Drug-Loaded Polymeric Particulated Systems for Ophthalmic Drugs Release
Abstract
:1. Introduction
2. Results
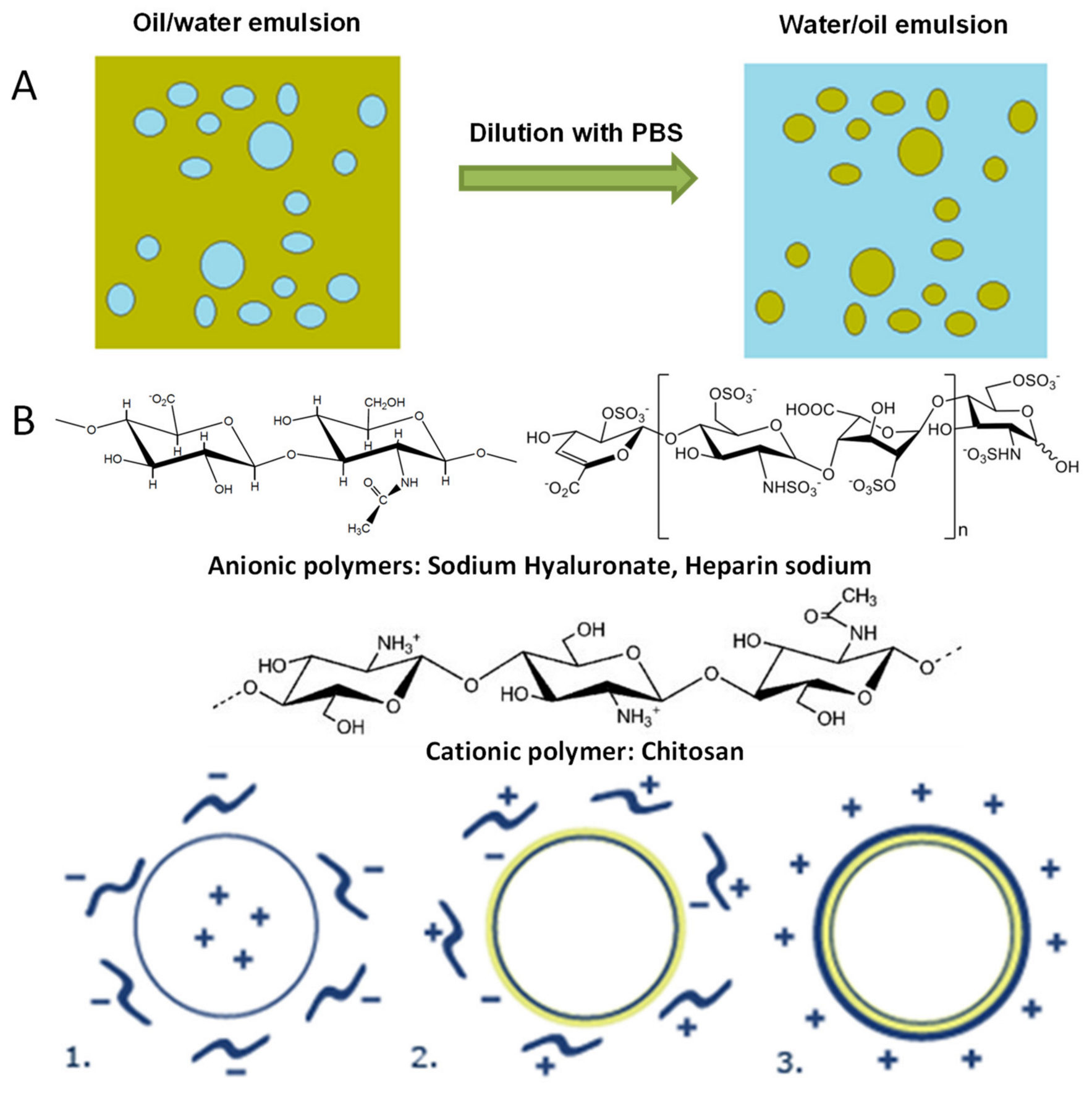
2.1. Particulate Systems Preparation Using Layer-by-layer Technique
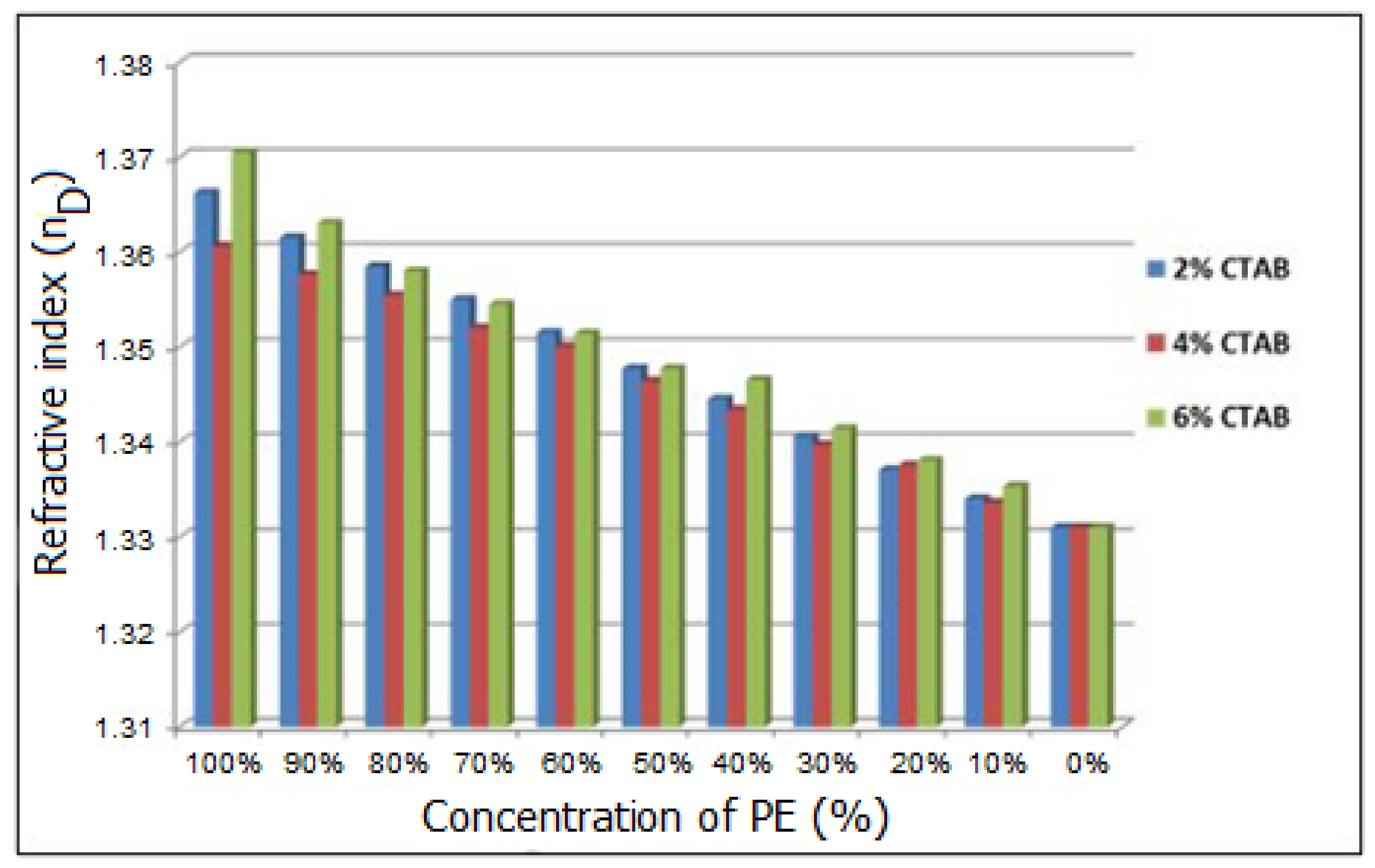
2.2. The Influence of CTAB Concentration on the Emulsion Characteristics
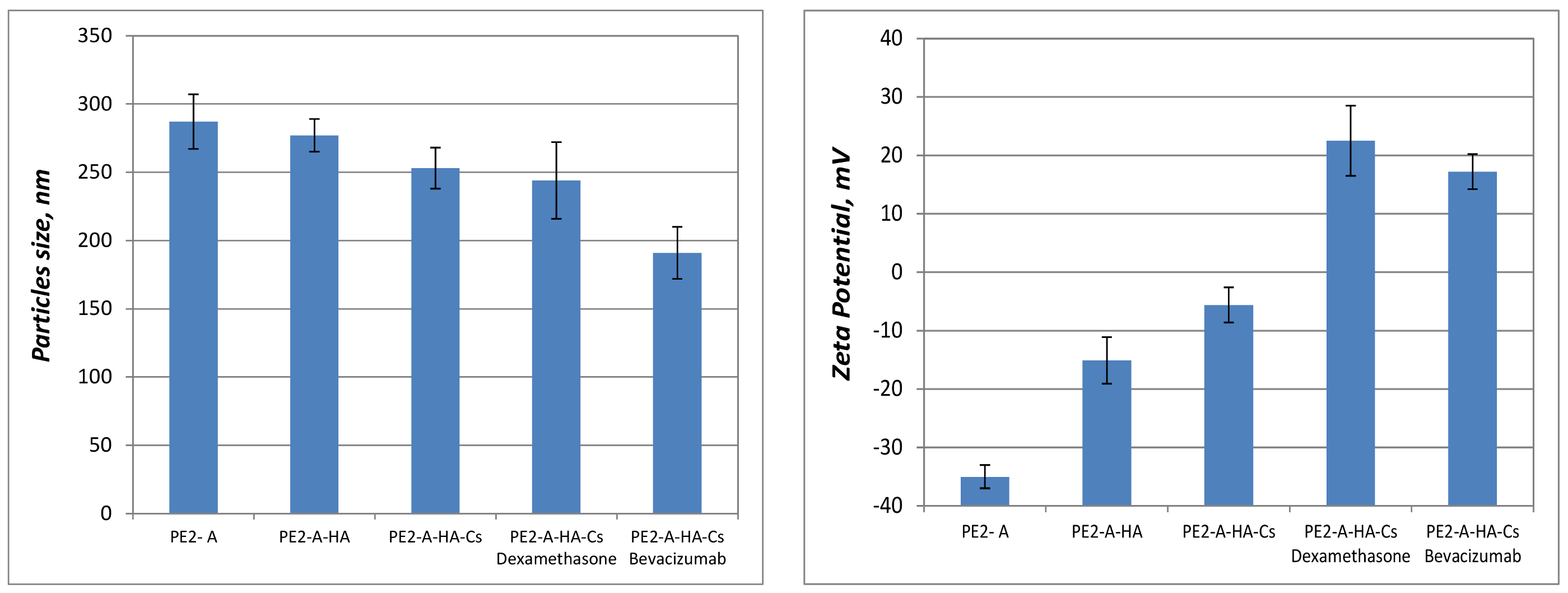
2.3. Characterization of Synthesized Particulated Systems
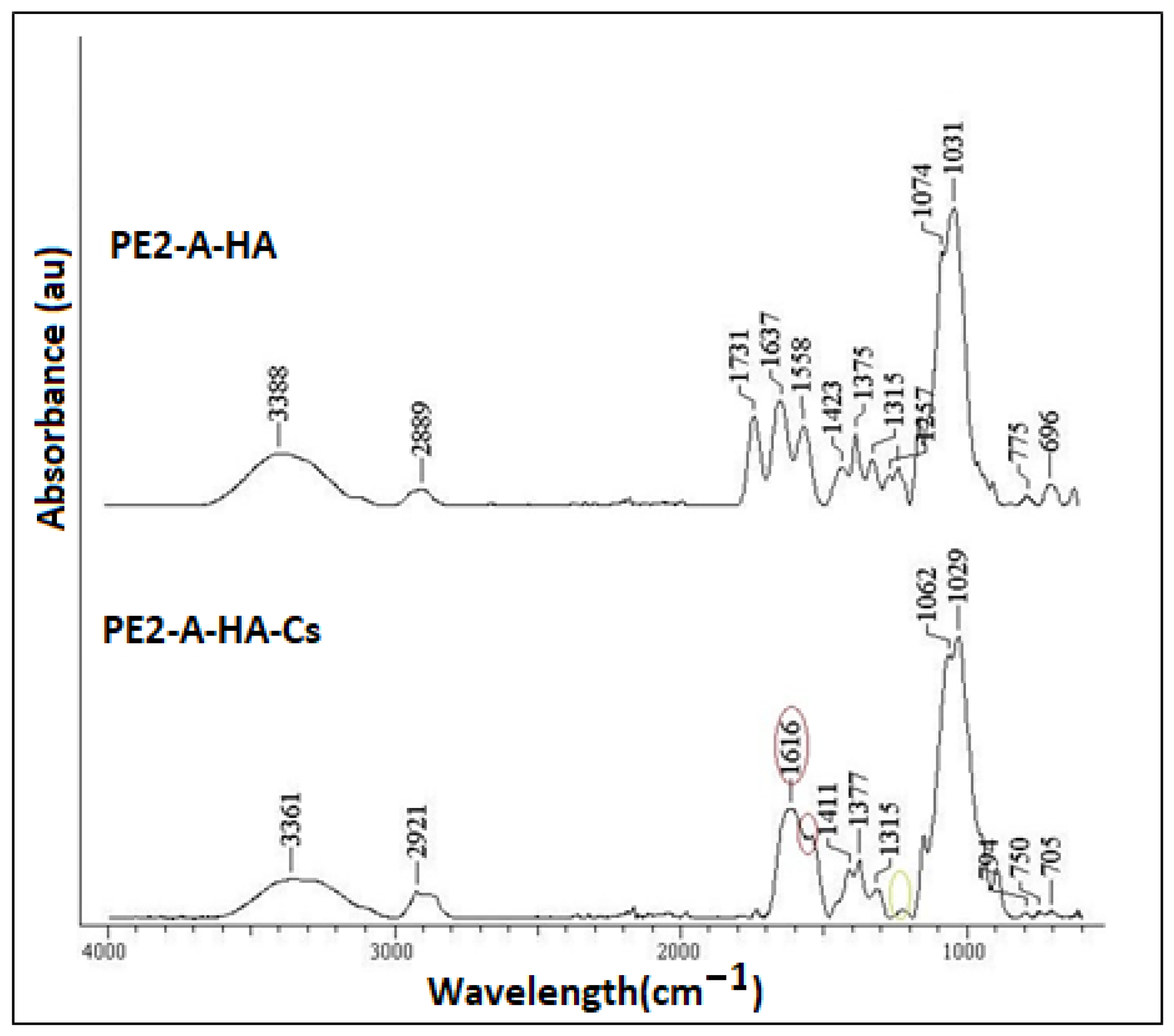
2.4. FT-IR Data
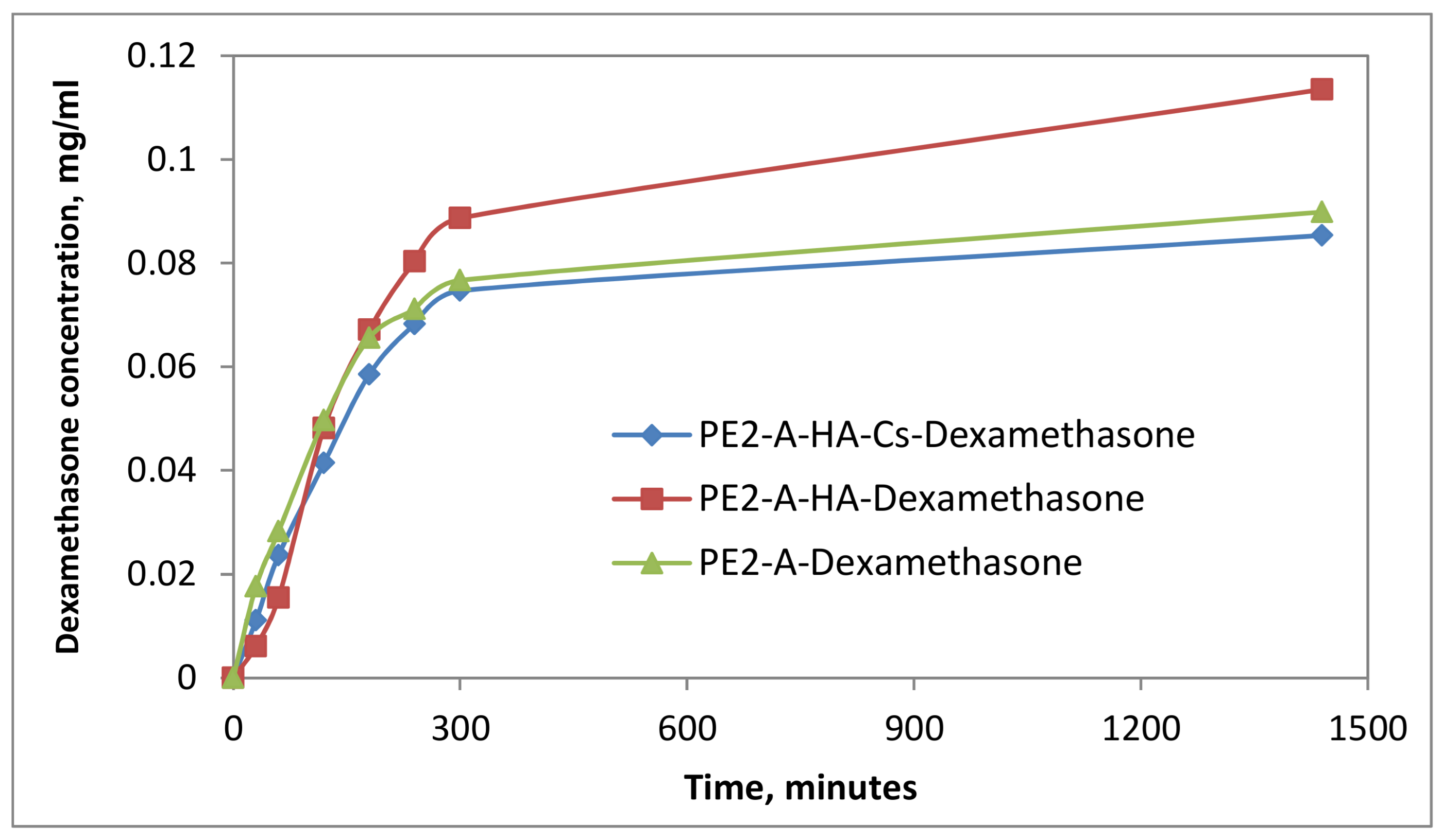
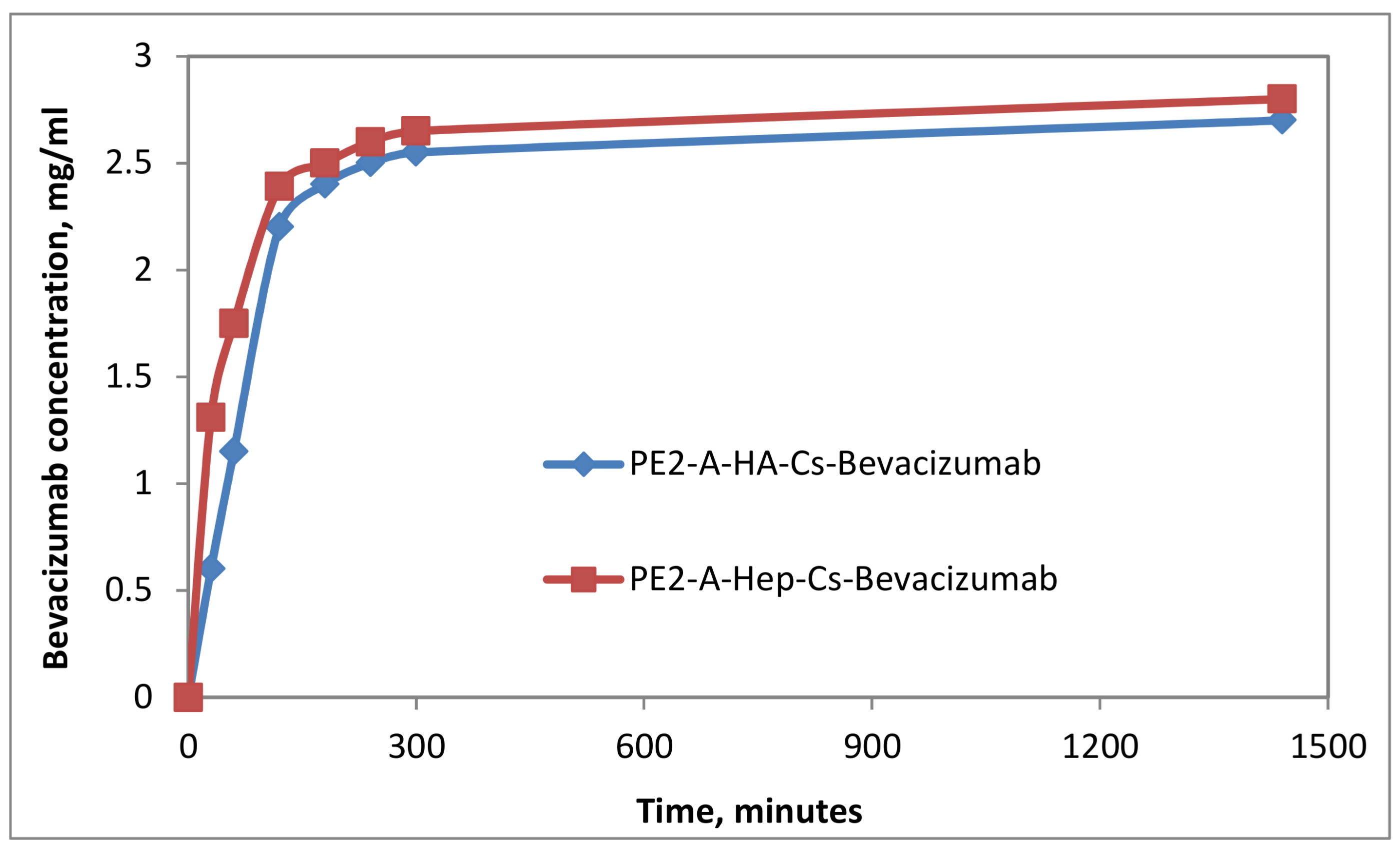
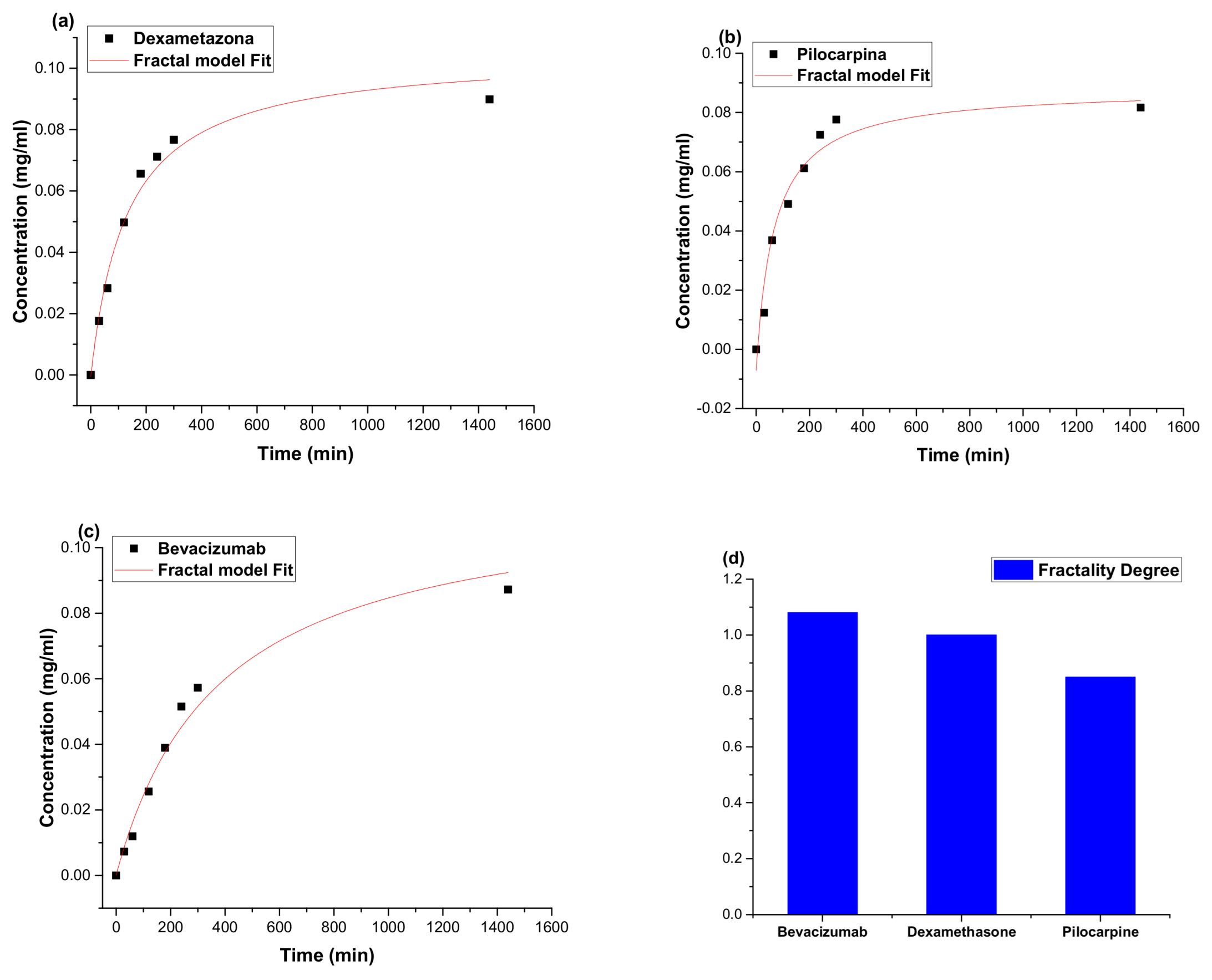
2.5. In Vitro Drug Release
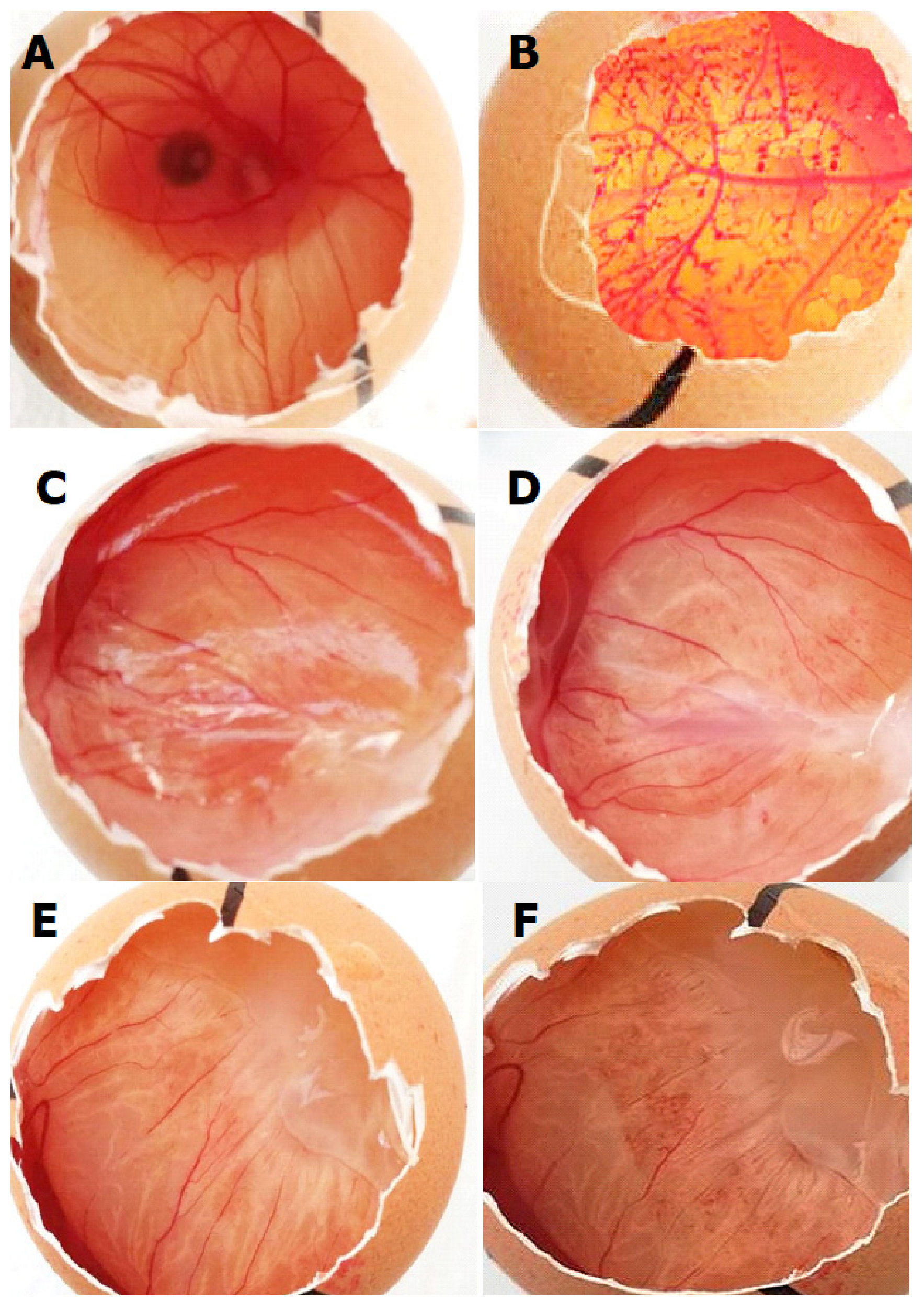
2.6. HET-CAM Tests
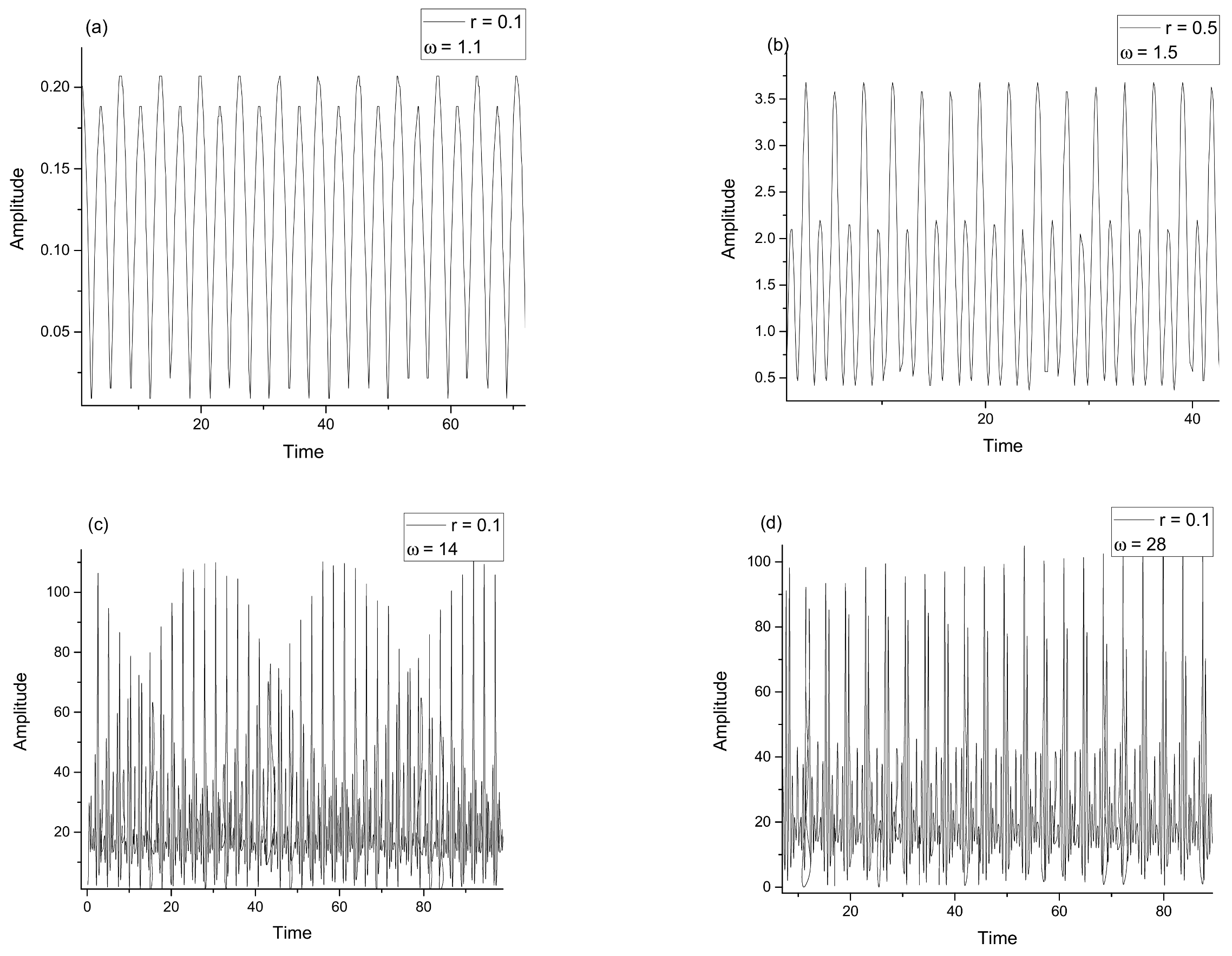
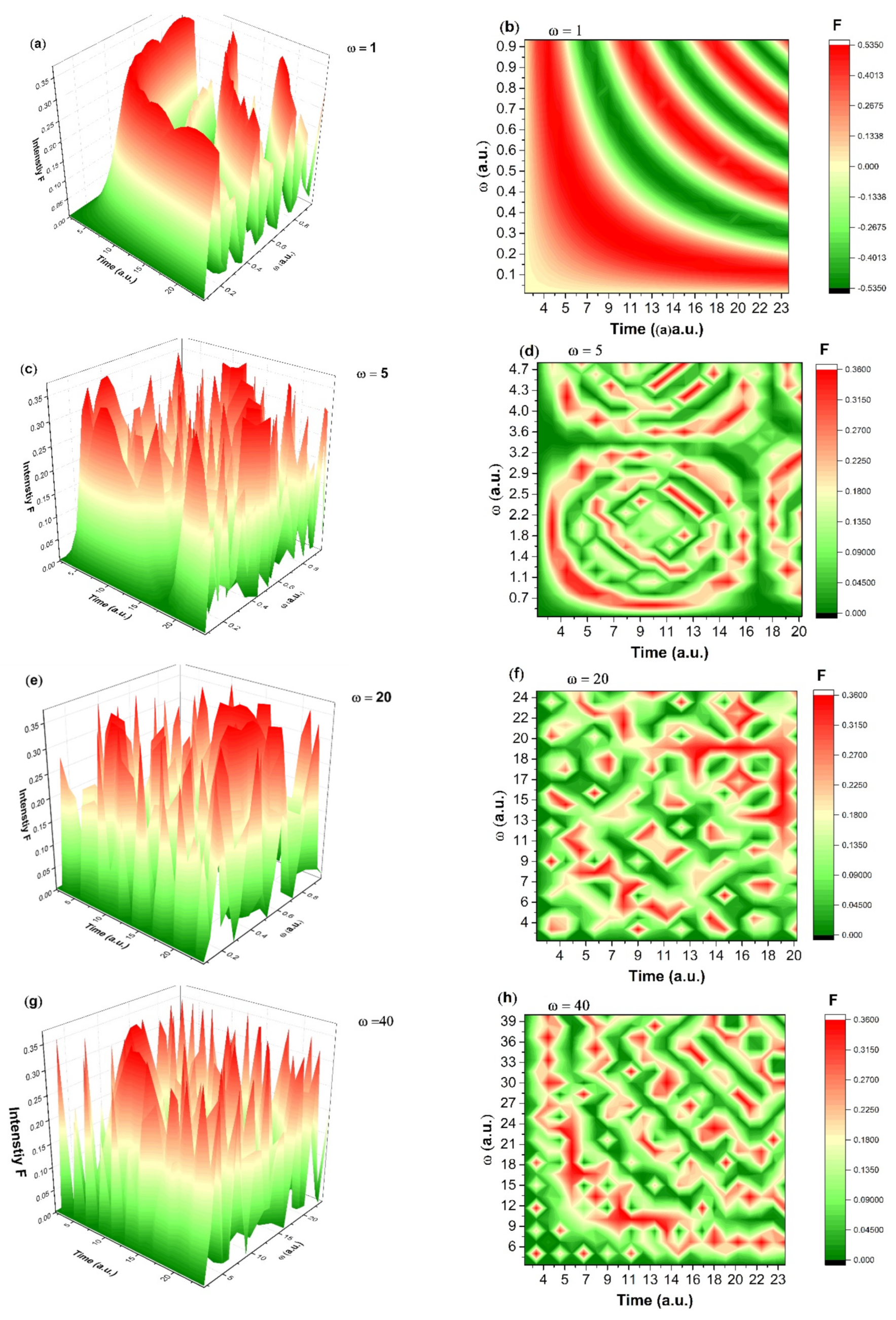
3. Discussions
4. Materials and Methods
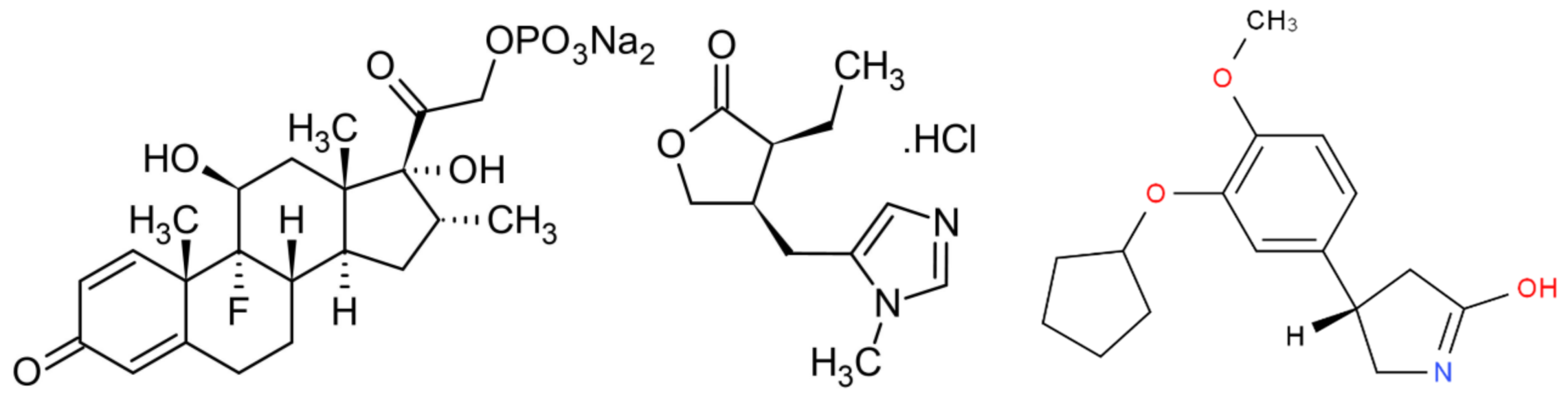
4.1. Materials
4.2. Preparation of Polymeric Particles
4.3. Characterization of Emulsions and Particles
4.3.1. Particles Size and Zeta Potential
4.3.2. Optical Properties of Particles Suspensions
4.3.3. Stability of the Emulsions and Particles
4.4. FT-IR Data
4.5. In Vitro Drug Release Studies
4.6. Mathematical Modelling for Drug Release
4.7. In Vitro Tests for Ocular Tolerability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Dosmar, E.; Walsh, J.; Doyel, M.; Bussett, K.; Oladipupo, A.; Amer, S.; Goebel, K. Targeting Ocular Drug Delivery: An Examination of Local Anatomy and Current Approaches. Bioengineering 2022, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular delivery of macromolecules. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Hao, J.-L.; Wang, S.; Zheng, Y.; Zhang, W.-S. Nanoparticles in the ocular drug delivery. Int. J. Ophthalmol. 2013, 6, 390–396. [Google Scholar] [CrossRef]
- Nakhlband, A.; Barar, J. Impacts of Nanomedicines in Ocular Pharmacotherapy. BioImpacts 2012, 1, 7–22. [Google Scholar]
- Ying, L.; Tahara, K.; Takeuchi, H. Drug delivery to the ocular posterior segment using lipid emulsion via eye drop administration: Effect of emulsion formulations and surface modification. Int. J. Pharm. 2013, 453, 329–335. [Google Scholar] [CrossRef]
