Medicinal Fungi with Antiviral Effect
Abstract
1. Introduction
2. Antiviral Activity
2.1. Human Viruses
2.1.1. SARS-CoV-2
2.1.2. Influenza Virus (IV)
2.1.3. Enterovirus 71(EV71)
2.1.4. Human Immunodeficiency Virus (HIV)
2.1.5. Human Papilloma Virus (HPV)
2.1.6. Dengue Virus (DENV)
2.1.7. Hepatitis Viruses (HV)
Hepatitis B Virus (HBV)
Hepatitis C Virus (HCV)
2.1.8. Herpes Viruses (HV)
Herpes Simplex Virus (HSV)
Epstein-Barr Virus (EBV)
2.1.9. Respiratory Syncytial Virus (RSV)
2.1.10. Poliovirus (PV)
2.1.11. Rabies Virus (RV)
2.1.12. Marburg Virus (MARV)
2.2. Animal Viruses
2.2.1. Infectious Hematopoietic Necrosis Virus (IHNV)
2.2.2. Muscovy Duck Reovirus (MDRV)
2.2.3. White Spot Syndrome Virus (WSSV)
2.2.4. Feline Immunodeficiency Virus (FIV)
2.2.5. Deformable Wing Virus (DWV)and Lake Sinai Virus (LSV)
2.2.6. Nerve Necrosis Virus (NNV)
2.2.7. Porcine Circovirus Type 2 (PCV-2)
2.2.8. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV)
2.2.9. Porcine Delta Coronavirus (PDCoV)
2.2.10. Bovine Herpesvirus 1 (BoHV-1)
2.2.11. Newcastle Disease Virus (NDV)
2.3. Plant Viruses
2.3.1. Tobacco Mosaic Virus (TMV)
2.3.2. Groundnut Bud Necrosis Virus (GBNV)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Theves, C.; Biagini, P.; Crubezy, E. The rediscovery of smallpox. Clin. Microbiol. Infect. 2014, 20, 210–218. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Enjuanes, L.; Berkhout, B. RNA virus replication, transcription and recombination. RNA Biol. 2011, 8, 182–183. [Google Scholar] [CrossRef]
- Milpied-Homsi, B.; Moran, E.M.; Phillips, E.J. Antiviral drug allergy. Immunol. Allergy Clin. N. Am. 2014, 34, 645–662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Izzedine, H.; Launay-Vacher, V.; Deray, G. Antiviral drug-induced nephrotoxicity. Am. J. Kidney Dis. 2005, 45, 804–817. [Google Scholar] [CrossRef]
- Collado Mateo, D.; Pazzi, F.; Dominguez Munoz, F.J.; Martin Martinez, J.P.; Olivares, P.R.; Gusi, N.; Adsuar, J.C. Ganoderma Lucidum Improves Physical Fitness in Women with Fibromyalgia. Nutr. Hosp. 2015, 32, 2126–2135. [Google Scholar]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.; Bisen, P.S. Ganoderma lucidum: A Potent Pharmacological Macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- Teplyakova, T.V.; Ilyicheva, T.N.; Kosogova, T.A.; Wasser, S.P. Medicinal Mushrooms against Influenza Viruses. Int. J. Med. Mushrooms 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal Mushrooms in Human Clinical Studies. Part, I. Anticancer, Oncoimmunological, and Immunomodulatory Activities: A Review. Int. J. Med. Mushrooms 2017, 19, 279–317. [Google Scholar] [CrossRef]
- Muszynska, B.; Grzywacz-Kisielewska, A.; Kala, K.; Gdula-Argasinska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.T.; Cheng, T.R.; Juang, Y.P.; Ma, H.H.; Wu, Y.T.; Yang, W.B.; Cheng, C.W.; Chen, X.; Chou, T.H.; Shie, J.J.; et al. Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2021579118. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.S.; Bashir, N.M.B.; Mia, R.; Hossain, A.; Saha, S.K.; Kakon, A.J.; Sarker, N.C. Rationalization of Mushroom-Based Preventive and Therapeutic Approaches to COVID-19: Review. Int. J. Med. Mushrooms 2021, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, K.J.; Gokila Vani, M.; Hsieh, H.W.; Lin, C.C.; Wang, S.Y. Antcins from Antrodia cinnamomea and Antrodia salmonea Inhibit Angiotensin-Converting Enzyme 2 (ACE2) in Epithelial Cells: Can Be Potential Candidates for the Development of SARS-CoV-2 Prophylactic Agents. Plants 2021, 10, 1736. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bertuccioli, A.; Cavecchia, I. Possible therapeutic role of a highly standardized mixture of active compounds derived from cultured Lentinula edodes mycelia (AHCC) in patients infected with 2019 novel coronavirus. Minerva Gastroenterol. Dietol. 2020, 66, 172–176. [Google Scholar] [CrossRef]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. beta-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef]
- Verma, A.K. Cordycepin: A bioactive metabolite of Cordyceps militaris and polyadenylation inhibitor with therapeutic potential against COVID-19. J. Biomol. Struct. Dyn. 2020, 40, 3745–3752. [Google Scholar] [CrossRef]
- Obi, N.; Hayashi, K.; Miyahara, T.; Shimada, Y.; Terasawa, K.; Watanabe, M.; Takeyama, M.; Obi, R.; Ochiai, H. Inhibitory Effect of TNF-alpha Produced by Macrophages Stimulated with Grifola frondosa Extract (ME) on the Growth of Influenza A/Aichi/2/68 Virus in MDCK Cells. Am. J. Chin. Med. 2008, 36, 1171–1183. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.W.; Hwang, B.S.; Woo, E.E.; Lee, Y.J.; Jeong, K.W.; Lee, I.K.; Yun, B.S. Neuraminidase Inhibitors from the Fruiting Body of Phellinus igniarius. Mycobiology 2016, 44, 117–120. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.I.; Heo, J.; Lee, I.; Park, S.; Hwang, M.W.; Bae, J.Y.; Park, M.S.; Park, H.J.; Park, M.S. The anti-influenza virus effect of Phellinus igniarius extract. J. Microbiol. 2013, 51, 676–681. [Google Scholar] [CrossRef]
- Kuroki, T.; Lee, S.; Hirohama, M.; Taku, T.; Kumakura, M.; Haruyama, T.; Nagata, K.; Kawaguchi, A. Inhibition of Influenza Virus Infection by Lentinus edodes Mycelia Extract Through Its Direct Action and Immunopotentiating Activity. Front. Microbiol. 2018, 9, 1164. [Google Scholar] [CrossRef]
- Gao, L.; Han, J.; Si, J.; Wang, J.; Wang, H.; Sun, Y.; Bi, Y.; Liu, J.; Cao, L. Cryptoporic acid E from Cryptoporus volvatus inhibits influenza virus replication in vitro. Antivir. Res. 2017, 143, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, Y.; Si, J.; Liu, J.; Sun, G.; Sun, X.; Cao, L. Cryptoporus volvatus extract inhibits influenza virus replication in vitro and in vivo. PLoS ONE 2014, 9, e113604. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Lee, J.B.; Hayashi, K.; Fujita, A.; Park, D.K.; Hayashi, T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Park, H.; Sung, G.H.; Lee, K.; Lee, T.; Lee, I.; Park, M.S.; Jung, Y.W.; Shin, Y.S.; Kang, H.; et al. Anti-influenza effect of Cordyceps militaris through immunomodulation in a DBA/2 mouse model. J. Microbiol. 2014, 52, 696–701. [Google Scholar] [CrossRef]
