Protein Attachment Mechanism for Improved Functionalization of Affinity Monolith Chromatography (AMC)
Abstract
1. Introduction
2. Results
2.1. Understanding Protein Attachment Mechanisms with APTES-Functionalized Alumina Monoliths
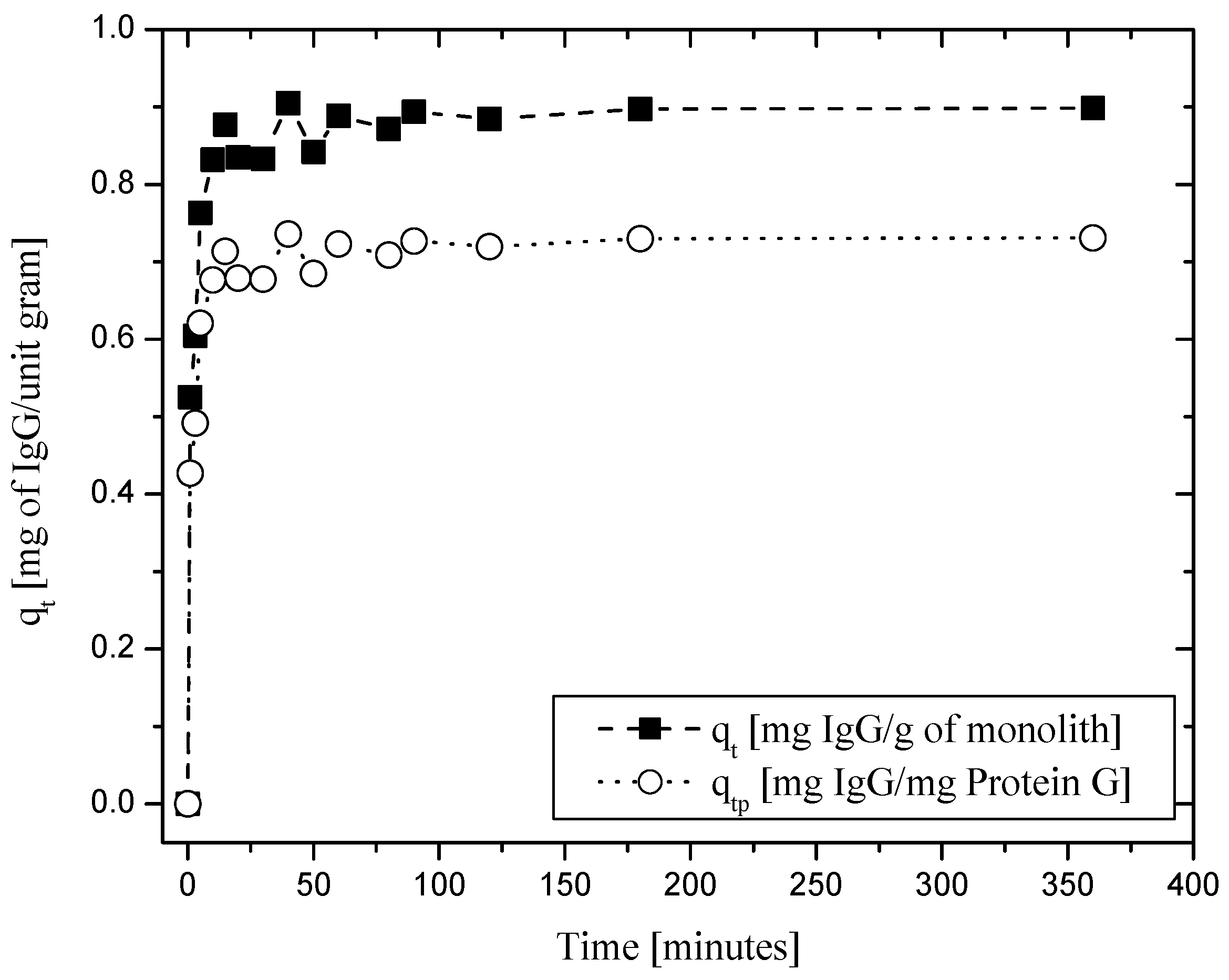
2.2. Performance of Protein-G-Functionalized Alumina Monoliths for IgG Binding and Elution
3. Materials and Methods
3.1. Materials
3.2. Fabrication of α-Alumina Monoliths
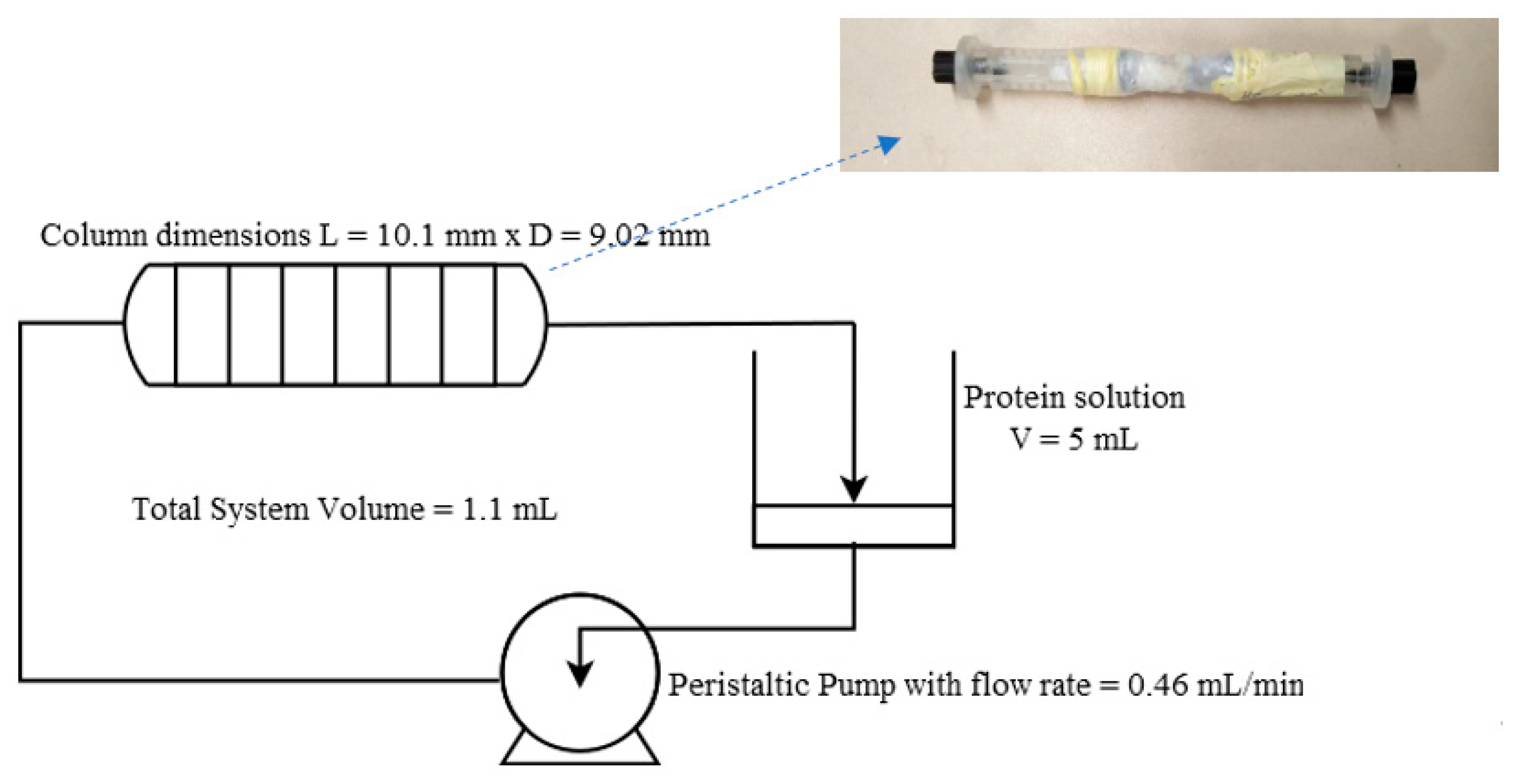
3.3. Preparation of Affinity Monolith Chromatography Setup
3.4. Interaction of Proteins with Amine-Functionalized Monoliths and Attachment Kinetics
3.5. Protein-Binding Stability
3.5.1. Desorption Using Glycine–HCl and Glycine–NaOH (0.1 M and 1 M)
3.5.2. Desorption Using NaCl (5 mM and 0.1 M) and Temperature
3.5.3. Quantification of the Total Protein Attached or Entrapped in the Monolith
3.6. Inspection of Protein Aggregation Effects
3.7. Evaluation of the Efficiency of Protein-G-Bound Amine-Functionalized Monolith for Purification of Immunoglobulin G (IgG)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Walters, R.R. Affinity chromatography. Anal. Chem. 1985, 57, 1099A–1114A. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cuatrecasas, P.; Wilchek, M.; Anfinsen, C.B. Selective enzyme purification by affinity chromatography. Proc. Natl. Acad. Sci. USA 1968, 61, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Schiel, J.E.; Mallik, R.; Soman, S.; Joseph, K.S.; Hage, D.S. Applications of silica supports in affinity chromatography. J. Sep. Sci. 2006, 29, 719–737. