1.25 | 85.58 ± 2.84 | 2.87 ± 0.29 |
| 15 | 10 | 30 | 4 | 1.25 | 85.33 ± 2.46 | 2.61 ± 0.26 |
| 16 | 12 | 30 | 4 | 1.25 | 85.50 ± 2.18 | 2.80 ± 0.34 |
| 17 | 11 | 30 | 4 | 1.25 | 85.81 ± 2.73 | 2.82 ± 0.42 |
| Variables | Levels Used | ||
|---|---|---|---|
| Low | Medium | High | |
| Factors: | |||
| A = the ratio of lipid and drug | 20:1 | 30:1 | 40:1 |
| B = the ratio of solid lipid and liquid lipid | 3:1 | 4:1 | 5:1 |
| C = surfactant concentration (%, w/v) | 1 | 1.25 | 1.5 |
| Responses: | Constraints | ||
| Y1 = EE (%) | Maximize | ||
| Y2 = DL (%) | Maximize |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Liu, J.; Cui, X.; Hou, J.; Yu, F.; Wang, J.; Wang, X.; Chen, C.; Tong, L. Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro. Molecules 2022, 27, 4553. https://doi.org/10.3390/molecules27144553
Ma Y, Liu J, Cui X, Hou J, Yu F, Wang J, Wang X, Chen C, Tong L. Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro. Molecules. 2022; 27(14):4553. https://doi.org/10.3390/molecules27144553
Chicago/Turabian StyleMa, Yufei, Jinli Liu, Xinyu Cui, Jiafu Hou, Fengbo Yu, Jinghua Wang, Xiaoxue Wang, Cong Chen, and Lei Tong. 2022. "Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro" Molecules 27, no. 14: 4553. https://doi.org/10.3390/molecules27144553
APA StyleMa, Y., Liu, J., Cui, X., Hou, J., Yu, F., Wang, J., Wang, X., Chen, C., & Tong, L. (2022). Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro. Molecules, 27(14), 4553. https://doi.org/10.3390/molecules27144553





