Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly
Abstract
1. Introduction
2. Results and Discussion
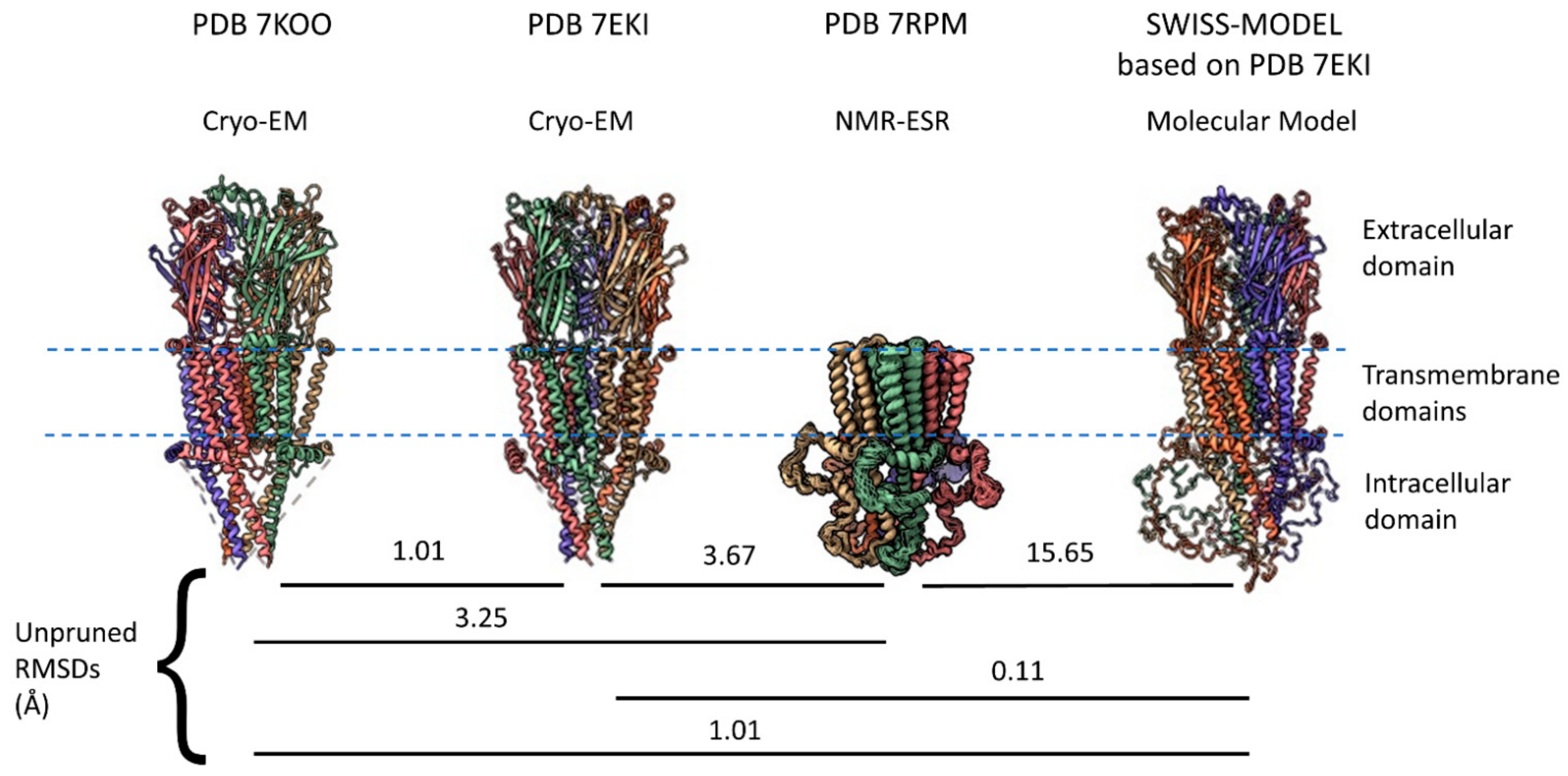
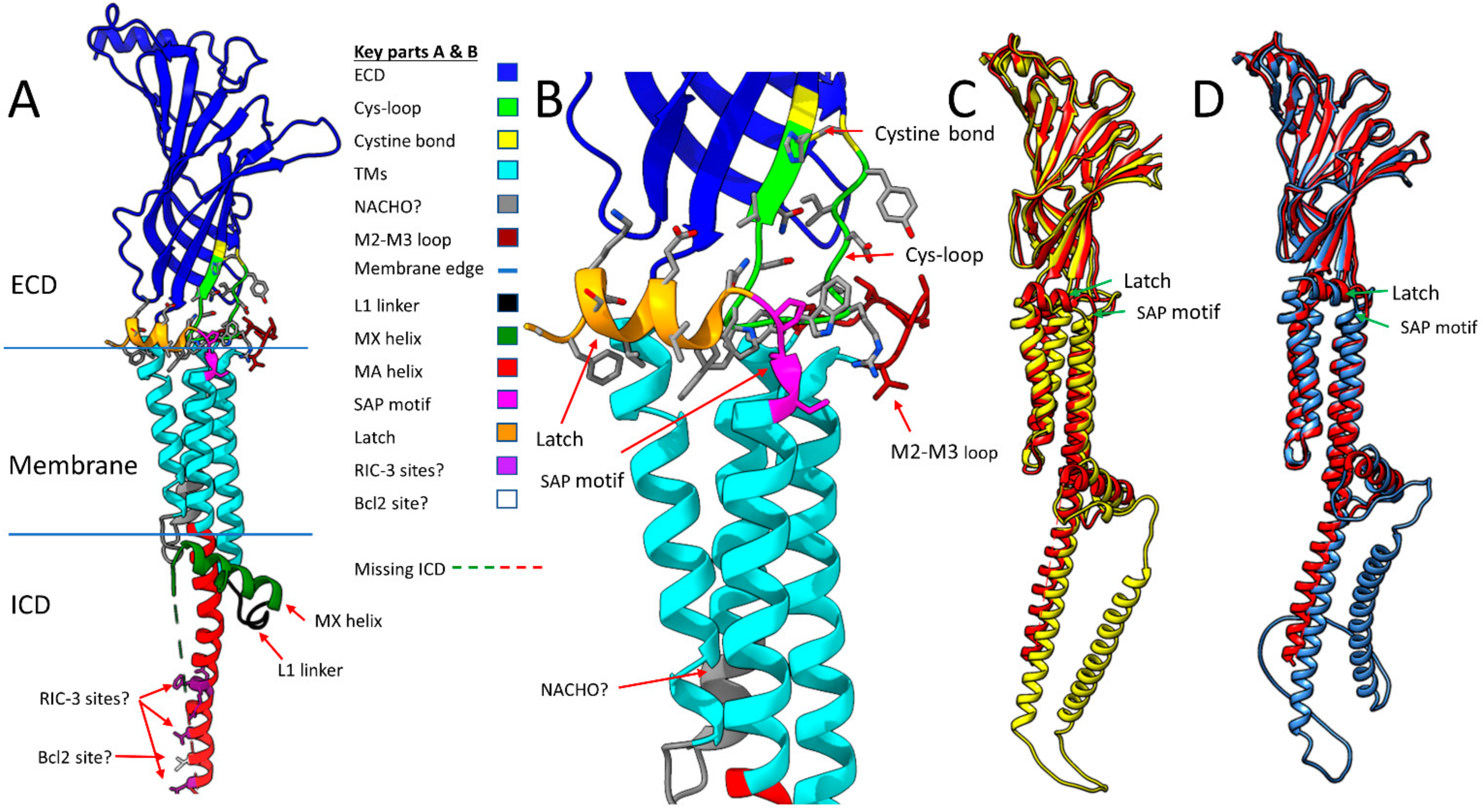
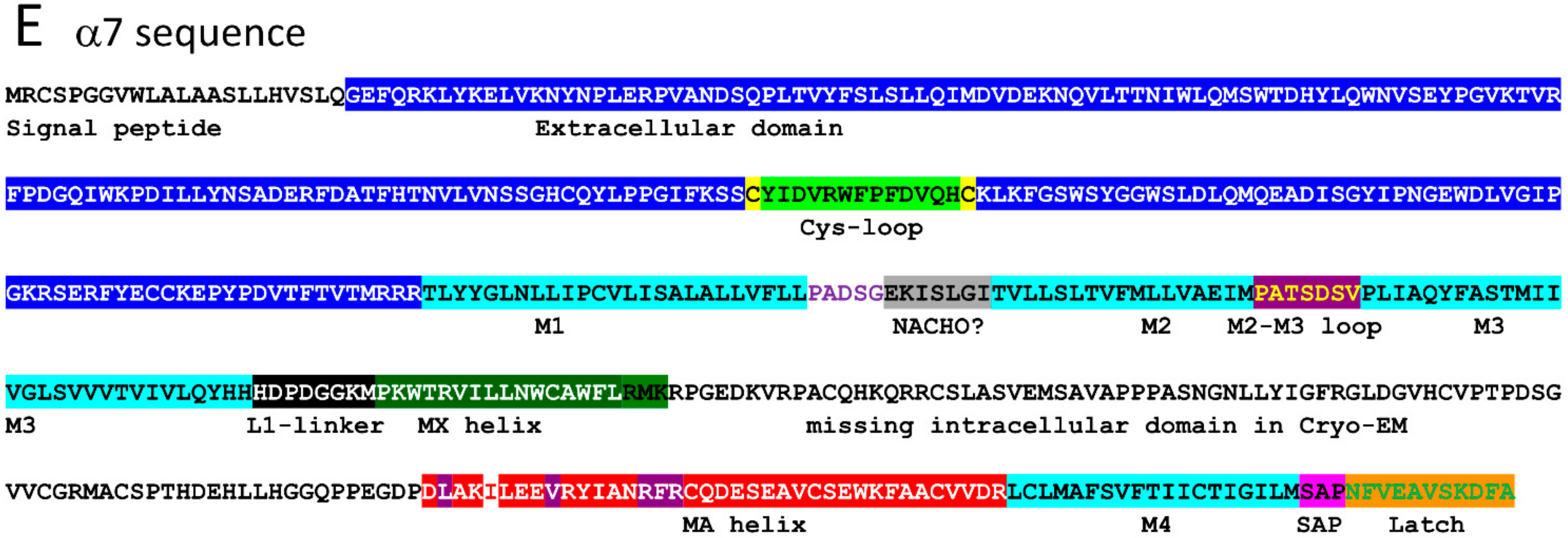
2.1. α7 Receptor Structure
2.2. How What We Know about Muscle Nicotinic Receptor Assembly Informs How We Think α7 Receptors Assemble
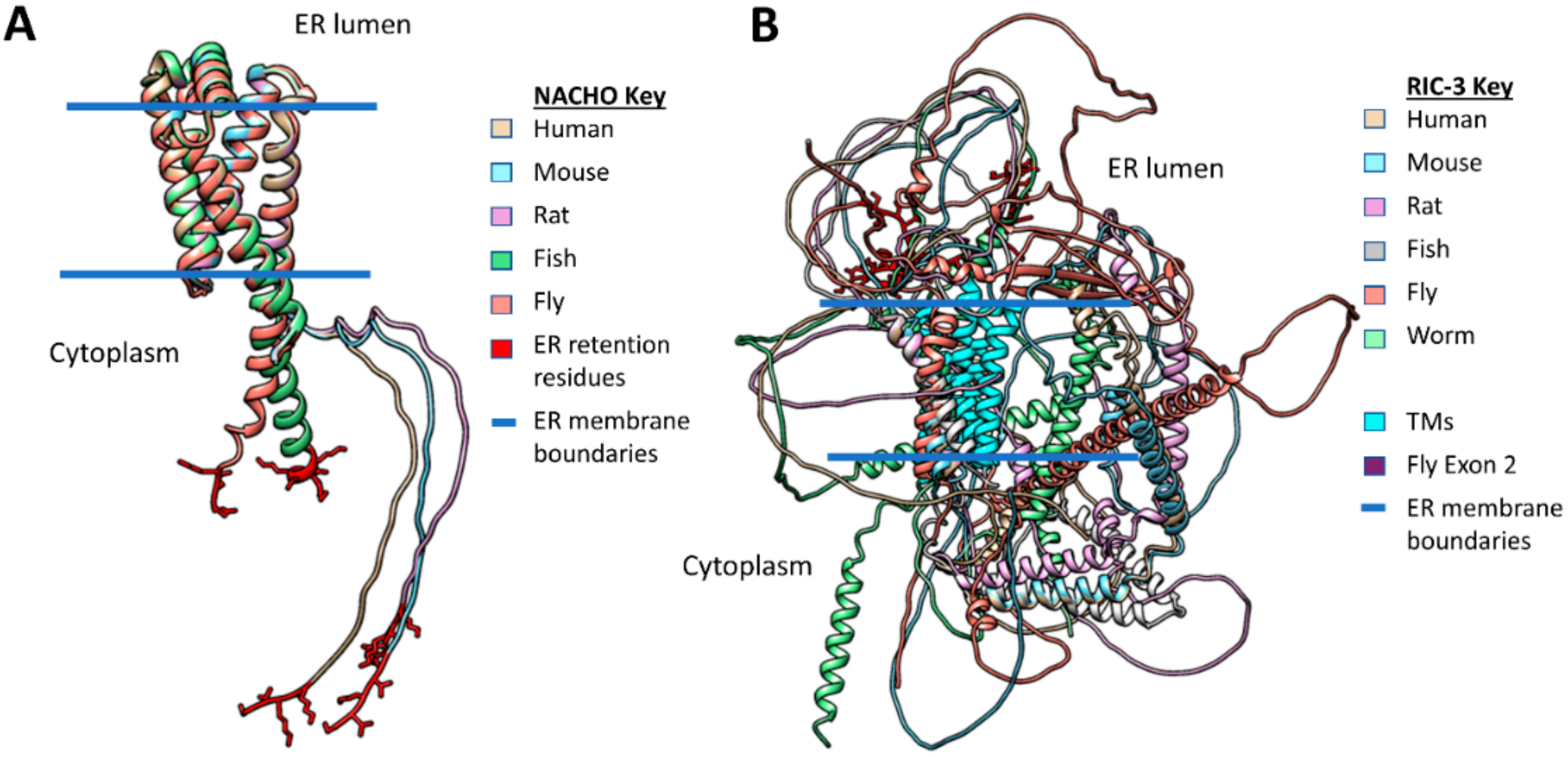
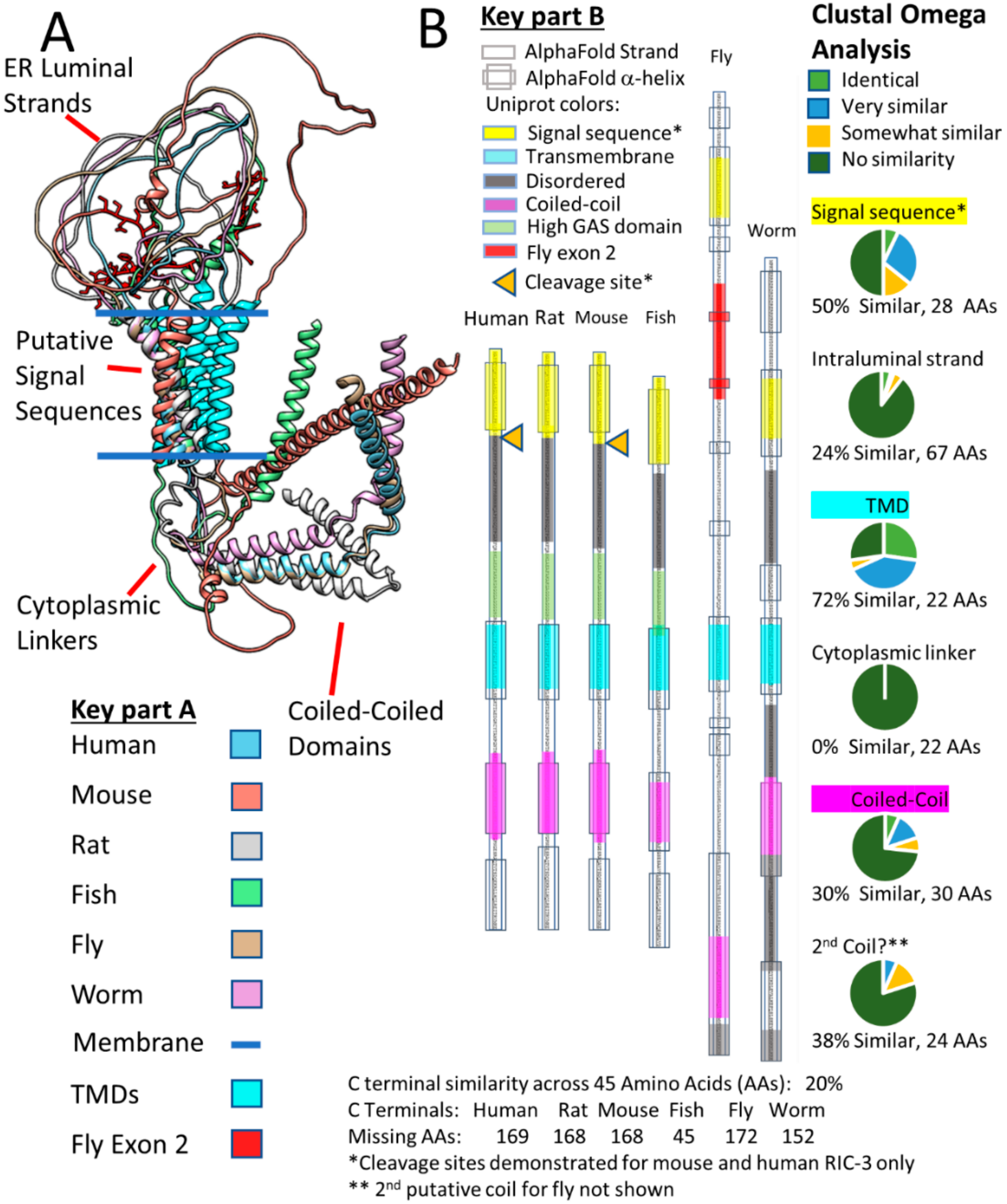
2.3. RIC-3 & NACHO Chaperone Effects and Structures (or Lack Thereof)
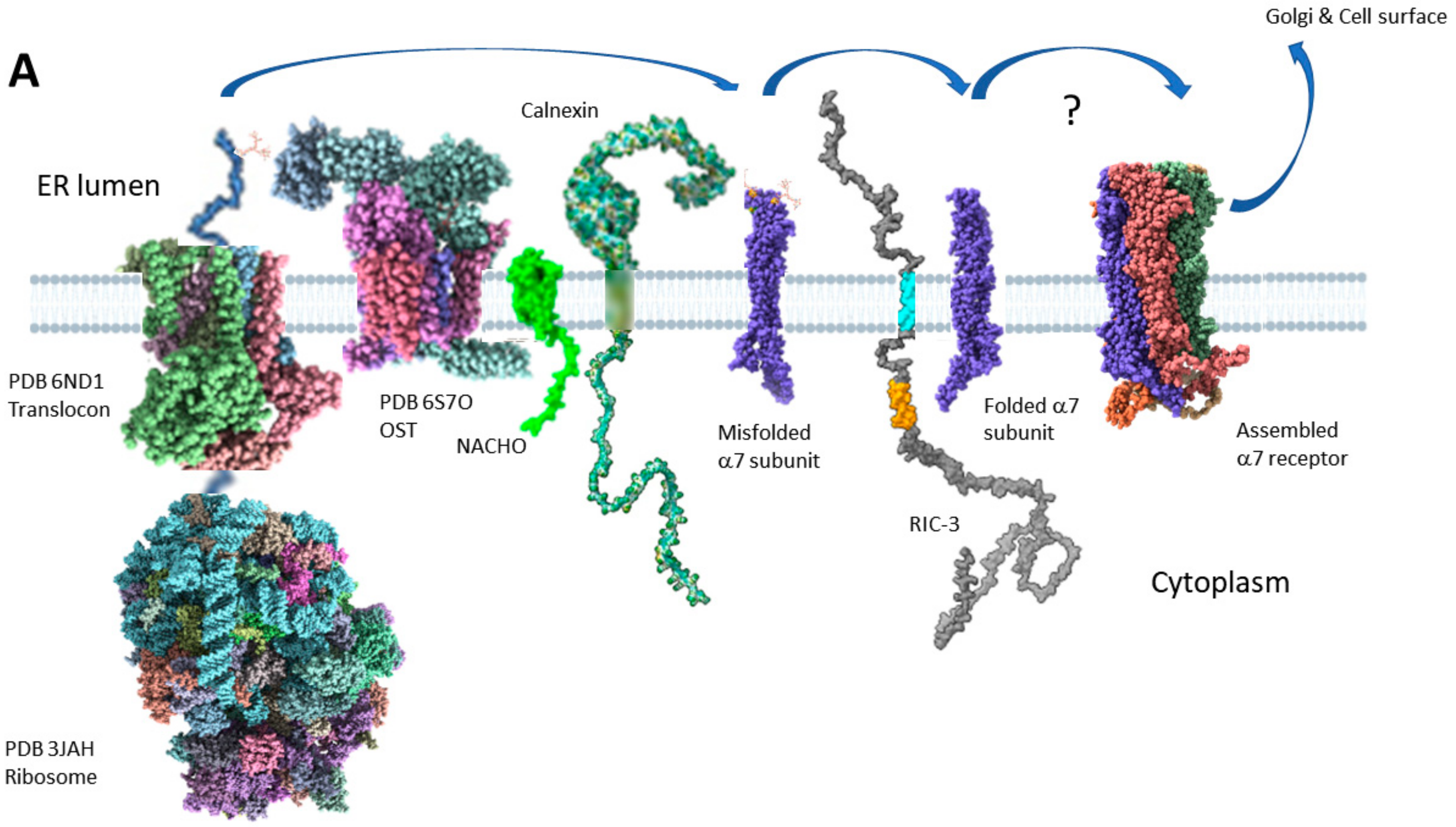
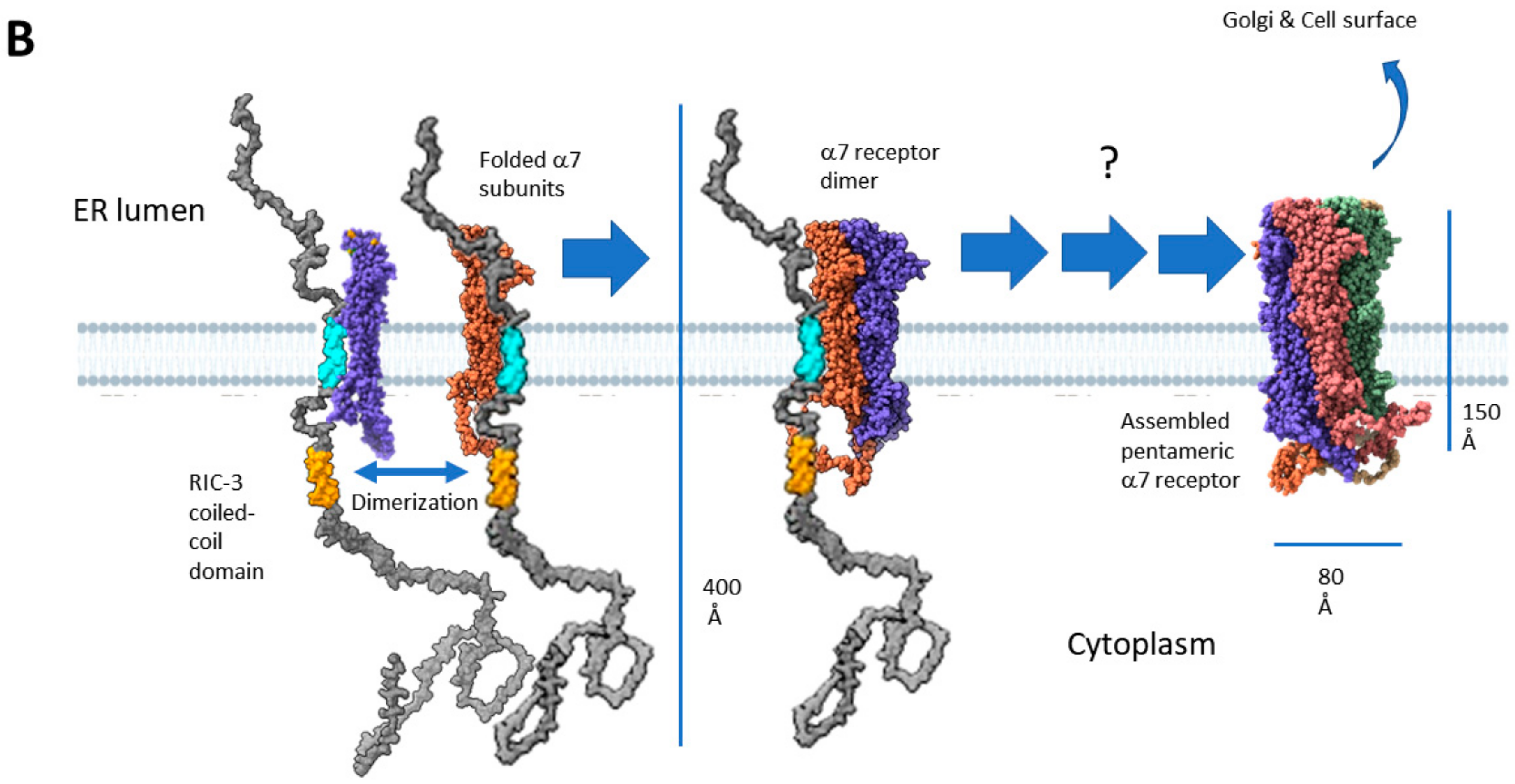
2.4. Two Models for How RIC-3 Helps Assemble α7 Receptors
2.5. Does RIC-3 Bind to α7 Receptors, and If So, Where?
2.5.1. RIC-3 Interactions with the α7 Receptor ECD
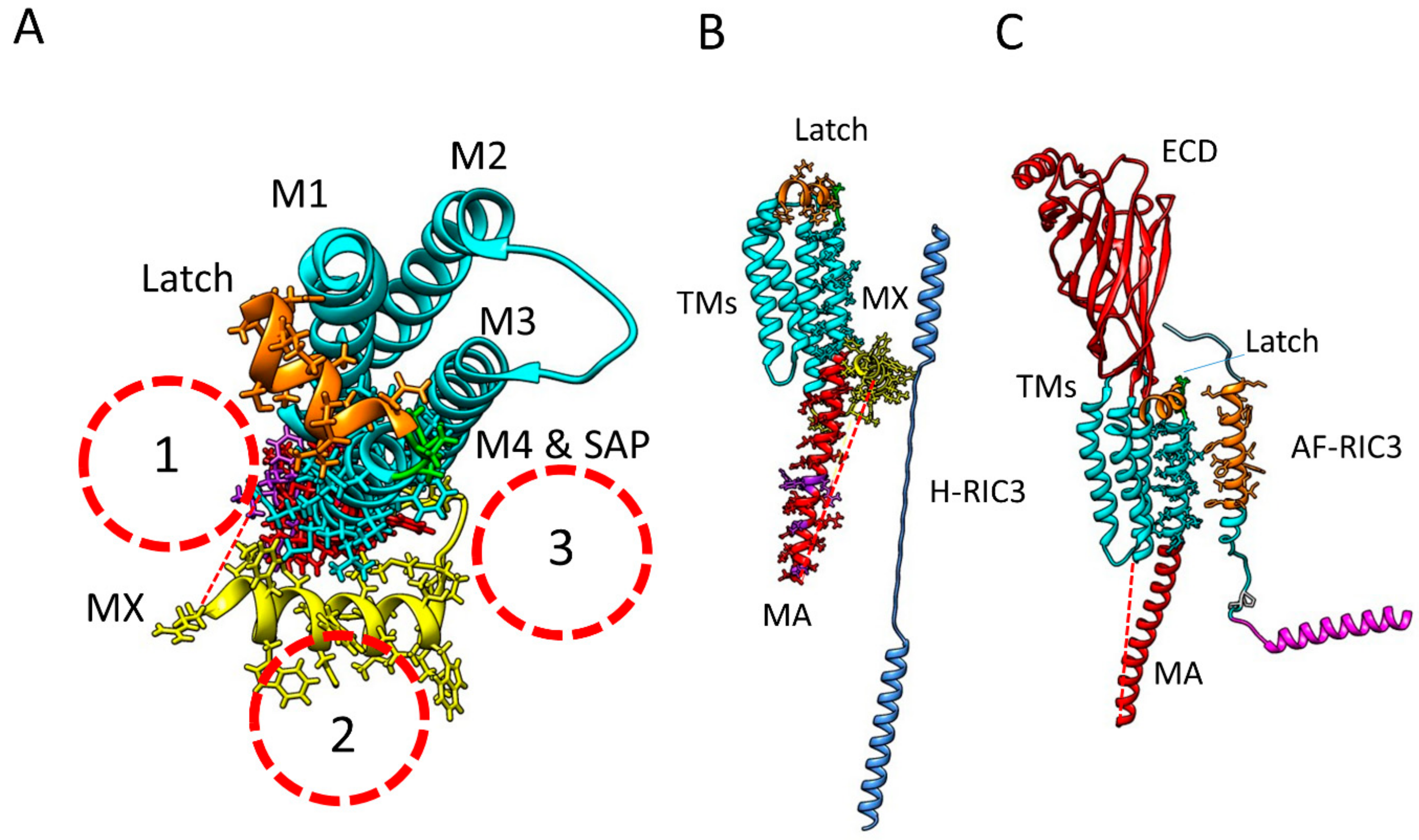
2.5.2. RIC-3 Interactions with α7 Receptor Transmembrane Domains
2.5.3. RIC-3 Interactions with the α7 Receptor Intracellular Domain
2.6. RIC-3 Integration with Other Chaperones and Regulators
3. Summary and Conclusions
4. Methods
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.S.; Harkness, P.C. Assembly and trafficking of nicotinic acetylcholine receptors (Review). Mol. Membr. Biol. 2008, 25, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and Extraneuronal Nicotinic Acetylcholine Receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Corringer, P.J.; Poitevin, F.; Prevost, M.S.; Sauguet, L.; Delarue, M.; Changeux, J.P. Structure and pharmacology of pentameric receptor channels: From bacteria to brain. Structure 2012, 20, 941–956. [Google Scholar] [CrossRef]
- Williams, M.E.; Burton, B.; Urrutia, A.; Shcherbatko, A.; Chavez-Noriega, L.E.; Cohen, C.J.; Aiyar, J. Ric-3 promotes functional expression of the nicotinic acetylcholine receptor alpha7 subunit in mammalian cells. J. Biol. Chem. 2005, 280, 1257–1263. [Google Scholar] [CrossRef]
- Gu, S.; Matta, J.A.; Lord, B.; Harrington, A.W.; Sutton, S.W.; Davini, W.B.; Bredt, D.S. Brain α7 Nicotinic Acetylcholine Receptor Assembly Requires NACHO. Neuron 2016, 89, 948–955. [Google Scholar] [CrossRef]
- Bertrand, D.; Lee, C.H.; Flood, D.; Marger, F.; Donnelly-Roberts, D. Therapeutic Potential of α7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2015, 67, 1025–1073. [Google Scholar] [CrossRef]
- Corradi, J.; Bouzat, C. Understanding the Bases of Function and Modulation of α7 Nicotinic Receptors: Implications for Drug Discovery. Mol. Pharmacol. 2016, 90, 288–299. [Google Scholar] [CrossRef]
- Papke, R.L.; Horenstein, N.A. Therapeutic Targeting of alpha7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2021, 73, 1118–1149. [Google Scholar] [CrossRef]
- Basak, S.; Gicheru, Y.; Rao, S.; Sansom, M.S.P.; Chakrapani, S. Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT. Nature 2018, 563, 270–274. [Google Scholar] [CrossRef]
- Morales-Perez, C.L.; Noviello, C.M.; Hibbs, R.E. X-ray structure of the human α4β2 nicotinic receptor. Nature 2016, 538, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, V.; Mowrey, D.D.; Tillman, T.S.; Seyoum, E.; Xu, Y.; Tang, P. NMR structures of the human α7 nAChR transmembrane domain and associated anesthetic binding sites. Biochim. Biophys. Acta 2014, 1838, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Brejc, K.; van Dijk, W.J.; Klaassen, R.V.; Schuurmans, M.; van Der Oost, J.; Smit, A.B.; Sixma, T.K. Crystal structure of an ACh-binding protein reveals the ligand-binding doma.ain of nicotinic receptors. Nature 2001, 411, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.B.; Syed, N.I.; Schaap, D.; van Minnen, J.; Klumperman, J.; Kits, K.S.; Lodder, H.; van der Schors, R.C.; van Elk, R.; Sorgedrager, B.; et al. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature 2001, 411, 261–268. [Google Scholar] [CrossRef]
- Ulens, C.; Akdemir, A.; Jongejan, A.; van Elk, R.; Bertrand, S.; Perrakis, A.; Leurs, R.; Smit, A.B.; Sixma, T.K.; Bertrand, D.; et al. Use of acetylcholine binding protein in the search for novel alpha7 nicotinic receptor ligands. In silico docking, pharmacological screening, and X-ray analysis. J. Med. Chem. 2009, 52, 2372–2383. [Google Scholar] [CrossRef] [PubMed]
- Noviello, C.M.; Gharpure, A.; Mukhtasimova, N.; Cabuco, R.; Baxter, L.; Borek, D.; Sine, S.M.; Hibbs, R.E. Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 2021, 184, 2121–2134.e2113. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Zhou, Y.; Zhang, M.; Chen, H.; Eric Xu, H.; Sun, D.; Liu, L.; Tian, C. Structural basis of human α7 nicotinic acetylcholine receptor activation. Cell Res. 2021, 31, 713–716. [Google Scholar] [CrossRef]
- Bondarenko, V.; Wells, M.M.; Chen, Q.; Tillman, T.S.; Singewald, K.; Lawless, M.J.; Caporoso, J.; Brandon, N.; Coleman, J.A.; Saxena, S.; et al. Structures of highly flexible intracellular domain of human α7 nicotinic acetylcholine receptor. Nat. Commun. 2022, 13, 793. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Bennett, H.M.; Lees, K.; Harper, K.M.; Jones, A.K.; Sattelle, D.B.; Wonnacott, S.; Wolstenholme, A.J. Xenopus laevis RIC-3 enhances the functional expression of the C. elegans homomeric nicotinic receptor, ACR-16, in Xenopus oocytes. J. Neurochem. 2012, 123, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, Y.; Tang, X.Q.; Wang, Z.Z. Mouse RIC-3, an endoplasmic reticulum chaperone, promotes assembly of the alpha7 acetylcholine receptor through a cytoplasmic coiled-coil domain. J. Neurosci. 2009, 29, 12625–12635. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.J.; Gu, S.; Witham, E.; Dhara, M.; Yu, H.; Mandon, E.D.; Jawhari, A.; Bredt, D.S. NACHO Engages N-Glycosylation ER Chaperone Pathways for α7 Nicotinic Receptor Assembly. Cell Rep. 2020, 32, 108025. [Google Scholar] [CrossRef] [PubMed]
