Advances in Fibrin-Based Materials in Wound Repair: A Review
Abstract
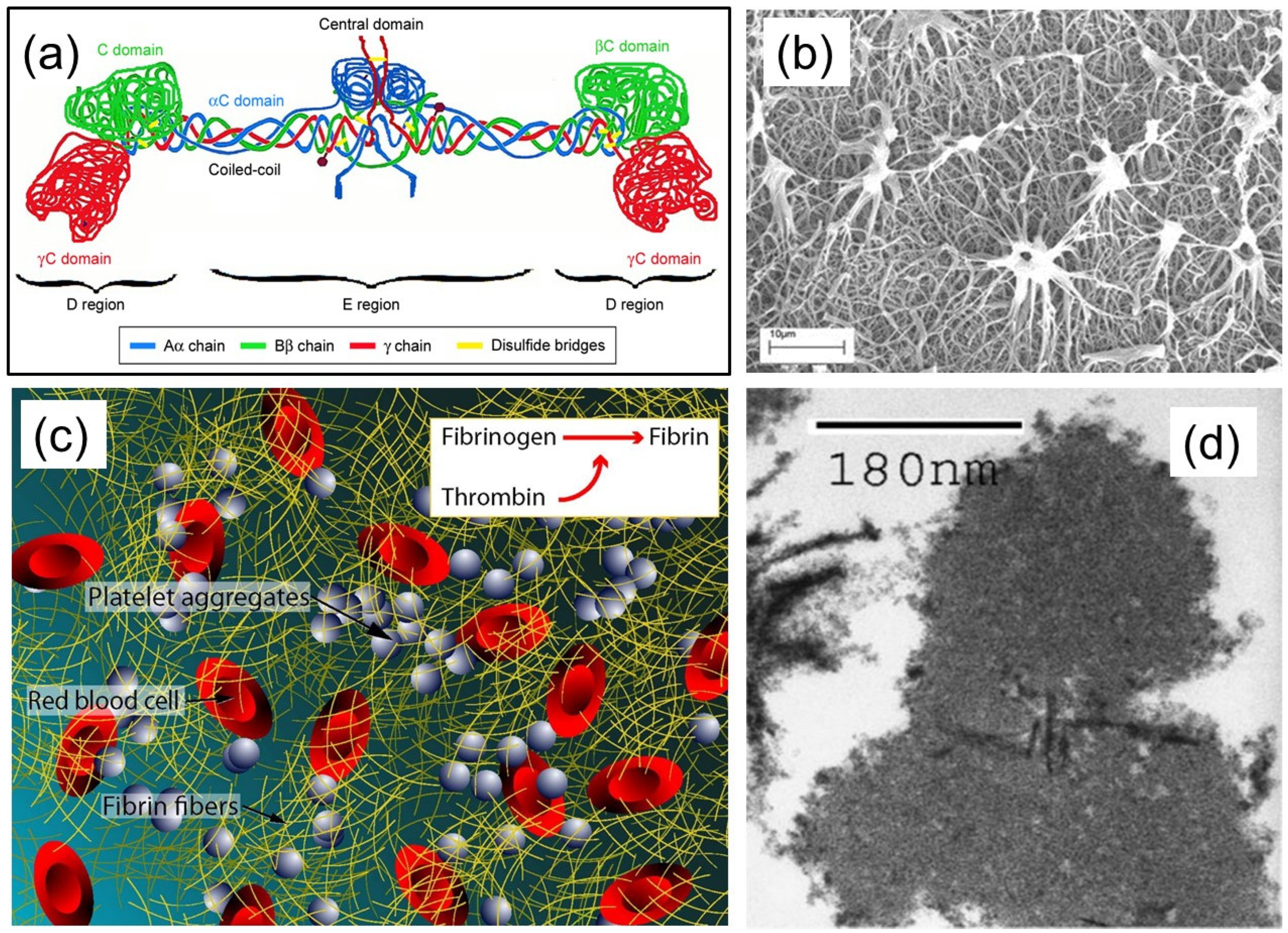
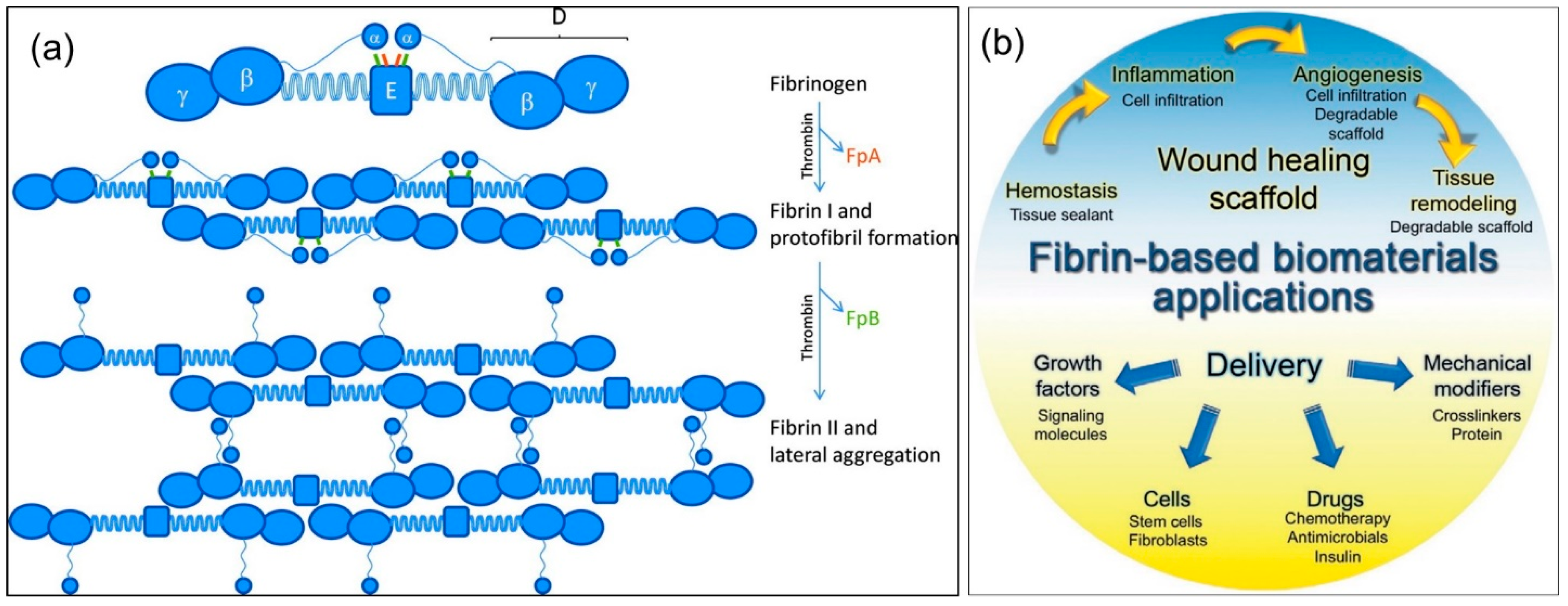
1. Fibrin(ogen) Structure and Function
2. Properties of Fibrin Fibers
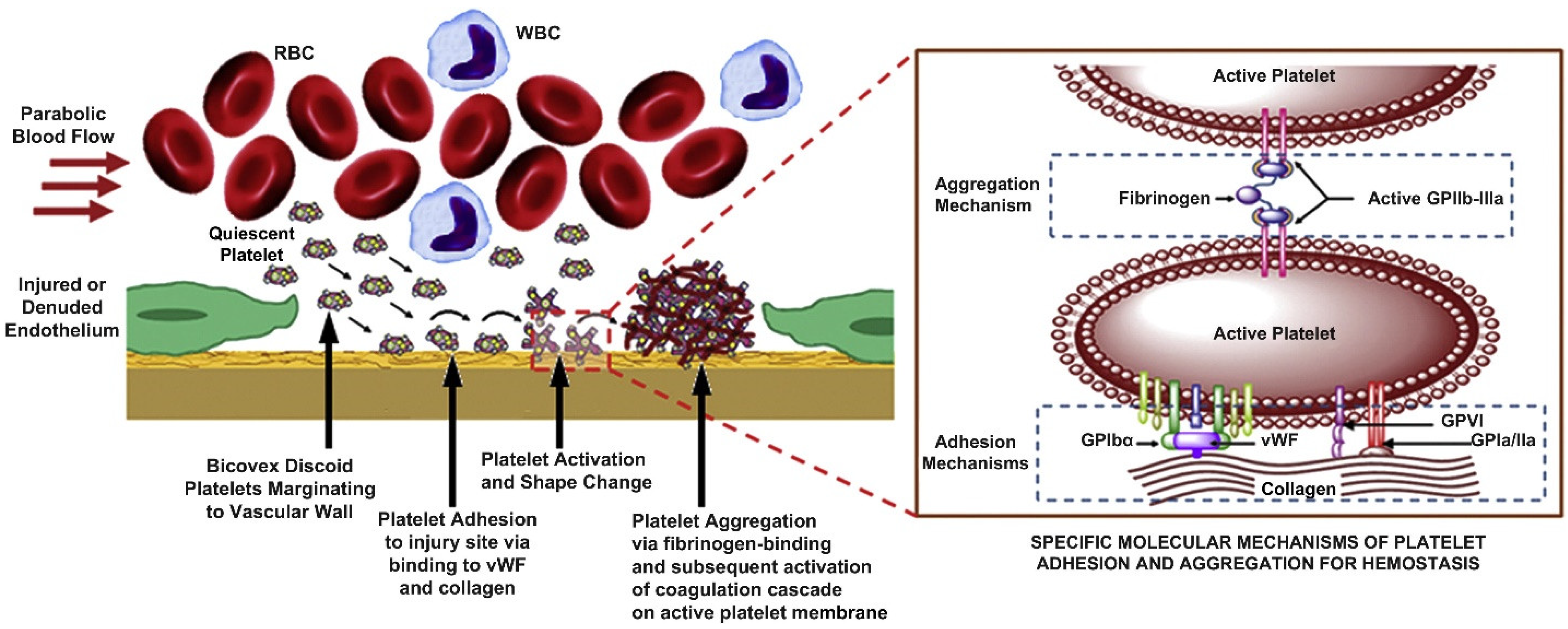
3. Mechanics of Platelet–Fibrin Interactions and Wound Healing
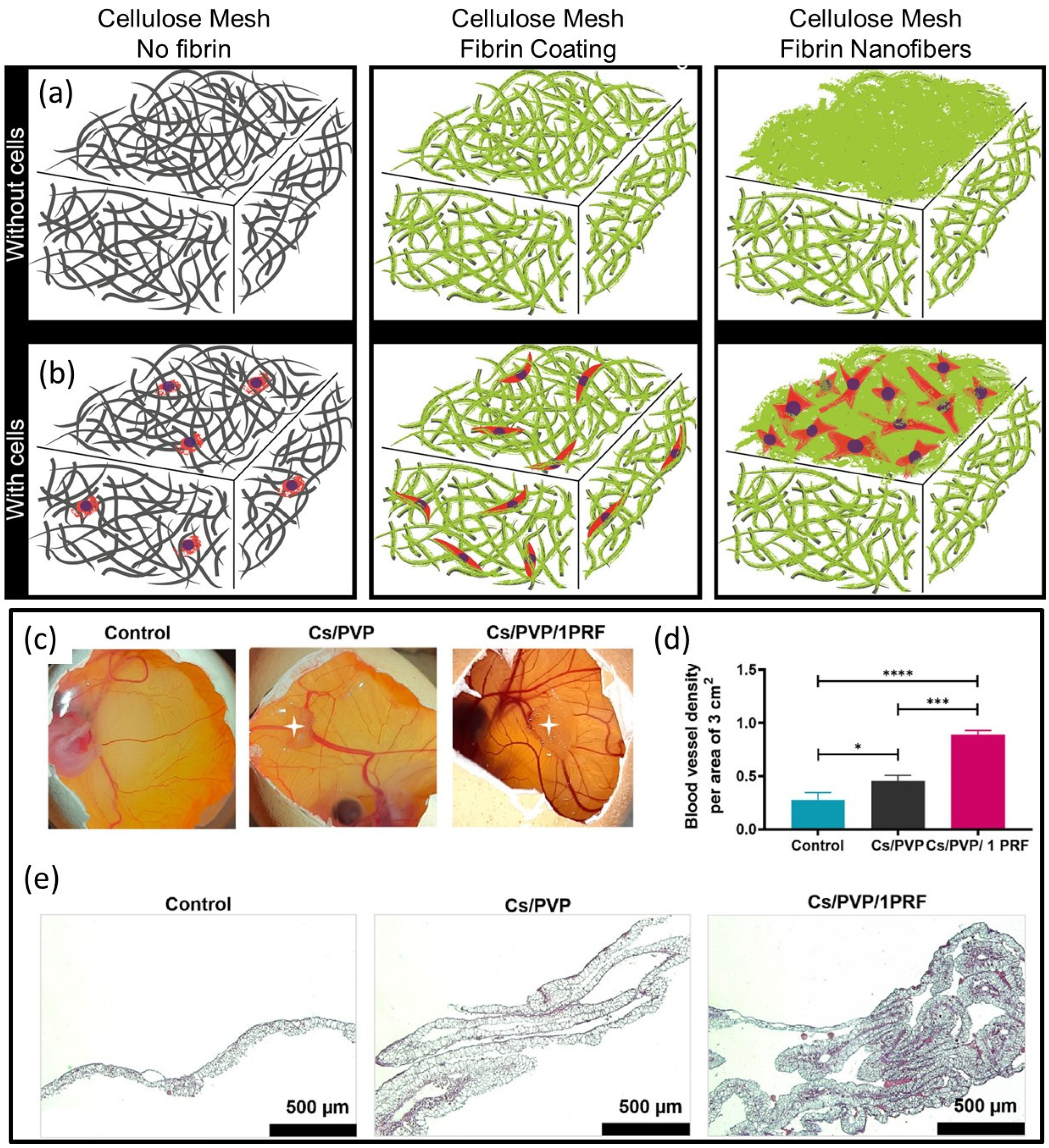
4. Fibrin Nanocomposites in Wound Management
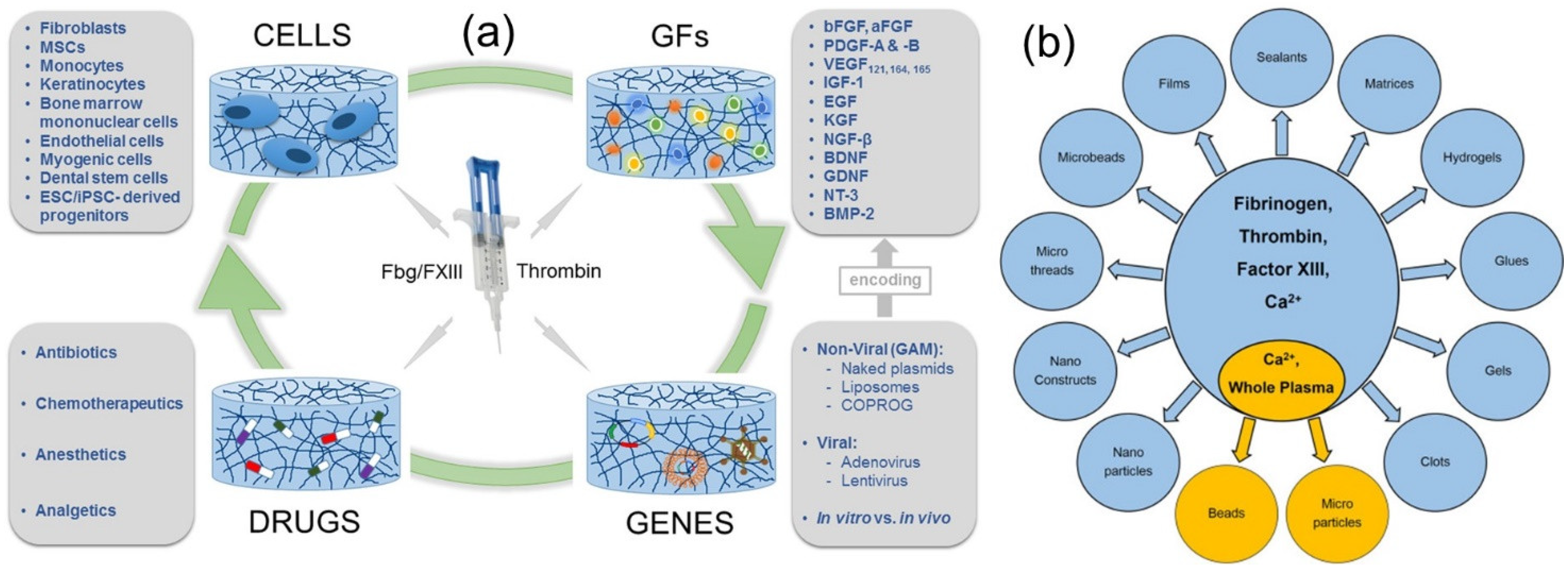
5. Fibrin-Based Drug Delivery Systems
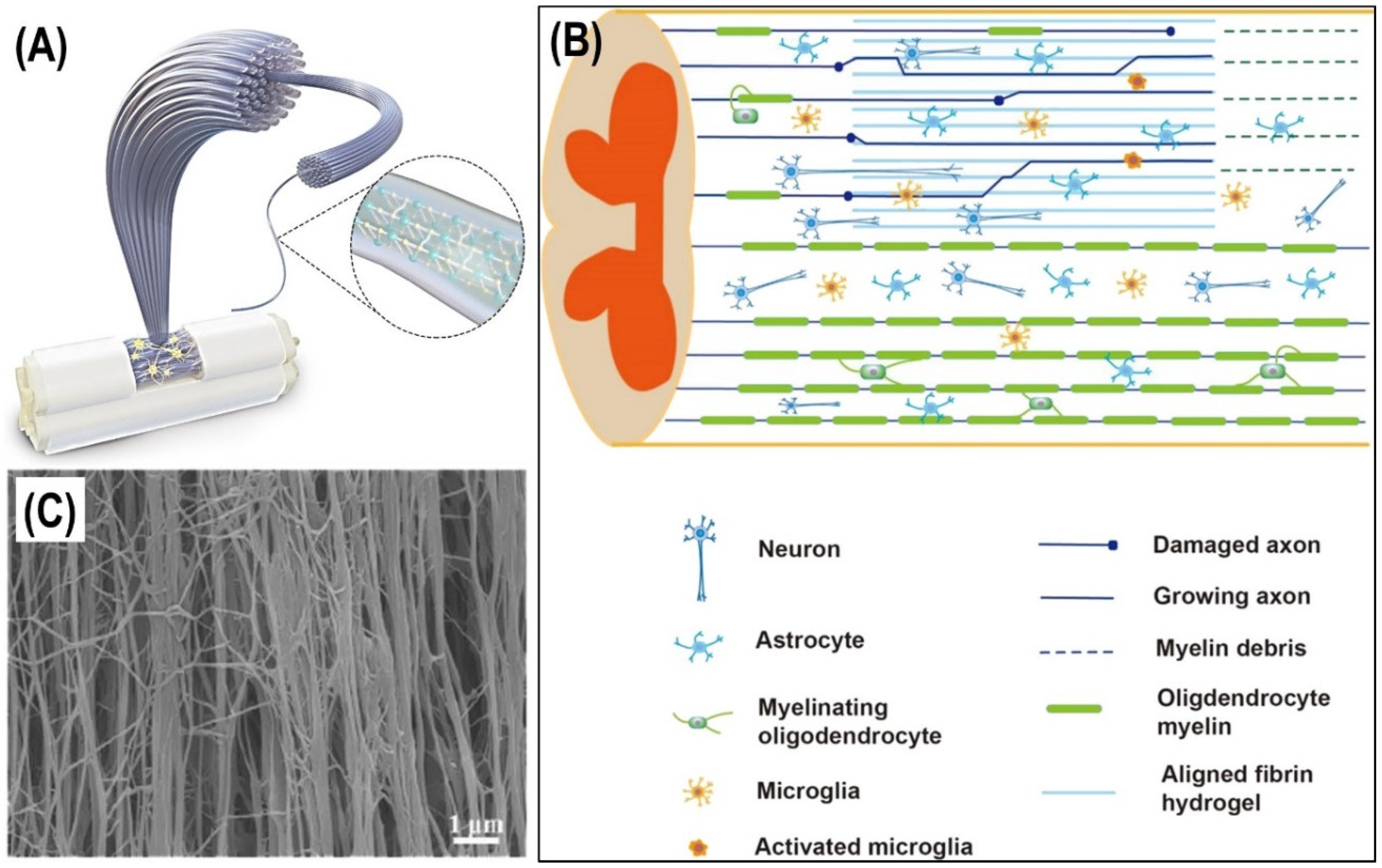
6. Fibrin and Cell Delivery
7. Fibrin and Skin Reconstruction
8. Applications of Drug-Loaded Fibrin-Based Materials
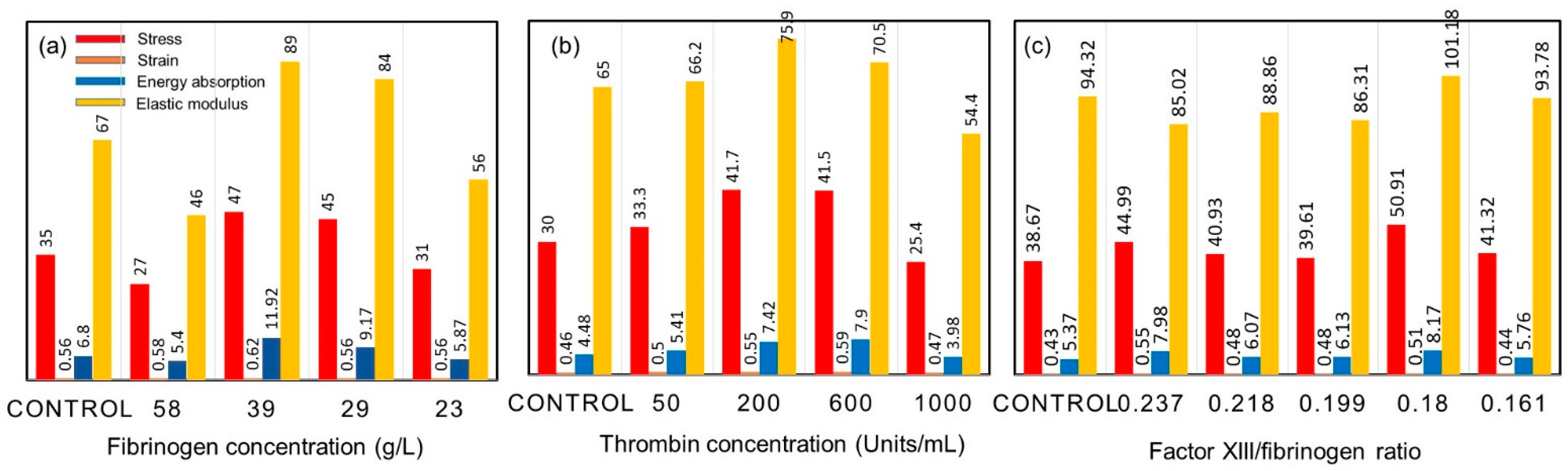
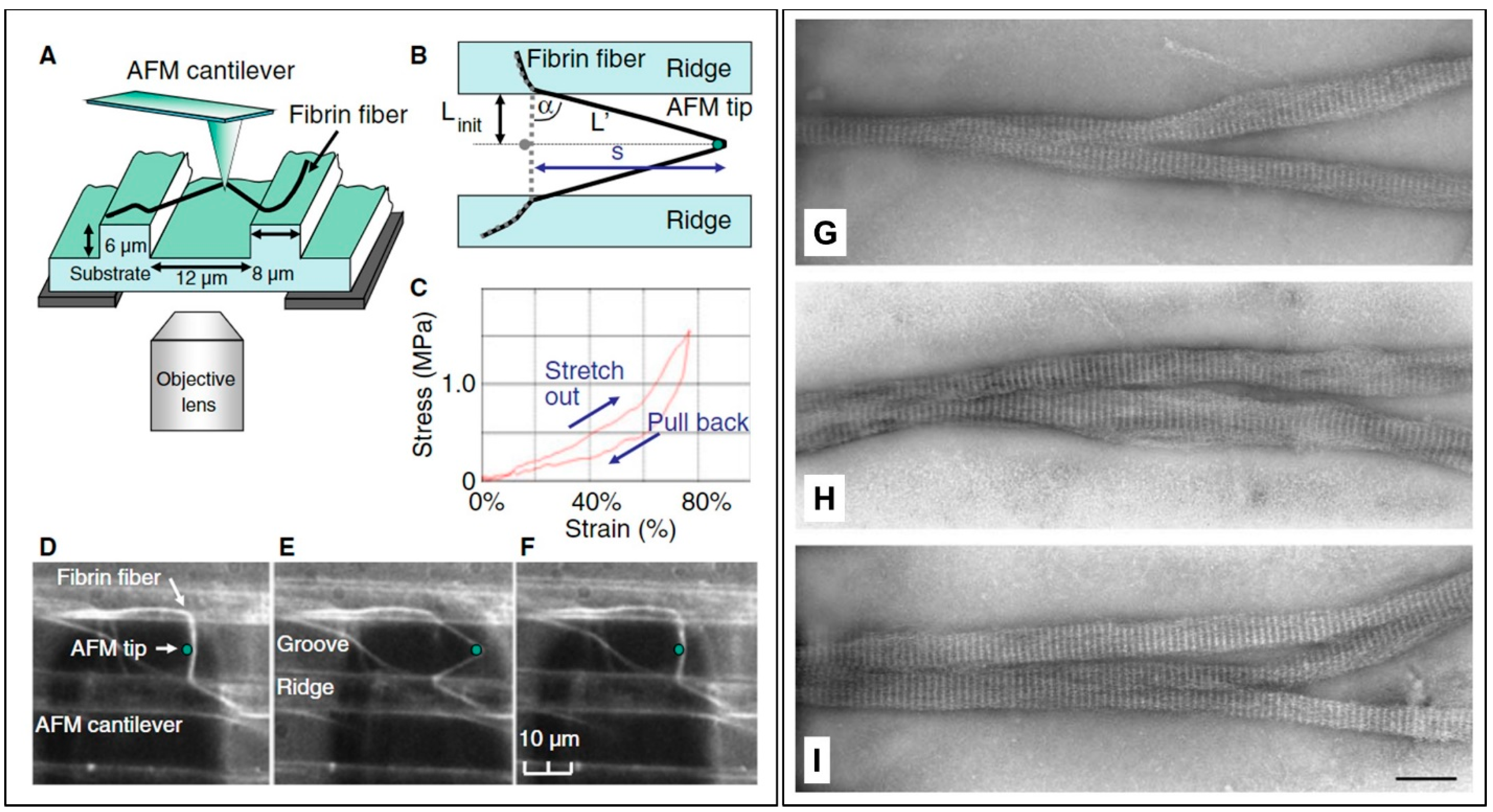
9. On the Mechanical Properties of Fibrin Fibers
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F. Fibrinogen and fibrin. Annu. Rev. Biochem. 1984, 53, 195–229. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Ariëns, R.A.; Grant, P.J. Genetic and environmental determinants of fibrin structure and function: Relevance to clinical disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937. [Google Scholar] [CrossRef]
- Longstaff, C.; Thelwell, C.; Williams, S.C.; Silva, M.M.; Szabó, L.; Kolev, K. The interplay between tissue plasminogen activator domains and fibrin structures in the regulation of fibrinolysis: Kinetic and microscopic studies. Blood J. Am. Soc. Hematol. 2011, 117, 661–668. [Google Scholar] [CrossRef]
- Tutwiler, V.; Wang, H.; Litvinov, R.I.; Weisel, J.W.; Shenoy, V.B. Interplay of platelet contractility and elasticity of fibrin/erythrocytes in blood clot retraction. Biophys. J. 2017, 112, 714–723. [Google Scholar] [CrossRef]
- Guthold, M.; Liu, W.; Sparks, E.A.; Jawerth, L.M.; Peng, L.; Falvo, M.; Lord, S.T. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem. Biophys. 2007, 49, 165–181. [Google Scholar] [CrossRef]
- Undas, A.; Ariëns, R.A. Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef]
- Sproul, E.; Nandi, S.; Brown, A. Fibrin biomaterials for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Woodhead Publishing: Sawston, UK, 2018; pp. 151–173. [Google Scholar]
- Chan, L.W.; Wang, X.; Wei, H.; Pozzo, L.D.; White, N.J.; Pun, S.H. A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci. Transl. Med. 2015, 7, 277ra29. [Google Scholar] [CrossRef]
- Yang, Z.; Mochalkin, I.; Doolittle, R.F. A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proc. Natl. Acad. Sci. USA 2000, 97, 14156–14161. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, T.C.; Sachweh, J.S.; Frese, J.; Schnöring, H.; Gronloh, N.; Koch, S.; Jockenhoevel, S. In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Eng. Part A 2009, 15, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.; Osipova, D.; Efremov, Y.; Bikmulina, P.; Kosheleva, N.; Lipina, M.; Timashev, P. Fibrin-based bioinks: New tricks from an old dog. Int. J. Bioprint. 2020, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Nurden, P.; Prado, R.; Nurden, A.T.; Padilla, S. Autologous fibrin scaffolds: When platelet-and plasma-derived biomolecules meet fibrin. Biomaterials 2019, 192, 440–460. [Google Scholar] [CrossRef]
- Illingworth, K.D.; Musahl, V.; Lorenz, S.G.; Fu, F.H. Use of fibrin clot in the knee. Oper. Tech. Orthop. 2010, 20, 90–97. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Weisel, J.W. Fibrin mechanical properties and their structural origins. Matrix Biol. 2017, 60, 110–123. [Google Scholar] [CrossRef]
- Collet, J.P.; Shuman, H.; Ledger, R.E.; Lee, S.; Weisel, J.W. The elasticity of an individual fibrin fiber in a clot. Proc. Natl. Acad. Sci. USA 2005, 102, 9133–9137. [Google Scholar] [CrossRef]
- Liu, W.; Carlisle, C.R.; Sparks, E.A.; Guthold, M. The mechanical properties of single fibrin fibers. J. Thromb. Haemost. 2010, 8, 1030–1036. [Google Scholar] [CrossRef]
- Yesudasan, S.; Averett, R.D. Recent advances in computational modeling of fibrin clot formation: A review. Comput. Biol. Chem. 2019, 83, 107148. [Google Scholar] [CrossRef]
- Xu, Z.; Christley, S.; Lioi, J.; Kim, O.; Harvey, C.; Sun, W.; Alber, M. Multiscale model of fibrin accumulation on the blood clot surface and platelet dynamics. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2012; Volume 110, pp. 367–388. [Google Scholar]
- Tosenberger, A.; Ataullakhanov, F.; Bessonov, N.; Panteleev, M.; Tokarev, A.; Volpert, V. Modelling of platelet–fibrin clot formation in flow with a DPD–PDE method. J. Math. Biol. 2016, 72, 649–681. [Google Scholar] [CrossRef]
- Byrne, D.J.; Hardy, J.; Wood RA, B.; McIntosh, R.; Cuschieri, A. Effect of fibrin glues on the mechanical properties of healing wounds. Br. J. Surg. 1991, 78, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Gersh, K.C.; Nagaswami, C.; Weisel, J.W. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb. Haemost. 2009, 102, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Cai, L.H.; Wu, H.; MacKintosh, F.C.; Weitz, D.A. Anomalous mechanics of Zn2+-modified fibrin networks. Proc. Natl. Acad. Sci. USA 2021, 118, e2020541118. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W. The mechanical properties of fibrin for basic scientists and clinicians. Biophys. Chem. 2004, 112, 267–276. [Google Scholar] [CrossRef]
- Weisel, J.W. Structure of fibrin: Impact on clot stability. J. Thromb. Haemost. 2007, 5, 116–124. [Google Scholar] [CrossRef]
- Liu WJ, L.M.; Jawerth, L.M.; Sparks, E.A.; Falvo, M.; Hantgan, R.R.; Superfine, R.; Guthold, M. Fibrin fibers have extraordinary extensibility and elasticity. Science 2006, 313, 634. [Google Scholar]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Bilgen, F.; Ural, A.; Bekerecioglu, M. Platelet-rich fibrin: An effective chronic wound healing accelerator. J. Tissue Viability 2021, 30, 616–620. [Google Scholar] [CrossRef]