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.O. High-performance liquid affinity chromatography. Methods Enzymol. 1984, 104, 212–223. [Google Scholar]
- Gustavsson, P.E.; Larsson, P.O. Handbook of Affinity Chromatography, 2nd ed.; Hage, D.S., Ed.; Taylor & Francis: New York, NY, USA, 2006; Volume 2. [Google Scholar]
- Mazzei, R.; Drioli, E.; Giorno, L. Biocatalytic Membranes and Membrane Bioreactors. In Comprehensive Membrane Science and Engineering; Elsevier: London, UK, 2010; Volume 3, pp. 195–212. [Google Scholar]
- Gustavsson, P.E.; Larsson, P.O. Monolithic Materials; Svec, F., Tennikova, T.B., Deyl, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 121–141. [Google Scholar]
- Tetala, K.K.; van Beek, T.A. Bioaffinity chromatography on monolithic supports. J. Sep. Sci. 2010, 33, 422–438. [Google Scholar] [CrossRef]
- Mallik, R.; Hage, D.S. Affinity monolith chromatography. J. Sep. Sci. 2006, 29, 1686–1704. [Google Scholar] [CrossRef]
- Yoo, M.J.; Hage, D.S. Monolithic Chromatography and Its Modern Applications; Wang, P., Ed.; ILM: St. Albans, UK, 2010; Volume 1. [Google Scholar]
- Pfaunmiller, E.L.; Paulemond, M.L.; Dupper, C.M.; Hage, D.S. Affinity monolith chromatography: A review of principles and recent analytical applications. Anal. Bioanal. Chem. 2013, 405, 2133–2145. [Google Scholar] [CrossRef]