- Crespi, A.; Colombo, S.F.; Gotti, C. Proteins and chemical chaperones involved in neuronal nicotinic receptor expression and function: An update. Br. J. Pharmacol 2018, 175, 1869–1879. [Google Scholar] [CrossRef]
- Miwa, J.M.; Anderson, K.R.; Hoffman, K.M. Lynx Prototoxins: Roles of Endogenous Mammalian Neurotoxin-Like Proteins in Modulating Nicotinic Acetylcholine Receptor Function to Influence Complex Biological Processes. Front. Pharmacol. 2019, 10, 343. [Google Scholar] [CrossRef]
- Matta, J.A.; Gu, S.; Davini, W.B.; Bredt, D.S. Nicotinic acetylcholine receptor redux: Discovery of accessories opens therapeutic vistas. Science 2021, 373, eabg6539. [Google Scholar] [CrossRef]
- Vallés, A.S.; Barrantes, F.J. Chaperoning α7 neuronal nicotinic acetylcholine receptors. Biochim. Biophys. Acta 2012, 1818, 718–729. [Google Scholar] [CrossRef]
- Bocquet, N.; Prado de Carvalho, L.; Cartaud, J.; Neyton, J.; Le Poupon, C.; Taly, A.; Grutter, T.; Changeux, J.P.; Corringer, P.J. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature 2007, 445, 116–119. [Google Scholar] [CrossRef]
- Corringer, P.J.; Baaden, M.; Bocquet, N.; Delarue, M.; Dufresne, V.; Nury, H.; Prevost, M.; Van Renterghem, C. Atomic structure and dynamics of pentameric ligand-gated ion channels: New insight from bacterial homologues. J. Physiol. 2010, 588, 565–572. [Google Scholar] [CrossRef]
- Hilf, R.J.; Dutzler, R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 2008, 452, 375–379. [Google Scholar] [CrossRef]
- Hilf, R.J.; Dutzler, R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 2009, 457, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, Q.; Willenbring, D.; Yoshida, K.; Tillman, T.; Kashlan, O.B.; Cohen, A.; Kong, X.P.; Xu, Y.; Tang, P. Structure of the pentameric ligand-gated ion channel ELIC cocrystallized with its competitive antagonist acetylcholine. Nat. Commun. 2012, 3, 714. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, A.; Iyer, L.M.; Jakobsson, E.; Aravind, L. Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol. 2005, 6, R4. [Google Scholar] [CrossRef]
- Althoff, T.; Hibbs, R.E.; Banerjee, S.; Gouaux, E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature 2014, 512, 333–337. [Google Scholar] [CrossRef]
- Hibbs, R.E.; Gouaux, E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 2011, 474, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Temburni, M.K.; Levey, M.S.; Bertrand, S.; Bertrand, D.; Jacob, M.H. The long internal loop of the alpha 3 subunit targets nAChRs to subdomains within individual synapses on neurons in vivo. Nat. Neurosci. 1998, 1, 557–562. [Google Scholar] [CrossRef][Green Version]
- Borges, L.S.; Yechikhov, S.; Lee, Y.I.; Rudell, J.B.; Friese, M.B.; Burden, S.J.; Ferns, M.J. Identification of a motif in the acetylcholine receptor beta subunit whose phosphorylation regulates rapsyn association and postsynaptic receptor localization. J. Neurosci. 2008, 28, 11468–11476. [Google Scholar] [CrossRef]
- Temburni, M.K.; Blitzblau, R.C.; Jacob, M.H. Receptor targeting and heterogeneity at interneuronal nicotinic cholinergic synapses in vivo. J. Physiol. 2000, 525 Pt 1, 21–29. [Google Scholar] [CrossRef]
- Tsetlin, V.; Kuzmin, D.; Kasheverov, I. Assembly of nicotinic and other Cys-loop receptors. J. Neurochem. 2011, 116, 734–741. [Google Scholar] [CrossRef]
- Stokes, C.; Treinin, M.; Papke, R.L. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2015, 36, 514–523. [Google Scholar] [CrossRef]
- King, J.R.; Nordman, J.C.; Bridges, S.P.; Lin, M.K.; Kabbani, N. Identification and Characterization of a G Protein-binding Cluster in α7 Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2015, 290, 20060–20070. [Google Scholar] [CrossRef] [PubMed]
- Hucho, F.; Tsetlin, V.I.; Machold, J. The emerging three-dimensional structure of a receptor. The nicotinic acetylcholine receptor. Eur. J. Biochem. 1996, 239, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Langlhofer, G.; Villmann, C. The Intracellular Loop of the Glycine Receptor: It’s not all about the Size. Front. Mol. Neurosci. 2016, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Ferns, M. An Inside Job: Molecular Determinants for Postsynaptic Localization of Nicotinic Acetylcholine Receptors. Molecules 2021, 26, 3065. [Google Scholar] [CrossRef] [PubMed]
- Valor, L.M.; Mulet, J.; Sala, F.; Sala, S.; Ballesta, J.J.; Criado, M. Role of the large cytoplasmic loop of the alpha 7 neuronal nicotinic acetylcholine receptor subunit in receptor expression and function. Biochemistry 2002, 41, 7931–7938. [Google Scholar] [CrossRef]