- Pancaldi, F.; Kim, O.V.; Weisel, J.W.; Alber, M.; Xu, Z. Computational Biomechanical Modeling of Fibrin Networks and Platelet-Fiber Network Interactions. Curr. Opin. Biomed. Eng. 2022, 22, 100369. [Google Scholar] [CrossRef]
- Sun, Y.; Myers, D.R.; Nikolov, S.V.; Oshinowo, O.; Baek, J.; Bowie, S.M.; Alexeev, A. Platelet heterogeneity enhances blood clot volumetric contraction: An example of asynchrono-mechanical amplification. Biomaterials 2021, 274, 120828. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Sproul, E.P.; Nellenbach, K.; Erb, M.; Gaffney, L.; Freytes, D.O.; Brown, A.C. Platelet-like particles dynamically stiffen fibrin matrices and improve wound healing outcomes. Biomater. Sci. 2019, 7, 669–682. [Google Scholar] [CrossRef]
- Modery-Pawlowski, C.L.; Tian, L.L.; Pan, V.; McCrae, K.R.; Mitragotri, S.; Gupta, A.S. Approaches to synthetic platelet analogs. Biomaterials 2013, 34, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Carrim, N.; Wang, Y.; Gallant, R.C.; Marshall, A.; Ni, H. Platelets in hemostasis and thrombosis: Novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. J. Biomed. Res. 2015, 29, 437. [Google Scholar]
- Naik, B.; Karunakar, P.; Jayadev, M.; Marshal, V.R. Role of Platelet rich fibrin in wound healing: A critical review. J. Conserv. Dent. JCD 2013, 16, 284. [Google Scholar]
- Rowe, S.L.; Lee, S.; Stegemann, J.P. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007, 3, 59–67. [Google Scholar] [CrossRef]
- Osathanon, T.; Linnes, M.L.; Rajachar, R.M.; Ratner, B.D.; Somerman, M.J.; Giachelli, C.M. Microporous nanofibrous fibrin-based scaffolds for bone tissue engineering. Biomaterials 2008, 29, 4091–4099. [Google Scholar] [CrossRef]
- Rajangam, T.; An, S.S.A. Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int. J. Nanomed. 2013, 8, 3641. [Google Scholar]
- Vedakumari, W.S.; Prabu, P.; Sastry, T.P. Chitosan-fibrin nanocomposites as drug delivering and wound healing materials. J. Biomed. Nanotechnol. 2015, 11, 657–667. [Google Scholar] [CrossRef]
- Vedakumari, W.S.; Ayaz, N.; Karthick, A.S.; Senthil, R.; Sastry, T.P. Quercetin impregnated chitosan–fibrin composite scaffolds as potential wound dressing materials—Fabrication, characterization and In vivo analysis. Eur. J. Pharm. Sci. 2017, 97, 106–112. [Google Scholar] [CrossRef]
- Mohandas, A.; Sun, W.; Nimal, T.R.; Shankarappa, S.A.; Hwang, N.S.; Jayakumar, R. Injectable chitosan-fibrin/nanocurcumin composite hydrogel for the enhancement of angiogenesis. Res. Chem. Intermed. 2018, 44, 4873–4887. [Google Scholar] [CrossRef]
- Wang, K.; Mosser, G.; Haye, B.; Baccile, N.; Le Griel, P.; Pernot, P.; Coradin, T. Cellulose Nanocrystal–Fibrin Nanocomposite Hydrogels Promoting Myotube Formation. Biomacromolecules 2021, 22, 2740–2753. [Google Scholar] [CrossRef] [PubMed]
- Pathmanapan, S.; Periyathambi, P.; Anandasadagopan, S.K. Fibrin hydrogel incorporated with graphene oxide functionalized nanocomposite scaffolds for bone repair—In vitro and study. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102251. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, M.; Pajorova, J.; Sopuch, T.; Bacakova, L. Fibrin-modified cellulose as a promising dressing for accelerated wound healing. Materials 2018, 11, 2314. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.N.; Krishnamoorthi Kaliannagounder, V.; Selvaprithiviraj, V.; Suresh, M.K.; Biswas, R.; Vasudevan, A.K.; Jayakumar, R. Bioadhesive, hemostatic, and antibacterial in situ chitin–fibrin Nanocomposite gel for controlling bleeding and preventing infections at mediastinum. ACS Sustain. Chem. Eng. 2018, 6, 7826–7840. [Google Scholar] [CrossRef]
- Tavakoli, M.; Mirhaj, M.; Labbaf, S.; Varshosaz, J.; Taymori, S.; Jafarpour, F.; Sepyani, A. Fabrication and evaluation of Cs/PVP sponge containing platelet-rich fibrin as a wound healing accelerator: An in vitro and In vivo study. Int. J. Biol. Macromol. 2022, 204, 245–257. [Google Scholar] [CrossRef]
- Talukder, M.E.; Hasan, K.M.; Wang, J.; Yao, J.; Li, C.; Song, H. Novel fibrin functionalized multilayered electrospun nanofiber membrane for burn wound treatment. J. Mater. Sci. 2021, 56, 12814–12834. [Google Scholar] [CrossRef]
- Filová, E.; Rampichová, M.; Litvinec, A.; Držík, M.; Míčková, A.; Buzgo, M.; Amler, E. A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Int. J. Pharm. 2013, 447, 139–149. [Google Scholar] [CrossRef]
- Ortiz, A.D.C.; Fideles, S.O.M.; Pomini, K.T.; Reis, C.H.B.; Bueno, C.R.D.S.; Pereira, E.D.S.B.M.; Buchaim, R.L. Effects of Therapy with Fibrin Glue combined with Mesenchymal Stem Cells (MSCs) on Bone Regeneration: A Systematic Review. Cells 2021, 10, 2323. [Google Scholar] [CrossRef]
- Cao, Z.; Yao, S.; Xiong, Y.; Zhang, Z.; Yang, Y.; He, F.; Wang, X. Directional axonal regrowth induced by an aligned fibrin nanofiber hydrogel contributes to improved motor function recovery in canine L2 spinal cord injury. J. Mater. Sci. Mater. Med. 2020, 31, 1–13. [Google Scholar] [CrossRef]
- Cao, Z.; Man, W.; Xiong, Y.; Guo, Y.; Yang, S.; Liu, D.; Wang, X. White matter regeneration induced by aligned fibrin nanofiber hydrogel contributes to motor functional recovery in canine T12 spinal cord injury. Regen. Biomater. 2022, 9, rbab069. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, H.; Xia, P.; Kong, W.; Chang, Y.; Fu, C.; Qi, Z. Application of fibrin-based hydrogels for nerve protection and regeneration after spinal cord injury. J. Biol. Eng. 2020, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Praveen, G.; Raj, M.; Chennazhi, K.P.; Jayakumar, R. Flexible, micro-porous chitosan–gelatin hydrogel/nanofibrin composite bandages for treating burn wounds. Rsc Adv. 2014, 4, 65081–65087. [Google Scholar] [CrossRef]