- Krupodorova, T.; Rybalko, S.; Barshteyn, V. Antiviral activity of Basidiomycete mycelia against influenza type A (serotype H1N1) and herpes simplex virus type 2 in cell culture. Virol. Sin. 2014, 29, 284–290. [Google Scholar] [CrossRef]
- Li, R.F.; Zhou, X.B.; Zhou, H.X.; Yang, Z.F.; Jiang, H.M.; Wu, X.; Li, W.J.; Qiu, J.J.; Mi, J.N.; Chen, M.; et al. Novel Fatty Acid in Cordyceps Suppresses Influenza A (H1N1) Virus-Induced Proinflammatory Response Through Regulating Innate Signaling Pathways. ACS Omega 2021, 6, 1505–1515. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Li, H.; Zhao, C.; Ma, H.; Zhao, X.; Wu, J.; Liu, K.; Shan, J.; Wang, Y. Effect of a polysaccharide from Poria cocos on humoral response in mice immunized by H1N1 influenza and HBsAg vaccines. Int. J. Biol. Macromol. 2016, 91, 248–257. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, M.; Wang, D.; Xu, G.; Yin, X.; Liu, X.; Ding, M.; Han, L. Mixed polysaccharides derived from Shiitake mushroom, Poriacocos, Ginger, and Tangerine peel enhanced protective immune responses in mice induced by inactivated influenza vaccine. Biomed. Pharm. 2020, 126, 110049. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef]
- Lin, Y.L.; Shih, C.; Cheng, P.Y.; Chin, C.L.; Liou, A.T.; Lee, P.Y.; Chiang, B.L. A Polysaccharide Purified from Ganoderma lucidum Acts as a Potent Mucosal Adjuvant That Promotes Protective Immunity Against the Lethal Challenge with Enterovirus A71. Front. Immunol. 2020, 11, 561758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tao, J.; Yang, X.; Yang, Z.; Zhang, L.; Liu, H.; Wu, K.; Wu, J. Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochem. Biophys. Res. Commun. 2014, 449, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Liu, Y.; Zhang, G.Q.; Zhao, S.; Xu, F.; Geng, X.L.; Wang, H.X. Cordysobin, a novel alkaline serine protease with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Cordyceps sobolifera. J. Biosci. Bioeng. 2012, 113, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ma, L.; Hu, Q.; Li, J.; Chen, Y.; Jia, R.; Shen, S.; Zeng, Y. In Vitro Anti-HIV-1 Activity of Cordyceps sinensis Extracts. Bing Du Xue Bao 2016, 32, 417–422. [Google Scholar] [PubMed]
- Jiang, Y.; Wong, J.H.; Fu, M.; Ng, T.B.; Liu, Z.K.; Wang, C.R.; Li, N.; Qiao, W.T.; Wen, T.Y.; Liu, F. Isolation of adenosine, iso-sinensetin and dimethylguanosine with antioxidant and HIV-1 protease inhibiting activities from fruiting bodies of Cordyceps militaris. Phytomedicine 2011, 18, 189–193. [Google Scholar] [CrossRef]
- Wong, J.H.; Wang, H.; Ng, T.B. A haemagglutinin from the medicinal fungus Cordyceps militaris. Biosci. Rep. 2009, 29, 321–327. [Google Scholar] [CrossRef]
- Li, Y.R.; Liu, Q.H.; Wang, H.X.; Ng, T.B. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim Biophys Acta 2008, 1780, 51–57. [Google Scholar] [CrossRef]
- Wong, J.H.; Wang, H.X.; Ng, T.B. Marmorin, a new ribosome inactivating protein with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from the mushroom Hypsizigus marmoreus. Appl. Microbiol. Biotechnol. 2008, 81, 669–674. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 2001, 68, 2151–2158. [Google Scholar] [CrossRef]
- Han, C.H.; Zhang, G.Q.; Wang, H.X.; Ng, T.B. Schizolysin, a hemolysin from the split gill mushroom Schizophyllum commune. FEMS Microbiol. Lett. 2010, 309, 115–121. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Zhang, G.Q.; Ng, T.B.; Wang, H.X. A novel ribonuclease with potent HIV-1 reverse transcriptase inhibitory activity from cultured mushroom Schizophyllum commune. J. Microbiol. 2011, 49, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.G.; Teoh, T.C.; Rizman-Idid, M. Chemical Compounds and Computational Prediction of Their Inhibitory Effects on the HIV-1 gp120 Receptor by Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), with Antler-Like Morphology of Fruiting Bodies. Int. J. Med. Mushrooms 2021, 23, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Akbar, R.; Yam, W.K. Interaction of ganoderic acid on HIV related target: Molecular docking studies. Bioinformation 2011, 7, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Min, B.S.; Nakamura, N.; Miyashiro, H.; Bae, K.W.; Hattori, M. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998, 46, 1607–1612. [Google Scholar] [CrossRef]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef]
- Collins, R.A.; Ng, T.B. Polysaccharopeptide from Coriolus versicolor has potential for use against human immunodeficiency virus type 1 infection. Life Sci. 1997, 60, PL383–PL387. [Google Scholar] [CrossRef]
- Rodriguez-Valentin, M.; Lopez, S.; Rivera, M.; Rios-Olivares, E.; Cubano, L.; Boukli, N.M. Naturally Derived Anti-HIV Polysaccharide Peptide (PSP) Triggers a Toll-Like Receptor 4-Dependent Antiviral Immune Response. J. Immunol. Res. 2018, 2018, 8741698. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.X.; Ng, T.B. Isolation of a laccase with HIV-1 reverse transcriptase inhibitory activity from fresh fruiting bodies of the Lentinus edodes (Shiitake mushroom). Indian J. Biochem. Biophys. 2011, 48, 88–94. [Google Scholar]
- Ngai, P.H.; Ng, T.B. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003, 73, 3363–3374. [Google Scholar] [CrossRef]
- Suzuki, H.; Okubo, A.; Yamazaki, S.; Suzuki, K.; Mitsuya, H.; Toda, S. Inhibition of the infectivity and cytopathic effect of human immunodeficiency virus by water-soluble lignin in an extract of the culture medium of Lentinus edodes mycelia (LEM). Biochem. Biophys. Res. Commun. 1989, 160, 367–373. [Google Scholar] [CrossRef]