- Dau, A.; Komal, P.; Truong, M.; Morris, G.; Evans, G.; Nashmi, R. RIC-3 differentially modulates α4β2 and α7 nicotinic receptor assembly, expression, and nicotine-induced receptor upregulation. BMC Neurosci. 2013, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Bali, M.; Akabas, M.H. Modular design of Cys-loop ligand-gated ion channels: Functional 5-HT3 and GABA rho1 receptors lacking the large cytoplasmic M3M4 loop. J. Gen. Physiol. 2008, 131, 137–146. [Google Scholar] [CrossRef]
- McKinnon, N.K.; Bali, M.; Akabas, M.H. Length and amino acid sequence of peptides substituted for the 5-HT3A receptor M3M4 loop may affect channel expression and desensitization. PLoS ONE 2012, 7, e35563. [Google Scholar] [CrossRef]
- Murray, T.A.; Liu, Q.; Whiteaker, P.; Wu, J.; Lukas, R.J. Nicotinic acetylcholine receptor alpha7 subunits with a C2 cytoplasmic loop yellow fluorescent protein insertion form functional receptors. Acta Pharmacol. Sin. 2009, 30, 828–841. [Google Scholar] [CrossRef]
- Pirayesh, E.; Stuebler, A.G.; Pandhare, A.; Jansen, M. Delineating the Site of Interaction of the 5-HT3a Receptor with the Chaperone Protein RIC-3. Biophys. J. 2020, 118, 934–943. [Google Scholar] [CrossRef]
- Gharpure, A.; Noviello, C.M.; Hibbs, R.E. Progress in nicotinic receptor structural biology. Neuropharmacology 2020, 171, 108086. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Lester, H.A.; Lummis, S.C. The structural basis of function in Cys-loop receptors. Q. Rev. Biophys. 2010, 43, 449–499. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.M.; Buisson, B.; Bertrand, S.; Corringer, P.J.; Galzi, J.L.; Changeux, J.P.; Bertrand, D. Ivermectin: A positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 1998, 53, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Dawe, G.B.; Yu, H.; Gu, S.; Blackler, A.N.; Matta, J.A.; Siuda, E.R.; Rex, E.B.; Bredt, D.S. α7 nicotinic acetylcholine receptor upregulation by anti-apoptotic Bcl-2 proteins. Nat. Commun. 2019, 10, 2746. [Google Scholar] [CrossRef]
- Castillo, M.; Mulet, J.; Gutiérrez, L.M.; Ortiz, J.A.; Castelán, F.; Gerber, S.; Sala, S.; Sala, F.; Criado, M. Dual role of the RIC-3 protein in trafficking of serotonin and nicotinic acetylcholine receptors. J. Biol. Chem. 2005, 280, 27062–27068. [Google Scholar] [CrossRef]
- Castillo, M.; Mulet, J.; Gutierrez, L.M.; Ortiz, J.A.; Castelan, F.; Gerber, S.; Sala, S.; Sala, F.; Criado, M. Role of the RIC-3 protein in trafficking of serotonin and nicotinic acetylcholine receptors. J. Mol. Neurosci. 2006, 30, 153–156. [Google Scholar] [CrossRef]
- Kaji, M.D.; Geary, T.G.; Beech, R.N. A Functional Comparison of Homopentameric Nicotinic Acetylcholine Receptors (ACR-16) Receptors from Necator americanus and Ancylostoma ceylanicum. Front. Mol. Neurosci. 2020, 13, 601102. [Google Scholar] [CrossRef]
- Hansen, T.V.A.; Grencis, R.K.; Issouf, M.; Neveu, C.; Charvet, C.L. Functional Characterization of the Oxantel-Sensitive Acetylcholine Receptor from. Pharmaceuticals 2021, 14, 698. [Google Scholar] [CrossRef]
- Hansen, T.V.A.; Cirera, S.; Neveu, C.; Courtot, E.; Charvet, C.L.; Calloe, K.; Klaerke, D.A.; Martin, R.J. The narrow-spectrum anthelmintic oxantel is a potent agonist of a novel acetylcholine receptor subtype in whipworms. PLoS Pathog. 2021, 17, e1008982. [Google Scholar] [CrossRef]
- Jones, A.K.; Raymond-Delpech, V.; Thany, S.H.; Gauthier, M.; Sattelle, D.B. The nicotinic acetylcholine receptor gene family of the honey bee, Apis mellifera. Genome Res. 2006, 16, 1422–1430. [Google Scholar] [CrossRef]
- Mongan, N.P.; Jones, A.K.; Smith, G.R.; Sansom, M.S.; Sattelle, D.B. Novel alpha7-like nicotinic acetylcholine receptor subunits in the nematode Caenorhabditis elegans. Protein Sci. 2002, 11, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Kashyap, S.S.; Martin, R.J.; Robertson, A.P. Advances in our understanding of nematode ion channels as potential anthelmintic targets. Int. J. Parasitol. Drugs Drug Resist. 2022, 18, 52–86. [Google Scholar] [CrossRef] [PubMed]
- Touroutine, D.; Fox, R.M.; Von Stetina, S.E.; Burdina, A.; Miller, D.M.; Richmond, J.E. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J. Biol. Chem. 2005, 280, 27013–27021. [Google Scholar] [CrossRef] [PubMed]
- Sattelle, D.B.; Buckingham, S.D.; Akamatsu, M.; Matsuda, K.; Pienaar, I.S.; Jones, A.K.; Sattelle, B.M.; Almond, A.; Blundell, C.D. Comparative pharmacology and computational modelling yield insights into allosteric modulation of human alpha7 nicotinic acetylcholine receptors. Biochem. Pharmacol. 2009, 78, 836–843. [Google Scholar] [CrossRef]
- Ballivet, M.; Alliod, C.; Bertrand, S.; Bertrand, D. Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J. Mol. Biol. 1996, 258, 261–269. [Google Scholar] [CrossRef]
- Abongwa, M.; Buxton, S.K.; Courtot, E.; Charvet, C.L.; Neveu, C.; McCoy, C.J.; Verma, S.; Robertson, A.P.; Martin, R.J. Pharmacological profile of Ascaris suum ACR-16, a new homomeric nicotinic acetylcholine receptor widely distributed in Ascaris tissues. Br. J. Pharmacol. 2016, 173, 2463–2477. [Google Scholar] [CrossRef]
- Bentley, G.N.; Jones, A.K.; Oliveros Parra, W.G.; Agnew, A. ShAR1alpha and ShAR1beta: Novel putative nicotinic acetylcholine receptor subunits from the platyhelminth blood fluke Schistosoma. Gene 2004, 329, 27–38. [Google Scholar] [CrossRef]