- Senderoff, R.I.; Sheu, M.T.; Sokoloski, T.D. Fibrin based drug delivery systems. PDA J. Pharm. Sci. Technol. 1991, 45, 2–6. [Google Scholar]
- Breen, A.; O'Brien, T.; Pandit, A. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng. Part B Rev. 2009, 15, 201–214. [Google Scholar] [CrossRef]
- Wong, C.; Inman, E.; Spaethe, R.; Helgerson, S. Fibrin-based biomaterials to deliver human growth factors. Thromb. Haemost. 2003, 89, 573–582. [Google Scholar] [CrossRef]
- Jackson, M.R. Fibrin sealants in surgical practice: An overview. Am. J. Surg. 2001, 182, S1–S7. [Google Scholar] [CrossRef]
- Ahmad, E.; Fatima, M.T.; Hoque, M.; Owais, M.; Saleemuddin, M. Fibrin matrices: The versatile therapeutic delivery systems. Int. J. Biol. Macromol. 2015, 81, 121–136. [Google Scholar] [CrossRef]
- Meyenburg, S.; Lilie, H.; Panzner, S.; Rudolph, R. Fibrin encapsulated liposomes as protein delivery system: Studies on the in vitro release behavior. J. Control. Release 2000, 69, 159–168. [Google Scholar] [CrossRef]
- Heher, P.; Mühleder, S.; Mittermayr, R.; Redl, H.; Slezak, P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 134–147. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, Y.B. Sustained release of antibiotic from a fibrin-gelatin-antibiotic mixture. Laryngoscope 1997, 107, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.; Bai, M.V.; Krishnan, L.K. A freeze-dried fibrin disc as a biodegradable drug release matrix. Biologicals 2004, 32, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kirsch, R.; Valente, K.P.; Perez, M.R.; Willerth, S.M. Physical and mechanical characterization of fibrin-based bioprinted constructs containing drug-releasing microspheres for neural tissue engineering applications. Processes 2021, 9, 1205. [Google Scholar] [CrossRef]
- Sharma, R.; Smits, I.P.; De La Vega, L.; Lee, C.; Willerth, S.M. 3D bioprinting pluripotent stem cell derived neural tissues using a novel fibrin bioink containing drug releasing microspheres. Front. Bioeng. Biotechnol. 2020, 8, 57. [Google Scholar] [CrossRef]
- Viale, M.; Monticone, M.; Maric, I.; Giglio, V.; Profumo, A.; Aprile, A.; Rocco, M. Characterization of drug release from fibrin gels loaded with different pharmaceutical and experimental doxorubicin formulations. Pharmacol. Rep. 2018, 70, 760–765. [Google Scholar] [CrossRef]
- Egle, K.; Salma, I.; Dubnika, A. From Blood to Regenerative Tissue: How Autologous Platelet-Rich Fibrin Can Be Combined with Other Materials to Ensure Controlled Drug and Growth Factor Release. Int. J. Mol. Sci. 2021, 22, 11553. [Google Scholar] [CrossRef]
- Willerth, S.M.; Johnson, P.J.; Maxwell, D.J.; Parsons, S.R.; Doukas, M.E.; Sakiyama-Elbert, S.E. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J. Biomed. Mater. Res. Part A 2007, 80, 13–23. [Google Scholar] [CrossRef]
- Ho, H.O.; Sheu, M.T.; Sokoloski, T.D.; Chen, C.Y. Drug release from glutaraldehyde-treated fibrin gels. Drug Des. Deliv. 1990, 7, 65–73. [Google Scholar]
- Praveen, G.; Sreerekha, P.R.; Menon, D.; Nair, S.V.; Chennazhi, K.P. Fibrin nanoconstructs: A novel processing method and their use as controlled delivery agents. Nanotechnology 2012, 23, 095102. [Google Scholar] [CrossRef]
- Whelan, D.; Caplice, N.M.; Clover, A.J.P. Fibrin as a delivery system in wound healing tissue engineering applications. J. Control. Release 2014, 196, 1–8. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Rubin, J.P.; Pfeifer, M.E.; Moore, L.R.; Donnenberg, V.S.; Donnenberg, A.D. Human adipose stromal vascular cell delivery in a fibrin spray. Cytotherapy 2013, 15, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hu, Q.; Braunlin, E.A.; Suggs, L.J.; Zhang, J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng. Part A 2008, 14, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Carrion, B.; Janson, I.A.; Kong, Y.P.; Putnam, A.J. A safe and efficient method to retrieve mesenchymal stem cells from three-dimensional fibrin gels. Tissue Eng. Part C Methods 2014, 20, 252–263. [Google Scholar] [CrossRef]
- Kopp, J.; Jeschke, M.G.; Bach, A.D.; Kneser, U.; Horch, R.E. Applied tissue engineering in the closure of severe burns and chronic wounds using cultured human autologous keratinocytes in a natural fibrin matrix. Cell Tissue Bank. 2004, 5, 89–96. [Google Scholar] [CrossRef]
- De La Puente, P.; Ludeña, D.; Fernández, A.; Aranda, J.L.; Varela, G.; Iglesias, J. Autologous fibrin scaffolds cultured dermal fibroblasts and enriched with encapsulated bFGF for tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99, 648–654. [Google Scholar] [CrossRef]
- Donaghue, I.E.; Tam, R.; Sefton, M.V.; Shoichet, M.S. Cell and biomolecule delivery for tissue repair and regeneration in the central nervous system. J. Control. Release 2014, 190, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, S.; Ramanathan, G.; Raja, M.D.; Nagiah, N.; Padmapriya, P.; Kaveri, K.; Sivagnanam, U.T. Biomimetic interconnected porous keratin–fibrin–gelatin 3D sponge for tissue engineering application. Int. J. Biol. Macromol. 2016, 86, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Tatara, A.; Shiu, A.; Sakiyama-Elbert, S.E. Controlled release of neurotrophin-3 and platelet-derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transplant. 2010, 19, 89–101. [Google Scholar] [CrossRef]
- Johnson, P.J.; Tatara, A.; McCreedy, D.A.; Shiu, A.; Sakiyama-Elbert, S.E. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter 2010, 6, 5127–5137. [Google Scholar] [CrossRef]
- Arulmoli, J.; Wright, H.J.; Phan, D.T.; Sheth, U.; Que, R.A.; Botten, G.A.; Flanagan, L.A. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016, 43, 122–138. [Google Scholar] [CrossRef]
