Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review
Abstract
1. Introduction
2. Effects of Berberine on Cancers
2.1. Breast Cancer
2.2. Lung Cancer
2.3. Gastric Cancer
2.4. Liver Cancer
2.5. Colorectal Cancer
2.6. Ovarian Cancer
2.7. Cervical Cancer
2.8. Prostate Cancer
2.9. Other Cancers
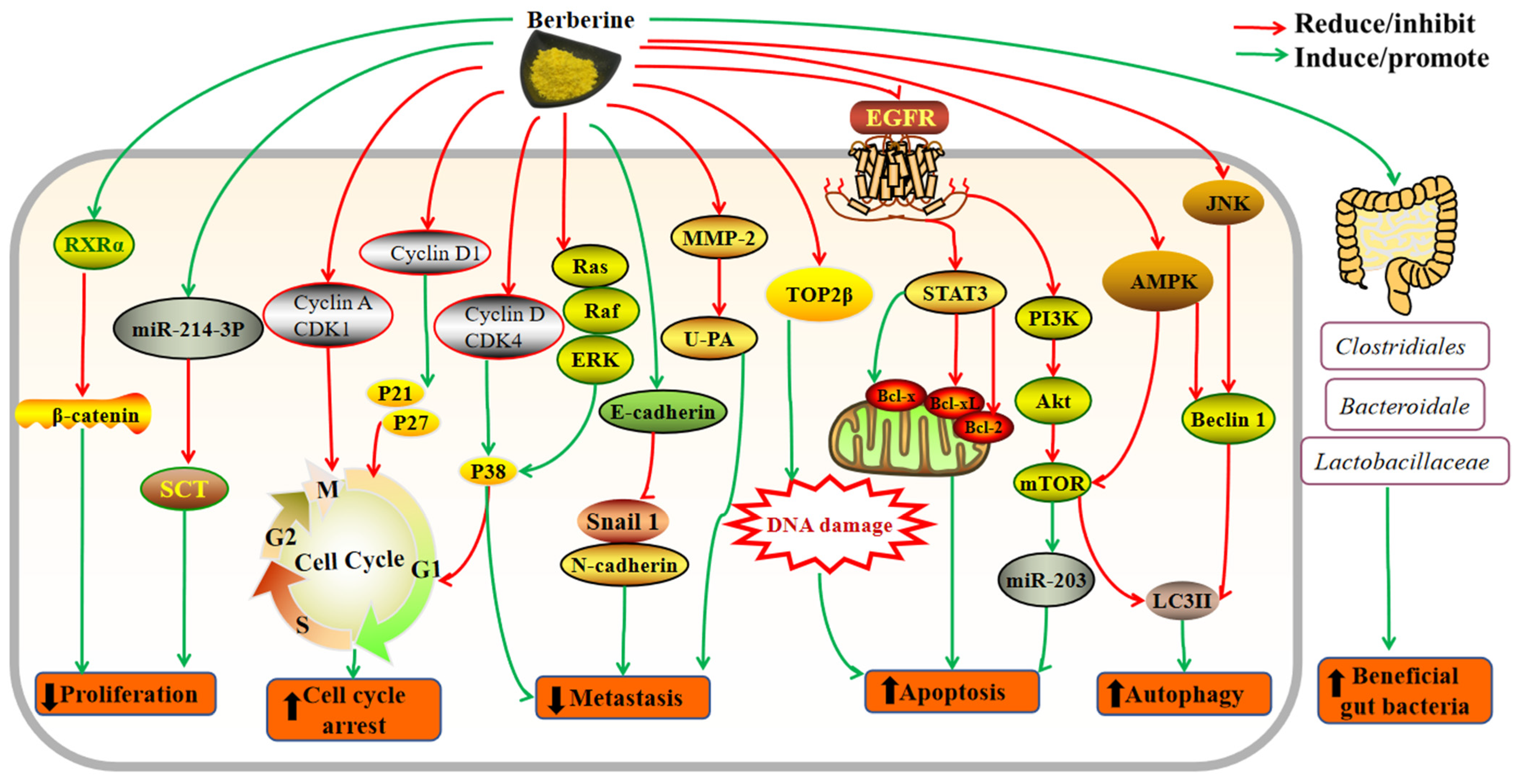
3. Mechanisms of Action
4. Bioavailability of Berberine
5. Safety of Berberine
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Gan, R.Y.; Li, H.B.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.J.; Xu, D.P.; Li, H.B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef]
- Saimaiti, A.; Zhou, D.D.; Li, J.; Xiong, R.G.; Gan, R.Y.; Huang, S.Y.; Shang, A.; Zhao, C.N.; Li, H.Y.; Li, H.B. Dietary sources, health benefits, and risks of caffeine. Crit. Rev. Food Sci. Nutr. 2022, in press. [CrossRef]
- Xu, X.Y.; Zhao, C.N.; Cao, S.Y.; Tang, G.Y.; Gan, R.Y.; Li, H.B. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1693–1705. [Google Scholar] [CrossRef]
- Zhao, C.N.; Li, Y.; Meng, X.; Li, S.; Liu, Q.; Tang, G.Y.; Gan, R.Y.; Li, H.B. Insight into the roles of vitamins C and D against cancer: Myth or truth? Cancer Lett. 2018, 431, 161–170. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for prevention and treatment of cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.C.; Chai, L.W.; Cao, S.J.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res. 2019, 33, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverria, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Vallega, K.A.; Chen, H.Y.; Zhou, J.; Ramalingam, S.S.; Sun, S.Y. The natural product berberine synergizes with osimertinib preferentially against MET-amplified osimertinib-resistant lung cancer via direct MET inhibition. Pharmacol. Res. 2022, 175, 105998. [Google Scholar] [CrossRef]
- Gan, R.Y. Bioactivities of berberine: An update. Int. J. Mod. Biol. Med. 2012, 1, 48–81. [Google Scholar]
- Ortiz, L.M.G.; Lombardi, P.; Tillhon, M.; Scovassi, A.I. Berberine, an epiphany against cancer. Molecules 2014, 19, 12349–12367. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.Y.; Li, L.; Yuen, M.F.; Feng, Y.B. Berberine and Coptidis rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef]
- Xia, Y.Y.; Chen, S.Z.; Cui, J.F.; Wang, Y.; Liu, X.C.; Shen, Y.L.; Gong, L.; Jiang, X.W.; Wang, W.F.; Zhu, Y.F.; et al. Berberine suppresses bladder cancer cell proliferation by inhibiting JAK1-STAT3 signaling via upregulation of miR-17-5p. Biochem. Pharmacol. 2021, 188, 114575. [Google Scholar] [CrossRef]
- Khan, S.; Hussain, A.; Attar, F.; Bloukh, S.H.; Edis, Z.; Sharifi, M.; Balali, E.; Nemati, F.; Derakhshankhah, H.; Zeinabad, H.A.; et al. A review of the berberine natural polysaccharide nanostructures as potential anticancer and antibacterial agents. Biomed. Pharmacother. 2022, 146, 112531. [Google Scholar] [CrossRef]
- Zhang, C.; Sheng, J.; Li, G.; Zhao, L.; Wang, Y.; Yang, W.; Yao, X.; Sun, L.; Zhang, Z.; Cui, R. Effects of berberine and its derivatives on cancer: A systems pharmacology review. Front. Pharmacol. 2019, 10, 1461. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Khalil, A.A.; Imran, M.; Shah, Z.A.; Emran, T.B.; Mitra, S.; Khan, Z.; Alhumaydhi, F.A.; Aljohani, A.S.M.; et al. Berberine as a potential anticancer agent: A comprehensive review. Molecules 2021, 26, 7368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The anti-cancer mechanisms of berberine: A review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary natural products for prevention and treatment of breast cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, W.T.; Tong, Y.W. Berberine inhibits proliferative ability of breast cancer cells by reducing metadherin. Med. Sci. Monit. 2019, 25, 9058–9066. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.J.; Fan, X.D.; Yuan, B.; Takagi, N.; Liu, S.; Han, X.; Ren, J.G.; Liu, J.X. Berberine inhibits NLRP3 inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement. Altern. Med. 2019, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Karin, M. Inflammatory cytokines in cancer: Tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011, 70, I104–I108. [Google Scholar] [CrossRef]
- Sefidabi, R.; Mortazavi, P.; Hosseini, S. Antiproliferative effect of berberine on canine mammary gland cancer cell culture. Biomed. Rep. 2017, 6, 95–98. [Google Scholar] [CrossRef][Green Version]
- Khalki, L.E.; Maire, V.; Dubois, T.; Zyad, A. Berberine impairs the survival of triple negative breast cancer cells: Cellular and molecular analyses. Molecules 2020, 25, 506. [Google Scholar] [CrossRef]
- Lin, Y.S.; Chiu, Y.C.; Tsai, Y.H.; Tsai, Y.F.; Wang, J.Y.; Tseng, L.M.; Chiu, J.H. Different mechanisms involved in the berberine-induced antiproliferation effects in triple-negative breast cancer cell lines. J. Cell. Biochem. 2019, 120, 13531–13544. [Google Scholar] [CrossRef]
- Ahmadiankia, N.; Moghaddam, H.K.; Mishan, M.A.; Bahrami, A.R.; Meshkin, H.N.; Bidkhori, H.R.; Moghaddam, M.; Mirfeyzi, S.J.A. Berberine suppresses migration of MCF-7 breast cancer cells through down-regulation of chemokine receptors. Iran. J. Basic Med. Sci. 2016, 19, 125–131. [Google Scholar] [PubMed]