- Jones, A.K.; Davis, P.; Hodgkin, J.; Sattelle, D.B. The nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans: An update on nomenclature. Invertebr. Neurosci. 2007, 7, 129–131. [Google Scholar] [CrossRef][Green Version]
- Ben-Ami, H.C.; Yassin, L.; Farah, H.; Michaeli, A.; Eshel, M.; Treinin, M. RIC-3 affects properties and quantity of nicotinic acetylcholine receptors via a mechanism that does not require the coiled-coil domains. J. Biol. Chem. 2005, 280, 28053–28060. [Google Scholar] [CrossRef]
- Ben-Ami, H.C.; Biala, Y.; Farah, H.; Elishevitz, E.; Battat, E.; Treinin, M. Receptor and Subunit Specific Interactions of RIC-3 with Nicotinic Acetylcholine Receptors. Biochemistry 2009, 48, 12329–12336. [Google Scholar] [CrossRef]
- Blanton, M.P.; Cohen, J.B. Mapping the lipid-exposed regions in the Torpedo californica nicotinic acetylcholine receptor. Biochemistry 1992, 31, 3738–3750. [Google Scholar] [CrossRef] [PubMed]
- Baenziger, J.E.; Hénault, C.M.; Therien, J.P.; Sun, J. Nicotinic acetylcholine receptor-lipid interactions: Mechanistic insight and biological function. Biochim. Biophys. Acta 2015, 1848, 1806–1817. [Google Scholar] [CrossRef] [PubMed]
- Hénault, C.M.; Sun, J.; Therien, J.P.; daCosta, C.J.; Carswell, C.L.; Labriola, J.M.; Juranka, P.F.; Baenziger, J.E. The role of the M4 lipid-sensor in the folding, trafficking, and allosteric modulation of nicotinic acetylcholine receptors. Neuropharmacology 2015, 96, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.J.; Domville, J.A.; Edrington, C.H.; Venes, A.; Giguère, P.M.; Baenziger, J.E. Distinct functional roles for the M4 α-helix from each homologous subunit in the hetero-pentameric ligand-gated ion channel nAChR. J. Biol. Chem. 2022, 298, 102104. [Google Scholar] [CrossRef] [PubMed]
- Mesoy, S.; Jeffreys, J.; Lummis, S.C.R. Characterization of Residues in the 5HT3 Receptor M4 Region That Contribute to Function. ACS Chem. Neurosci. 2019, 10, 3167–3172. [Google Scholar] [CrossRef]
- Mesoy, S.M.; Lummis, S.C.R. M4, the Outermost Helix, is Extensively Involved in Opening of the α4β2 nACh Receptor. ACS Chem. Neurosci. 2021, 12, 133–139. [Google Scholar] [CrossRef]
- da Costa Couto, A.R.G.M.; Price, K.L.; Mesoy, S.; Capes, E.; Lummis, S.C.R. The M4 Helix Is Involved in α7 nACh Receptor Function. ACS Chem. Neurosci. 2020, 11, 1406–1412. [Google Scholar] [CrossRef]
- Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005, 346, 967–989. [Google Scholar] [CrossRef]
- Unwin, N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: Insights from Torpedo postsynaptic membranes. Q. Rev. Biophys. 2013, 46, 283–322. [Google Scholar] [CrossRef]
- Rahman, M.M.; Teng, J.; Worrell, B.T.; Noviello, C.M.; Lee, M.; Karlin, A.; Stowell, M.H.B.; Hibbs, R.E. Structure of the Native Muscle-type Nicotinic Receptor and Inhibition by Snake Venom Toxins. Neuron 2020, 106, 952–962.e5. [Google Scholar] [CrossRef]
- Anderson, D.J.; Blobel, G. In vitro synthesis, glycosylation, and membrane insertion of the four subunits of Torpedo acetylcholine receptor. Proc. Natl. Acad. Sci. USA 1981, 78, 5598–5602. [Google Scholar] [CrossRef]
- Anderson, D.J.; Walter, P.; Blobel, G. Signal recognition protein is required for the integration of acetylcholine receptor delta subunit, a transmembrane glycoprotein, into the endoplasmic reticulum membrane. J. Cell. Biol. 1982, 93, 501–506. [Google Scholar] [CrossRef]
- Green, W.N.; Claudio, T. Acetylcholine receptor assembly: Subunit folding and oligomerization occur sequentially. Cell 1993, 74, 57–69. [Google Scholar] [CrossRef]
- Green, W.N.; Millar, N.S. Ion-channel assembly. Trends Neurosci. 1995, 18, 280–287. [Google Scholar] [CrossRef]
- Green, W.N. Ion channel assembly: Creating structures that function. J. Gen. Physiol. 1999, 113, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Lindstrom, J.; Merlie, J.P. Formation of the alpha-bungarotoxin binding site and assembly of the nicotinic acetylcholine receptor subunits occur in the endoplasmic reticulum. J. Biol. Chem. 1987, 262, 4367–4376. [Google Scholar] [CrossRef]
- Karlin, A.; Holtzman, E.; Yodh, N.; Lobel, P.; Wall, J.; Hainfeld, J. The arrangement of the subunits of the acetylcholine receptor of Torpedo californica. J. Biol. Chem. 1983, 258, 6678–6681. [Google Scholar] [CrossRef]
- Blount, P.; Merlie, J.P. Mutational analysis of mu.u.u.u.uscle nicotinic acetylcholine receptor subunit assembly. J. Cell. Biol. 1990, 111, 2613–2622. [Google Scholar] [CrossRef]
- Green, W.N.; Wanamaker, C.P. The role of the cystine loop in acetylcholine receptor assembly. J. Biol. Chem. 1997, 272, 20945–20953. [Google Scholar] [CrossRef]
- Rickert, K.W.; Imperiali, B. Analysis of the conserved glycosylation site in the nicotinic acetylcholine receptor: Potential roles in complex assembly. Chem. Biol. 1995, 2, 751–759. [Google Scholar] [CrossRef]
- Dellisanti, C.D.; Yao, Y.; Stroud, J.C.; Wang, Z.Z.; Chen, L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat. Neurosci. 2007, 10, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.N.; Karlin, A. Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues. J. Biol. Chem. 1986, 261, 8085–8088. [Google Scholar] [CrossRef]