- Kubin, M.; Spacek, P.; Chromecek, R. Gel permeation chromatography on porous poly(ethylene glycol methacrylate). Collect. Czech. Chem. Commun. 1967, 32, 3881–3887. [Google Scholar] [CrossRef]
- Lynn, T.R.; Rushneck, D.R.; Cooper, A.R. High Resolution-Low Pressure Liquid Chromatography. J. Chromatogr. Sci. 1974, 12, 76–79. [Google Scholar] [CrossRef]
- Hileman, F.D.; Sievers, R.E.; Hess, G.G.; Ross, W.D. In situ preparation and evaluation of open pore polyurethane chromatographic columns. Anal. Chem. 1973, 45, 1126–1130. [Google Scholar] [CrossRef]
- Ross, W.D.; Jefferson, R.T. In situ-formed open-pore polyurethane as chromatography supports. J. Chromatogr. Sci. 1970, 8, 386–389. [Google Scholar] [CrossRef]
- Hansen, L.C.; Sievers, R.E. Highly permeable open-pore polyurethane columns for liquid chromatography. J. Chromatogr. 1974, 99, 123–133. [Google Scholar] [CrossRef]
- Josic, D.; Reusch, J.; Loester, K.; Baum, O.; Reutter, W. High-performance membrane chromatography of serum and plasma membrane proteins. J. Chromatogr. 1992, 590, 59–76. [Google Scholar] [CrossRef]
- Merhar, M.; Podgornik, A.; Barut, M.; Zigon, M.; Strancer, A. Methacrylate monoliths prepared from various hydrophobic and hydrophilic monomers–Structural and chromatographic characteristics. J. Sep. Sci. 2003, 26, 322–330. [Google Scholar] [CrossRef]
- Tennikova, T.B.; Belenkii, B.G.; Svec, F. High-performance membrane chromatography. A novel method of protein separation. J. Liq. Chromatogr. 1990, 13, 63–70. [Google Scholar] [CrossRef]