- Zhu, C.Y.; Li, J.P.; Hua, Y.M.; Wang, J.L.; Wang, K.; Sun, J.Q. Berberine inhibits the expression of SCT through miR-214-3p stimulation in breast cancer cells. Evid. Based Complement. Altern. Med. 2020, 2020, 2817147. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; You, D.; Kang, H.G.; Yu, J.; Kim, S.W.; Nam, S.J.; Lee, J.E.; Kim, S. Berberine suppresses fibronectin expression through inhibition of c-Jun phosphorylation in breast cancer cells. J. Breast Cancer 2018, 21, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, F.; Zhao, Y.W.; Shao, D.; Zheng, X.; Chen, Y.J.; He, K.; Li, J.; Chen, L. Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast cancer. J. Cancer 2017, 8, 1679–1689. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Kitaguchi, D.; Morinami, S.; Kurashiki, Y.; Hashida, H.; Miyata, S.; Yamaguchi, M.; Sakai, M.; Murata, N.; Tanaka, S. Berberine-induced nucleolar stress response in a human breast cancer cell line. Biochem. Biophys. Res. Commun. 2020, 528, 227–233. [Google Scholar] [CrossRef]
- Tak, J.; Sabarwal, A.; Shyanti, R.K.; Singh, R.P. Berberine enhances posttranslational protein stability of p21/cip1 in breast cancer cells via down-regulation of Akt. Mol. Cell. Biochem. 2019, 458, 49–59. [Google Scholar] [CrossRef]
- Gao, X.J.; Wang, J.; Li, M.Q.; Wang, J.; Lv, J.; Zhang, L.; Sun, C.F.; Ji, J.M.; Yang, W.B.; Zhao, Z.N.; et al. Berberine attenuates XRCC1-mediated base excision repair and sensitizes breast cancer cells to the chemotherapeutic drugs. J. Cell. Mol. Med. 2019, 23, 6797–6804. [Google Scholar] [CrossRef]
- Ponnusamy, L.; Kothandan, G.; Manoharan, R. Berberine and Emodin abrogates breast cancer growth and facilitates apoptosis through inactivation of SIK3-induced mTOR and Akt signaling pathway. BBA Mol. Basis Dis. 2020, 1866, 165897. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.Y. Berberine and chemotherapeutic drugs synergistically inhibits cell proliferation and migration of breast cancer cells. Int. J. Clin. Exp. Med. 2018, 11, 13243–13250. [Google Scholar]
- Zhao, Y.W.; Jing, Z.L.; Li, Y.; Mao, W.F. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncol. Rep. 2016, 36, 567–572. [Google Scholar] [CrossRef]
- Qian, K.; Tang, C.Y.; Chen, L.Y.; Zheng, S.; Zhao, Y.; Ma, L.S.; Xu, L.; Fan, L.H.; Yu, J.D.; Tan, H.S.; et al. Berberine reverses breast cancer multidrug resistance based on fluorescence pharmacokinetics in vitro and in vivo. ACS Omega 2021, 6, 10645–10654. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Yao, B.; Dong, T.; Chen, Y.; Yao, J.; Liu, Y.; Li, H.; Bai, H.; Liu, X.; Zhang, Y.; et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 2022, 185, 1356–1372.e1326. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Muthupalani, S.; Erdman, S.; Liu, H.; Niu, Z.; Wang, T.C.; Fox, J.G. Translocation of Helicobacter hepaticus synergizes with myeloid-derived suppressor cells and contributes to breast carcinogenesis. Oncoimmunology 2022, 11, 2057399. [Google Scholar] [CrossRef]
- Wolf, P.G.; Devendran, S.; Doden, H.L.; Ly, L.K.; Moore, T.; Takei, H.; Nittono, H.; Murai, T.; Kurosawa, T.; Chlipala, G.E.; et al. Berberine alters gut microbial function through modulation of bile acids. BMC Microbiol. 2021, 21, 24. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, F.; Miao, P.; Mao, Z.; Redfearn, D.P.; Cao, R.Y. Antimicrobial natural product berberine is efficacious for the treatment of atrial fibrillation. BioMed Res. Int. 2017, 2017, 3146791. [Google Scholar] [CrossRef]
- Huang, X.X.; Yi, Y.L.; Yong, J.Y.; Sun, J.Y.; Song, Z.; Li, D.M.; Li, Y. Inhibitory effect of berberine hydrochloride against Candida albicans and the role of the HOG-MAPK pathway. J. Antibiot. 2021, 74, 807–816. [Google Scholar] [CrossRef]
- Kocic, B.D.; Dimitrijevic, M.V.; Miladinovic, L.C.; Markovic, M.S.; Rankovic, G.Z.; Miladinovic, D.L. In vitro anti-Helicobacter pylori activity of berberine and barberry extracts: A preliminary report. Nat. Prod. Commun. 2019, 14, 1934578X19857905. [Google Scholar] [CrossRef]
- Li, Y.; Wen, H.L.; Ge, X.Z. Hormesis effect of berberine against klebsiella pneumoniae is mediated by up-regulation of the efflux pump kmrA. J. Nat. Prod. 2021, 84, 2885–2892. [Google Scholar] [CrossRef]
- Tan, J.; Wang, J.; Yang, C.; Zhu, C.; Guo, G.; Tang, J.; Shen, H. Antimicrobial characteristics of berberine against prosthetic joint infection-related Staphylococcus aureus of different multi-locus sequence types. BMC Complement. Altern. Med. 2019, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Y.; Li, Y.; Meng, X.; Zhao, C.N.; Li, S.; Gan, R.Y.; Li, H.B. Dietary natural products and lung cancer: Effects and mechanisms of action. J. Funct. Foods 2019, 52, 316–331. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.F.; Tao, C.; Wang, L.; Chen, Z.D.; Li, X.P.; Zeng, Q.; Ma, M.; Zhang, R.; Wu, Z.Z. Berberine chloride suppresses non-small cell lung cancer by deregulating Sin3A/TOP2B pathway in vitro and in vivo. Cancer Chemother. Pharmacol. 2020, 86, 151–161. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Jiang, S.L.; Liu, J.; Chen, X.H.; Zhang, S.N.A.; Zhao, H.B. Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non-small cell lung cancer via the suppression of the MMP2 and Bcl-2/Bax signaling pathways. Oncol. Lett. 2018, 15, 7409–7414. [Google Scholar] [CrossRef]
- James, M.A.; Fu, H.; Liu, Y.; Chen, D.R.; You, M. Dietary administration of berberine or Phellodendron amurense extract inhibits cell cycle progression and lung tumorigenesis. Mol. Carcinog. 2011, 50, 1–7. [Google Scholar] [CrossRef]