- Harwansha, R.K.; Deshmukha, R.; Rahman, M.A. Nanoemulsion: Promising nanocarrier system for delivery of herbal bioactives. J. Drug Deliv. Sci. Technol. 2019, 51, 224–233. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Preparation of microemulsions and nanoemulsions by membrane emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123–709. [Google Scholar] [CrossRef]
- Coty, J.-B.; Vauthier, C. Characterization of nanomedicines: A reflection on a field under construction needed for clinical translation success. J. Control. Release 2018, 275, 254–268. [Google Scholar] [CrossRef]
- Álvarez-Álvarez, L.; Barral, L. Hydrocortisone loaded poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) nanoparticles for topical ophthalmic administration: Preparation, characterization and evaluation of ophthalmic toxicity. Int. J. Pharm. 2019, 568, 118519. [Google Scholar] [CrossRef]
- Ruseva, V.; Jankevics, H.; Corbett, J. An optimized filling method for capillary DLS. MethodsX 2019, 6, 606–614. [Google Scholar] [CrossRef]
- Ameeduzzafara Javed, A.; Aseem, B.; Kumar, N.; Ali, A. Chitosan nanoparticles amplify the ocular hypotensive effect of cateolol in rabbits. Int. J. Biol. Macromol. 2014, 65, 479–491. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Ahmed, T.; Aljaeid, B. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [Green Version]
- Pontillo, A.R.N.; Detsi, A. Nanoparticles for ocular drug delivery: Modified and non-modified chitosan as a promising biocompatible carrier. Nanomedicine 2019, 14, 1889–1909. [Google Scholar] [CrossRef]
- Deng, F.; Hu, W.; Chen, H.; Tang, Y.; Zhang, L. Development of a Chitosan-Based Nanoparticle Formulation for Ophthalmic Delivery of Honokiol. Curr. Drug Deliv. 2018, 15, 594–600. [Google Scholar] [CrossRef]
- Başaran, E.; Yazan, Y. Ocular application of chitosan. Expert Opin. Drug Deliv. 2012, 9, 701–712. [Google Scholar] [CrossRef]