- Lei, P.; Padmashali, R.M.; Andreadis, S.T. Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels. Biomaterials 2009, 30, 3790–3799. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Tawil, B.; Dunn, J.C.; Wu, B.M. The behavior of human mesenchymal stem cells in 3D fibrin clots: Dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006, 12, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Eaglstein, W.H.; Sullivan, T. Cyanoacrylates for skin closure. Dermatol. Clin. 2005, 23, 193–198. [Google Scholar] [CrossRef]
- Mele, E.; Heredia-Guerrero, J.A.; Bayer, I.S.; Ciofani, G.; Genchi, G.G.; Ceseracciu, L.; Athanassiou, A. Zwitterionic nanofibers of super-glue for transparent and biocompatible multi-purpose coatings. Sci. Rep. 2015, 5, 14019. [Google Scholar] [CrossRef]
- Bayer, I.S. Nanostructured Cyanoacrylates: Biomedical Applications. In Smart Nanoparticles for Biomedicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 65–81. [Google Scholar]
- Gulalp, B.; Seyhan, T.; Gursoy, S.; Altinors, M.N. Emergency wounds treated with cyanoacrylate and long-term results in pediatrics: A series of cases; what are the advantages and boards? BMC Res. Notes 2009, 2, 132. [Google Scholar] [CrossRef] [PubMed]
- Trott, A.T. Cyanoacrylate tissue adhesives: An advance in wound care. JAMA 1997, 277, 1559–1560. [Google Scholar] [CrossRef]
- Park, W.; Kim, W.H.; Lee, C.H.; Kim, D.Y.; Choi, J.H.; Huh, J.W.; Kweon, O.K. Comparison of two fibrin glues in anastomoses and skin closure. J. Vet. Med. Ser. A 2002, 49, 385–389. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Fibrin formation, structure and properties. In Fibrous Proteins: Structures and Mechanisms; Springer: Cham, Switzerland, 2017; pp. 405–456. [Google Scholar]
- Bencherif, S.A.; Gsib, O.; Egles, C. Fibrin: An underrated biopolymer for skin tissue engineering. J. Mol. Biol. Biotech 2017, 2, 1–5. [Google Scholar]
- Sánchez-Muñoz, I.; Granados, R.; Holguin Holgado, P.; García-Vela, J.A.; Casares, C.; Casares, M. The use of adipose mesenchymal stem cells and human umbilical vascular endothelial cells on a fibrin matrix for endothelialized skin substitute. Tissue Eng. Part A 2015, 21, 214–223. [Google Scholar] [CrossRef]
- Law, J.X.; Musa, F.; Ruszymah BH, I.; El Haj, A.J.; Yang, Y. A comparative study of skin cell activities in collagen and fibrin constructs. Med. Eng. Phys. 2016, 38, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. A Review of Sustained Drug Release Studies from Nanofiber Hydrogels. Biomedicines 2021, 9, 1612. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Pérez, D.; Correa, L.; Restrepo, L. Evaluation of fibrin-based dermal-epidermal organotypic cultures for in vitro skin corrosion and irritation testing of chemicals according to OECD TG 431 and 439. Toxicol. Vitr. 2016, 36, 89–96. [Google Scholar] [CrossRef]
- Wei, H.; Gu, S.X.; Liang, Y.D.; Liang, Z.J.; Chen, H.; Zhu, M.G.; Li, H.M. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget 2017, 8, 68542. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.P.; Wang, O.; do Prado, T.P.; Ismail, A.; Fabian, F.M.; Li, H.; Araújo, E.P. Novel fibrin-fibronectin matrix accelerates mice skin wound healing. Bioact. Mater. 2020, 5, 949–962. [Google Scholar] [CrossRef]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef]
- Desai, C.B.; Mahindra, U.R.; Kini, Y.K.; Bakshi, M.K. Use of platelet-rich fibrin over skin wounds: Modified secondary intention healing. J. Cutan. Aesthet. Surg. 2013, 6, 35. [Google Scholar] [CrossRef]
- Gode, S.; Ozturk, A.; Kısmalı, E.; Berber, V.; Turhal, G. The effect of platelet-rich fibrin on nasal skin thickness in rhinoplasty. Fac. Plast. Surg. 2019, 35, 400–403. [Google Scholar] [CrossRef]
- Ionescu, A.M.; Chato-Astrain, J.; Pérez, J.D.L.C.C.; Campos, F.; Gómez, M.M.P.; Alaminos, M.; Bello, I.G. Evaluation of the optical and biomechanical properties of bioengineered human skin generated with fibrin-agarose biomaterials. J. Biomed. Opt. 2020, 25, 055002. [Google Scholar] [CrossRef]
- Kljenak, A.; Tominac Trcin, M.; Bujić, M.; Dolenec, T.; Jevak, M.; Mršić, G.; Popović, M. Fibrin gel as a scaffold for skin substitute–production and clinical experience. Acta Clin. Croat. 2016, 55, 279–288. [Google Scholar] [CrossRef]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2018, 21, 326–340. [Google Scholar] [CrossRef]
- Ding, Y.; Cui, L.; Zhao, Q.; Zhang, W.; Sun, H.; Zheng, L. Platelet-rich fibrin accelerates skin wound healing in diabetic mice. Ann. Plast. Surg. 2017, 79, e15–e19. [Google Scholar] [CrossRef] [PubMed]
- Van Cleemput, T.; Hendrikx, S.; Politis, C.; Spaey, Y. Leukocyte-and Platelet-Rich Fibrin: A New Method for Scalp Defect Reconstruction. Dermatol. Surg. 2022, 48, 261–262. [Google Scholar] [CrossRef]
- Bacakova, M.; Pajorova, J.; Broz, A.; Hadraba, D.; Lopot, F.; Zavadakova, A.; Bacakova, L. A two-layer skin construct consisting of a collagen hydrogel reinforced by a fibrin-coated polylactide nanofibrous membrane. Int. J. Nanomed. 2019, 14, 5033. [Google Scholar] [CrossRef]
- Frisman, I.; Seliktar, D.; Bianco-Peled, H. Nanostructuring of PEG–fibrinogen polymeric scaffolds. Acta Biomater. 2010, 6, 2518–2524. [Google Scholar] [CrossRef]
- Kober, J.; Gugerell, A.; Schmid, M.; Kamolz, L.P.; Keck, M. Generation of a fibrin based three-layered skin substitute. BioMed Res. Int. 2015, 2015, 104. [Google Scholar] [CrossRef] [PubMed]
- Kouhbananinejad, S.M.; Derakhshani, A.; Vahidi, R.; Dabiri, S.; Fatemi, A.; Armin, F.; Farsinejad, A. A fibrinous and allogeneic fibroblast-enriched membrane as a biocompatible material can improve diabetic wound healing. Biomater. Sci. 2019, 7, 1949–1961. [Google Scholar] [CrossRef]