- Ni, L.L.; Li, Z.J.; Ren, H.L.; Kong, L.Z.; Chen, X.; Xiong, M.R.; Zhang, X.Q.; Ning, B.B.; Li, J.A. Berberine inhibits non-small cell lung cancer cell growth through repressing DNA repair and replication rather than through apoptosis. Clin. Exp. Pharmacol. Physiol. 2022, 49, 134–144. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Shi, J.M.; Ding, Z.; Xia, Q.; Zheng, T.S.; Ren, Y.B.; Li, M.; Fan, L.H. Berberine induces apoptosis in non-small-cell lung cancer cells by upregulating miR-19a targeting tissue factor. Cancer Manag. Res. 2019, 11, 9005–9015. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Hou, Y.Q.; Li, D.; Ding, Z.; Xu, X.; Hao, B.J.; Xia, Q.; Li, M.; Fan, L.H. Berberine induces non-small cell lung cancer apoptosis via the activation of the ROS/ASK1/JNK pathway. Ann. Transl. Med. 2022, in press. [CrossRef]
- Chen, P.; Dai, C.H.; Shi, Z.H.; Wang, Y.; Wu, J.N.; Chen, K.; Su, J.Y.; Li, J. Synergistic inhibitory effect of berberine and icotinib on non-small cell lung cancer cells via inducing autophagic cell death and apoptosis. Apoptosis 2021, 26, 639–656. [Google Scholar] [CrossRef]
- Zheng, F.; Li, J.; Ma, C.J.; Tang, X.J.; Tang, Q.; Wu, J.J.; Chai, X.S.; Xie, J.H.; Yang, X.B.; Hann, S.S. Novel regulation of miR-34a-5p and HOTAIR by the combination of berberine and gefitinib leading to inhibition of EMT in human lung cancer. J. Cell. Mol. Med. 2020, 24, 5578–5592. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Shang, A.; Gan, R.Y.; Wu, D.T.; Atanasov, A.G.; Li, H.B. Phytochemicals for the prevention and treatment of gastric cancer: Effects and mechanisms. Int. J. Mol. Sci. 2020, 21, 570. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.T.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Yang, Y.H.; Zhang, N.; Li, K.D.; Chen, J.; Qiu, L.; Zhang, J.F. Integration of microRNA-mRNA profiles and pathway analysis of plant isoquinoline alkaloid berberine in SGC-7901 gastric cancers cells. Drug Des. Dev. Ther. 2018, 12, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Wu, H.; Zhang, B.B.; Shi, H.L.; Wu, X.J. MAPK pathways are involved in the inhibitory effect of berberine hydrochloride on gastric cancer MGC 803 cell proliferation and IL-8 secretion in vitro and in vivo. Mol. Med. Rep. 2016, 14, 1430–1438. [Google Scholar] [CrossRef]
- Wang, J.X.; Yang, S.; Cai, X.Q.; Dong, J.Q.; Chen, Z.Q.; Wang, R.; Zhang, S.; Cao, H.C.; Lu, D.; Jin, T.; et al. Berberine inhibits EGFR signaling and enhances the antitumor effects of EGFR inhibitors in gastric cancer. Oncotarget 2016, 7, 76076–76086. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.B.; Cao, S.J.; Sun, Y.J.; He, X.Y.; Jiang, B.K.; Yu, Y.Q.; Duan, J.S.; Qiu, F.; Kang, N. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.T.; Wang, L.; Sun, Y.N. Effects of berberine on circular RNA expression profiles in human gastric cancer cells. Evid. Based Complement. Altern. Med. 2021, 2021, 6688629. [Google Scholar] [CrossRef]
- Kou, Y.Y.; Tong, B.D.; Wu, W.Q.; Liao, X.Q.; Zhao, M. Berberine improves chemo-sensitivity to cisplatin by enhancing cell apoptosis and repressing PI3K/AKT/mTOR signaling pathway in gastric cancer. Front. Pharmacol. 2020, 11, 616251. [Google Scholar] [CrossRef]
- You, H.Y.; Xie, X.M.; Zhang, W.J.; Zhu, H.L.; Jiang, F.Z. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cell Dev. Biol. Anim. 2016, 52, 857–863. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef]
- Zhang, P.C.; Wang, Q.C.; Lin, Z.B.; Yang, P.J.; Dou, K.F.; Zhang, R.H. Berberine inhibits growth of liver cancer cells by suppressing glutamine uptake. OncoTargets Ther. 2019, 12, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Mohamed, H.; Shah, A.M.; Yu, S.X.; Akhlaq, M.; Xiao, H.F.; Li, S.Q.; Naz, T.; Nosheen, S.; Bai, X.Y.; et al. In vitro anticancer potential of Berberis lycium Royle extracts against human hepatocarcinoma (HepG2) cells. BioMed Res. Int. 2020, 2020, 8256809. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Wang, K.Y.; Gu, C.X.; Yu, G.X.; Zhao, D.; Mai, W.J.; Zhong, Y.; Liu, S.M.; Nie, Y.Q.; Yang, H. Berberine, a natural plant alkaloid, synergistically sensitizes human liver cancer cells to sorafenib. Oncol. Rep. 2018, 40, 1525–1532. [Google Scholar] [CrossRef]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Shu, X.B.; Li, M.; Cao, Y.; Li, C.L.; Zhou, W.J.; Ji, G.; Zhang, L. Berberine alleviates non-alcoholic steatohepatitis through modulating gut microbiota mediated intestinal FXR activation. Front. Pharmacol. 2021, 12, 750826. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Tan, H.Y.; Chueng, F.; Zhang, Z.J.; Yuen, M.F.; Feng, Y.B. Modulation of gut microbiota mediates berberine-induced expansion of immuno-suppressive cells to against alcoholic liver disease. Clin. Transl. Med. 2020, 10, e112. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Tao, J.; Li, Y.; Li, S.; Li, H.B. Plant foods for the prevention and management of colon cancer. J. Funct. Foods 2018, 42, 95–110. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Zeng, J.H.; Guo, Q.; Pu, K.M.; Yang, Y.; Chen, N.Z.; Zhang, G.; Zhao, M.Y.; Zheng, Q.; Tang, J.Y.; et al. Berberine suppresses stemness and tumorigenicity of colorectal cancer stem-like cells byinhibiting m(6)A methylation. Front. Oncol. 2021, 11, 775418. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.H.; Peng, W.W.; Wang, Y.L.; Zhong, L.T.; Zhang, X.B.; Zeng, L. β-catenin correlates with the progression of colon cancers and berberine inhibits the proliferation of colon cancer cells by regulating the β-catenin signaling pathway. Gene 2022, 818, 146207. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Zhan, Y.Y.; Hou, J.; Xu, B.; Chen, B.; Tian, Y.; Wu, D.; Zhao, Y.; Zhang, Y.; Chen, X.; et al. Berberine binds RXR alpha to suppress beta-catenin signaling in colon cancer cells. Oncogene 2017, 36, 6906–6918. [Google Scholar] [CrossRef] [PubMed]