- Kumara, B.; Shambhu, R.; Prasad, K.S. Why chitosan could be apt candidate for glaucoma drug delivery—An overview. Int. J. Biol. Macromol. 2021, 176, 47–65. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urimi, D.; Widenbring, R.; García, R.O.P.; Gedda, L.; Edwards, K.; Loftsson, T.; Schipper, N. Formulation development and upscaling of lipid nanocapsules as a drug delivery system for a novel cyclic GMP analogue intended for retinal drug delivery. Int. J. Pharm. 2021, 602, 120640. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Kaur, G.; Mehta, S. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef]
- Liu, W.; Lee, B.-S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr. Eye Res. 2019, 44, 264–274. [Google Scholar] [CrossRef]
- Mobaraki, M.; Soltani, M.; Harofte, S.Z.; Zoudani, E.L.; Daliri, R.; Aghamirsalim, M.; Raahemifar, K. Biodegradable Nanoparticle for Cornea Drug Delivery: Focus Review. Pharmaceutics 2020, 12, 1232. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Doll, T.A.P.F.; Raman, S.; Dey, R.; Burkhard, P. Nanoscale assemblies and their biomedical applications. J. R. Soc. Interface 2013, 10, 20120740. [Google Scholar] [CrossRef] [Green Version]
- Vichare, R.; Garner, I.; Paulson, R.J.; Tzekov, R.; Sahiner, N.; Panguluri, S.K.; Mohapatra, S.; Mohapatra, S.S.; Ayyala, R.; Sneed, K.B.; et al. Biofabrication of Chitosan-Based Nanomedicines and Its Potential Use for Translational Ophthalmic Applications. Appl. Sci. 2020, 10, 4189. [Google Scholar] [CrossRef]
- Sarheed, O.; Dibi, M.; Ramesh, K.V.R.N.S. Studies on the Effect of Oil and Surfactant on the Formation of Alginate-Based O/W Lidocaine Nanocarriers Using Nanoemulsion Template. Pharmaceutics 2020, 12, 1223. [Google Scholar] [CrossRef]
- Peetla, C.; Labhasetwar, V. Effect of Molecular Structure of Cationic Surfactants on Biophysical Interactions of Surfactant-Modified Nanoparticles with a Model Membrane and Cellular Uptake. Langmuir 2009, 25, 2369–2377. [Google Scholar] [CrossRef] [Green Version]