- Tennikova, T.B.; Svec, F. High-performance membrane chromatography: Highly efficient separation method for proteins in ion-exchange, hydrophobic interaction and reversed phase modes. J. Chromatogr. 1993, 646, 279–288. [Google Scholar] [CrossRef]
- Hjerten, S.; Liao, J.L.; Zhang, R. High-performance liquid chromatography on continuous polymer beds. J. Chromatogr. 1989, 473, 273–275. [Google Scholar] [CrossRef]
- Svec, F.; Frechet, J.M. Continuous rods of macroporous polymer as high-performance liquid chromatography separation media. Anal. Chem. 1992, 64, 820–822. [Google Scholar] [CrossRef]
- Jiang, T.; Mallik, R.; Hage, D.S. Affinity Monoliths for Ultrafast Immunoextraction. Anal. Chem. 2005, 15, 2362–2372. [Google Scholar] [CrossRef]
- Mallik, R.; Jiang, T.; Hage, D.S. High-Performance Affinity Monolith Chromatography: Development and Evaluation of Human Serum Albumin Columns. Anal. Chem. 2004, 76, 7013–7022. [Google Scholar] [CrossRef]
- Pfaunmiller, E.L.; Hartmann, M.; Dupper, C.M.; Soman, S.; Hage, D.S. Optimization of human serum albumin monoliths for chiral separations and high-performance affinity chromatography. J. Chromatogr. A 2012, 126, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Nayak, N.; Vitorino, N.; Frade, J.R.; Kovalevsky, A.V.; Alves, V.D.; Crespo, J.G.; Portugal, C.A.M. Design of alumina monoliths by emulsion-gel casting: Understanding the monolith structure from a rheological approach. Mater. Des. 2018, 157, 119–129. [Google Scholar] [CrossRef]