- Tochikura, T.S.; Nakashima, H.; Ohashi, Y.; Yamamoto, N. Inhibition (in vitro) of replication and of the cytopathic effect of human immunodeficiency virus by an extract of the culture medium of Lentinus edodes mycelia. Med. Microbiol. Immunol. 1988, 177, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Marquez, E.; Lagunas-Martinez, A.; Bermudez-Morales, V.H.; Burgete-Garcia, A.I.; Leon-Rivera, I.; Montiel-Arcos, E.; Garcia-Villa, E.; Gariglio, P.; Madrid-Marina, V.; Ondarza-Vidaurreta, R.N. Inhibitory Activity of Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Higher Basidiomycetes) on Transformed Cells by Human Papillomavirus. Int. J. Med. Mushrooms 2014, 16, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Losa, F.; Dexeus, D.; Cortes, J. Beneficial effects of a Coriolus versicolor-based vaginal gel on cervical epithelization, vaginal microbiota and vaginal health: A pilot study in asymptomatic women. BMC Womens Health 2017, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Morton, J.C., Jr.; Ramirez, J.L.; Souza-Neto, J.A.; Dimopoulos, G. The entomopathogenic fungus Beauveria bassiana activate toll and JAK-STAT pathway-controlled effector genes and anti-dengue activity in Aedes aegypti. Insect Biochem. Mol. Biol. 2012, 42, 126–132. [Google Scholar] [CrossRef]
- Panya, A.; Songprakhon, P.; Panwong, S.; Jantakee, K.; Kaewkod, T.; Tragoolpua, Y.; Sawasdee, N.; Lee, V.S.; Nimmanpipug, P.; Yenchitsomanus, P.T. Cordycepin Inhibits Virus Replication in Dengue Virus-Infected Vero Cells. Molecules 2021, 26, 3118. [Google Scholar] [CrossRef]
- Ellan, K.; Thayan, R.; Raman, J.; Hidari, K.; Ismail, N.; Sabaratnam, V. Anti-viral activity of culinary and medicinal mushroom extracts against dengue virus serotype 2: An in-vitro study. BMC Complement. Altern. Med. 2019, 19, 260. [Google Scholar] [CrossRef]
- Ellan, K.; Thayan, R.; Phan, C.W.; Sabaratnam, V. Anti-inflammatory effect of mushrooms in dengue-infected human monocytes. Trop. Biomed. 2019, 36, 1087–1098. [Google Scholar]
- Lim, W.Z.; Cheng, P.G.; Abdulrahman, A.Y.; Teoh, T.C. The identification of active compounds in Ganoderma lucidum var. antler extract inhibiting dengue virus serine protease and its computational studies. J. Biomol. Struct. Dyn. 2020, 38, 4273–4288. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Lee, K.E.; Dwivedi, V.D.; Yadava, U.; Panwar, A.; Lucas, S.J.; Pandey, A.; Kang, S.G. Discovery of Ganoderma lucidum triterpenoids as potential inhibitors against Dengue virus NS2B-NS3 protease. Sci. Rep. 2019, 9, 19059. [Google Scholar] [CrossRef]
- He, P.; Lei, J.; Miao, J.N.; Wu, D.; Wang, C. Cordyceps sinensis attenuates HBx-induced cell apoptosis in HK-2 cells through suppressing the PI3K/Akt pathway. Int. J. Mol. Med. 2020, 45, 1261–1269. [Google Scholar] [CrossRef]
- Gu, C.Q.; Li, J.; Chao, F.H. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: Synergistic effect of combination with interferon-alpha in HepG2 2.2.15. Antiviral. Res. 2006, 72, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zang, Y.; Zhang, L. The efficacy of Polyporus Umbellatus polysaccharide in treating hepatitis B in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 329–360. [Google Scholar] [PubMed]
- Kakumu, S.; Ishikawa, T.; Wakita, T.; Yoshioka, K.; Ito, Y.; Shinagawa, T. Effect of sizofiran, a polysaccharide, on interferon gamma, antibody production and lymphocyte proliferation specific for hepatitis B virus antigen in patients with chronic hepatitis B. Int. J. Immunopharmacol. 1991, 13, 969–975. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yang, Y.L.; Fang, L.; Zhang, Z.B.; Jin, J.; Zhang, K.C. Anti-hepatitis activities in the broth of Ganoderma lucidum supplemented with a Chinese herbal medicine. Am. J. Chin. Med. 2006, 34, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Wang, S.F. Anti-hepatitis B activities of ganoderic acid from Ganoderma lucidum. Biotechnol. Lett. 2006, 28, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Yang, J.M.; Liu, Y.H.; Zhao, M.; Wang, J. Ultrasound assisted extraction of polysaccharides from Lentinus edodes and its anti-hepatitis B activity in vitro. Int. J. Biol. Macromol. 2018, 107 Pt B, 2217–2223. [Google Scholar] [CrossRef]
- Matsuhisa, K.; Yamane, S.; Okamoto, T.; Watari, A.; Kondoh, M.; Matsuura, Y.; Yagi, K. Anti-HCV effect of Lentinula edodes mycelia solid culture extracts and low-molecular-weight lignin. Biochem. Biophys. Res. Commun. 2015, 462, 52–57. [Google Scholar] [CrossRef]
- Ueda, Y.; Mori, K.; Satoh, S.; Dansako, H.; Ikeda, M.; Kato, N. Anti-HCV activity of the Chinese medicinal fungus Cordyceps militaris. Biochem. Biophys. Res. Commun. 2014, 447, 341–345. [Google Scholar] [CrossRef]
- Gu, C.Q.; Li, J.W.; Chao, F.; Jin, M.; Wang, X.W.; Shen, Z.Q. Isolation, identification and function of a novel anti-HSV-1 protein from Grifola frondosa. Antivir. Res. 2007, 75, 250–257. [Google Scholar] [CrossRef]
- Sarkar, S.; Koga, J.; Whitley, R.J.; Chatterjee, S. Antiviral effect of the extract of culture medium of Lentinus edodes mycelia on the replication of herpes simplex virus type 1. Antivir. Res. 1993, 20, 293–303. [Google Scholar] [CrossRef]
- Eo, S.K.; Kim, Y.S.; Lee, C.K.; Han, S.S. Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses. J. Ethnopharmacol. 2000, 72, 475–481. [Google Scholar] [CrossRef]
- Kim, Y.S.; Eo, S.K.; Oh, K.W.; Lee, C.K.; Han, S.S. Antiherpetic activities of acidic protein bound polysaccharide isolated from Ganoderma lucidum alone and in combinations with interferons. J. Ethnopharmacol. 2000, 72, 451–458. [Google Scholar] [CrossRef]
- Oh, K.W.; Lee, C.K.; Kim, Y.S.; Eo, S.K.; Han, S.S. Antiherpetic activities of acidic protein bound polysacchride isolated from Ganoderma lucidum alone and in combinations with acyclovir and vidarabine. J. Ethnopharmacol. 2000, 72, 221–227. [Google Scholar] [CrossRef]
- Eo, S.K.; Kim, Y.S.; Lee, C.K.; Han, S.S. Antiviral activities of various water and methanol soluble substances isolated from Ganoderma lucidum. J. Ethnopharmacol. 1999, 68, 129–136. [Google Scholar] [CrossRef]