- Li, P.F.; Hao, Z.F.; Liu, H.H.; Zhu, B.J.; Dang, L.Y.; Ma, C.; Xu, Y.T.; Zhang, Y.Y.; Fan, D.D.; Sun, S.S. Quantitative proteomics analysis of berberine-treated colon cancer cells reveals potential therapy targets. Biology 2021, 10, 250. [Google Scholar] [CrossRef]
- Samad, M.A.; Saiman, M.Z.; Majid, N.A.; Karsani, S.A.; Yaacob, J.S. Berberine inhibits telomerase activity and induces cell cycle arrest and telomere erosion in colorectal cancer cell line, HCT 116. Molecules 2021, 26, 376. [Google Scholar] [CrossRef]
- Liu, Y.X.; Hua, W.W.; Li, Y.; Xian, X.R.; Zhao, Z.; Liu, C.; Zou, J.J.; Li, J.; Fang, X.J.; Zhu, Y.B. Berberine suppresses colon cancer cell proliferation by inhibiting the SCAP/SREBP-1 signaling pathway-mediated lipogenesis. Biochem. Pharmacol. 2020, 174, 113776. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Liu, X.J.; Yu, M.; Xu, M.M.; Xiao, Y.P.; Ma, W.Y.; Huang, L.; Li, X.G.; Ye, X.L. Berberine inhibits proliferation and induces G0/G1 phase arrest in colorectal cancer cells by downregulating IGF2BP3. Life Sci. 2020, 260, 118413. [Google Scholar] [CrossRef]
- Liu, Y.X.; Fang, X.J.; Li, Y.; Bing, L.; Li, Y.; Fang, J.; Xian, X.R.; Zhang, W.; Li, J.; Zhao, Z.; et al. Berberine suppresses the migration and invasion of colon cancer cells by inhibition of lipogenesis through modulation of promyelocytic leukemia zinc finger-mediated sterol-regulatory element binding proteins cleavage-activating protein ubiquitination. J. Pharm. Pharmacol. 2022, in press. [CrossRef]
- Gong, C.X.; Hu, X.; Xu, Y.L.; Yang, J.H.; Zong, L.; Wang, C.; Zhu, J.; Li, Z.Y.; Lu, D.Z. Berberine inhibits proliferation and migration of colorectal cancer cells by downregulation of GRP78. Anti-Cancer Drugs 2020, 31, 141–149. [Google Scholar] [CrossRef]
- Lü, Y.; Han, B.; Yu, H.; Cui, Z.; Li, Z.; Wang, J. Berberine regulates the microRNA-21-ITGΒ4-PDCD4 axis and inhibits colon cancer viability. Oncol. Lett. 2018, 15, 5971–5976. [Google Scholar] [CrossRef]
- Dai, W.; Mu, L.Y.; Cui, Y.L.; Li, Y.Y.; Chen, P.; Xie, H.J.; Wang, X. Berberine promotes apoptosis of colorectal cancer via regulation of the long non-coding RNA (lncRNA) cancer susceptibility candidate 2 (CASC2)/AU-binding factor 1 (AUF1)/B-cell CLL/Lymphoma 2 (Bcl-2) axis. Med. Sci. Monit. 2019, 25, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Coulter, S.; Yoshihara, E.; Oh, T.G.; Fang, S.; Cayabyab, F.; Zhu, Q.Y.; Zhang, T.; Leblanc, M.; Liu, S.H.; et al. FXR regulates intestinal cancer stem cell proliferation. Cell 2019, 176, 1098–1112.e1018. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cai, J.W.; Gui, W.; Nichols, R.G.; Koo, I.; Zhang, J.T.; Anitha, M.; Patterson, A.D. Berberine directly affects the gut microbiota to promote intestinal farnesoid X receptor activation. Drug Metab. Dispos. 2019, 47, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, L.; Yuan, X.; Li, Y.; Shi, J.; Zhang, H.; Zhao, Y.; Han, L.; Wang, H.; Yan, Y.; et al. Pre-administration of berberine exerts chemopreventive effects in AOM/DSS-induced colitis-associated carcinogenesis mice via modulating inflammation and intestinal microbiota. Nutrients 2022, 14, 726. [Google Scholar] [CrossRef]
- Okuno, K.; Garg, R.; Yuan, Y.C.; Tokunaga, M.; Kinugasa, Y.; Goel, A. Berberine and oligomeric proanthocyanidins exhibit synergistic efficacy through regulation of PI3K-Akt signaling pathway in colorectal cancer. Front. Oncol. 2022, 12, 855860. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061. [Google Scholar] [CrossRef]
- Li, J.; Zou, Y.L.; Pei, M.L.; Zhang, Y.; Jiang, Y. Berberine inhibits the Warburg effect through TET3/miR-145/HK2 pathways in ovarian cancer cells. J. Cancer 2021, 12, 207–216. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Yang, X.H.; Zhao, J.T.; Gao, M.H.; Zhang, M.; Shi, T.F.; Zhang, F.; Zheng, X.; Pan, Y.; Shao, D.; et al. Berberine inhibits chemotherapy-exacerbated ovarian cancer stem cell-like characteristics and metastasis through GLI1. Eur. J. Pharmacol. 2021, 895, 173887. [Google Scholar] [CrossRef]
- Parashar, D.; Nair, B.; Geethadevi, A.; George, J.; Nair, A.; Tsaih, S.W.; Kadamberi, I.P.; Nair, G.K.G.; Lu, Y.L.; Ramchandran, R.; et al. Peritoneal spread of ovarian cancer harbors therapeutic vulnerabilities regulated by FOXM1 and EGFR/ERBB2 signaling. Cancer Res. 2020, 80, 5554–5568. [Google Scholar] [CrossRef]
- Chuang, T.C.; Wu, K.H.; Lin, Y.Y.; Kuo, H.P.; Kao, M.C.; Wang, V.; Hsu, S.C.; Lee, S.L. Dual down-regulation of EGFR and ErbB2 by berberine contributes to suppression of migration and invasion of human ovarian cancer cells. Environ. Toxicol. 2021, 36, 737–747. [Google Scholar] [CrossRef]
- Liu, L.; Fan, J.Y.; Ai, G.H.; Liu, J.; Luo, N.; Li, C.X.; Cheng, Z.P. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.W.; Cui, L.Z.; Pan, Y.; Shao, D.; Zheng, X.; Zhang, F.; Zhang, H.S.; He, K.; Chen, L. Berberine inhibits the chemotherapy-induced repopulation by suppressing the arachidonic acid metabolic pathway and phosphorylation of FAK in ovarian cancer. Cell Prolif. 2017, 50, e12393. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Xu, G.W.; Zhang, C.B.; Li, B.X.; Qin, J.C.; Hao, X.H.; Liu, Q.; Zhang, X.Y.; Liu, J.S.; Wei, J.J.; et al. Berberine induces oxidative DNA damage and impairs homologous recombination repair in ovarian cancer cells to confer increased sensitivity to PARP inhibition. Cell Death Dis. 2017, 8, e3070. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Liu, L.P.; Sun, L.; Zheng, J.; Cui, L. Berberine modulates Keratin 17 to inhibit cervical cancer cell viability and metastasis. J. Recept. Signal Transduct. 2021, 41, 521–531. [Google Scholar] [CrossRef]