- Chiaravalloti, A.J.; Zubkov, B.; Zubkov, A. Treatment of a chronic cutaneous surgical wound with platelet-rich fibrin. Dermatol. Surg. 2018, 44, 449–452. [Google Scholar] [CrossRef]
- Soldatova, L.; Campbell, R.G.; Elkhatib, A.H.; Schmidt, T.W.; Pinto, N.R.; Pinto, J.M.; Carrau, R.L. Role of leukocyte–platelet-rich fibrin in endoscopic endonasal skull base surgery defect reconstruction. J. Neurol. Surg. Part B Skull Base 2017, 78, 59–62. [Google Scholar] [CrossRef]
- Natesan, S.; Zamora, D.O.; Wrice, N.L.; Baer, D.G.; Christy, R.J. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J. Burn Care Res. 2013, 34, 18–30. [Google Scholar] [CrossRef]
- Kim, D.Y.; Pyun, J.; Choi, J.W.; Kim, J.H.; Lee, J.S.; Shin, H.A.; Kim, C.H. Tissue-engineered allograft tracheal cartilage using fibrin/hyaluronan composite gel and its in vivo implantation. Laryngoscope 2010, 120, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Klar, A.S.; Güven, S.; Biedermann, T.; Luginbühl, J.; Böttcher-Haberzeth, S.; Meuli-Simmen, C.; Reichmann, E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 2014, 35, 5065–5078. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.J.; Chang, J.W.; Park, J.K.; Choi, J.W.; Kim, Y.S.; Shin, Y.S.; Choi, E.C. Tracheal reconstruction using chondrocytes seeded on a poly (l-lactic-co-glycolic acid)–fibrin/hyaluronan. J. Biomed. Mater. Res. Part A 2014, 102, 4142–4150. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, M.; Musilkova, J.; Riedel, T.; Stranska, D.; Brynda, E.; Zaloudkova, M.; Bacakova, L. The potential applications of fibrin-coated electrospun polylactide nanofibers in skin tissue engineering. Int. J. Nanomed. 2016, 11, 771. [Google Scholar] [CrossRef]
- Sharma, K.; Bullock, A.J.; Giblin, V.; MacNeil, S. Identification of a fibrin concentration that promotes skin cell outgrowth from skin explants onto a synthetic dermal substitute. JPRAS Open 2020, 25, 8–17. [Google Scholar] [CrossRef]
- Reksodiputro, M.H.; Harba’i, H.M.; Koento, T.; Harahap, A.R. Platelet-rich fibrin enhances wound epithelialization in the skin graft donor site. J. Phys. Conf. Ser. 2018, 1073, 032046. [Google Scholar] [CrossRef]
- Przekora, A. A concise review on tissue engineered artificial skin grafts for chronic wound treatment: Can we reconstruct functional skin tissue in vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef]
- Gürer, B.; Kertmen, H.; Akturk, U.D.; Kalan, M.; Sekerci, Z. Use of the bovine pericardial patch and fibrin sealant in meningomyelocele closure. Acta Neurochir. 2014, 156, 1345–1350. [Google Scholar] [CrossRef]
- Bujoli, B.; Scimeca, J.C.; Verron, E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef]
- Roberts, I.V.; Bukhary, D.; Valdivieso CY, L.; Tirelli, N. Fibrin matrices as (injectable) biomaterials: Formation, clinical use, and molecular engineering. Macromol. Biosci. 2020, 20, 1900283. [Google Scholar] [CrossRef]
- Brown, A.C.; Barker, T.H. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014, 10, 1502–1514. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kyung, M.W. Fibrin-based biomaterial applications in tissue engineering and regenerative medicine. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 253–261. [Google Scholar]
- Losi, P.; Briganti, E.; Errico, C.; Lisella, A.; Sanguinetti, E.; Chiellini, F.; Soldani, G. Fibrin-based scaffold incorporating VEGF-and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013, 9, 7814–7821. [Google Scholar] [CrossRef] [PubMed]
- Storozhylova, N.; Crecente-Campo, J.; Cabaleiro, D.; Lugo, L.; Dussouy, C.; Simões, S.; Alonso, M.J. An in situ hyaluronic acid-fibrin hydrogel containing drug-loaded nanocapsules for intra-articular treatment of inflammatory joint diseases. Regen. Eng. Transl. Med. 2020, 6, 201–216. [Google Scholar] [CrossRef]
- Tredwell, S.; Jackson, J.K.; Hamilton, D.; Lee, V.; Burt, H.M. Use of fibrin sealants for the localized, controlled release of cefazolin. Can. J. Surg. 2006, 49, 347. [Google Scholar] [PubMed]
- Thattaruparambil Raveendran, N.; Mohandas, A.; Ramachandran Menon, R.; Somasekharan Menon, A.; Biswas, R.; Jayakumar, R. Ciprofloxacin-and fluconazole-containing fibrin-nanoparticle-incorporated chitosan bandages for the treatment of polymicrobial wound infections. ACS Appl. Bio Mater. 2018, 2, 243–254. [Google Scholar] [CrossRef]
- Seetharaman, S.; Natesan, S.; Stowers, R.S.; Mullens, C.; Baer, D.G.; Suggs, L.J.; Christy, R.J. A PEGylated fibrin-based wound dressing with antimicrobial and angiogenic activity. Acta Biomater. 2011, 7, 2787–2796. [Google Scholar] [CrossRef]
- Viale, M.; Vecchio, G.; Monticone, M.; Bertone, V.; Giglio, V.; Maric, I.; Rocco, M. Fibrin gels entrapment of a poly-cyclodextrin nanocarrier as a doxorubicin delivery system in an orthotopic model of neuroblastoma: Evaluation of in vitro activity and In vivo toxicity. Pharm. Res. 2019, 36, 115. [Google Scholar] [CrossRef]
- Alphonsa, B.M.; Kumar, S.; Praveen, G.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Antimicrobial drugs encapsulated in fibrin nanoparticles for treating microbial infested wounds. Pharm. Res. 2014, 31, 1338–1351. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Hu, L.; Han, X.; Zou, X.; Chen, Q.; Wang, C. In situ formed fibrin scaffold with cyclophosphamide to synergize with immune checkpoint blockade for inhibition of cancer recurrence after surgery. Adv. Funct. Mater. 2020, 30, 1906922. [Google Scholar] [CrossRef]
- Al Kayal, T.; Buscemi, M.; Cavallo, A.; Foffa, I.; Soldani, G.; Losi, P. Plasminogen-Loaded Fibrin Scaffold as Drug Delivery System for Wound Healing Applications. Pharmaceutics 2022, 14, 251. [Google Scholar] [CrossRef]