- Daull, P.; Lallemand, F.; Garrigue, J.S. Benefits of cetalkonium chloride cationic oil-in-water nanoemulsions for topical ophthalmic drug delivery. J. Pharm. Pharmacol. 2014, 66, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Ailincai, D.; Dorobantu, A.M.; Dima, B.; Irimiciuc, S.A.; Lupascu, C.; Agop, M.; Olguta, O. Poly(vinyl alcohol boric acid)-Diclofenac Sodium Salt Drug Delivery Systems: Experimental and Theoretical Studies. J. Immunol. Res. 2020, 2020, 3124304. [Google Scholar] [CrossRef]
- Patel, N.; Nakrani, H.; Raval, M.; Sheth, N. Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability. Drug Deliv. 2016, 23, 3712–3723. [Google Scholar] [CrossRef] [Green Version]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Bucolo, C.; Drago, F.; Salomone, S. Ocular drug delivery: A clue from nanotechnology. Front. Pharmacol. 2012, 3, 188. [Google Scholar] [CrossRef] [Green Version]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 787644. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Ikuta, Y.; Aoyagi, S.; Tanaka, Y.; Sato, K.; Inada, S.; Koseki, Y.; Onodera, T.; Oikawa, H.; Kasai, H. Creation of nano eye-drops and effective drug delivery to the interior of the eye. Sci. Rep. 2017, 7, 44229. [Google Scholar] [CrossRef] [Green Version]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef]
- Youssef, A.; Cai, C.; Dudhipala, N.; Majumdar, S. Design of Topical Ocular Ciprofloxacin Nanoemulsion for the Management of Bacterial Keratitis. Pharmaceuticals 2021, 14, 210. [Google Scholar] [CrossRef]
- Gentimir, C.; Tatarciuc, D.; Zaharia, C.A.; Chelaru, L.; Costin, A.; Jitareanu, A.; Bejinariu, C.; Costuleanu, M. Biochemical Effects of Some Tyrosine Kinase Inhibitors on Pro-B Cells-Induced Apoptosis. J. Chem. 2017, 68, 1864–1867. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Q.; Zhang, B.; Song, Q. Estimating Preferred Alkane Carbon Numbers of Nonionic Surfactants in Normalized Hydrophilic–Lipophilic Deviation Theory from Dissipative Particle Dynamics Modeling. J. Phys. Chem. B 2022, 126, 3593–3606. [Google Scholar] [CrossRef]