- Hijikata, Y.; Yamada, S.; Yasuhara, A. Herbal mixtures containing the mushroom Ganoderma lucidum improve recovery time in patients with herpes genitalis and labialis. J. Altern. Complement. Med. 2007, 13, 985–987. [Google Scholar] [CrossRef]
- Ryu, E.; Son, M.; Lee, M.; Lee, K.; Cho, J.Y.; Cho, S.; Lee, S.K.; Lee, Y.M.; Cho, H.; Sung, G.H.; et al. Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication. Oncoscience 2014, 1, 866–881. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Akihisa, T.; Tokuda, H.; Ukiya, M.; Oshikubo, M.; Kimura, Y.; Asano, T.; Nomura, A.; Nishino, H. Lucidenic acids P and Q, methyl lucidenate P, and other triterpenoids from the fungus Ganoderma lucidum and their inhibitory effects on Epstein-Barr virus activation. J. Nat. Prod. 2003, 66, 1582–1585. [Google Scholar] [CrossRef]
- Zheng, D.S.; Chen, L.S. Triterpenoids from Ganoderma lucidum inhibit the activation of EBV antigens as telomerase inhibitors. Exp. Ther. Med. 2017, 14, 3273–3278. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chow, Y.H.; Sun, H.L.; Liu, Y.F.; Lee, Y.T.; Lue, K.H.; Ko, J.L. Alleviation of respiratory syncytial virus replication and inflammation by fungal immunomodulatory protein FIP-fve from Flammulina velutipes. Antivir. Res. 2014, 110, 124–131. [Google Scholar] [CrossRef]
- Rincao, V.P.; Yamamoto, K.A.; Ricardo, N.M.; Soares, S.A.; Meirelles, L.D.; Nozawa, C.; Linhares, R.E. Polysaccharide and extracts from Lentinula edodes: Structural features and antiviral activity. Virol. J. 2012, 9, 37. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, N.; Wang, Y.; Zheng, X.; Zhao, Y.; Wang, H.; Wang, C.; Han, Q.; Gao, Y.; Shan, J.; et al. Adjuvant activity of PCP-II, a polysaccharide from Poria cocos, on a whole killed rabies vaccine. Virus Res. 2019, 270, 197638. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.; Zheng, X.; Wang, C.; Wang, H.; Zhao, Y.; Wang, Q.; Wong, G.; Zhang, W.; Feng, N.; Qiu, B.; et al. Marburg virus-like particles by co-expression of glycoprotein and matrix protein in insect cells induces immune responses in mice. Virol. J. 2017, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Weiwei, G.; Xuexing, Z.; Chong, W.; Yongkun, Z.; Qi, W.; Hualei, W.; Gary, W.; Ying, X.; Haijun, W.; Zengguo, C.; et al. Marburg virus-like particles produced in insect cells induce neutralizing antibodies in rhesus macaques. J. Med. Virol. 2017, 89, 2069–2074. [Google Scholar] [CrossRef]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, H.; Zhu, E.; Li, J.; Wang, Q.; Zhou, W.; Qin, T.; Wu, X.; Wu, B.; Huang, Y. Hericium erinaceus polysaccharide facilitates restoration of injured intestinal mucosal immunity in Muscovy duck reovirus-infected Muscovy ducklings. Int. J. Biol. Macromol. 2018, 107 Pt A, 1151–1161. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, L.; Chen, Q.; Lin, S.; Luo, Y.; Qin, T.; Li, J.; Wang, Q.; Wu, B.; Huang, Y.; et al. Effects of Hericium erinaceus polysaccharide on immunity and apoptosis of the main immune organs in Muscovy duck reovirus-infected ducklings. Int. J. Biol. Macromol. 2021, 171, 448–456. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Yan, P.; Zhu, Z.; Liao, L.; Chen, Q.; Luo, Y.; Li, H.; Li, J.; Wang, Q.; et al. Transcriptome analysis of the effects of Hericium erinaceus polysaccharide on the lymphocyte homing in Muscovy duck reovirus-infected ducklings. Int. J. Biol. Macromol. 2019, 140, 697–708. [Google Scholar] [CrossRef]
- Chang, C.-F.; Su, M.-S.; Chen, H.-Y.; Liao, I.C. Dietary β-1,3-glucan effectively improves immunity and survival of Penaeus monodon challenged with white spot syndrome virus. Fish Shellfish Immunol. 2003, 15, 297–310. [Google Scholar] [CrossRef]
- Seetaha, S.; Ratanabunyong, S.; Tabtimmai, L.; Choowongkomon, K.; Rattanasrisomporn, J.; Choengpanya, K. Anti-feline immunodeficiency virus reverse transcriptase properties of some medicinal and edible mushrooms. Vet. World 2020, 13, 1798–1806. [Google Scholar] [CrossRef]
- Forzan, M.; Pacini, M.I.; Bonaccini, P.; Mazzei, M. Antiviral effect of a commercially phytotherapeutic compound against feline immunodeficiency virus. Nat. Prod. Res. 2021. [Google Scholar] [CrossRef]
- Stamets, P.E.; Naeger, N.L.; Evans, J.D.; Han, J.O.; Hopkins, B.K.; Lopez, D.; Moershel, H.M.; Nally, R.; Sumerlin, D.; Taylor, A.W.; et al. Extracts of Polypore Mushroom Mycelia Reduce Viruses in Honey Bees. Sci. Rep. 2018, 8, 13936. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.C.; Sheu, F.; Lee, G.C.; Tsai, M.W.; Hung, C.L.; Nan, F.H. Administration of recombinant Reishi immunomodulatory protein (rLZ-8) diet enhances innate immune responses and elicits protection against nervous necrosis virus in grouper Epinephelus coioides. Fish Shellfish Immunol. 2012, 32, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, T.; He, J.; Zhang, Y.; Gu, P.; Qiu, T.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Adjuvanticity of Ganoderma lucidum polysaccharide liposomes on porcine circovirus type-II in mice. Int. J. Biol. Macromol. 2019, 141, 1158–1164. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, W.; Wang, L.; Zhu, M.; Wang, H.; Feng, W.H.; Ng, T.B. A novel compound from the mushroom Cryptoporus volvatus inhibits porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. PLoS ONE 2013, 8, e79333. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Lv, N.; Gao, L.; Cao, L.; Shen, L.; Si, J. Chemical constituents from the fruiting bodies of Cryptoporus volvatus. Arch. Pharm. Res. 2016, 39, 747–754. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, W.; Sun, Y.; Yang, Q.; Ren, J.; Liu, J.; Wang, H.; Feng, W.H. Cryptoporus volvatus extract inhibits porcine reproductive and respiratory syndrome virus (PRRSV) in vitro and in vivo. PLoS ONE 2013, 8, e63767. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Ge, X.; Wang, J.; Wei, Z.; Feng, W.H.; Wang, J. Ergosterol peroxide exhibits antiviral and immunomodulatory abilities against porcine deltacoronavirus (PDCoV) via suppression of NF-kappaB and p38/MAPK signaling pathways in vitro. Int. Immunopharmacol. 2021, 93, 107317. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Wang, J.; Liu, Y.; Zhang, J.; Si, J.; Hao, Z.; Wang, J. Antiviral effects of ergosterol peroxide in a pig model of porcine deltacoronavirus (PDCoV) infection involves modulation of apoptosis and tight junction in the small intestine. Vet. Res. 2021, 52, 86. [Google Scholar] [CrossRef]