- Yang, H.S.; La, W.G.; Bhang, S.H.; Jeon, J.Y.; Lee, J.H.; Kim, B.S. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng. Part A 2010, 16, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Samal, J.; Hoban, D.B.; Naughton, C.; Concannon, R.; Dowd, E.; Pandit, A. Fibrin-based microsphere reservoirs for delivery of neurotrophic factors to the brain. Nanomedicine 2015, 10, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, M.; Zhao, Y.; Mou, Y. A fibrin gel loaded with chitosan nanoparticles for local delivery of rhEGF: Preparation and in vitro release studies. J. Mater. Sci. Mater. Med. 2011, 22, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Vedakumari, W.S.; Sastry, T.P. Physiologically clotted fibrin–preparation and characterization for tissue engineering and drug delivery applications. Biologicals 2014, 42, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Dubnika, A.; Egle, K.; Skrinda-Melne, M.; Skadins, I.; Rajadas, J.; Salma, I. Development of Vancomycin Delivery Systems Based on Autologous 3D Platelet-Rich Fibrin Matrices for Bone Tissue Engineering. Biomedicines 2021, 9, 814. [Google Scholar] [CrossRef]
- Viale, M.; Rossi, M.; Russo, E.; Cilli, M.; Aprile, A.; Profumo, A.; Rocco, M. Fibrin gels loaded with cisplatin and cisplatin-hyaluronate complexes tested in a subcutaneous human melanoma model. Investig. New Drugs 2015, 33, 1151–1161. [Google Scholar] [CrossRef]
- Vedakumari, W.S.; Prabu, P.; Babu, S.C.; Sastry, T.P. Fibrin nanoparticles as Possible vehicles for drug delivery. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4244–4253. [Google Scholar] [CrossRef]
- Itokazu, M.; Yamamoto, K.; Yang, W.Y.; Aoki, T.; Kato, N.; Watanabe, K. The sustained release of antibiotic from freeze-dried fibrin-antibiotic compound and efficacies in a rat model of osteomyelitis. Infection 1997, 25, 359–363. [Google Scholar] [CrossRef]
- Kim, S.N.; Choi, B.H.; Kim, H.K.; Choy, Y.B. Poly (lactic-co-glycolic acid) microparticles in fibrin glue for local, sustained delivery of bupivacaine. J. Ind. Eng. Chem. 2019, 75, 86–92. [Google Scholar] [CrossRef]
- Wang, S.S.; Yang, M.C.; Chung, T.W. Liposomes/chitosan scaffold/human fibrin gel composite systems for delivering hydrophilic drugs—release behaviors of Tirofiban in vitro. Drug Deliv. 2008, 15, 149–157. [Google Scholar] [CrossRef]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Aleshkin, A.; Bochkareva, S.; Modin, E.; Haverich, A. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 2019, 9, 2091. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010, 223, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, V.; Mittermayr, R.; Hartinger, J.; Martino, M.M.; Lorentz, K.M.; Wolbank, S.; Banfi, A. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc. Natl. Acad. Sci. USA 2014, 111, 6952–6957. [Google Scholar] [CrossRef] [PubMed]
- Losi, P.; Briganti, E.; Magera, A.; Spiller, D.; Ristori, C.; Battolla, B.; Soldani, G. Tissue response to poly (ether) urethane-polydimethylsiloxane-fibrin composite scaffolds for controlled delivery of pro-angiogenic growth factors. Biomaterials 2010, 31, 5336–5344. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, R.; Slezak, P.; Haffner, N.; Smolen, D.; Hartinger, J.; Hofmann, A.; Redl, H. Controlled release of fibrin matrix-conjugated platelet derived growth factor improves ischemic tissue regeneration by functional angiogenesis. Acta Biomater. 2016, 29, 11–20. [Google Scholar] [CrossRef]
- Morton, T.J.; Fuerst, W.; Griensven, M.V.; Redl, H. Controlled release of substances bound to fibrin-anchors or of DNA. Drug Deliv. 2009, 16, 102–107. [Google Scholar] [CrossRef]
- Taylor, S.J.; McDonald, J.W., III; Sakiyama-Elbert, S.E. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J. Control. Release 2004, 98, 281–294. [Google Scholar] [CrossRef]
- Wilems, T.S.; Sakiyama-Elbert, S.E. Sustained dual drug delivery of anti-inhibitory molecules for treatment of spinal cord injury. J. Control. Release 2015, 213, 103–111. [Google Scholar] [CrossRef]
- Falvo, M.R.; Gorkun, O.V.; Lord, S.T. The molecular origins of the mechanical properties of fibrin. Biophys. Chem. 2010, 152, 15–20. [Google Scholar] [CrossRef]
- Helms, C.C.; Ariëns, R.A.; De Willige, S.U.; Standeven, K.F.; Guthold, M. α−α Cross-links increase fibrin fiber elasticity and stiffness. Biophys. J. 2012, 102, 168–175. [Google Scholar] [CrossRef]
- Münster, S.; Jawerth, L.M.; Leslie, B.A.; Weitz, J.I.; Fabry, B.; Weitz, D.A. Strain history dependence of the nonlinear stress response of fibrin and collagen networks. Proc. Natl. Acad. Sci. USA 2013, 110, 12197–12202. [Google Scholar] [CrossRef] [PubMed]
- Yesudasan, S.; Averett, R.D. Multiscale network modeling of fibrin fibers and fibrin clots with protofibril binding mechanics. Polymers 2020, 12, 1223. [Google Scholar] [CrossRef] [PubMed]
- Maksudov, F.; Daraei, A.; Sesha, A.; Marx, K.A.; Guthold, M.; Barsegov, V. Strength, deformability and toughness of uncrosslinked fibrin fibers from theoretical reconstruction of stress-strain curves. Acta Biomater. 2021, 136, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, O.V.; Machlus, K.R.; Liu, X.; Kupaev, T.; Lioi, J.; Alber, M. Correlation between fibrin network structure and mechanical properties: An experimental and computational analysis. Soft Matter 2011, 7, 4983–4992. [Google Scholar] [CrossRef]
- Li, W.; Sigley, J.; Pieters, M.; Helms, C.C.; Nagaswami, C.; Weisel, J.W.; Guthold, M. Fibrin fiber stiffness is strongly affected by fiber diameter, but not by fibrinogen glycation. Biophys. J. 2016, 110, 1400–1410. [Google Scholar] [CrossRef]
- Krasokha, N.; Theisen, W.; Reese, S.; Mordasini, P.; Brekenfeld, C.; Gralla, J.; Monstadt, H. Mechanical properties of blood clots—A new test method. Mater. Werkst. 2010, 41, 1019–1024. [Google Scholar] [CrossRef]