- Olson, E.N.; Glaser, L.; Merlie, J.P. Alpha and beta subunits of the nicotinic acetylcholine receptor contain covalently bound lipid. J. Biol. Chem. 1984, 259, 5364–5367. [Google Scholar] [CrossRef]
- Alexander, J.K.; Govind, A.P.; Drisdel, R.C.; Blanton, M.P.; Vallejo, Y.; Lam, T.T.; Green, W.N. Palmitoylation of nicotinic acetylcholine receptors. J. Mol. Neurosci. 2010, 40, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Blount, P.; Merlie, J.P. BIP associates with newly synthesized subunits of the mouse muscle nicotinic receptor. J. Cell. Biol. 1991, 113, 1125–1132. [Google Scholar] [CrossRef]
- Paulson, H.L.; Ross, A.F.; Green, W.N.; Claudio, T. Analysis of early events in acetylcholine receptor assembly. J. Cell. Biol. 1991, 113, 1371–1384. [Google Scholar] [CrossRef]
- Gelman, M.S.; Chang, W.; Thomas, D.Y.; Bergeron, J.J.; Prives, J.M. Role of the endoplasmic reticulum chaperone calnexin in subunit folding and assembly of nicotinic acetylcholine receptors. J. Biol. Chem. 1995, 270, 15085–15092. [Google Scholar] [CrossRef]
- Keller, S.H.; Lindstrom, J.; Taylor, P. Involvement of the chaperone protein calnexin and the acetylcholine receptor beta-subunit in the assembly and cell surface expression of the receptor. J. Biol. Chem. 1996, 271, 22871–22877. [Google Scholar] [CrossRef][Green Version]
- Wanamaker, C.P.; Green, W.N. N-linked glycosylation is required for nicotinic receptor assembly but not for subunit associations with calnexin. J. Biol. Chem. 2005, 280, 33800–33810. [Google Scholar] [CrossRef]
- Merlie, J.P.; Smith, M.M. Synthesis and assembly of acetylcholine receptor, a multisubunit membrane glycoprotein. J. Membr. Biol. 1986, 91, 1–10. [Google Scholar] [CrossRef]
- Smith, M.M.; Schlesinger, S.; Lindstrom, J.; Merlie, J.P. The effects of inhibiting oligosaccharide trimming by 1-deoxynojirimycin on the nicotinic acetylcholine receptor. J. Biol. Chem. 1986, 261, 14825–14832. [Google Scholar] [CrossRef]
- Moretti, M.; Zoli, M.; George, A.A.; Lukas, R.J.; Pistillo, F.; Maskos, U.; Whiteaker, P.; Gotti, C. The novel α7β2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: Biochemical and pharmacological characterization. Mol. Pharmacol. 2014, 86, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Borroni, V.; Barrantes, F.J. Homomeric and Heteromeric α7 Nicotinic Acetylcholine Receptors in Health and Some Central Nervous System Diseases. Membranes 2021, 11, 664. [Google Scholar] [CrossRef]
- Rex, E.B.; Shukla, N.; Gu, S.; Bredt, D.; DiSepio, D. A Genome-Wide Arrayed cDNA Screen to Identify Functional Modulators of α7 Nicotinic Acetylcholine Receptors. SLAS Discov. 2017, 22, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.S.; Hajós, M.; Raggenbass, M.; Wall, T.M.; Higdon, N.R.; Lawson, J.A.; Rutherford-Root, K.L.; Berkenpas, M.B.; Hoffmann, W.E.; Piotrowski, D.W.; et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: In vitro and in vivo characterization. J. Neurosci. 2005, 25, 4396–4405. [Google Scholar] [CrossRef]
- Yamauchi, J.G.; Nemecz, Á.; Nguyen, Q.T.; Muller, A.; Schroeder, L.F.; Talley, T.T.; Lindstrom, J.; Kleinfeld, D.; Taylor, P. Characterizing ligand-gated ion channel receptors with genetically encoded Ca2++ sensors. PLoS ONE 2011, 6, e16519. [Google Scholar] [CrossRef]
- Roncarati, R.; Seredenina, T.; Jow, B.; Jow, F.; Papini, S.; Kramer, A.; Bothmann, H.; Dunlop, J.; Terstappen, G.C. Functional properties of alpha7 nicotinic acetylcholine receptors co-expressed with RIC-3 in a stable recombinant CHO-K1 cell line. Assay Drug Dev. Technol. 2008, 6, 181–193. [Google Scholar] [CrossRef]
- Andersen, N.; Corradi, J.; Sine, S.M.; Bouzat, C. Stoichiometry for activation of neuronal α7 nicotinic receptors. Proc. Natl. Acad. Sci. USA 2013, 110, 20819–20824. [Google Scholar] [CrossRef]
- Eisele, J.L.; Bertrand, S.; Galzi, J.L.; Devillers-Thiery, A.; Changeux, J.P.; Bertrand, D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature 1993, 366, 479–483. [Google Scholar] [CrossRef]
- Gee, V.J.; Kracun, S.; Cooper, S.T.; Gibb, A.J.; Millar, N.S. Identification of domains influencing assembly and ion channel properties in alpha 7 nicotinic receptor and 5-HT3 receptor subunit chimaeras. Br. J. Pharmacol. 2007, 152, 501–512. [Google Scholar] [CrossRef]
- Kracun, S.; Harkness, P.C.; Gibb, A.J.; Millar, N.S. Influence of the M3-M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. Br. J. Pharmacol. 2008, 153, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.J.; Bose, S.; Zwart, R.; Beattie, R.E.; Folly, E.A.; Johnson, L.R.; Bell, E.; Evans, N.M.; Benedetti, G.; Pearson, K.H.; et al. Stable expression and characterisation of a human alpha 7 nicotinic subunit chimera: A tool for functional high-throughput screening. Eur. J. Pharmacol. 2004, 502, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Campos-Caro, A.; Sala, S.; Ballesta, J.J.; Vicente-Agulló, F.; Criado, M.; Sala, F. A single residue in the M2-M3 loop is a major determinant of coupling between binding and gating in neuronal nicotinic receptors. Proc. Natl. Acad. Sci. USA 1996, 93, 6118–6123. [Google Scholar] [CrossRef]