- Shamaki, B.U.; Sandabe, U.K.; Ogbe, A.O.; Abdulrahman, F.I.; El-Yuguda, A.D. Methanolic Soluble Fractions of Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Higher Basidiomycetes) Extract Inhibit Neuraminidase Activity in Newcastle Disease Virus (LaSota). Int. J. Med. Mushrooms 2014, 16, 579–583. [Google Scholar] [CrossRef]
- Zhang, P.; Ding, R.; Jiang, S.; Ji, L.; Pan, M.; Liu, L.; Zhang, W.; Gao, X.; Huang, W.; Zhang, G.; et al. The adjuvanticity of Ganoderma lucidum polysaccharide for Newcastle disease vaccine. Int. J. Biol. Macromol. 2014, 65, 431–435. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Chen, J.; Hu, Y.; Wang, D.; Fan, Y.; Wang, J.; Abula, S.; Zhang, J.; Qin, T.; Chen, X.; et al. In vitro antiviral activity of sulfated Auricularia auricula polysaccharides. Carbohydr. Polym. 2012, 90, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, X.; Yang, R.; Qin, T.; Li, Y.; Zhang, L.; Fei, C.; Zhen, W.; Zhang, K.; Wang, X.; et al. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Biol. Macromol. 2013, 59, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Rong, X.; Chen, P.T.; Zhang, X.; Ma, Z.Q. Two new steroids from sclerotia of the fungus Omphalia lapidescens. J. Asian Nat. Prod. Res. 2014, 16, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Tan, M.; Fukushima, A.; Hieda, T.; Kubo, S.; Takabayashi, M.; Ono, K.; Mikami, Y. Antiviral Substances with Systemic Effects Produced by Basidiomycetes such as Fomes fomentarius. Biosci. Biotechnol. Biochem. 1993, 57, 278–282. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Wang, H.Y.; Xia, X.M.; Li, P.P.; Wang, K.Y. Inhibitory effect of sulfated lentinan and lentinan against tobacco mosaic virus (TMV) in tobacco seedlings. Int. J. Biol. Macromol. 2013, 61, 264–269. [Google Scholar] [CrossRef]
- Sangeetha, B.; Krishnamoorthy, A.S.; Renukadevi, P.; Malathi, V.G.; Jeya Sundara Sharmila, D.; Amirtham, D. Antiviral activity of basidiomycetous fungi against Groundnut bud necrosis virus in tomato. Pestic. Biochem. Physiol. 2020, 166, 104570. [Google Scholar] [CrossRef]
- Del Rio, C.; Malani, P.N. COVID-19-New Insights on a Rapidly Changing Epidemic. JAMA 2020, 323, 1339–1340. [Google Scholar] [CrossRef]
- Gao, S.J.; Guo, H.; Luo, G. Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert! J. Med. Virol. 2022, 94, 1255–1256. [Google Scholar] [CrossRef]
- Pleschka, S. Overview of influenza viruses. Curr. Top. Microbiol. Immunol. 2013, 370, 1–20. [Google Scholar]
- Sullivan, S.J.; Jacobson, R.M.; Dowdle, W.R.; Poland, G.A. 2009 H1N1 influenza. Mayo. Clin. Proc. 2010, 85, 64–76. [Google Scholar] [CrossRef]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar] [PubMed]
- Wang, L.; Hou, Y. Determination of trace elements in anti-influenza virus mushrooms. Biol. Trace Elem. Res. 2011, 143, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lu, J.; Lu, J. Enterovirus 71 vaccine: Close but still far. Int. J. Infect. Dis. 2010, 14, e739–e743. [Google Scholar] [CrossRef]
- Fowlkes, A.L.; Honarmand, S.; Glaser, C.; Yagi, S.; Schnurr, D.; Oberste, M.S.; Anderson, L.; Pallansch, M.A.; Khetsuriani, N. Enterovirus-associated encephalitis in the California encephalitis project, 1998–2005. J. Infect. Dis. 2008, 198, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Ortblad, K.F.; Lozano, R.; Murray, C.J. The burden of HIV: Insights from the Global Burden of Disease Study 2010. AIDS 2013, 27, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Z.; Wu, X.; Wu, M.; Fang, Q.; Zhang, X.; Shi, T.; Tully, D.C.; Zhang, T. Prevalence of transmitted HIV-1 drug resistance among treatment-naive individuals in China, 2000–2016. Arch. Virol. 2021, 166, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.F.; Park, I.U. HPV and HPV-associated diseases. Infect. Dis. Clin. N. Am. 2013, 27, 765–778. [Google Scholar] [CrossRef]
- Yang, A.; Farmer, E.; Wu, T.C.; Hung, C.F. Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci. 2016, 23, 75. [Google Scholar] [CrossRef]
- Criscuolo, A.A.; Sesti, F.; Piccione, E.; Mancino, P.; Belloni, E.; Gullo, C.; Ciotti, M. Therapeutic Efficacy of a Coriolusversicolor-Based Vaginal Gel in Women with Cervical Uterine High-Risk HPV Infection: A Retrospective Observational Study. Adv. Ther. 2021, 38, 1202–1211. [Google Scholar] [CrossRef]
- Salazar, M.I.; Richardson, J.H.; Sanchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef]
- Tian, J.N.; Yang, C.C.; Chuang, C.K.; Tsai, M.H.; Wu, R.H.; Chen, C.T.; Yueh, A. A Dengue Virus Type 2 (DENV-2) NS4B-Interacting Host Factor, SERP1, Reduces DENV-2 Production by Suppressing Viral RNA Replication. Viruses 2019, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Nanaware, N.; Banerjee, A.; Mullick Bagchi, S.; Bagchi, P.; Mukherjee, A. Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses. Viruses 2021, 13, 1967. [Google Scholar] [CrossRef] [PubMed]
- Rechenchoski, D.Z.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Herpesvirus: An underestimated virus. Folia Microbio. 2017, 62, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Widener, R.W.; Whitley, R.J. Herpes simplex virus. Handb. Clin. Neurol. 2014, 123, 251–263. [Google Scholar] [PubMed]