- Nayak, N.; Huertas, R.; Crespo, J.G.; Portugal, C.A.M. Surface Modification of Alumina Monolithic Columns with 3-Aminopropyltetraethoxysilane (APTES) for Protein Attachment. Sep. Purif. Technol. 2019, 229, 115674. [Google Scholar] [CrossRef]
- Lynch, K.B.; Ren, J.; Beckner, M.A.; He, C.; Liu, S. Monolith columns for liquid chromatographic separations of intact proteins: A review of recent advances and applications. Anal. Chim. Acta 2019, 1046, 48–68. [Google Scholar] [CrossRef]
- Lalli, E.; Silva, J.S.; Boi, C.; Sarti, G.C. Affinity membranes and monoliths for protein purifica-tion. Membranes 2019, 10, 1. [Google Scholar] [CrossRef]
- Poddar, S.; Sharmeen, S.; Hage, D.S. Affinity monolith chromatography: A review of general principles and recent developments. Electrophoresis 2021, 42, 2577–2598. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Mielczarski, J.A.; Mielczarski, E.; Rai, B. Efficiency of blocking of non-specific interaction of different proteins by BSA adsorbed on hydrophobic and hydrophilic surfaces. J. Colloid Interface Sci. 2010, 341, 136–142. [Google Scholar] [CrossRef]
- Williams, E.H.; Davydov, A.V.; Motayed, A.; Sundaresan, S.G.; Bocchini, P.; Richter, L.J.; Stan, G.; Steffens, K.; Zangmeister, R.; Schreifels, J.A.; et al. Immobilization of streptavidin on 4H–SiC for biosensor development. Appl. Surf. Sci. 2012, 258, 6056–6063. [Google Scholar] [CrossRef]