- Abrego, C.J.G.; Dedroog, L.; Deschaume, O.; Wellens, J.; Vananroye, A.; Lettinga, M.P.; Bartic, C. Multiscale Characterization of the Mechanical Properties of Fibrin and Polyethylene Glycol (PEG) Hydrogels for Tissue Engineering Applications. Macromol. Chem. Phys. 2022, 223, 2100366. [Google Scholar] [CrossRef]
- Bayer, G.; Grasselli, S.; Malchiodi, A.; Bayer, I.S. Antiseptic povidone-iodine encapsulating edible phospholipid gels. Coll. Surf. A Physicochem. Eng. Asp. 2021, 619, 126537. [Google Scholar] [CrossRef]
- Rancan, F.; Contardi, M.; Jurisch, J.; Blume-Peytavi, U.; Vogt, A.; Bayer, I.S.; Schaudinn, C. Evaluation of drug delivery and efficacy of ciprofloxacin-loaded povidone foils and nanofiber mats in a wound-infection model based on ex vivo human skin. Pharmaceutics 2019, 11, 527. [Google Scholar] [CrossRef]
- Bayer, I.S. Recent Advances in Mucoadhesive Interface Materials, Mucoadhesion Characterization, and Technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Wang, Y.; Armato, U.; Wu, J. Targeting tunable physical properties of materials for chronic wound care. Front. Bioeng. Biotechnol. 2020, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xiao, Z.; Zhou, Y.; Chen, A.; Xuan, X.; Li, Y.; Wu, J. Zwitterionic poly (sulfobetaine methacrylate) hydrogels with optimal mechanical properties for improving wound healing In vivo. J. Mater. Chem. B 2019, 7, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, E.; Mehdikhani-Nahrkhalaji, M.; Hashemi-Beni, B.; Zargar-Kharazi, A.; Kharaziha, M.J.P.M.S. Preparation, characterization and mechanical assessment of poly (lactide-co-glycolide)/hyaluronic acid/fibrin/bioactive glass nano-composite scaffolds for cartilage tissue engineering applications. Proced. Mater. Sci. 2015, 11, 124–130. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Schneider-Barthold, C.; Baganz, S.; Wilhelmi, M.; Scheper, T.; Pepelanova, I. Hydrogels based on collagen and fibrin–frontiers and applications. BioNanoMaterials 2016, 17, 3–12. [Google Scholar] [CrossRef]
- Laurens, N.; Koolwijk, P.D.; De Maat, M.P.M. Fibrin structure and wound healing. J. Thromb. Haemost. 2006, 4, 932–939. [Google Scholar] [CrossRef]
- Kearney, K.J.; Ariëns, R.A.; Macrae, F.L. The role of fibrin (ogen) in wound healing and infection control. Semin. Thromb. Hemost. 2022, 48, 174–187. [Google Scholar] [CrossRef]
- Amrani, D.L.; Diorio, J.P.; Delmotte, Y. Wound healing: Role of commercial fibrin sealants. Ann. N. Y. Acad. Sci. 2001, 936, 566–579. [Google Scholar] [CrossRef]
- Mazlyzam, A.L.; Aminuddin, B.S.; Fuzina, N.H.; Norhayati, M.M.; Fauziah, O.; Isa, M.R.; Ruszymah, B.H.I. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns 2007, 33, 355–363. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Mechanisms of fibrin polymerization and clinical implications. Blood J. Am. Soc. Hematol. 2013, 121, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, I.N.; Nagaswami, C.; Purohit, P.K.; Weisel, J.W. Fibrin clots are equilibrium polymers that can be remodeled without proteolytic digestion. Sci. Rep. 2012, 2, 879. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Triveni, M.G.; Kumar, A.T.; Mehta, D.S. Role of platelet-rich-fibrin in enhancing palatal wound healing after free graft. Contemp. Clin. Dent. 2012, 3 (Suppl. S2), S240. [Google Scholar] [PubMed]
- Gaule, T.G.; Ajjan, R.A. Fibrin (ogen) as a Therapeutic Target: Opportunities and Challenges. Int. J. Mol. Sci. 2021, 22, 6916. [Google Scholar] [CrossRef]
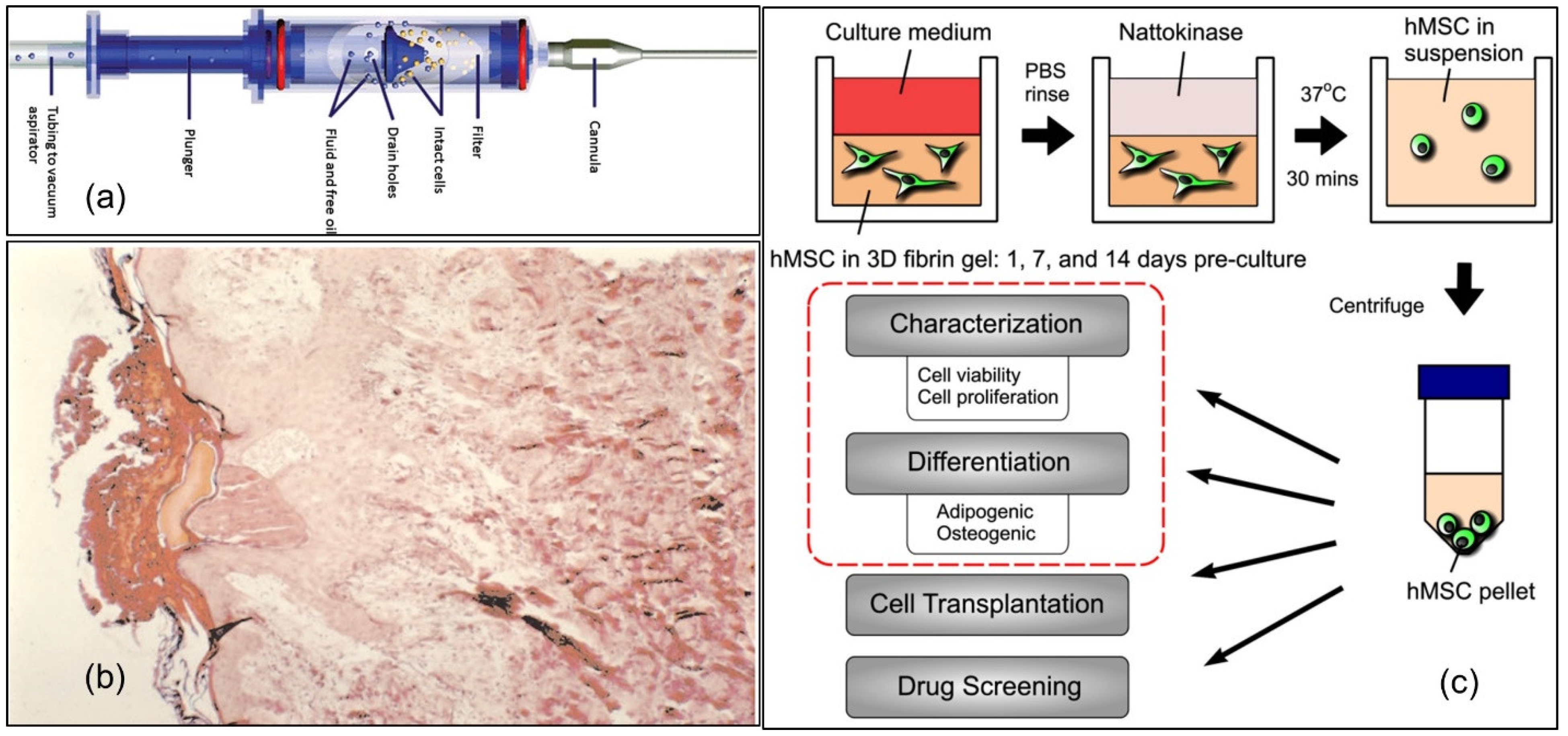
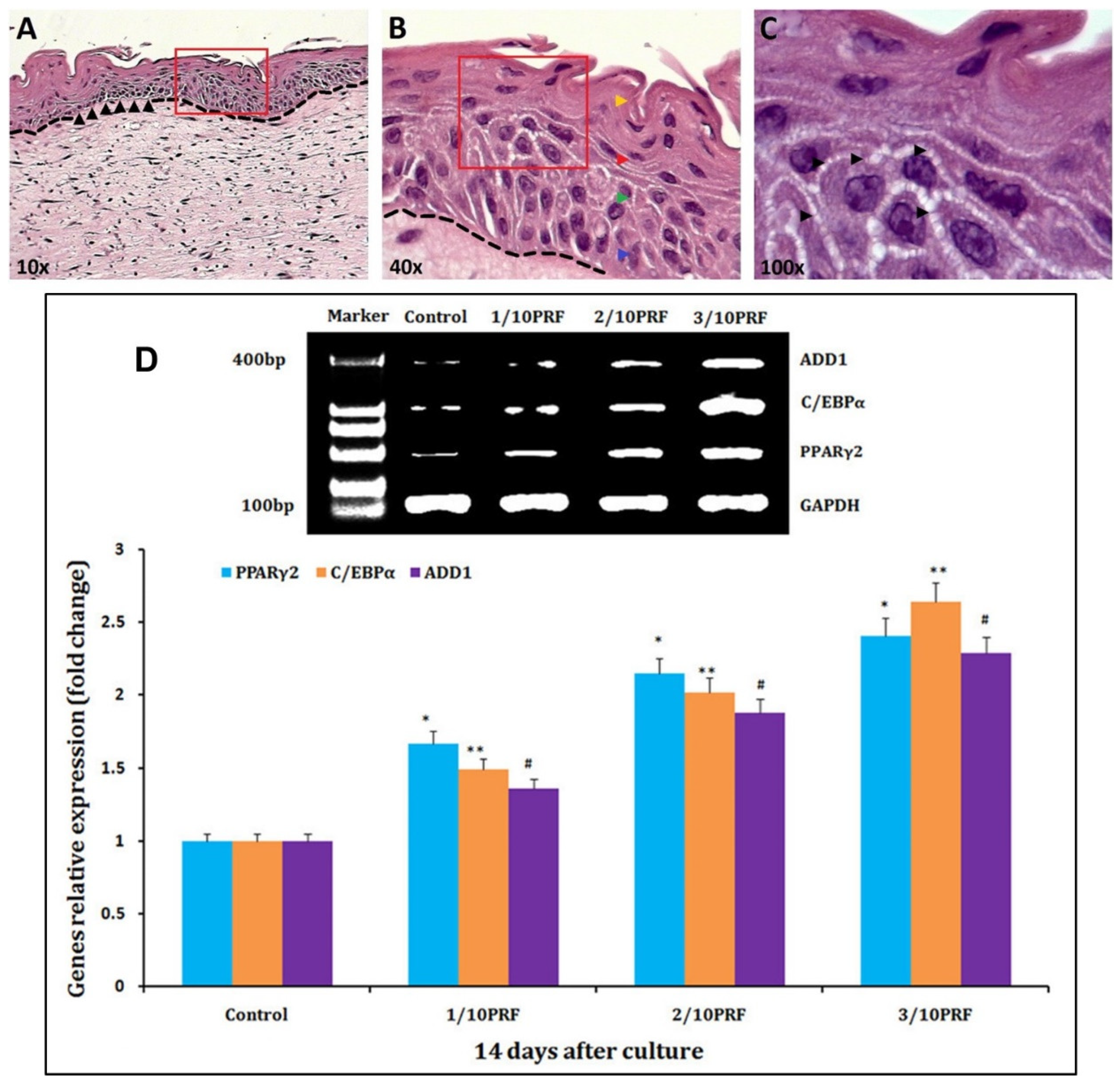
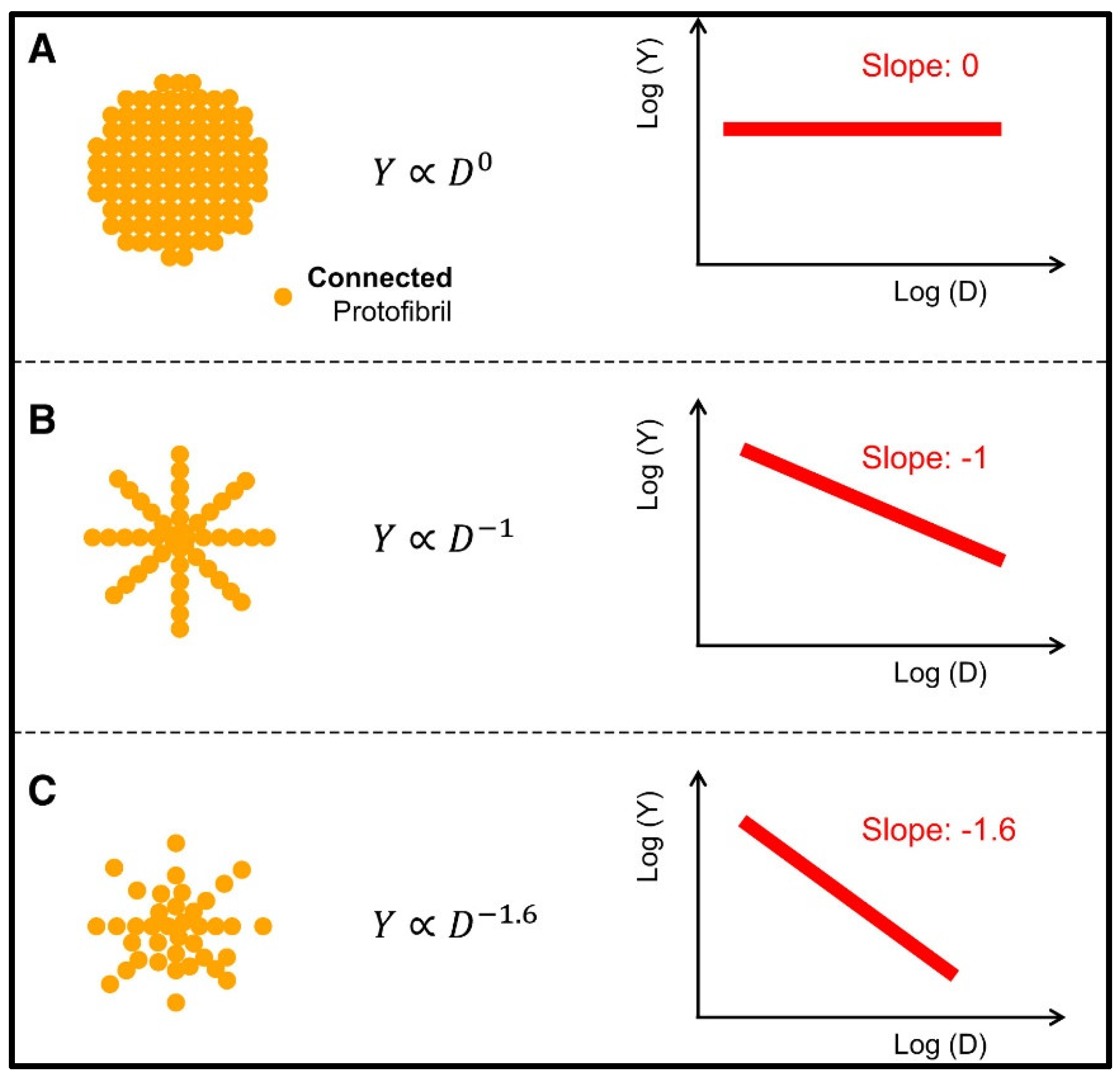
| Fibrin-Based Material Description | Targeted Biomedical Application | Wound Type | Type of Cells Used | Animal Models | Clinical Application | Bio-Mechanical Tests | Comments | Reference |
|---|---|---|---|---|---|---|---|---|
| Pure fibrin gel | Skin substitute | Burn | Human keratinocytes | n/a | Yes | n/a | 3-year patient follow up | [104] |
| Fibrin–succinimidyl glutarate blends | Skin-on-chip/skin reconstruction | Toxic wounds | Fibroblasts and keratinocytes | n/a | n/a | Permeation and swelling tests | Good potential for wound healing | [105] |
| Platelet-rich fibrin | Diabetic foot ulcers | Diabetic skin wound | n/a | Male nude mice | n/a | n/a | Promoting angiogenesis | [106] |
| Leukocyte- and platelet-rich fibrin | Scalp Defect Reconstruction | Surgical open wounds | n/a | n/a | Yes | n/a | Effective toward skin malignancy on the scalp | [107] |
| Collagen hydrogel/fibrin-coated polylactide | Skin repair | Deep skin wounds | Fibroblasts | n/a | n/a | n/a | Keratinocytes formed basal layers | [108] |
| Poly(ethylene glycol)–fibrinogen conjugates | Tissue engineering | n/a | Smooth muscle cells | n/a | n/a | Stress–sweep rheological testing | Cell proliferation control with fibrin nano-fibers | [109] |
| Fibrin hydrogel | Skin substitutes | Subcutaneous replacement | Adipose-derived stem cells (ASCs) and mature adipocytes | n/a | Patients undergoing body contouring surgery | n/a | Artificial hypodermis similar to native adipose tissue | [110] |
| Fibrin membrane | Skin scaffolds | Diabetic wound regeneration | Fibroblasts | Diabetic rats | n/a | n/a | Good collagen deposition in the wounds | [111] |
| Platelet-rich fibrin | Excisions of skin cancers | Dermatologic surgery | n/a | n/a | Patient with multiple nonmelanoma skin cancer. | n/a | Exuberant granulation tissue formation over ulcers | [112] |
| Leukocyte–Platelet-Rich Fibrin | Skull defect reconstruction | Endoscopic skull base surgery | n/a | n/a | Patients underwent endoscopic endonasal resection | n/a | Healthy crust formation occurred | [113] |