- Vittal, R.; Gomathi, H.; Kim, K.-J. Beneficial role of surfactants in electrochemistry and in the modification of electrodes. Adv. Colloid Interface Sci. 2005, 119, 55–68. [Google Scholar] [CrossRef]
- Bordag, N.; Klie, S.; Jürchott, K.; Vierheller, J.; Schiewe, H.; Albrecht, V.; Tonn, J.-C.; Schwartz, C.; Schichor, C.; Selbig, J. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci. Rep. 2015, 5, 15954. [Google Scholar] [CrossRef]
- Qian, T.; Zhao, M.; Wan, Y.; Li, M.; Xu, X. Comparison of the efficacy and safety of drug therapies for macular edema secondary to central retinal vein occlusion. BMJ Open 2018, 8, e022700. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Woo, S. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Farsiu, S.; Chiu, S.J.; Gonzalez, P.; Lütjen-Drecoll, E.; Overby, D.R.; Stamer, W.D. Pilocarpine-Induced Dilation of Schlemm’s Canal and Prevention of Lumen Collapse at Elevated Intraocular Pressures in Living Mice Visualized by OCT. Investig. Opthalmol. Vis. Sci. 2014, 55, 3737–3746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alotaibi, H.F.; Al Thaher, Y.; Perni, S.; Prokopovich, P. Role of processing parameters on surface and wetting properties controlling the behaviour of layer-by-layer coated nanoparticles. Curr. Opin. Colloid Interface Sci. 2018, 36, 130–142. [Google Scholar] [CrossRef]
- Bortnowska, G. Multilayer Oil-in-Water Emulsions: Formation, Characteristics and Application as the Carriers for Lipophilic Bioactive Food Components—A Review. Pol. J. Food Nutr. Sci. 2015, 65, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.; Vikulina, A.S. Layer-By-Layer Assemblies of Biopolymers: Build-Up, Mechanical Stability and Molecular Dynamics. Polymers 2020, 12, 1949. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Cadogan, S.P.; Hahn, C.J.; Rausch, M.H.; Fröba, A.P. Study on the applicability of dynamic light scattering (DLS) to microemulsions including supercritical carbon dioxide-swollen micelles. J. Colloid Interface Sci. 2017, 499, 202–208. [Google Scholar] [CrossRef]