- Karlsson, E.; Ryden, L.; Brewer, J. Ion exchange chromatography. In Protein Purification: Principles, High-Resolution Methods, and Application; Janson, J.C., Ryden, L., Eds.; John Wiley & Sons: New York, NY, USA, 1998; pp. 145–205. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Frank. Inst. 1917, 183, 102–105. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, e470. [Google Scholar]
- Tempkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chem. URSS 1940, 12, 327–356. [Google Scholar]
- Militano, F.; Poerio, T.; Mazzei, R.; Piacentini, E.; Gugliuzza, A.; Giorno, L. Influence of protein bulk properties on membrane surface coverage during immobilization. Colloids Surf. B Biointerfaces 2016, 143, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Militano, F.; Poerio, T.; Mazzei, R.; Salerno, S.; De Bartolo, L.; Giorno, L. Development of biohybrid immuno-selective membranes for target antigen recognition. Biosens. Bioelectron. 2017, 92, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Gentili, D.; D’Angelo, P.; Militano, F.; Mazzei, R.; Poerio, T.; Brucale, M.G.; Tarabella, S.; Bonetti, S.L.; Marasso, M.; Cocuzza, L.; et al. Integration of organic electrochemical transistors and immuno-affinity membranes for label-free detection of interleukin-6 in the physiological concentration range through antibody–antigen recognition. J. Mater. Chem. B 2018, 6, 5400–5406. [Google Scholar] [CrossRef] [PubMed]