- Quiram, P.A.; Sine, S.M. Identification of residues in the neuronal alpha7 acetylcholine receptor that confer selectivity for conotoxin ImI. J. Biol. Chem. 1998, 273, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- García-Guzmán, M.; Sala, F.; Sala, S.; Campos-Caro, A.; Criado, M. Role of two acetylcholine receptor subunit domains in homomer formation and intersubunit recognition, as revealed by alpha 3 and alpha 7 subunit chimeras. Biochemistry 1994, 33, 15198–15203. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.K.; Loring, R.H. Evaluating Commercially Available Antibodies for Rat α7 Nicotinic Acetylcholine Receptors. J. Histochem. Cytochem. 2017, 65, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Couturier, S.; Bertrand, D.; Matter, J.M.; Hernandez, M.C.; Bertrand, S.; Millar, N.; Valera, S.; Barkas, T.; Ballivet, M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron 1990, 5, 847–856. [Google Scholar] [CrossRef]
- Kassner, P.D.; Berg, D.K. Differences in the fate of neuronal acetylcholine receptor protein expressed in neurons and stably transfected cells. J. Neurobiol. 1997, 33, 968–982. [Google Scholar] [CrossRef]
- Cooper, S.T.; Millar, N.S. Host cell-specific folding and assembly of the neuronal nicotinic acetylcholine receptor alpha7 subunit. J. Neurochem. 1997, 68, 2140–2151. [Google Scholar] [CrossRef]
- Rangwala, F.; Drisdel, R.C.; Rakhilin, S.; Ko, E.; Atluri, P.; Harkins, A.B.; Fox, A.P.; Salman, S.S.; Green, W.N. Neuronal alpha-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. J. Neurosci. 1997, 17, 8201–8212. [Google Scholar] [CrossRef]
- Sweileh, W.; Wenberg, K.; Xu, J.; Forsayeth, J.; Hardy, S.; Loring, R.H. Multistep expression and assembly of neuronal nicotinic receptors is both host-cell- and receptor-subtype-dependent. Brain Res. Mol. Brain Res. 2000, 75, 293–302. [Google Scholar] [CrossRef]
- Nguyen, M.; Alfonso, A.; Johnson, C.D.; Rand, J.B. Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 1995, 140, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Halevi, S.; McKay, J.; Palfreyman, M.; Yassin, L.; Eshel, M.; Jorgensen, E.; Treinin, M. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002, 21, 1012–1020. [Google Scholar] [CrossRef]
- Cheng, A.; Bollan, K.A.; Greenwood, S.M.; Irving, A.J.; Connolly, C.N. Differential subcellular localization of RIC-3 isoforms and their role in determining 5-HT3 receptor composition. J. Biol. Chem. 2007, 282, 26158–26166. [Google Scholar] [CrossRef]
- Millar, N.S. RIC-3: A nicotinic acetylcholine receptor chaperone. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S177–S183. [Google Scholar] [CrossRef]
- Halevi, S.; Yassin, L.; Eshel, M.; Sala, F.; Sala, S.; Criado, M.; Treinin, M. Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J. Biol. Chem. 2003, 278, 34411–34417. [Google Scholar] [CrossRef] [PubMed]
- Treinin, M.; Jin, Y. Cholinergic transmission in C. elegans: Functions, diversity, and maturation of ACh-activated ion channels. J. Neurochem. 2021, 158, 1274–1291. [Google Scholar] [CrossRef]
- Quik, M.; Choremis, J.; Komourian, J.; Lukas, R.J.; Puchacz, E. Similarity between rat brain nicotinic alpha-bungarotoxin receptors and stably expressed alpha-bungarotoxin binding sites. J. Neurochem. 1996, 67, 145–154. [Google Scholar] [CrossRef]
- Koperniak, T.M.; Garg, B.K.; Boltax, J.; Loring, R.H. Cell-specific effects on surface α7 nicotinic receptor expression revealed by over-expression and knockdown of rat RIC3 protein. J. Neurochem. 2013, 124, 300–309. [Google Scholar] [CrossRef]
- Matta, J.A.; Gu, S.; Davini, W.B.; Lord, B.; Siuda, E.R.; Harrington, A.W.; Bredt, D.S. NACHO Mediates Nicotinic Acetylcholine Receptor Function throughout the Brain. Cell Rep. 2017, 19, 688–696. [Google Scholar] [CrossRef]
- Castelán, F.; Castillo, M.; Mulet, J.; Sala, S.; Sala, F.; Domínguez Del Toro, E.; Criado, M. Molecular characterization and localization of the RIC-3 protein, an effector of nicotinic acetylcholine receptor expression. J. Neurochem. 2008, 105, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Vinayakamoorthy, R.M.; Garg, B.K.; Thummapudi, J.P.; Oza, G.; Adhikari, K.; Agarwal, A.; Dalvi, P.; Iyer, S.; Thulasi Raman, S.; et al. Why Does Knocking out NACHO, but not RIC3, Completely Block Expression of α7 Nicotinic Receptors in Mouse Brain? Biomolecules 2020, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Termini, C.M.; Gillette, J.M. Tetraspanins Function as Regulators of Cellular Signaling. Front. Cell Dev. Biol. 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chetkovich, D.M.; Petralia, R.S.; Sweeney, N.T.; Kawasaki, Y.; Wenthold, R.J.; Bredt, D.S.; Nicoll, R.A. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000, 408, 936–943. [Google Scholar] [CrossRef]
- Vandenberghe, W.; Nicoll, R.A.; Bredt, D.S. Interaction with the unfolded protein response reveals a role for stargazin in biosynthetic AMPA receptor transport. J. Neurosci. 2005, 25, 1095–1102. [Google Scholar] [CrossRef]
- Biala, Y.; Liewald, J.F.; Ben-Ami, H.C.; Gottschalk, A.; Treinin, M. The conserved RIC-3 coiled-coil domain mediates receptor-specific interactions with nicotinic acetylcholine receptors. Mol. Biol. Cell 2009, 20, 1419–1427. [Google Scholar] [CrossRef]