- Dogan, H.H.; Karagoz, S.; Duman, R. In Vitro Evaluation of the Antiviral Activity of Some Mushrooms from Turkey. Int. J. Med. Mushrooms 2018, 20, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Mammas, I.N.; Drysdale, S.B.; Rath, B.; Theodoridou, M.; Papaioannou, G.; Papatheodoropoulou, A.; Koutsounaki, E.; Koutsaftiki, C.; Kozanidou, E.; Achtsidis, V.; et al. Update on current views and advances on RSV infection (Review). Int. J. Mol. Med. 2020, 46, 509–520. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mavunda, K.; Krilov, L.R. Current State of Respiratory Syncytial Virus Disease and Management. Infect. Dis. Ther. 2021, 10 (Suppl. S1), 5–16. [Google Scholar] [CrossRef]
- Racaniello, V.R. One hundred years of poliovirus pathogenesis. Virology 2006, 344, 9–16. [Google Scholar] [CrossRef]
- Nathanson, N. Chapter 1 The Pathogenesis of Poliomyelitis: What We Don’t Know. Adv. Virus Res. 2008, 71, 1–50. [Google Scholar]
- Davis, B.M.; Rall, G.F.; Schnell, M.J. Everything You Always Wanted to Know About Rabies Virus (But Were Afraid to Ask). Annu. Rev. Virol. 2015, 2, 451–471. [Google Scholar] [CrossRef]
- Stitz, L.; Vogel, A.; Schnee, M.; Voss, D.; Rauch, S.; Mutzke, T.; Ketterer, T.; Kramps, T.; Petsch, B. A thermostable messenger RNA based vaccine against rabies. PLoS Negl. Trop. Dis. 2017, 11, e0006108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, S.; Xie, H.; Li, Q.; Liu, S.; Liu, Q.; Huang, W.; Xiao, X.; Wang, Y. Screening and Identification of Marburg Virus Entry Inhibitors Using Approved Drugs. Virol. Sin. 2020, 35, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Paley, R.; Alegria-Moran, R.; Oidtmann, B. Epidemiological characteristics of infectious hematopoietic necrosis virus (IHNV): A review. Vet. Res. 2016, 47, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zheng, M.; Huang, M.; Xiao, S.; Lin, F.; Chen, S.; Chen, S. Muscovy Duck Reovirus Infection Disrupts the Composition of Intestinal Microbiota in Muscovy Ducklings. Curr. Microbiol. 2020, 77, 769–778. [Google Scholar] [CrossRef]
- Zheng, S.C.; Xu, J.Y.; Liu, H.P. Cellular entry of white spot syndrome virus and antiviral immunity mediated by cellular receptors in crustaceans. Fish Shellfish Immunol. 2019, 93, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, L.I.B.; Looney, D.J. Feline immunodeficiency virus: A concise review. Front. Biosci. -Landmark 2004, 9, 370–377. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103 (Suppl. S1), S48–S61. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Zhang, X.; Yu, Y.; Huang, X.; Wei, J.; Qin, Q. Red grouper nervous necrosis virus (RGNNV) induces autophagy to promote viral replication. Fish Shellfish Immunol. 2020, 98, 908–916. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, H.; Hao, Q.; Li, M.; Liu, J.; Fan, H. Coinfection with porcine circovirus type 2 and Streptococcus suis serotype 2 enhances pathogenicity by dysregulation of the immune responses in piglets. Vet. Microbiol. 2020, 243, 108653. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Zhang, J. Porcine deltacoronavirus: Overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus. Res. 2016, 226, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.S.; Chen, Q.S.; Zheng, Q.X.; Shen, J.J.; Luo, Z.P.; Fan, K.; Xu, S.H.; Shen, Q.; Liu, P.P. Proteomic and Phosphoproteomic Analysis in Tobacco Mosaic Virus-Infected Tobacco (Nicotiana tabacum). Biomolecules 2019, 9, 39. [Google Scholar] [CrossRef]
- Sangeetha, B.; Krishnamoorthy, A.S.; Sharmila, D.J.S.; Renukadevi, P.; Malathi, V.G.; Amirtham, D. Molecular modelling of coat protein of the Groundnut bud necrosis tospovirus and its binding with Squalene as an antiviral agent: In vitro and in silico docking investigations. Int. J. Biol. Macromol. 2021, 189, 618–634. [Google Scholar] [CrossRef]
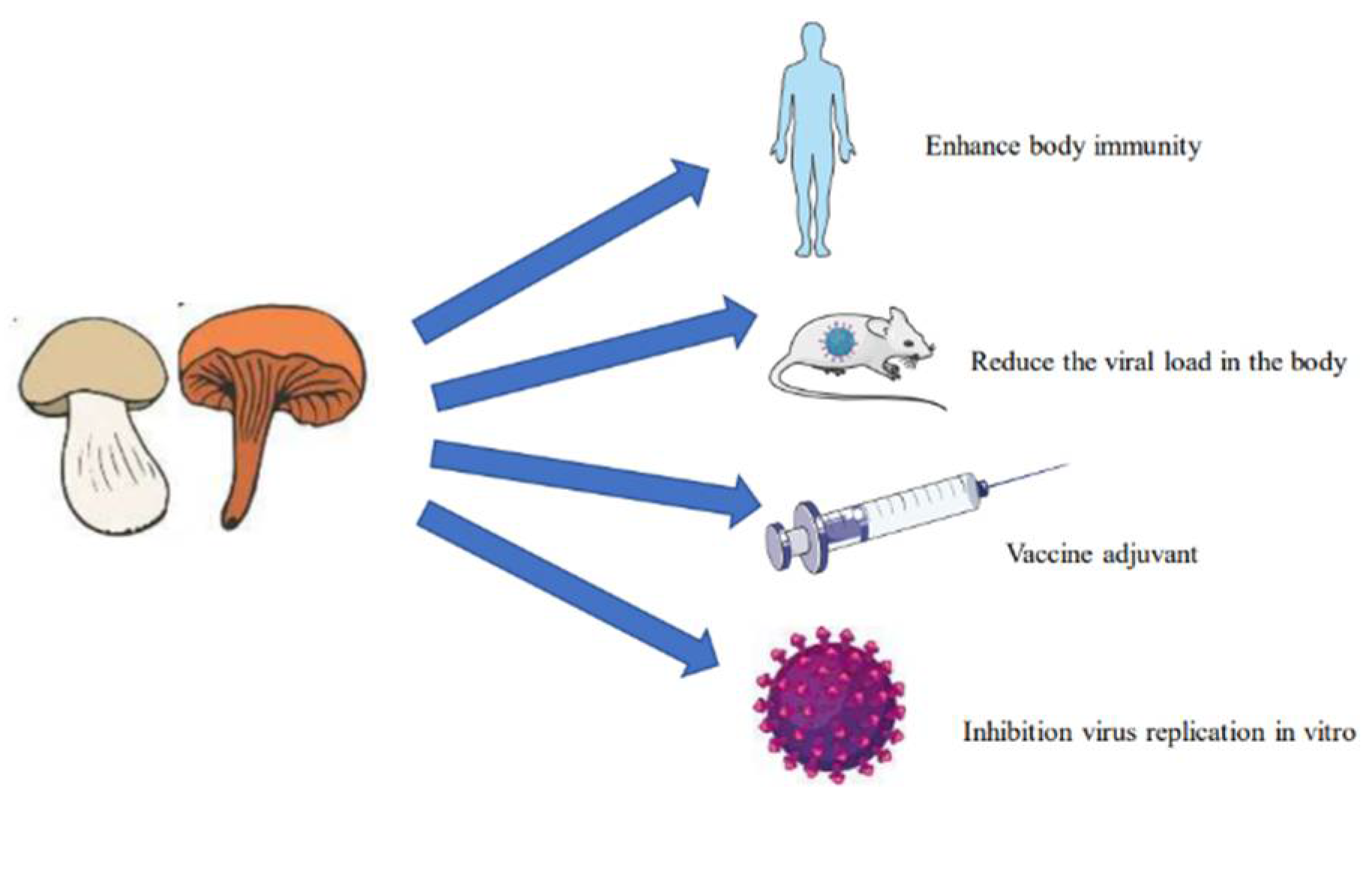
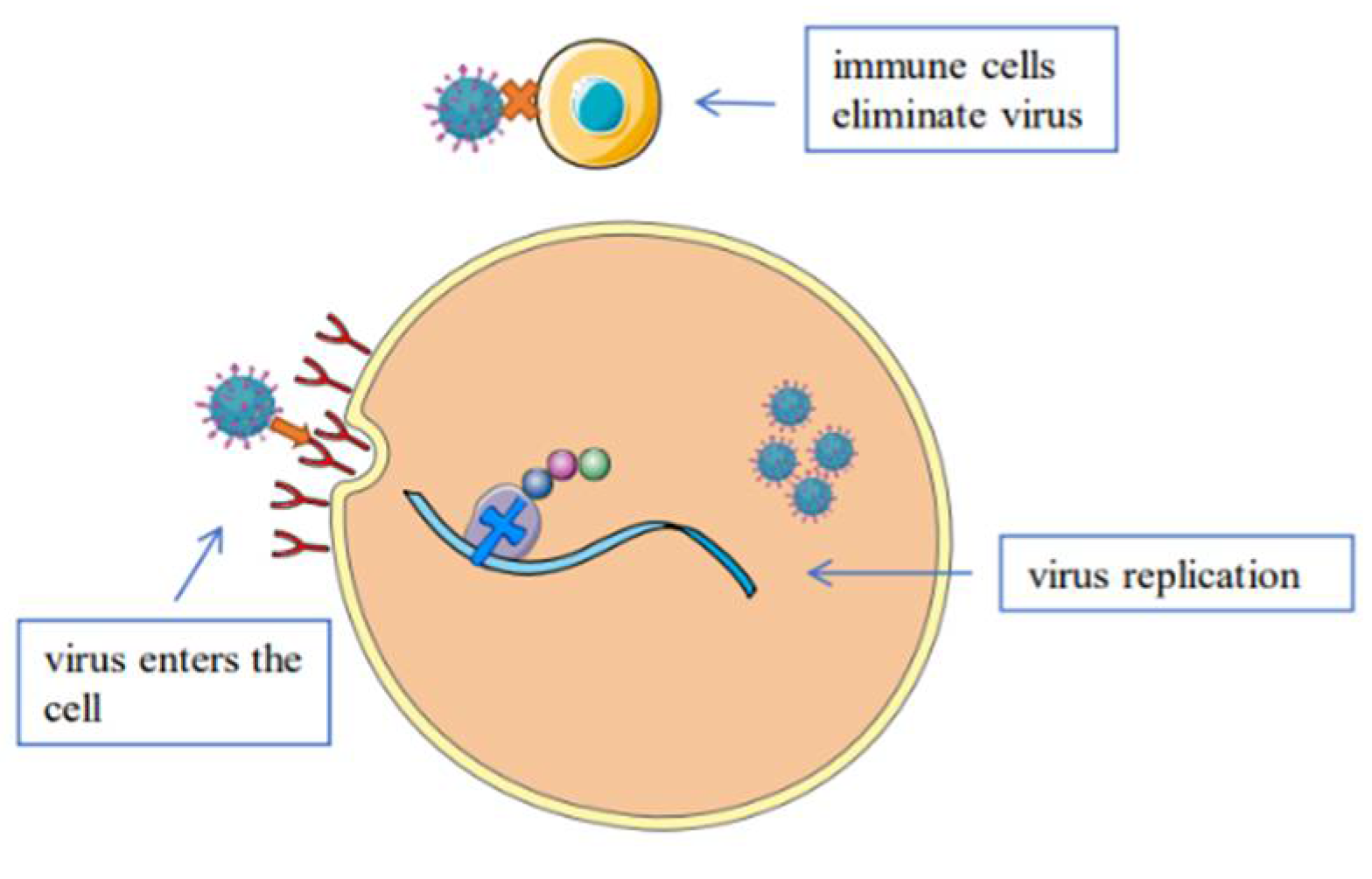

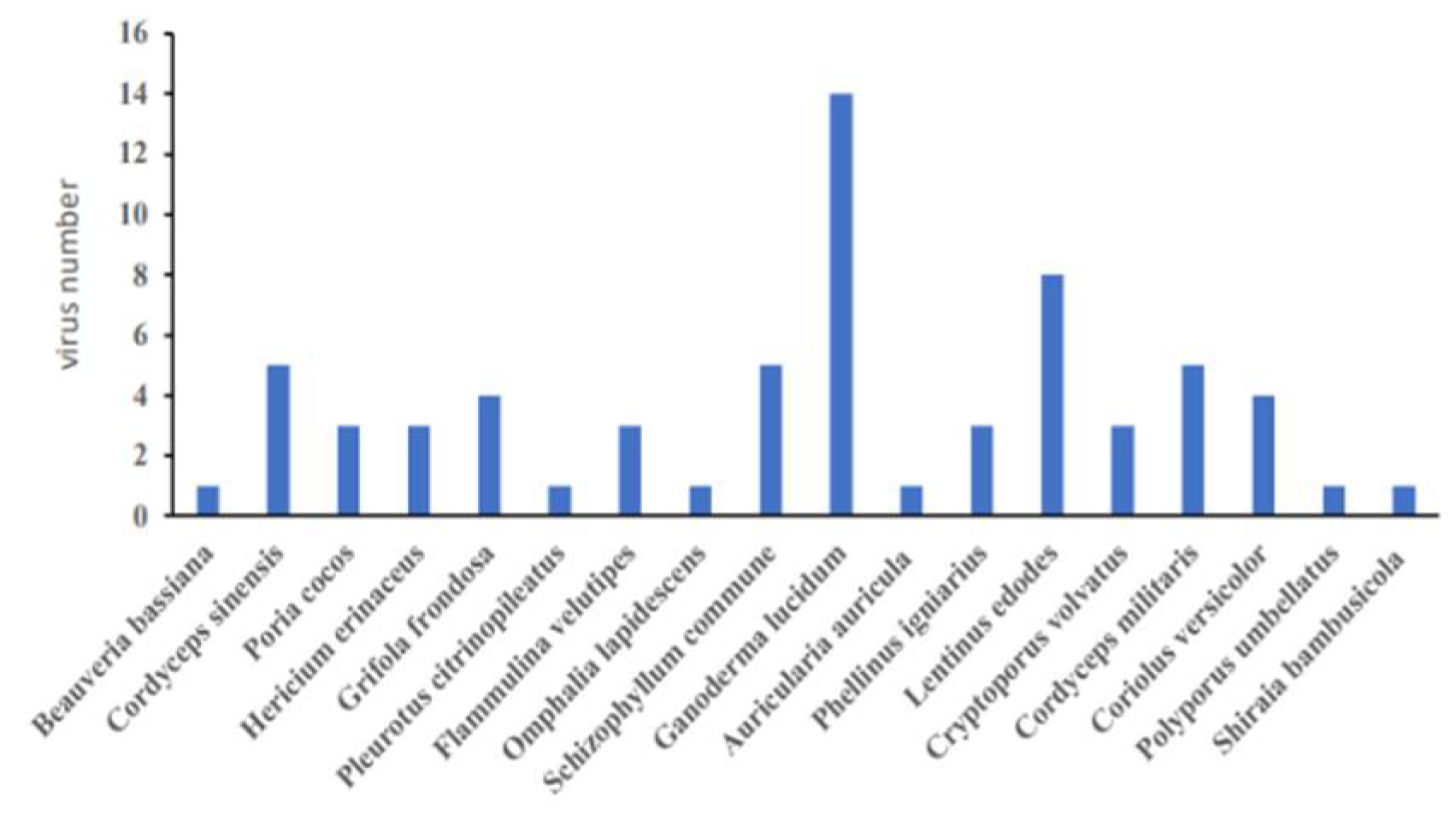
| Virus | Chemical Class | Antiviral Agent | Source | Action Mechanism | Reference | |
|---|---|---|---|---|---|---|
| SARS-CoV-2 | Polysaccharide | L-fucose–containing polysaccharides | Ganoderma lucidum | Vitro | [12] | |
| Triterpenes, protein | Ganoderma lucidum, Grifola frondosa et al. | Inhibition ACE activity | [13] | |||
| Steroid | Antcins | Antrodia cinnamomea | ACE2 inhibitory effect | [14] | ||
| Polysaccharide | 20% α-glucans | Lentinus edodes | Regulating the immune system | [15] | ||
| Polysaccharide | Carbosynth-lentinan (50%β-glucan 12%α-glucan) | Lentinus edodes | Reduce the level of inflammation in the lung | [16] | ||
| Polysaccharide | In-house Lentinan (72%β-glucan) | Lentinus edodes | Reduce the level of inflammation in the lung | [16] | ||
| Adenosine | Cordycepin(C10H13N5O3) | Cordyceps sinensis | Spike protein inhibitor | [17] | ||
| IV | Crude extract | ME, MFs (MF1, MF2 and MF3) | Grifola frondosa | Vitro induce the production of cytokines such as TNF-α, which inhibit the growth of virus in vitro. | [18] | |
| Pyranone | Phelligridins E, G | Phellinus igniarius | Vitro neuraminidase inhibitory activity | [19] | ||
| Crude extract | Aqueous extract PIW | Phellinus igniarius | Vitro interfering with the early replication process of the virus | [20] | ||
| Crude extract | Lentinus edodes mycelium extract LEM | Lentinus edodes | Vitro Inhibit virus entry into host cells activate the immune response through the type I IFN pathway | [21] | ||
| Terpenoids | Cryptoporic acid E | Cryptoporus volvatus | Vitro | [22] | ||
| Crude extract | Water extract | Cryptoporus volvatus | Vitro | [23] | ||
| Polysaccharide | APS (D-galactose, L-arabinose, D-xylose, L-rhamnose, and D-galacturonic acid) | Cordyceps militaris | Vitro | [24] | ||
| Crude extract | Cordyceps militaris | Vivo increased IL-12 expression and greater number of NK cells | [25] | |||
| Crude extract | Auuriporia aurea, Flammulina velutipes, Fomes fomentarius, Ganoderma lucidum, Lentinus edodes, Lyophyllum shimeji, Pleurotus eryngii, Pleurotus ostreatus, Schizophyllum commune, and Trametes versicolor | Vitro | [26] | |||