- Mirchell, M. Complexity: A Guided Tour; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Badii, R.; Politi, A. Complexity: Hierarchical Structure and Scaling in Physics; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Craciun, A.M.; Tartau, L.; Pinteala, M.; Marin, L. Nitrosalicyl-imine-chitosan hydrogels based drug delivery systems for long term sustained release in local therapy. J. Colloid Interface Sci. 2019, 536, 196–207. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature; W. H. Freeman: New York, NY, USA, 1983. [Google Scholar]
- Schrittwieser, R.W.; Ionita, C.; Teodorescu-Soare, C.T.; Vasilovici, O.; Gurlui, S.; Irimiciuc, S.A.; Dimitriu, D.G. Spectral and electrical diagnosis of complex space-charge structures excited by a spherical grid cathode with orifice. Phys. Scr. 2017, 92, 044001. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Chertopalov, S.; Lancok, J.; Craciun, V. Langmuir Probe Technique for Plasma Characterization during Pulsed Laser Deposition Process. Coatings 2021, 11, 762. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Chertopalov, S.; Craciun, V.; Novotny, M.; Lancok, J. Investigation of laser-produced plasma multistructuring by floating probe measurements and optical emission spectroscopy. Plasma Process. Polym. 2020, 17, e2000136. [Google Scholar] [CrossRef]
- Nottale, L. Scale Relativity and Fractal Space-Time—A New Approach to Unifying Relativity and Quantum Mechanics; Imperial College Press: London, UK, 2011. [Google Scholar]
- Gottlieb, I.; Agop, M.; Ciobanu, G.; Stroe, A. El Naschie’s ε (∞) space-time and new results in scale relativity theories. Chaos Solitons Fractals 2006, 30, 380–398. [Google Scholar] [CrossRef]
- Agop, M.; Nica, P.; Ioannou, P.D.; Malandraki, O.; Gavanas-Pahomi, I. El Naschie’s ε (∞) space-time, hydrodynamic model of scale relativity theory and some applications. Chaos Solitons Fractals 2007, 34, 1704–1723. [Google Scholar] [CrossRef]