- Padwal, P.; Finger, C.; Fraga-García, P.; Kaveh-Baghbaderani, Y.; Schwaminger, S.P.; Berensmeier, S. Seeking Innovative Affinity Approaches: A Performance Comparison between Magnetic Nanoparticle Agglomerates and Chromatography Resins for Antibody Recovery. ACS Appl. Mater. Interfaces 2020, 12, 39967–39978. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Pierce Protein. Protein Purification Technical Handbook. 2010. Available online: http://tools.thermofisher.com/content/sfs/brochures/1602015-Protein-Purification-Handbook.pdf (accessed on 7 June 2022).
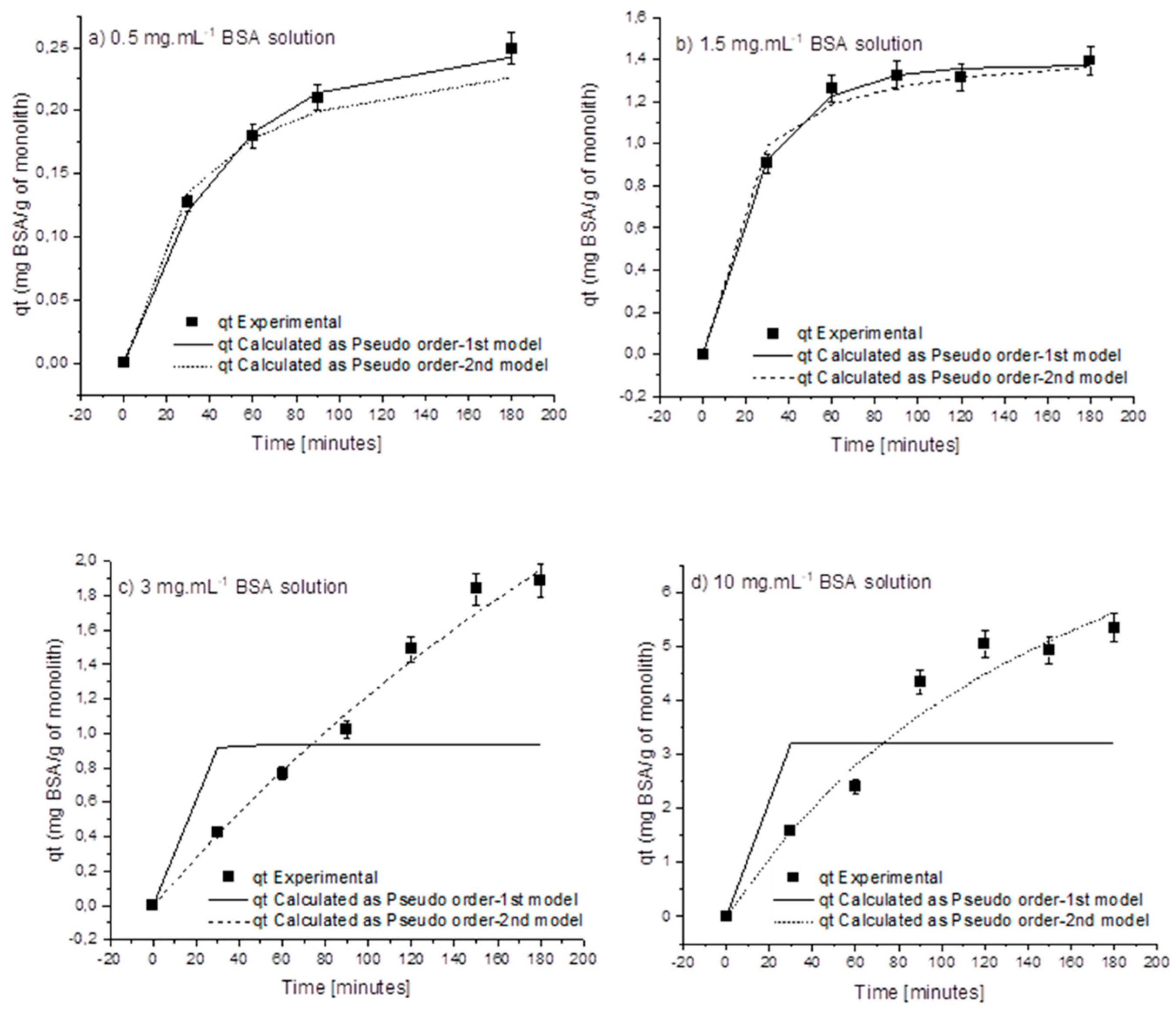
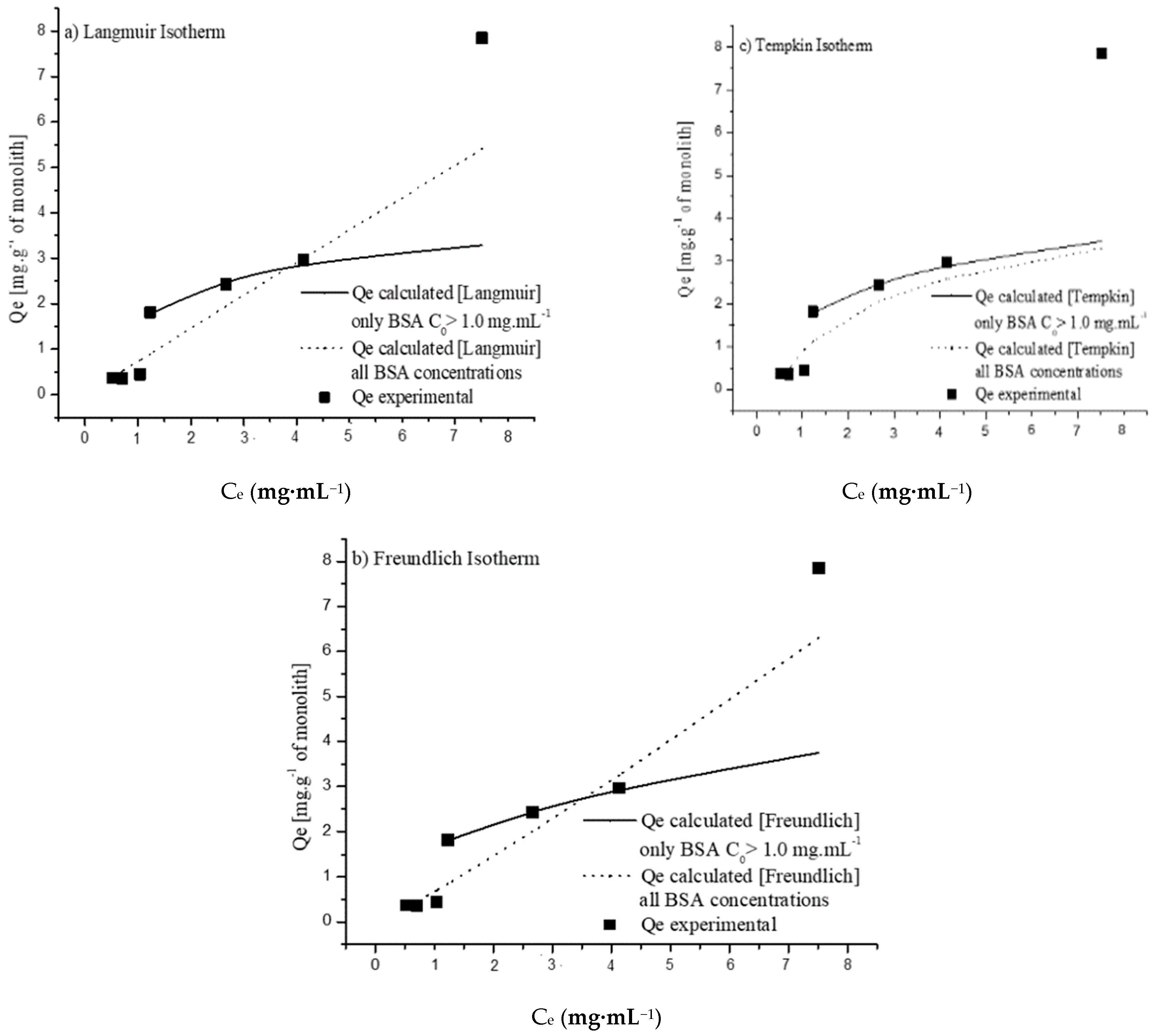
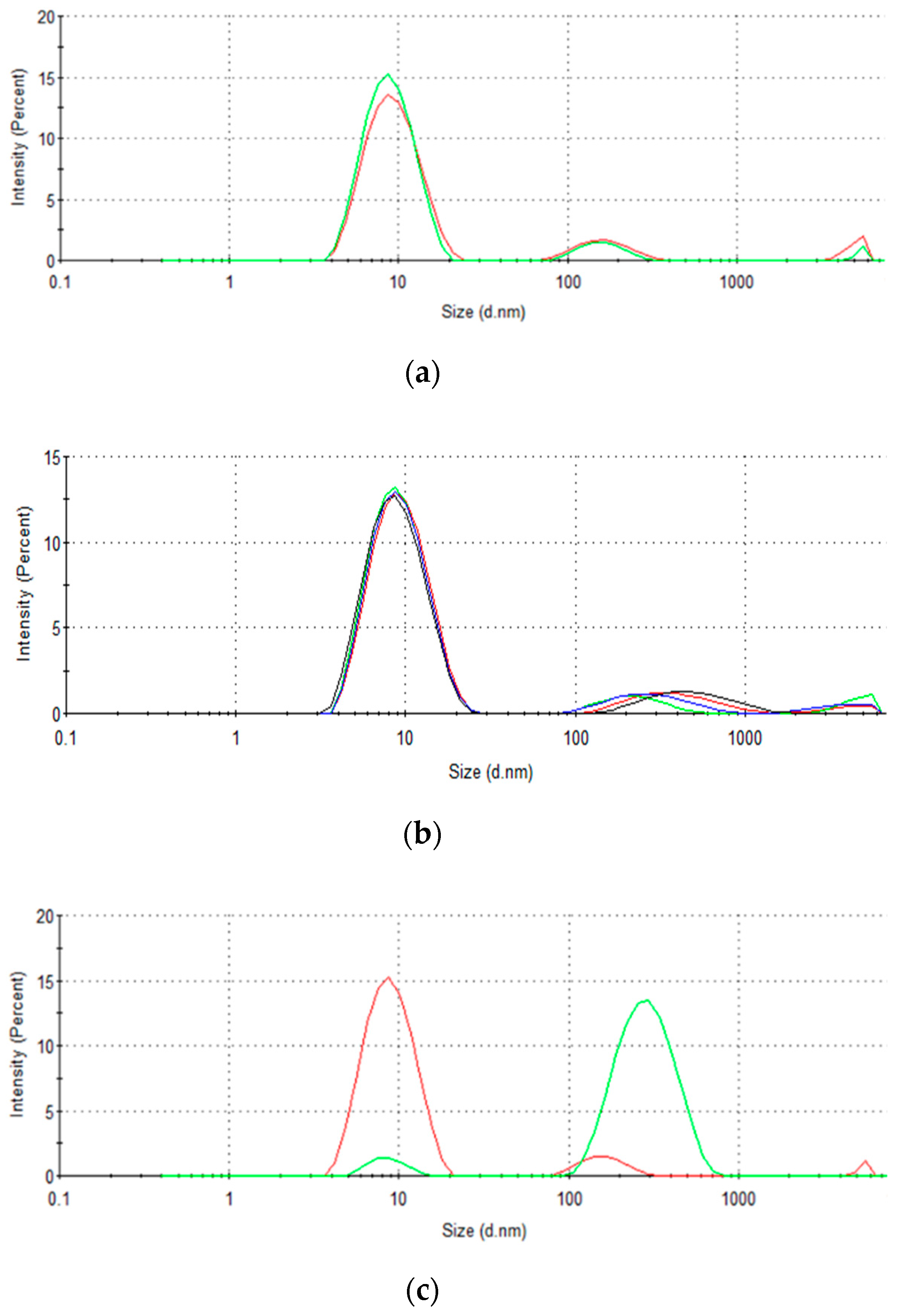
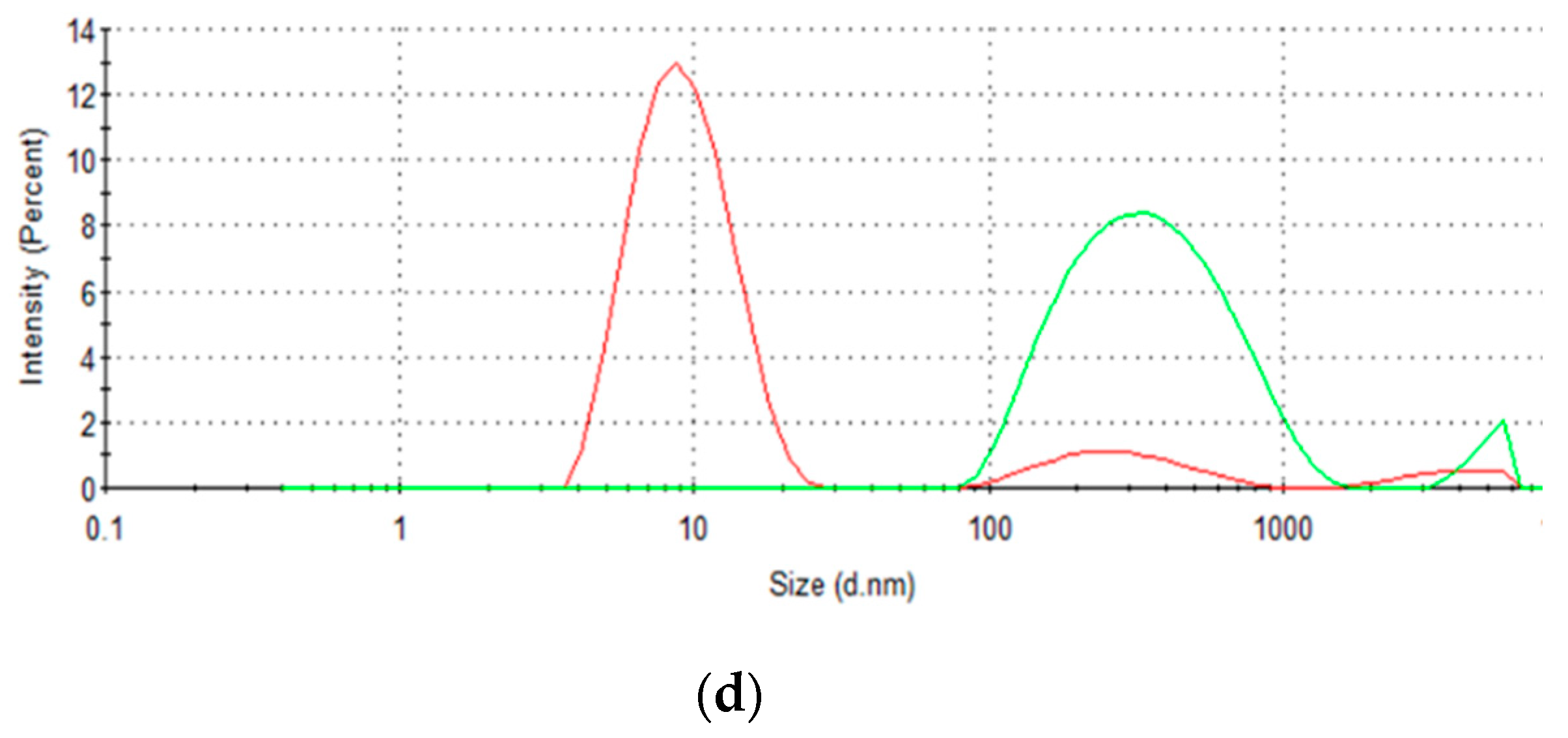
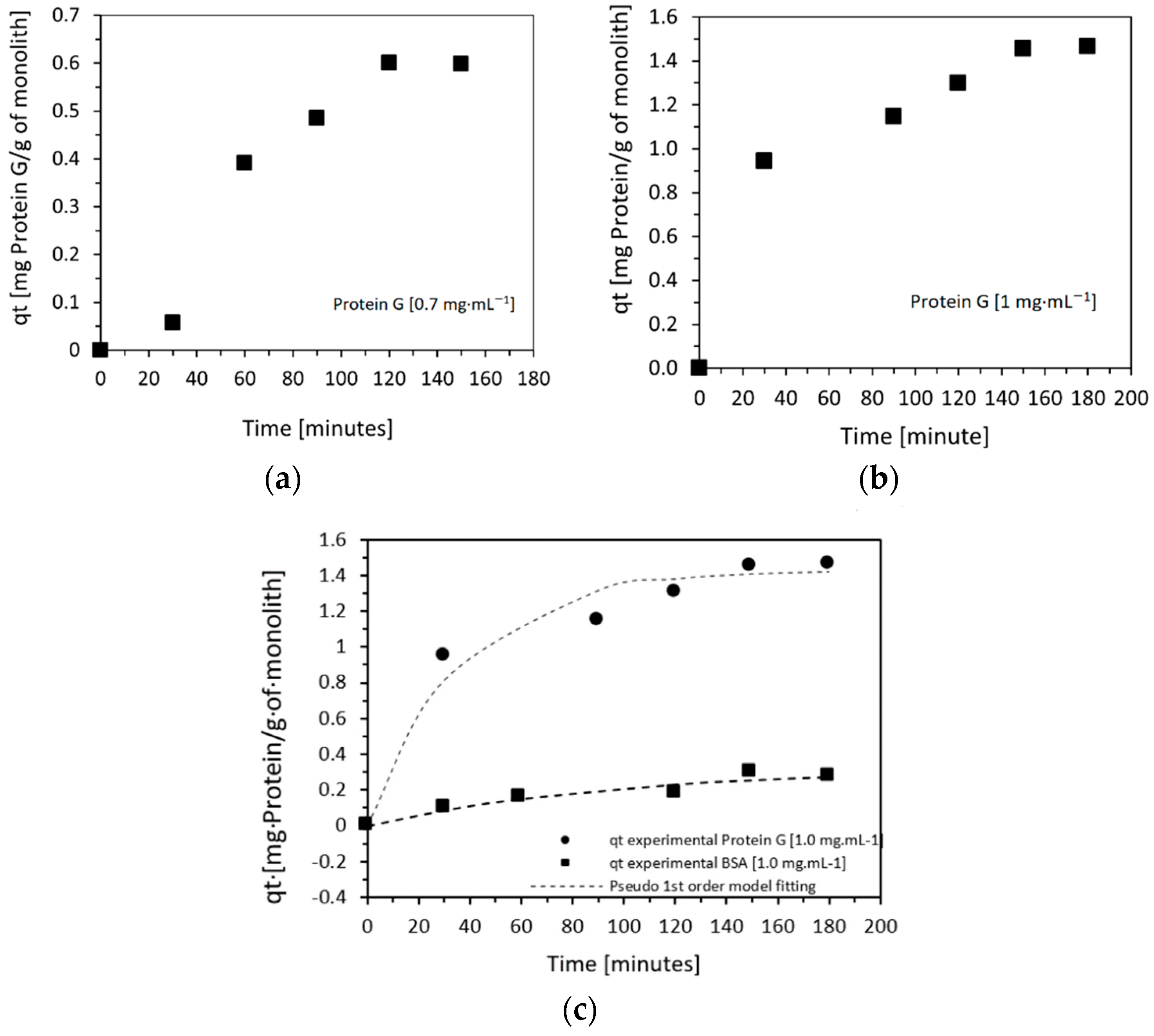
| Initial Concentration (mg·mL−1) | QExpt (mg·g−1) | Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| QPred (mg·g−1) | k1 | R2 | χ2 | QPred | k2 | R2 | χ2 | ||
| (mg·g−1) | |||||||||
| 0.5 | 0.249 | 0.242 | 0.022 | 0.990 | 0.001 | 0.261 | 0.137 | 0.989 | 0.003 |
| 1 | 0.276 | 0.319 | 0.011 | 0.925 | 0.022 | 0.461 | 0.0175 | 0.928 | 0.020 |
| 1.5 | 1.395 | 1.374 | 0.037 | 0.997 | 0.003 | 1.474 | 0.0466 | 0.989 | 0.016 |
| 3 | 1.887 | 0.938 | 0.783 | 0.425 | 1.803 | 1.951 | 0.0003 | 0.988 | 0.025 |
| 5 | 2.239 | 0.678 | 0.783 | 0.275 | 3.729 | 2.037 | 0.0004 | 0.905 | 0.434 |