- Lansdell, S.J.; Collins, T.; Yabe, A.; Gee, V.J.; Gibb, A.J.; Millar, N.S. Host-cell specific effects of the nicotinic acetylcholine receptor chaperone RIC-3 revealed by a comparison of human and Drosophila RIC-3 homologues. J. Neurochem. 2008, 105, 1573–1581. [Google Scholar] [CrossRef]
- Lansdell, S.J.; Gee, V.J.; Harkness, P.C.; Doward, A.I.; Baker, E.R.; Gibb, A.J.; Millar, N.S. RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol. Pharmacol. 2005, 68, 1431–1438. [Google Scholar] [CrossRef]
- Akopian, D.; Shen, K.; Zhang, X.; Shan, S.O. Sign.nal recognition particle: An essential protein-targeting machine. Annu. Rev. Biochem. 2013, 82, 693–721. [Google Scholar] [CrossRef]
- Wu, X.; Cabanos, C.; Rapoport, T.A. Structure of the post-translational protein translocation machinery of the ER membrane. Nature 2019, 566, 136–139. [Google Scholar] [CrossRef]
- Voss, M.; Schröder, B.; Fluhrer, R. Mechanism, specificity, and physiology of signal peptide peptidase (SPP) and SPP-like proteases. Biochim. Biophys. Acta 2013, 1828, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Chaudhary, B.P.; Zoetewey, D. Structural Insight into the Mechanism of N-Linked Glycosylation by Oligosaccharyltransferase. Biomolecules 2020, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dang, H.; Patrick, J.W. Contributions of N-linked glycosylation to the expression of a functional alpha7-nicotinic receptor in Xenopus oocytes. J. Neurochem. 1998, 70, 349–357. [Google Scholar] [CrossRef]
- Bañó-Polo, M.; Baeza-Delgado, C.; Tamborero, S.; Hazel, A.; Grau, B.; Nilsson, I.; Whitley, P.; Gumbart, J.C.; von Heijne, G.; Mingarro, I. Transmembrane but not soluble helices fold inside the ribosome tunnel. Nat. Commun. 2018, 9, 5246. [Google Scholar] [CrossRef]
- Egea, P.F.; Stroud, R.M. Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes. Proc. Natl. Acad. Sci. USA 2010, 107, 17182–17187. [Google Scholar] [CrossRef]
- Adams, B.M.; Canniff, N.P.; Guay, K.P.; Hebert, D.N. The Role of Endoplasmic Reticulum Chaperones in Protein Folding and Quality Control. Prog. Mol. Subcell. Biol. 2021, 59, 27–50. [Google Scholar] [CrossRef]
- Schrag, J.D.; Bergeron, J.J.; Li, Y.; Borisova, S.; Hahn, M.; Thomas, D.Y.; Cygler, M. The Structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol. Cell 2001, 8, 633–644. [Google Scholar] [CrossRef]
- Kozlov, G.; Gehring, K. Calnexin cycle—Structural features of the ER chaperone system. FEBS J. 2020, 287, 4322–4340. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Pocanschi, C.L.; Rosenauer, A.; Bastos-Aristizabal, S.; Gorelik, A.; Williams, D.B.; Gehring, K. Structural basis of carbohydrate recognition by calreticulin. J. Biol. Chem. 2010, 285, 38612–38620. [Google Scholar] [CrossRef] [PubMed]
- Dunckley, T.; Wu, J.; Zhao, L.; Lukas, R.J. Mutational analysis of roles for extracellular cysteine residues in the assembly and function of human alpha 7-nicotinic acetylcholine receptors. Biochemistry 2003, 42, 870–876. [Google Scholar] [CrossRef]
- Drisdel, R.C.; Manzana, E.; Green, W.N. The role of palmitoylation in functional expression of nicotinic alpha7 receptors. J. Neurosci. 2004, 24, 10502–10510. [Google Scholar] [CrossRef] [PubMed]
- Dhara, M.; Matta, J.A.; Lei, M.; Knowland, D.; Yu, H.; Gu, S.; Bredt, D.S. Polyamine regulation of ion channel assembly and implications for nicotinic acetylcholine receptor pharmacology. Nat. Commun. 2020, 11, 2799. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.K.; Sagher, D.; Krivoshein, A.V.; Criado, M.; Jefford, G.; Green, W.N. Ric-3 promotes alpha7 nicotinic receptor assembly and trafficking through the ER subcompartment of dendrites. J. Neurosci. 2010, 30, 10112–10126. [Google Scholar] [CrossRef] [PubMed]
- Lansdell, S.J.; Millar, N.S. Molecular characterization of Dalpha6 and Dalpha7 nicotinic acetylcholine receptor subunits from Drosophila: Formation of a high-affinity alpha-bungarotoxin binding site revealed by expression of subunit chimeras. J. Neurochem. 2004, 90, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Seredenina, T.; Ferraro, T.; Terstappen, G.C.; Caricasole, A.; Roncarati, R. Molecular cloning and characterization of a novel human variant of RIC-3, a putative chaperone of nicotinic acetylcholine receptors. Biosci. Rep. 2008, 28, 299–306. [Google Scholar] [CrossRef]
- Ben-David, Y.; Mizrachi, T.; Kagan, S.; Krisher, T.; Cohen, E.; Brenner, T.; Treinin, M. RIC-3 expression and splicing regulate nAChR functional expression. Mol. Brain 2016, 9, 47. [Google Scholar] [CrossRef]
- Mulcahy, M.J.; Paulo, J.A.; Hawrot, E. Proteomic Investigation of Murine Neuronal α7-Nicotinic Acetylcholine Receptor Interacting Proteins. J. Proteome Res. 2018, 17, 3959–3975. [Google Scholar] [CrossRef]
- Paulo, J.A.; Brucker, W.J.; Hawrot, E. Proteomic analysis of an alpha7 nicotinic acetylcholine receptor interactome. J. Proteome Res. 2009, 8, 1849–1858. [Google Scholar] [CrossRef]
- Mulcahy, M.J.; Blattman, S.B.; Barrantes, F.J.; Lukas, R.J.; Hawrot, E. Resistance to Inhibitors of Cholinesterase 3 (Ric-3) Expression Promotes Selective Protein Associations with the Human α7-Nicotinic Acetylcholine Receptor Interactome. PLoS ONE 2015, 10, e0134409. [Google Scholar] [CrossRef]