- Nedeff, V.; Moşneguţu, E.; Panainte, M.; Ristea, M.; Lazăr, G.; Scurtu, D.; Ciobanu, B.; Timofte, A.; Toma, Ş.; Agop, M. Dynamics in the boundary layer of a flat particle. Powder Technol. 2012, 221, 312–317. [Google Scholar] [CrossRef]
- Agop, M.; Craciun, P. El Naschie’s Cantorian space-time and general relativity by means of Barbilian’s group. A Cantorian fractal axiomatic model of space-time. Chaos Solitons Fractals 2004, 19, 705–730. [Google Scholar]
- Bacaita, E.S.; Agop, M. A multiscale mechanism of drug release from polymeric matrices: Confirmation through a nonlinear theoretical model. Phys. Chem. Chem. Phys. 2016, 18, 21809–21816. [Google Scholar] [CrossRef]
- Balaita, L.; Chailanb, J.F.; Nguyenc, X.H.; Bacaita, S.; Popa, M. Hybrid Chitosan-Gelatine magnetic polymer particles for drug release. J. Optoelectron. Adv. M. 2014, 1612, 1463–1471. [Google Scholar]
- Radu, V.; Băcăiţă, S.; Uliniuc, A.; Popa, M.; Susanu, S. Fractal hydrodynamic model for drug release processes from starch based hydrogels. Mater. Plast. 2013, 50, 18–22. [Google Scholar]
- Agop, M.; Merches, I. Operational Procedures Describing Physical Systems; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Mazilu, N.; Agop, M. Skyrmions: A Great Finishing Touch to Classical Newtonian Philosophy; World Philosophy Series; Nova: New York, NY, USA, 2012. [Google Scholar]
- Cristescu, C.P. Nonlinear Dynamics and Chaos. Theoretical Fundaments and Applications; Romanian Academy Publishing House: Bucharest, Romania, 2008. [Google Scholar]
- Cazedey, E.C.L.; Carvalho, F.C. Corrositex®, BCOP and HET-CAM as Alternative Methods to Animal Experimentation. Braz. J. Pharm. Sci. 2009, 45, 759–766. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The hen’s egg chorioallantoic membrane (HET-CAM) test to predict the ophthalmic irritation potential of a cysteamine-containing gel: Quantification using Photoshop and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef]