| 10 | 5.333 | 3.208 | 0.783 | 0.521 | 4.332 | 5.619 | 0.0005 | 0.964 | 0.229 |
| Langmuir | Freundlich | Tempkin | |||
|---|---|---|---|---|---|
| Qm | 3.947068 | KF | 0.861167 | Kt | 1.138941 |
| Ka | 0.133524 | nF | 2.463419 | bT | 2735.478 |
| χ2 | 0.006007 | χ2 | 0.000692 | B | 0.920915 |
| R2 | 0.97742 | R2 | 0.997444 | χ2 | 0.003577 |
| R2 | 0.986508 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayak, N.; Mazzei, R.; Giorno, L.; Crespo, J.G.; Portugal, C.A.M.; Poerio, T. Protein Attachment Mechanism for Improved Functionalization of Affinity Monolith Chromatography (AMC). Molecules 2022, 27, 4496. https://doi.org/10.3390/molecules27144496
Nayak N, Mazzei R, Giorno L, Crespo JG, Portugal CAM, Poerio T. Protein Attachment Mechanism for Improved Functionalization of Affinity Monolith Chromatography (AMC). Molecules. 2022; 27(14):4496. https://doi.org/10.3390/molecules27144496
Chicago/Turabian StyleNayak, Nayan, Rosalinda Mazzei, Lidietta Giorno, João G. Crespo, Carla A. M. Portugal, and Teresa Poerio. 2022. "Protein Attachment Mechanism for Improved Functionalization of Affinity Monolith Chromatography (AMC)" Molecules 27, no. 14: 4496. https://doi.org/10.3390/molecules27144496
APA StyleNayak, N., Mazzei, R., Giorno, L., Crespo, J. G., Portugal, C. A. M., & Poerio, T. (2022). Protein Attachment Mechanism for Improved Functionalization of Affinity Monolith Chromatography (AMC). Molecules, 27(14), 4496. https://doi.org/10.3390/molecules27144496