| Fatty acid | (2Z,4E)-deca-2,4-dienoic acid (DDEA) | Cordyceps sinensis | Reduce the level of inflammation caused by infection with H1N1 | [27] | ||
| Polysaccharide | PCP-II (fucose, mannose, glucose and galactose in molar ration of 1.00:1.63:0.16:6.29) | Poria cocos | Vaccine adjuvant | [28] | ||
| Polysaccharide | Mixed polysaccharides (MPs) | shiitake mushroom, poriacocos, ginger, tangerine peel | Enhanced the level of cellular immunity and humoral immunity | [29] | ||
| EV71 | Polysaccharide | Neutral polysaccharide having a β-1,6-linked Glcp backbone with 1,3-α-glucan units | Grifola frondosa | Vitro Inhibition of VP1 protein expression and genomic RNA synthesis | [30] | |
| Polysaccharide | 95%polysaccharides (glucose 79% and mannose 21%) and 5% proteins | Ganoderma lucidum | Vaccine adjuvant | [31] | ||
| Triterpenoid | Lanosta-7,9(11),24-trien-3one,15;26-dihydroxy (GLTA) and Ganoderic acid Y (GLTB) | Ganoderma lucidum | Vitro inhibits the replication of the viral RNA replication through blocking EV71 uncoating | [32] | ||
| HIV | Adenosine | Cordycepin(C10H13N5O3) | Cordyceps sinensis | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 8.2 × 10−3 μM | [33] | |
| Water extract | Cordyceps sinensis | Vitro inhibitory activity on HIV-1 reverse transcriptase | [34] | |||
| Adenosine | L3a(C10H13N5O4), L3b(C20H20O7), L3c(C12H17N5O5) | Cordyceps militaris | Vitro inhibitory activity on HIV-1 reverse transcriptase | [35] | ||
| Lectin | N-terminal amino acid sequence (NSTDISLNHG) Molecular mass 30 kDa | Cordyceps militaris | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 10 μM | [36] | ||
| Lectin | N-terminal amino acid sequence (QYSQMAQVME) Molecular mass 32.4 kDa | Pleurotus ostreatus | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 0.93 μM | [37] | ||
| Protein | N-terminal amino acid sequence (AEGTLLGSRA TCESGNSMY) Molecular mass 9567 Da, | Hypsizigus marmoreus | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 30 nM | [38] | ||
| Protein | N-terminal amino acid sequence(XHPDLFXX) molecular mass 13.8 kDa | Flammulina velutipes | Vitro inhibitory activity on HIV-1 reverse transcriptase | [39] | ||
| Hemolysin | N-terminal amino acid sequence (ATNYNKCPGA) Molecular mass 29 kDa | Schizophyllum commune | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 1.8 mM | [40] | ||
| Ribonuclease | N-terminal amino acid sequence (TPYLDYLAAL QADGPVVPFIRNWEGALSIS) Molecular mass 20 kDa | Schizophyllum commune | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 65 mM | [41] | ||
| Crude extract | NGCs and AGCs | Ganoderma lucidum | Vitro inhibitory effects on the attachment of HIV-1 glycoprotein 120 to cluster of differentiation 4 | [42] | ||
| Triterpenoid | Ganoderic acid B | Ganoderma lucidum | Vitro inhibitory activity on HIV-1 reverse transcriptase | [43] | ||
| Triterpenoid | Ganoderic acid β, lucidumol B, ganodermanondiol, ganodermanontriol, ganolucidic acid A | Ganoderma lucidum | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 20, 50, 90, 70, 70 μM | [44] | ||
| Triterpenoid | Ganoderiol F and ganodermanontriol | Ganoderma lucidum | Vitro anti-hiv-1 activity with an inhibition concentration of 7.8 μg/mL | [45] | ||
| Triterpenoid | Ganoderic acid B, ganoderiol B, ganoderic acid C1, 3b-5a-dihydroxy-6b-methoxyergosta-7,22-diene, ganoderic acid a, ganoderic acid H and ganoderiol A | Ganoderma lucidum | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 0.17–0.23 mM | [45] | ||
| Polysaccharide | PSP (28% polysaccharide-to-peptide ratio and a composition of 60.23 mg/g beta-1,3/1,6-glucan) | Coriolus versicolor | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 6.25 μg/mL inhibition of the interaction between HIV-l gp120 and immobilized CD4 receptor | [46,47] | ||
| Laccase | N-terminal amino acid sequence (AGTSHFADL) molecular mass 67 kDa | Lentinus edodes | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 7.5 µM | [48] | ||
| Protein | N-terminal amino acid sequence (CQRAFNNPRDDAIRW) molecular mass 27.5 kDa | Lentinus edodes | Vitro inhibitory activity on HIV-1 reverse transcriptase IC50 1.5 µM | [49] | ||
| Lignin | The elemental analysis; C, 44.6%; H, 4.68%; N, 1.74% | Lentinus edodes | Vitro | [50] | ||
| Water extract | E-P-LEM and LEM | Lentinus edodes | Vitro | [51] | ||
| HPV | Crude extract | Fruit body powders | Trametes versicolor, Ganoderma lucidum | Vivo | [52] | |
| Crude extract | Non-hormonal gel | Trametes versicolor | Vivo | [53] | ||
| DENV | Conidia | Conidia | Beauveria bassiana | Vivo activation of the mosquito’s anti-dengue Toll and JAK-STAT pathways | [54] | |
| Adenosine | Cordycepin(C10H13N5O3) | Cordyceps sinensis | Vitro inhibition of viral RNA replication | [55] | ||
| Crude extract | Hot aqueous extracts, and aqueous soluble extracts | rhinocerotis, Pleurotus giganteus, Hericium erinaceus, Schizophyllum commune | Vitro | [56,57] | ||
| Aqueous extract | Hesperidin | Ganoderma lucidum | Vitro anti-DENV NS2B-NS3 protease activity | [58] | ||
| Triterpene | Ganodermanontriol | Ganoderma lucidum | Vitro | [59] | ||
| HV | HBV | Crude extract | Crude extract | Cordyceps sinensis | Reduce apoptosis of renal tubular epithelial cells | [60] |
| Crude extract | GF-D extract | Grifola frondosa | Vitro directly interferes with HBV replication at the level of DNA polymerase | [61] | ||
| Polysaccharide | β-glucan with a (1-3)-β-glucose backbone and (1-6)-β-glucose side chains | Polyporus umbellatus | Treat hepatitis caused by HBV | [62] | ||
| Polysaccharide | Glucan | Schizophyllum commune | Modulate both cellular and humoral immune responses | [63] | ||
| Crude extract | Co-fermented broth | Ganoderma lucidum and Radix Sophorae flavescentis | Vitro | [64] | ||
| Triterpene | Ganoderic | Ganoderma lucidum | Vitro | [65] | ||