- Teixeira, L.; Bolon, B. Fundamentals of Toxicologic Pathology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Vinardell, M.; Macián, M. Comparative study of the HET-CAM test and the Draize eye test for assessment of irritancy potential. Toxicol. In Vitro 1994, 8, 467–470. [Google Scholar] [CrossRef]


| Pre-Emulsion | CTAB (%) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| PE0 | 0 | 181 ± 33 | 0.072 ± 0.015 | −6.92 ± 0.8 |
| PE2 | 2 | 136 ± 12 | 0.153 ± 0.040 | +36.9 ± 8.4 |
| PE4 | 4 | 168 ± 23 | 0.090 ± 0.021 | +12.8 ± 1.5 |
| PE5 | 6 | 174 ± 29 | 0.171 ± 0.027 | +49.5 ± 5.2 |
| Effect | Score | Cumulative Score | Irritation Degree | ||
|---|---|---|---|---|---|
| 0.5 min | 2 min | 5 min | 0–0.9 | Non-irritant | |
| Lysis/Hyperemia | 5 | 3 | 1 | 1.0–4.9 | Less irritant |
| Hemorrhage | 7 | 5 | 3 | 5.0–8.9 | Moderate irritant |
| Coagulation | 9 | 7 | 5 | 9.0–21.0 | Sever irritant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihailovici, R.; Croitoriu, A.; Nedeff, F.; Nedeff, V.; Ochiuz, L.; Vasincu, D.; Popa, O.; Agop, M.; Moraru, A.; Costin, D.; et al. Drug-Loaded Polymeric Particulated Systems for Ophthalmic Drugs Release. Molecules 2022, 27, 4512. https://doi.org/10.3390/molecules27144512
Mihailovici R, Croitoriu A, Nedeff F, Nedeff V, Ochiuz L, Vasincu D, Popa O, Agop M, Moraru A, Costin D, et al. Drug-Loaded Polymeric Particulated Systems for Ophthalmic Drugs Release. Molecules. 2022; 27(14):4512. https://doi.org/10.3390/molecules27144512
Chicago/Turabian StyleMihailovici, Ruxandra, Alexandra Croitoriu, Florin Nedeff, Valentin Nedeff, Lacramioara Ochiuz, Decebal Vasincu, Ovidiu Popa, Maricel Agop, Andreea Moraru, Danut Costin, and et al. 2022. "Drug-Loaded Polymeric Particulated Systems for Ophthalmic Drugs Release" Molecules 27, no. 14: 4512. https://doi.org/10.3390/molecules27144512
APA StyleMihailovici, R., Croitoriu, A., Nedeff, F., Nedeff, V., Ochiuz, L., Vasincu, D., Popa, O., Agop, M., Moraru, A., Costin, D., Costuleanu, M., & Verestiuc, L. (2022). Drug-Loaded Polymeric Particulated Systems for Ophthalmic Drugs Release. Molecules, 27(14), 4512. https://doi.org/10.3390/molecules27144512