- Chu, S.C.; Yu, C.C.; Hsu, L.S.; Chen, K.S.; Su, M.Y.; Chen, P.N. Berberine reverses epithelial-to-mesenchymal transition and inhibits metastasis and tumor-induced angiogenesis in human cervical cancer cells. Mol. Pharmacol. 2014, 86, 609–623. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Huang, Y.; Sun, J.; Zhao, C.; Song, Y.; Wu, J. Combination of berberine and matrine affects apoptosis and cellcycle in human cervical cancer cells. Acta Pol. Pharm. 2019, 76, 1089–1097. [Google Scholar] [CrossRef]
- Zeng, X.; Wan, L.; Wang, Y.; Xue, J.; Yang, H.; Zhu, Y. Effect of low dose of berberine on the radioresistance of cervical cancer cells via a PI3K/HIF-1 pathway under nutrient-deprived conditions. Int. J. Radiat. Biol. 2020, 96, 1060–1067. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Prim. 2021, 7, 9. [Google Scholar] [CrossRef]
- Li, X.N.; Zhang, A.H.; Sun, H.; Liu, Z.D.; Zhang, T.L.; Qiu, S.; Liu, L.; Wang, X.J. Metabolic characterization and pathway analysis of berberine protects against prostate cancer. Oncotarget 2017, 8, 65022–65041. [Google Scholar] [CrossRef]
- Zenata, O.; Dvorak, Z.; Vrzal, R. Pharmacologically relevant concentrations of berberine transiently stimulate dihydrotestosterone-inducible androgen receptor-mediated luciferase activity in human prostate cancer cells. Transl. Cancer Res. 2018, 7, 383–390. [Google Scholar] [CrossRef]
- Tian, Y.T.; Zhao, L.J.; Wang, Y.; Zhang, H.T.; Xu, D.; Zhao, X.J.; Li, Y.; Li, J. Berberine inhibits androgen synthesis by interaction with aldo-keto reductase 1C3 in 22Rv1 prostate cancer cells. Asian J. Androl. 2016, 18, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Zheng, H.F.; Wang, W.L.; Wang, Y.; Zhong, L.F.; Wu, J.L.; Li, Q.X. Berberine targets epidermal growth factor receptor signaling to suppress prostate cancer proliferation in vitro. Mol. Med. Rep. 2015, 11, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Tang, W.C.; Sia, P.; Huang, C.C.; Yang, P.M.; Wu, M.H.; Lai, I.L.; Lee, K.H. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing epithelial-to-mesenchymal transition (EMT)-associated genes with predictive and prognostic relevance. Int. J. Med. Sci. 2015, 12, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, C.; Yang, X.; Yang, B.; Wang, J.; Kang, Y.; Wang, Z.; Li, D.; Huang, G.; Ma, Z.; et al. Berberine inhibits the expression of hypoxia induction factor-1alpha and increases the radiosensitivity of prostate cancer. Diagn. Pathol. 2014, 9, 98. [Google Scholar] [CrossRef]
- Gao, X.H.; Liu, J.K.; Fan, D.M.; Li, X.F.; Fang, Z.Q.; Yan, K.Q.; Fan, Y.D. Berberine enhances gemcitabine-induced cytotoxicity in bladder cancer by downregulating Rad51 expression through inactivating the PI3K/Akt pathway. Oncol. Rep. 2022, 47, 33. [Google Scholar] [CrossRef]
- Zhuo, Y.M.; Chen, Q.B.; Chen, B.; Zhan, X.Y.; Qin, X.P.; Huang, J.; Lv, X.X. Berberine promotes antiproliferative effects of epirubicin in T24 bladder cancer cells by enhancing apoptosis and cell cycle arrest. Int. J. Clin. Pharmacol. Ther. 2017, 55, 32–40. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.L. Berberine suppresses growth and metastasis of endometrial cancer cells via miR-101/COX-2. Biomed. Pharmacother. 2018, 103, 1287–1293. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Guo, R.; Jing, W.; Lu, H. Cell metabolomics reveals berberine-inhibited pancreatic cancer cell viability and metastasis by regulating citrate metabolism. J. Proteome Res. 2020, 19, 3825–3836. [Google Scholar] [CrossRef]
- Och, A.; Zalewski, D.; Komsta, Ł.; Kołodziej, P.; Kocki, J.; Bogucka-Kocka, A. Cytotoxic and proapoptotic activity of sanguinarine, berberine, and extracts of Chelidonium majus L. and Berberis thunbergii DC. toward hematopoietic cancer cell lines. Toxins 2019, 11, 485. [Google Scholar] [CrossRef]
- Jiang, S.X.; Qi, B.; Yao, W.J.; Gu, C.W.; Wei, X.F.; Zhao, Y.; Liu, Y.Z.; Zhao, B.S. Berberine displays antitumor activity in esophageal cancer cells in vitro. World J. Gastroenterol. 2017, 23, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhang, W.; Wu, G.; Ren, J.; Lu, H.; Li, Z.; Han, X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed. Pharmacother. 2016, 84, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Naveen, C.R.; Gaikwad, S.; Agrawal-Rajput, R. Berberine induces neuronal differentiation through inhibition of cancer stemness and epithelial-mesenchymal transition in neuroblastoma cells. Phytomedicine 2016, 23, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, C.; Wang, Y.; Zhang, P.; Zhang, J.; Hong, T. Berberine and cisplatin exhibit synergistic anticancer effects on osteosarcoma MG-63 cells by inhibiting the MAPK pathway. Molecules 2021, 26, 1666. [Google Scholar] [CrossRef]
- Tong, M.F.; Liu, H.M.; Hao, J.Y.; Fan, D.M. Comparative pharmacoproteomics reveals potential targets for berberine, a promising therapy for colorectal cancer. Biochem. Biophys. Res. Commun. 2020, 525, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, C.; Wu, L.Y.; Wen, B. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. OncoTargets Ther. 2016, 9, 4121–4126. [Google Scholar] [CrossRef]
- Lin, J.P.; Yang, J.S.; Chang, N.W.; Chiu, T.H.; Su, C.C.; Lu, K.W.; Ho, Y.T.; Yeh, C.C.; Mei, D.; Lin, H.J.; et al. GADD153 mediates berberine-induced apoptosis in human cervical cancer Ca ski cells. Anticancer Res. 2007, 27, 3379–3386. [Google Scholar]
- Bhanumathi, R.; Vimala, K.; Shanthi, K.; Thangaraj, R.; Kannan, S. Bioformulation of silver nanoparticles as berberine carrier cum anticancer agent against breast cancer. New J. Chem. 2017, 41, 14466–14477. [Google Scholar] [CrossRef]