| Polysaccharide | LEP-1 (glucose72.15%, galactose12.12%, mannose10.02%) LEP-2(glucose79.49%, galactose7.12%, mannose6.92%) | Lentinus edodes | Vitro | [66] | ||
| HCV | Crude extract | MSCE and LM-lignin | Lentinula edodes | Inhibit the entry of HCVpv into cells | [67] | |
| Adenosine | Cordycepin(C10H13N5O3) | Cordyceps militaris | Inhibit HCV RNA replication in vitro inhibit NS5B polymerase activity | [68] | ||
| HV | HSV | Protein | N-terminal amino acid sequence (NH2-REQDNAPCGLN-COOH) molecular mass 29.5 kDa | Grifola frondosa | Vitro and vivo | [69] |
| Crude extract | JLS-SO01 | Lentinus edodes | Vitro blocks HSV-I replication at the later stage of the virus replication cycle | [70] | ||
| Polysaccharide | APBP polysacchride (approximately 40.6%) and protein (approximately 7.80%) carbohydrates molar ratio (C:H:O = 1:2:1) | Ganoderma lucidum | Vitro | [71,72,73] | ||
| Crude extract | Water-soluble extracts GLhw and GLlw and eight kinds of methanol-soluble extracts GLMe-1-8 | Ganoderma lucidum | Vitro | [74] | ||
| Crude extract | Herbal mixture WTTCGE | Ganoderma lucidum | Vivo | [75] | ||
| EBV | Adenosine | Cordycepin(C10H13N5O3) | Cordyceps sinensis | Vitro Affect the synthesis of viral proteins by acting on the genes of EBV | [76] | |
| Terpenoid | lucidenic acid P, methyl lucidenates P, methyl lucidenates Q | Ganoderma lucidum | Inhibitory effects on Epstein-Barr virus early antigen induction | [77] | ||
| Terpenoid | Ganoderic acid A, ganoderic acid B, ganoderol B, ganodermanontriol, ganodermanondiol | Ganoderma lucidum | Vitro | [78] | ||
| RSV | Protein | 114 amino acid residues molecular mass 13 kDa | Flammulina velutipes | In vivo reduce the viral titers of RSV reducing NF-jB translocation | [79] | |
| PV | Crude extract polysaccharide | LeP(β-D-glucan) aqueous (AqE) and ethanol (EtOHE) extracts | Lentinus edodes | Vitro | [80] | |
| RV | Polysaccharide | Fucose, mannose, glucose and galactose at a molar ratio 1.00:1.63:0.16:6.29. | Poria cocos | Adjuvant for rabies vaccine | [81] | |
| MARV | Polysaccharide | PCP-II (fucose, mannose, glucose and galactose in molar ration of 1.00:1.63:0.16:6.29) | Poria cocos | Vaccine adjuvant | [82,83] | |
| Virus | Chemical Class | Antiviral Agent | Source | Action Mechanism | Reference | |
|---|---|---|---|---|---|---|
| Animal viruses | IHNV | Polysaccharide | LNT-I β-(1→3)-glucan backbone with -(1→6)-glucosyl side-branching units glucose, mannose and galactose with the molar ratio of 19.26:1.20:1.00 | Lentinus edodes | Vitro directly inactivate virus and inhibit virus replication | [84] |
| MDRV | Polysaccharide | HEP glucose (51.02%), galactose (42.24%), mannose (4.5%) and arabinose (2.2%) | Hericium Erinaceus | Vivo improve the number of intestinal mucosal immune-related cells regulate the homing process of muscovy duck lymphocytes | [85,86,87] | |
| WSSV | Polysaccharide | β-1,3 glucan BG | Schizophyllum commune | Enhance the immune level of shrimp | [88] | |
| FIV | Crude extract | Ethanol extract | Inonotus obliquus | Vitro FIV reverse transcriptase inhibitory effect | [89] | |
| Crude extract | Commercially available compound HELP-TH1 | Ganoderma lucidum, Cordyceps sinensis, Trametes versicolor | Vitro | [90] | ||
| DWV LSV | Crude extract | Fomes fomentarius, Ganoderma applanatum | Vivo | [91] | ||
| NNV | Protein | Recombined protein rLZ-8 | Ganoderma lucidum | Vivo activate the immune defense | [92] | |
| PCV-2 | Polysaccharide | PS (D-glucose, D-xylose, D-galactose, L-fucose, D-mannose, and L-rhamnose at a molar ratio of 5.35:2.67:1:1.19:0.38:0.37) | Ganoderma lucidum | Vaccine adjuvant | [93] | |
| PRRSV | CM-H-L-5 | Cryptoporus volvatus | Vitro | [94] | ||
| Ergosterol | 5α,8α-epidioxy-22E-ergosta6,22-dien-3β-ol | Cryptoporus volvatus | Vitro | [95] | ||
| Crude extract | Water extract | Cryptoporus volvatus | Vitro and vivo inhibited the entry of PRRSV and the synthesis of PRRSV RNA | [96] | ||
| PDCoV | Triterpene | Ergosterol peroxide EP | Cryptoporus volvatus | Vitro and vivo activate p38/MAPK and NF- κB signal pathways | [97,98] | |
| BoHV-1 | Crude extract polysaccharide | LeP(β-D-glucan) aqueous (AqE) and ethanol (EtOHE) extracts | Lentinus edodes | Vitro | [80] | |
| NDV | Crude extract | Methanol and n-butanol fractions | Ganoderma lucidum | Vitro antineuraminidase activity | [99] | |
| Polysaccharide | GLP | Ganoderma lucidum | Vaccine adjuvant | [100] | ||
| Polysaccharide | AAP and sulfated polysaccharide sAAP | Auricularia auricula | Vitro | [101] | ||
| Polysaccharide | CMP40, CMP50 | Cordyceps militaris | Vaccine adjuvant | [102] | ||
| Plant viruses | TMV | Steroid | Leiwansterols A, B | Omphalia lapidescens | Vitro | [103] |
| Polysaccharide | BAS-F acidic polysaccharide contain carbon and hydrogen | Fomes fomentarius | Completely inhibit leaf infection and no toxic effect | [104] | ||
| Polysaccharide | LNT β-(1→3)-linked backbone of d-glucose residues, two β-(1→6)-d-glucosyl residues are attached for everyfive main-chain d-glucose residues | Lentinus edodes | Vitro affects the affinity of TMV coat protein to host and activate some defense genes | [105] | ||
| GBNV | Crude extract | Mixed culture filtrate | Coprinopsis cinerea, Ganoderma lucidum, Lentinula edodes | Vitro | [106] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, G.; Ling, J. Medicinal Fungi with Antiviral Effect. Molecules 2022, 27, 4457. https://doi.org/10.3390/molecules27144457
Zhang Y, Zhang G, Ling J. Medicinal Fungi with Antiviral Effect. Molecules. 2022; 27(14):4457. https://doi.org/10.3390/molecules27144457
Chicago/Turabian StyleZhang, Yu, Guoying Zhang, and Jianya Ling. 2022. "Medicinal Fungi with Antiviral Effect" Molecules 27, no. 14: 4457. https://doi.org/10.3390/molecules27144457
APA StyleZhang, Y., Zhang, G., & Ling, J. (2022). Medicinal Fungi with Antiviral Effect. Molecules, 27(14), 4457. https://doi.org/10.3390/molecules27144457