- Rudell, J.C.; Borges, L.S.; Yarov-Yarovoy, V.; Ferns, M. The MX-Helix of Muscle nAChR Subunits Regulates Receptor Assembly and Surface Trafficking. Front. Mol. Neurosci. 2020, 13, 48. [Google Scholar] [CrossRef]
- Margeta-Mitrovic, M.; Jan, Y.N.; Jan, L.Y. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 2000, 27, 97–106. [Google Scholar] [CrossRef]
- Walstab, J.; Hammer, C.; Lasitschka, F.; Möller, D.; Connolly, C.N.; Rappold, G.; Brüss, M.; Bönisch, H.; Niesler, B. RIC-3 exclusively enhances the surface expression of human homomeric 5-hydroxytryptamine type 3A (5-HT3A) receptors despite direct interactions with 5-HT3A, -C, -D, and -E subunits. J. Biol. Chem. 2010, 285, 26956–26965. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Salahudeen, A.A.; Jansen, M. Engineering a prokaryotic Cys-loop receptor with a third functional domain. J. Biol. Chem. 2011, 286, 34635–34642. [Google Scholar] [CrossRef] [PubMed]
- Nishtala, S.N.; Mnatsakanyan, N.; Pandhare, A.; Leung, C.; Jansen, M. Direct interaction of the resistance to inhibitors of cholinesterase type 3 protein with the serotonin receptor type 3A intracellular domain. J. Neurochem. 2016, 137, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jeffery, C.J. Moonlighting Proteins in the Fuzzy Logic of Cellular Metabolism. Molecules 2020, 25, 3440. [Google Scholar] [CrossRef]
- Shteingauz, A.; Cohen, E.; Biala, Y.; Treinin, M. The BTB-MATH protein BATH-42 interacts with RIC-3 to regulate maturation of nicotinic acetylcholine receptors. J. Cell Sci. 2009, 122, 807–812. [Google Scholar] [CrossRef][Green Version]
- Safdie, G.; Liewald, J.F.; Kagan, S.; Battat, E.; Gottschalk, A.; Treinin, M. RIC-3 phosphorylation enables dual regulation of excitation and inhibition of Caenorhabditis elegans muscle. Mol. Biol. Cell 2016, 27, 2994–3003. [Google Scholar] [CrossRef]
- Séguéla, P.; Wadiche, J.; Dineley-Miller, K.; Dani, J.A.; Patrick, J.W. Molecular cloning, functional properties, and distribution of rat brain alpha 7: A nicotinic cation channel highly permeable to calcium. J. Neurosci. 1993, 13, 596–604. [Google Scholar] [CrossRef]
- Bertrand, D.; Galzi, J.L.; Devillers-Thiéry, A.; Bertrand, S.; Changeux, J.P. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 6971–6975. [Google Scholar] [CrossRef]
- Fucile, S.; Palma, E.; Mileo, A.M.; Miledi, R.; Eusebi, F. Human neuronal threonine-for-leucine-248 alpha 7 mutant nicotinic acetylcholine receptors are highly Ca2+ permeable. Proc. Natl. Acad. Sci. USA 2000, 97, 3643–3648. [Google Scholar] [CrossRef]
- Trump, B.F.; Berezesky, I.K. Calcium-mediated cell injury and cell death. FASEB J. 1995, 9, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, C.Z.; Barrall, E.A.; Rissman, R.A.; Joiner, W.J. Unbalanced Regulation of α7 nAChRs by Ly6h and NACHO Contributes to Neurotoxicity in Alzheimer’s Disease. J. Neurosci. 2021, 41, 8461–8474. [Google Scholar] [CrossRef] [PubMed]
- Loughner, C.L.; Bruford, E.A.; McAndrews, M.S.; Delp, E.E.; Swamynathan, S.; Swamynathan, S.K. Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum. Genom. 2016, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Puddifoot, C.A.; Wu, M.; Sung, R.J.; Joiner, W.J. Ly6h regulates trafficking of alpha7 nicotinic acetylcholine receptors and nicotine-induced potentiation of glutamatergic signaling. J. Neurosci. 2015, 35, 3420–3430. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Huang, C.C.; Ferrin, T.E. Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinform. 2006, 7, 339. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef]
- Studer, G.; Rempfer, C.; Waterhouse, A.M.; Gumienny, R.; Haas, J.; Schwede, T. QMEANDisCo-distance constraints applied on model quality estimation. Bioinformatics 2020, 36, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc 2007, 2, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Lupas, A.; Van Dyke, M.; Stock, J. Predicting coiled coils from protein sequences. Science 1991, 252, 1162–1164. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry 1994, 33, 3038–3049. [Google Scholar] [CrossRef]
- Käll, L.; Krogh, A.; Sonnhammer, E.L. Advantages of combined transmembrane topology and signal peptide prediction--the Phobius web server. Nucleic Acids Res. 2007, 35, W429–W432. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Erdős, G.; Dosztányi, Z. Analyzing Protein Disorder with IUPred2A. Curr. Protoc. Bioinform. 2020, 70, e99. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loring, R.H. Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly. Molecules 2022, 27, 4527. https://doi.org/10.3390/molecules27144527
Loring RH. Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly. Molecules. 2022; 27(14):4527. https://doi.org/10.3390/molecules27144527
Chicago/Turabian StyleLoring, Ralph H. 2022. "Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly" Molecules 27, no. 14: 4527. https://doi.org/10.3390/molecules27144527
APA StyleLoring, R. H. (2022). Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly. Molecules, 27(14), 4527. https://doi.org/10.3390/molecules27144527