- Hu, S.W.; Zhao, R.C.; Liu, Y.H.; Chen, J.Z.; Zheng, Z.J.; Wang, S.S. Preventive and therapeutic roles of berberine in gastrointestinal cancers. BioMed Res. Int. 2019, 2019, 6831520. [Google Scholar] [CrossRef]
- Paudel, K.R.; Mehta, M.; Yin, G.H.S.; Yen, L.L.; Malyla, V.; Patel, V.K.; Panneerselvam, J.; Madheswaran, T.; MacLoughlin, R.; Jha, N.K.; et al. Berberine-loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Environ. Sci. Pollut. Res. Int. 2022, 29, 46830–46847. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Z.; Shao, D.; Chang, Z.; Hu, R.; Li, L.; Luo, S.Z.; Dong, W.F. Cancer cell membrane-modified biodegradable mesoporous silica nanocarriers for berberine therapy of liver cancer. RSC Adv. 2018, 8, 40288–40297. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Kim, J.J.; Yao, M.; Elbayoumi, T.A. Development and evaluation of vitamin E d-α-tocopheryl polyethylene glycol 1000 succinate-mixed polymeric phospholipid micelles of berberine as an anticancer nanopharmaceutical. Int. J. Nanomed. 2016, 11, 1687–1700. [Google Scholar] [CrossRef]
- Othman, M.S.; Obeidat, S.T.; Al-Bagawi, A.H.; Fareid, M.A.; Fehaid, A.; Moneim, A.E.A. Green-synthetized selenium nanoparticles using berberine as a promising anticancer agent. J. Integr. Med. 2022, 20, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Kamble, S.; Deshkar, S.; Kothapalli, L.; Chitlange, S. Bioavailability of berberine: Challenges and solutions. Istanb. J. Pharm. 2021, 51, 141–153. [Google Scholar] [CrossRef]
- Och, A.; Podgorski, R.; Nowak, R. Biological activity of berberine-a summary update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Guo, S.; Du, S.; Cao, Z.; Yang, Y.; Su, X.; Wei, W. Efficacy and safety of berberine in preventing recurrence of colorectal adenomas: A systematic review and meta-analysis. J. Ethnopharmacol. 2022, 282, 114617. [Google Scholar] [CrossRef]
- Mirzaee, F.; Razmjouei, P.; Shahrahmani, H.; Vafisani, F.; Najafi, M.N.; Ghazanfarpour, M. The effect and safety of Berberine on polycystic ovary syndrome: A systematic review. J. Obstet. Gynaecol. 2021, 41, 684–689. [Google Scholar] [CrossRef] [PubMed]
| Study Types | Models | Dosages | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| Breast cancer | ||||
| In vitro | MCF-7 and MDA-MB-231 cells | 0, 1, 10, 50, 100, and 200 μM | Inhibit proliferative ability of breast cancer cells by reducing metadherin | [25] |
| In vitro | MCF7 and MCF12A cells | 1, 10, and 100 μM | Induce the nucleolar stress response Upregulate the p53 | [36] |
| In vitro | MCF-7 and MDA-MB-231 cells | 25 and 50 µM | Upregulate miR-214-3p Reduce mRNA expression and protein levels of SCT | [32] |
| In vitro | MCF-7 and MDA-MB-231 cells | 20, 40 and 80 µM | Upregulate p21/cip1 and p27/kip1 Increase nuclear localization and post-translational protein stability | [37] |
| In vitro | MDA-MB-468, MDA-MB-231, HCC70, HCC38, HCC1937, HCC1143, BT-20, and BT-549 cells | 0.5 and 1 µM | Induce cell cycle arrest Induce cell apoptosis | [29] |
| In vitro | MDA-MB-231 cells | 2.5, 5, 10, 20, 40, 60, 80, 100 μg/mL | Reduce cell viability Increase LDH release Reduce the secretion of TNF-α, IL-1α, IL-1β, and IL-6 | [26] |
| In vitro | MDA-MB-231, MDA-MB-468, MDA-MB-453, and BT-549 cells | MDA-MB-231: 0, 6.25, 12.5, and 25 µM; MDA-MB-468: 0, 3, 6, and 12 µM; MDA-MB-453: 0, 2.5, 5, and 10 µM; BT-549 cells: 0, 5, 10, and 20 µM | Inhibit cell proliferation Induce cell cycle arrest Reduce the expression of cyclin A and CDK1 Reduce cyclin D and CDK4 expression | [30] |
| In vitro | MDA-MB-453, BT20, BT549, MDA-MB-231, Hs578T, and MDA-MB-157 cells | 50 µM | Decrease fibronectin expression through inhibition of AP-1 activity | [33] |
| In vitro | Canine mammary gland carcinoma cell line | 10, 25, 50, 100 and 200 µM | Inhibit cell proliferation Decrease cell viability | [28] |
| In vitro | MCF-7/MDR cells | 5, 10, 20 μmol/L | Induce cell apoptosis via AMPK-p53 signaling pathway | [35] |
| In vitro | MCF-7 cells | 10, 20, 40 and 80 μg/mL | Decrease cell migration through downregulation of several chemokine receptors | [31] |
| In vitro | MCF-7, T47D, MDA-MB-468, and MDA-MB-231 cells | Berberine: 0–40 µM; emodin: 0–40 µM | Inhibit cell growth via inhibiting SIK3 activity Induce G0/G1 phase cell cycle arrest and apoptosis | [39] |
| Colorectal cancer | ||||
| In vitro | HCT116 cells | 1, 10 and 100 µM | Regulate the three-gene network miR21-ITGβ4-PDCD4 | [90] |
| In vitro | CACO2 and LOVO CRC cell lines | 0, 10, 20, 40, 60, 80 µM | Inhibit mitochondrial protein synthesis, TCA, and respiratory electron transportation | [125] |
| In vitro | SW480 and HT-29 cells | 0, 20, 50, 100, 200, and 300 µM | Inhibit GRP78 expression Upregulate cytokeratin expression. | [89] |
| In vitro | SW480 and HT-29 cells | 0, 25, 50, 100, 200, 400 and 800 µM | Inhibit cell proliferation Induce cell cycle arrest at G0/G1 phase | [87] |
| In vitro | HT29 and HCT116 cells | 0, 10, 20, 40, 60, 80, 100 µM | Induce cell apoptosis via modulation of lncRNA CASC2/AUF1/Bcl-2 axis | [91] |
| In vitro | HCT 116 cells | 10.54 µg/mL | Decrease the activity and the level of telomerase Inhibit cell proliferation | [85] |
| In vitro | DLD-1 and Caco-2 cells | 6.25, 12.5, 25, 50 µM | Induce cell cycle arrest at G0/G1 phase Modulate lipogenesis through targeting the SCAP/SREBP-1 pathway | [86] |
| In vitro | KM12C cell | 6.25, 12.5, 25, 50 µM | Suppress β-catenin function through binding RXRα Inhibit cell proliferation | [83] |