| Collagen–fibrin–polyethylene glycol (PEG) scaffolds | Vascular skin reconstruction | Burn-induced wound debridement | Stem cells from adipose tissue layer | Athymic rats | n/a | n/a | Effective against vascularized dermal equivalent for severe trauma cases | [114] |
| Fibrin/hyaluronan gels | Tracheal defects | Cartilage regeneration | Chondrocytes from rabbit | Female rabbits | n/a | n/a | No graft rejection recorded | [115] |
| 3D fibrin constructs | Skin grafting | Dermo-epidermal skin substitutes | Adipose-derived cells | Immuno-incompetent female nu/nu rats | n/a | n/a | Successful prevascularizion of wound bed | [116] |
| Fibrin/hyaluronic acid (HA) hydrogel with poly(l-lactic-co-glycolic acid) (PLGA) | Reconstruction of trachea | Thyroid/laryngeal malignancies | Allogeneic chondrocytes | New Zealand white male rabbits | n/a | n/a | Successful neocartilage formation with minimal granulation tissue | [117] |
| Poly(l-lactide) modified fibrin | Ascorbic acid rich skin constructs | Heart valve | Human dermal fibroblasts | n/a | n/a | n/a | Promoted collagen production in the cells | [118] |
| Thrombin/fibrinogen embedded skin explants | Skin substitute | Skin explants in wound repair | Skeletal muscle explants | n/a | n/a | n/a | Excellent cell outgrowth from skin explants onto dermal substitute | [119] |
| Fibrin-Based Material Description | Drug Inclusion | Targeted Application | Sustained Release Experiments | Animal Models | Clinical Application | Growth Factors/Cells | Reference |
|---|---|---|---|---|---|---|---|
| Poly(ether)urethane-polydimethylsiloxane/fibrin-based scaffold | Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) | Diabetic skin ulcers | n/a | Male diabetic mice | n/a | Human growth factors | [127] |
| Hyaluronic acid–fibrin hydrogel | Dexamethasone and galectin-3 inhibitor | Inflammatory joint diseases | n/a | Rats (not specified) | n/a | n/a | [128] |
| Commercial fibrin sealant | Erythromycin and cefazolin | Postoperative antibiotic delivery | 120 h release | n/a | n/a | n/a | [129] |
| Fibrin nanoparticles in chitosan | Ciprofloxacin and fluconazole | Polymicrobial wound infections | 30 days release | Female SD rats and pig skin | n/a | n/a | [130] |
| PEGylated fibrin/chitosan gel | Silver sulfadiazine | Burn wounds | 72 h release | n/a | n/a | n/a | [131] |
| Fibrin gel | Doxorubicin | Neuroblastoma | n/a | Female nude mice | n/a | Human LAN5, IMR32Luc+ and SHSY5YLuc+ cells | [132] |
| Fibrin nanoparticles | Ciprofloxacin and fluconazole | Diabetes therapy | 150 h release | n/a | n/a | HDF Cell lines | [133] |
| Fibrin hydrogel | Cyclophosphamide | Unsatisfied cytoreductive surgery | 100 h release | Female C57BL/6 and BALB/c mice | n/a | PD-L1 antibody and Cell line 4T1-luc | [134] |
| Fibrin gel | Plasminogen | Tympanic perforations | 7-day release | Male diabetic mice | n/a | Mouse fibroblast cell line L929 and human keratinocytes HaCaT | [135] |
| Heparin-conjugated fibrin | Bone morphogenetic protein-2 (BMP-2) | Bone regeneration | 30-day release | Sprague Dawley rats | n/a | Carvarial osteoblasts | [136] |
| Hollow fibrin microspheres | Human β nerve growth factor (NGF) | Neuronal dysfunctions | 8-day release | Male Sprague Dawley rats | n/a | Rat mesenchymal stem cells | [137] |
| Fibrin–chitosan gel | Recombinant human epidermal growth factor (rhEGF) | General wound healing | 14-day release | n/a | n/a | BALB/c 3T3 cells | [138] |
| Physiologically clotted fibrin | Gallic acid | Bone tissue engineering | 80 h release | n/a | n/a | MG-63 cells | [139] |
| Autologous platelet-rich fibrin | Vancomycin | Bone tissue engineering | 350 h release | n/a | n/a | n/a | [140] |
| Fibrin gel | Cisplatin and cisplatin–hyaluronate complexes | Tumor growth inhibition | 70 h release | NOD-SCID mice | n/a | Murine B16 melanoma cells and human SK-Mel-28 melanoma cells | [141] |
| Fibrin nanoparticles | Metal nanoparticles | Not specified | n/a | Female balb/c mice | n/a | RAW 264.7 and NIH 3T3 cells | [142] |
| Freeze-dried fibrin | Arbekacin sulfate | Osteomyelitis | 18-day release | Male outbred Wistar rats | n/a | n/a | [143] |
| Poly(lactic-co-glycolic acid) microparticles in fibrin glue | Bupivacaine | Postoperative pain | 35-day release | Female Sprague Dawley rats | n/a | L929 mouse fibroblast cells | [144] |
| Liposomes/chitosan fibrin | Tirofiban | Antithrombosis | 25-day release | n/a | n/a | n/a | [145] |
| Protein | Young’s Modulus (MPa) | Elongation before Break (%) |
|---|---|---|
| Fibrin fiber, non-cross-linked | 1.0–2.0 | 226 |
| Fibrin fiber, cross-linked | 11.0–15.0 | 332 |
| Elastin | 1 | 150 |
| Myofibrils | 1 | 200 |
| Resilin | 1–2 | 190, 313 |
| Fibronectin | 0.1–3.5 | 700 |
| Spider silk (Araneus Flag) | 3 | 270 |
| Fibrillin | 0.2–100 | >185 |
| Intermediate filament | 6–300 | 160–220 |
| Mussel byssus | 10–500 | 109 |
| Collagen, tendon | 160–7500 | 12 |
| Microtubules | 1000–1500 | ≤20 |
| α-Keratin wet | 2000 | 45 |
| Actin | 1800–2500 | ≤15 |
| Collagen, cross-linked | 5000–7000 | 12–16 |
| Spider silk (Araneus MA) | 10,000 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayer, I.S. Advances in Fibrin-Based Materials in Wound Repair: A Review. Molecules 2022, 27, 4504. https://doi.org/10.3390/molecules27144504
Bayer IS. Advances in Fibrin-Based Materials in Wound Repair: A Review. Molecules. 2022; 27(14):4504. https://doi.org/10.3390/molecules27144504
Chicago/Turabian StyleBayer, Ilker S. 2022. "Advances in Fibrin-Based Materials in Wound Repair: A Review" Molecules 27, no. 14: 4504. https://doi.org/10.3390/molecules27144504
APA StyleBayer, I. S. (2022). Advances in Fibrin-Based Materials in Wound Repair: A Review. Molecules, 27(14), 4504. https://doi.org/10.3390/molecules27144504