| In vivo | BALB/c nude mice | 10 mg/kg | Inhibit the xenograft tumor growth | [83] |
| In vitro | Colorectal cancer tissues | 4, 8, 16 µM | Downregulate miR-429 expression | [126] |
| In vitro | HCT116 and HT29 cells | 10, 20, and 40 μM | Induce cancer cell apoptosis Decrease m6A methylation via decreasing β-catenin and increasing FTO Inhibit cell proliferation via decreasing cyclin D1, increasing p27 and p21 Induce cell cycle arrest at G1/G0 phase | [81] |
| In vivo | BALB/c nude mice | 5, 10, or 20 mg/kg | Inhibit the tumor growth | [81] |
| In vitro | HT-29, HCT116, and SW620 cell lines | 2, 10 and 50 μM | Inhibit cell proliferation via regulating β-catenin | [82] |
| In vivo | C57BL/6 nude mice | 5 and 50 mg/kg | Increase the survival rates Decrease β-catenin expression Inhibit the tumor growth of tumor-bearing mice | [82] |
| In vivo | C57BL/6 male mice | 7.5 and 15 mg/kg | Inhibit the development of precancerous lesions Improve intestinal barrier function Increase the ratio of Firmicutes/Bacteroidetes Decrease the abundance of cancer-related bacteria | [94] |
| In vitro | HCT-8, HCT-116, and HT-29 cells | 6.25, 12.5, 25, 50 μM | Suppress lipogenesis via promotion of PLZF-mediated SCAP ubiquitination Inhibit colon cancer cell metastasis | [88] |
| Gastric cancer | ||||
| In vitro | AGS and HGC27 GC cells | 0, 20, 50, 80 μM | Decrease cell viability Inhibit cell proliferation Induce cell apoptosis Modulate circRNA expression and their corresponding target genes | [68] |
| In vitro | SGC-7901 cells | 2.5, 5, 10, 20, and 30 µM | Inhibit cell proliferation; Induce cell cycle arrest at G1 phase; Induce apoptosis Induce autophagy | [64] |
| In vitro | BGC-823 cells | 14, 21, 32, 48, 72, and 108 μM | Induce cytostatic autophagy via inhibition of mTOR, Akt, and MAPK (ERK, JNK, and p38) pathways | [67] |
| In vivo | BALB/c nude mice | 5, 10, 20 mg/kg | Induce cytostatic autophagy via inhibition of mTOR, Akt, and MAPK pathways | [67] |
| In vitro | SGC-7901, BGC-823, SGC-7901/DDP, and BGC-823/DDP cells | Berberine:10 μM; cisplatin: 2, 4, 8, 16, 32, 64 μg/mL | Increase cisplatin sensitivity cancer cells by upregulating miR-203 expression | [70] |
| In vitro | MKN45, BGC823, and SGC7901 cells | Berberine: 15 to 90 μM; cetuximab: 0.03, 0.06, 0.13, 0.25, 0.50, 1.00, 2.00 mg/mL | Inhibit the activation of STAT3 via inhibiting the phosphorylation of EGFR Downregulate the expression of Bcl-xL and cyclin D1 Synergistic effect with erlotinib and cetuximab | [66] |
| In vivo | BALB/C-nu/nu nude mice | Berberine: 50 mg/kg; cetuximab: 0.8 mg/mouse/ day | Enhance the growth inhibitory activity of cetuximab Inhibit EGFR signaling | [66] |
| In vitro | MGC 803 cells | 0, 7.5, 15, 30 and 60 µM | Inhibit cell proliferation Reduce IL-8 secretion Deactivate MAPK signaling pathway | [65] |
| In vivo | BALB/C nude mice | 15 mg/kg | Reduce tumor weight and volume Reduce IL-8 secretion | [65] |
| Hepatic cancer | ||||
| In vitro | Hep3B and BEL-7404 cells | 12.5, 25, 50, 75, 100 and 125 μM | Suppress cell proliferation by inhibiting glutamine uptake via suppressing SLC1A5 | [73] |
| In vivo | BALB/C nude mice | 20 mg/kg | Suppress xenografts tumor growth Reduce SLC1A5 expression | [73] |
| In vitro | HepG2 and HUVEC cells | 0.0625 to 8 mg/mL | Inhibit cell proliferation Induce cell cycle arrest at G1 and S phase Upregulate the intracellular ROS level Downregulate mitochondrial membrane potential | [74] |
| Lung cancer | ||||
| In vitro | A549, PC9, H460, H1299, Beas-2b, and 293T cells | 0, 20, 40, 80, 120, and 160 μM | Promote cell apoptosis through miR19a/TF/MAPK signaling pathway | [58] |
| In vitro | A549, H1299, and H1975 cells | 0, 60, 120 μmol/L | Inhibit cancer cell growth via suppressing DNA repair and replication | [57] |
| In vivo | C57BL/6 mice | 200 mg/kg | Enlarge tumor necrosis area | [57] |
| In vitro | A549 cells | 0, 30, 60, 90, 150 and 200 µM | Inhibit cell proliferation through MMP-2, Bcl-2/Bax and Jak2/VEGF/NF-κB/AP-1 signaling pathways | [55] |
| In vitro | NCI-H460, A549 and NCI-H1299 cells | 10, 20, 40 and 80 µM | Suppress the proliferation and colony formation of cancer cells; Induce cell apoptosis by inducing DNA damage via downregulating the level of TOP2β | [54] |
| In vivo | BALB/c nude mice | 25 mg/kg | Suppress tumor growth by deregulating Sin3A/TOP2β pathway | [54] |
| In vitro | EGFRm NSCLC cell lines and their derived resistant cell lines | Berberine: 12.5, 25, 50, 100, 200 μM; Osimertinib: 31.25, 62.5, 125, 250, 500 nM | Help osimertinib overcoming the acquired resistance caused by MET gene amplification | [15] |
| In vivo | nu/nu nude mice | Berberine: 25 mg/kg; osimertinib: 5 mg/kg | Enhance inhibitory activity against the growth of MET-amplified osimertinib-resistant tumors | [15] |
| In vitro | H1299 and A549 cells | Berberine: 25 and 50 μM; P. amurense extract: 2.5 and 5 μg/mL | Arrest cell cycle at G1 phase via Akt/CREB signaling axis Suppress cell proliferation via inhibiting proliferative kinase signaling | [56] |
| In vivo | Athymic nude mice | Berberine: 1000 or 1800 ppm; P. amurense extract: 3000 or 5400 ppm | Inhibit tumor growth | [56] |
| In vitro | A549 and PC9 cells | 0, 40, and 80 µM | Induce cell apoptosis via activation of the ROS/ASK1/JNK pathway | [59] |
| Ovarian cancer | ||||
| In vitro | MDAH-2774 and SKOV-3 cells | 0, 25, 50, 75 μM | Consume EGFR and ERBB2 in ovarian cancer cells; Inhibit the activation of EGFR and ERBB2 downstream targets cyclin D1, MMPs, and VEGF via EGFR-ERBB2/PI3K/Akt signaling pathway | [100] |
| In vitro | SKOV3 and 3AO cells | SKOV3: 40 μM; 3AO: 80 μM | Suppress Warburg effect by increasing TET3-related demethylation and upregulating miR-145 | [97] |
| In vitro | SKOV3 cells | Bebrerine: 5 μmol/L; VP16: 5 μmol/L | Reverse chemotherapy drug VP16 induced repopulation of ovarian cancer cells by blocking the iPLA2-AA-COX-2-PGE2 pathway Reverse the increased phosphorylation of FAK | [102] |
| In vitro | A2780, HEY, SKOV3, FTE-187, HO8910, and OVCAR3 cells | 5, 10, 20 μM | Sensitize cancer cells to PARP inhibitors Induce oxidative DNA damage Downregulate HRR | [103] |
| Cervical cancer | ||||
| In vitro | Ca Ski cells | 0, 50, 100, 150 µM | Increase GADD153 expression by inducing ROS production; Induce mitochondria dysfunction; Activate caspase-3 and cytochrome C release | [127] |
| In vitro | SiHa, HeLa, and CaSki cells | 5, 10, 15, 20 µM | Reduce cell invasion and migration; Reduce transcriptional activities of MMP-2 and u-PA; Reverse EMT via upregulating E-cadherin and inhibiting N-cadherin and snail-1 Reduce angiogenesis by downregulating VEGF | [106] |
| In vivo | BALB/c nude mice | 20 mg/kg | Inhibit tumor growth Reduce tumor-induced angiogenesis | [106] |
| In vitro | HeLa and SiHa cells | 3, 10, 30, 100, and 300 µmol/L | Inhibit cell proliferation Trigger cell apoptosis Induce cell cycle arrest at G1 phase | [107] |
| In vitro | Hela cells | 0.098, 0.195, 0.391, 0.781, 1.563, 3.125, 6.25, 12.5, 25, and 50 µM | Overcome the radio-resistance Regulate glucose metabolism via PI3K/HIF-1 pathway | [108] |
| Prostate cancer | ||||
| In vitro | AIZ-AR cells | 0.01–50 µM; 0.001–1000 nM | Induce cell apoptosis Inhibit cell proliferation | [111] |
| In vitro | 22RV1 cell | 1, 2.5, 5, 10, 20, 50 μM | Inhibit cell proliferation Induce cell apoptosis Downregulate the expression of AR, PSA, COX-2, and Bcl-2 | [110] |
| In vivo | BALB/c nude mice | 0.01136g/kg | Inhibit xenograft tumor growth | [110] |
| In vitro | LNCaP, PC3, PC3M, and 22RV1 cells | 12.5, 25, 50 μmol/L | Suppress the intracellular androgen synthesis via inhibiting the AKR1C3 enzyme activity | [112] |
| In vitro | LNCaP and PC-3 cells | 20, 100 and 200 μM | Arrest cell cycle at G1 phase Inhibit cell growth via inhibiting the activation of EGFR Induce cell apoptosis | [113] |
| In vitro | PC-3 and LNCaP cells | 10, 25, 50, 75 μM | Inhibit cell invasion and migration by downregulating EMT-related genes | [114] |
| In vitro | LNCaP and DU-145 cells | 20, 50, 100, 150, 200, 250, 300, 400 μM | Increase radiosensitivity cancer cells through inhibiting the expression of HIF-1α and VEGF | [115] |
| Bladder cancer | ||||
| In vitro | T24, 5637, SV-HUC-1 cells | 20, 40, 60 µM | Disturb AK2-STAT3 signaling pathway via up-regulating miR-17-5p | [19] |
| In vivo | BALB/c nude mice | 200 mg/kg | Promote miR-17-5p expression Suppress JAK1 and STAT3 | [19] |
| In vitro | T24 and 5637 cells | 1, 5, 10, 20, 40, 80, 160 µM | Enhance gemcitabine-induced cytotoxicity | [116] |
| Endometrial cancer | ||||
| In vitro | AN3 CA and HEC-1-A cells | 0, 10, 20, 40, 80, 160 µM | Inhibit cell progression and migration via miR-101/COX-2/PGE2 signaling pathway | [118] |
| In vivo | nude mice | 50 mg/kg or 100 mg/kg | Inhibit cell invasion and migration | [118] |
| Pancreatic cancer | ||||
| In vitro | Panc-1 and hTERT-HPNE cells | 2.5, 3.75, 5, 10 μM | Inhibit cell viability and migration by regulating citrate metabolism and transportation | [119] |
| Hematopoietic Cancer | ||||
| In vitro | HL-60, HL-60/MX1, HL-60/MX2, CCRF/CEM, CEM/C1, J45.01, and U266B1 cells | 40–160 μM | Stimulate cell apoptosis | [120] |
| Esophageal cancer | ||||
| In vitro | KYSE-70 and SKGT4 cells | 20, 40, 60 and 80 μmol/L | Arrest cell cycle at G2 phase Induce cell apoptosis, | [121] |
| In vitro | Eca9706, TE-1, and EC109 cells | Berberine: 90 μM; galangin: 30 μM | Synergistically inhibit cell growth Synergistically arrest cell cycle at G2/M phase Synergistically induce cell apoptosis | [122] |
| Neuroblastoma | ||||
| In vitro | N2a cells | 0–20 µg/mL | Inhibit cancer stemness; Reverse the EMT | [123] |
| Osteosarcoma | ||||
| In vitro | MG-63 and HBMSC cells | Berberine: 2.5, 5, or 10 μM; cisplatin: 0, 1.25, 2.5, 5, or 10 μM | Induce apoptosis and cell cycle arrest at G0/G1 phase Inhibit MMP-2/9, Bcl-2, cyclin D1, and CDK4 expression Enhance Bax expression Regulate MAPK pathway | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, R.-G.; Huang, S.-Y.; Wu, S.-X.; Zhou, D.-D.; Yang, Z.-J.; Saimaiti, A.; Zhao, C.-N.; Shang, A.; Zhang, Y.-J.; Gan, R.-Y.; et al. Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review. Molecules 2022, 27, 4523. https://doi.org/10.3390/molecules27144523
Xiong R-G, Huang S-Y, Wu S-X, Zhou D-D, Yang Z-J, Saimaiti A, Zhao C-N, Shang A, Zhang Y-J, Gan R-Y, et al. Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review. Molecules. 2022; 27(14):4523. https://doi.org/10.3390/molecules27144523
Chicago/Turabian StyleXiong, Ruo-Gu, Si-Yu Huang, Si-Xia Wu, Dan-Dan Zhou, Zhi-Jun Yang, Adila Saimaiti, Cai-Ning Zhao, Ao Shang, Yun-Jian Zhang, Ren-You Gan, and et al. 2022. "Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review" Molecules 27, no. 14: 4523. https://doi.org/10.3390/molecules27144523
APA StyleXiong, R.-G., Huang, S.-Y., Wu, S.-X., Zhou, D.-D., Yang, Z.-J., Saimaiti, A., Zhao, C.-N., Shang, A., Zhang, Y.-J., Gan, R.-Y., & Li, H.-B. (2022). Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review. Molecules, 27(14), 4523. https://doi.org/10.3390/molecules27144523







