Lipid Peroxidation in Algae Oil: Antagonist Effects of Natural Antioxidants
Abstract
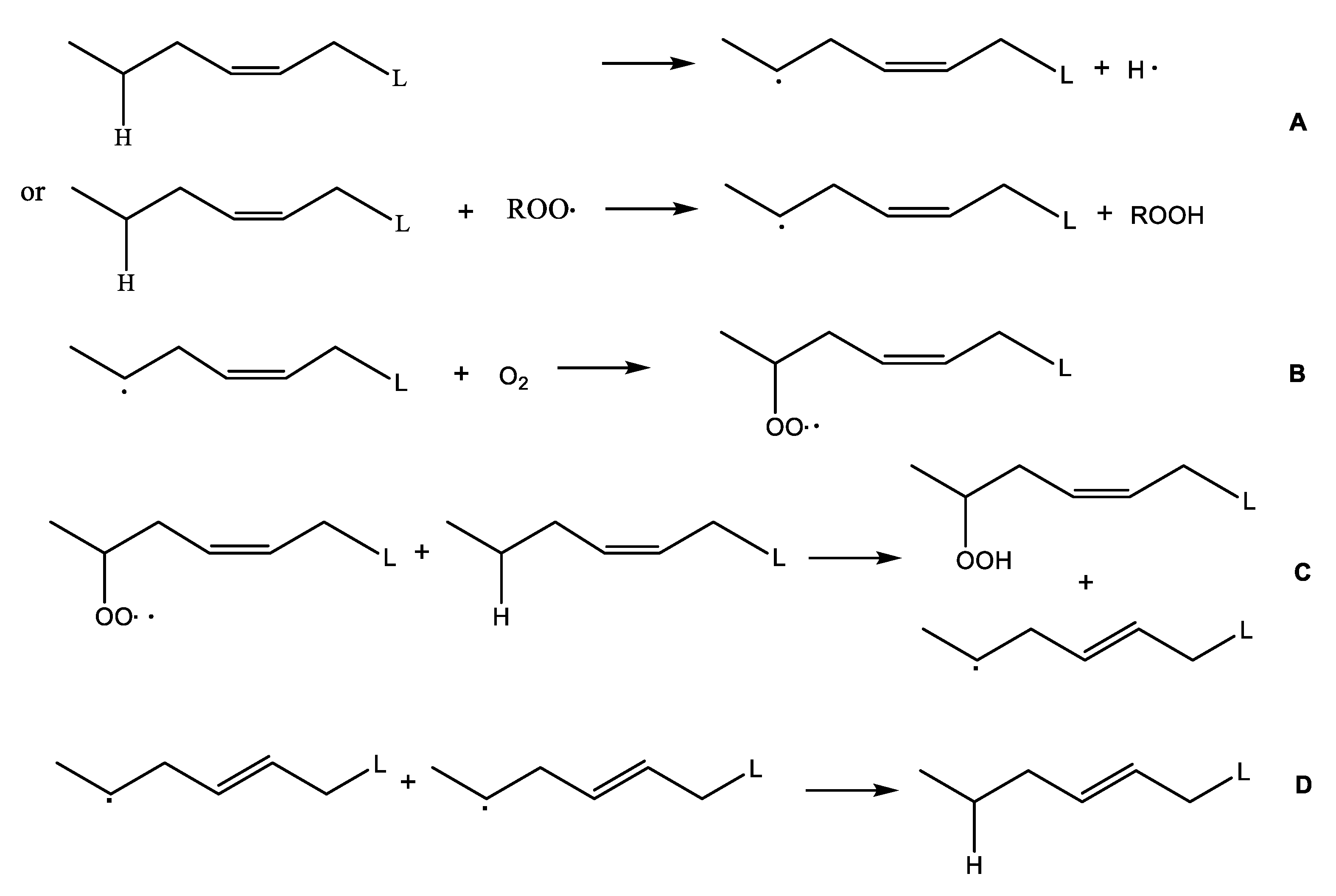
:1. Introduction
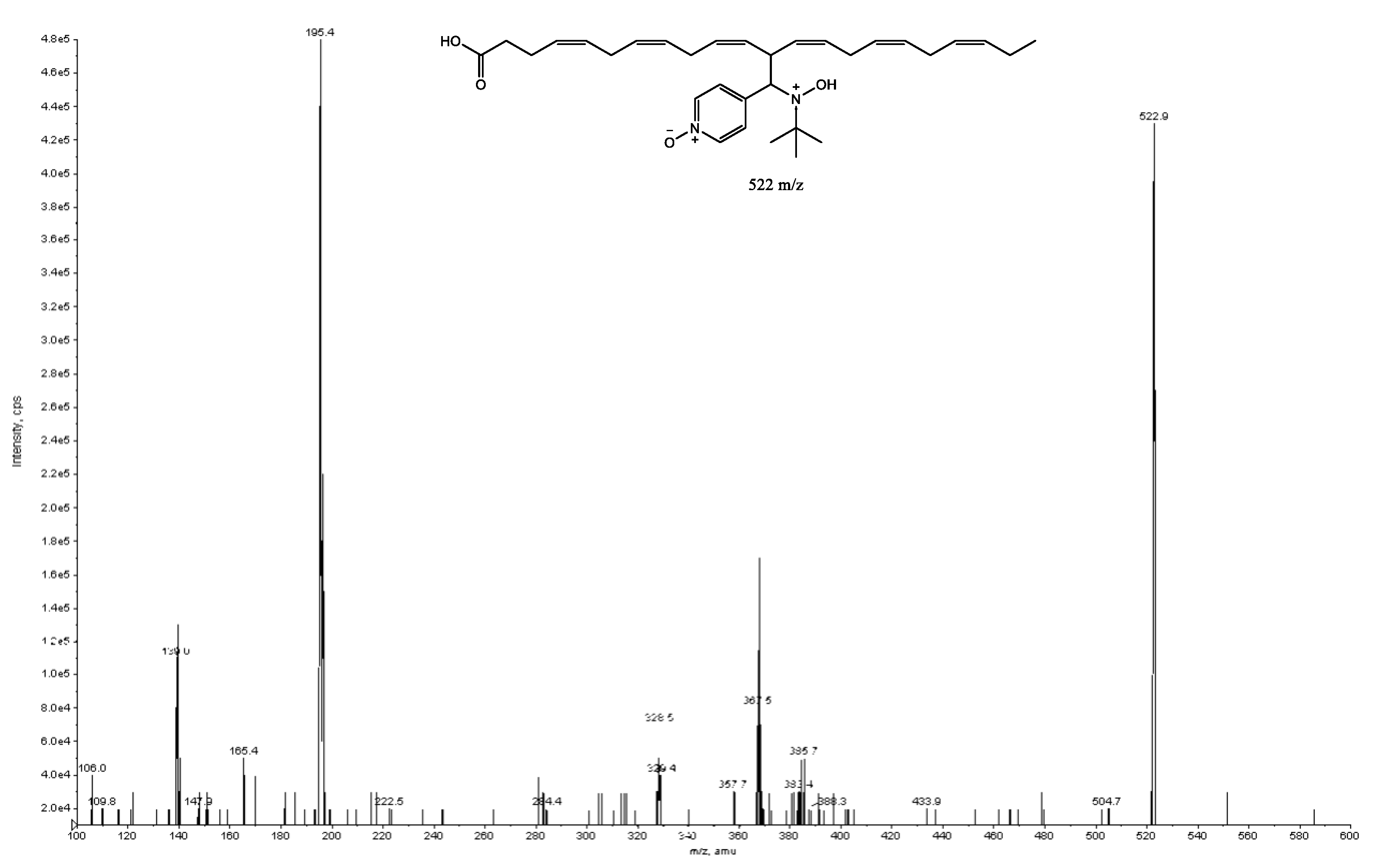
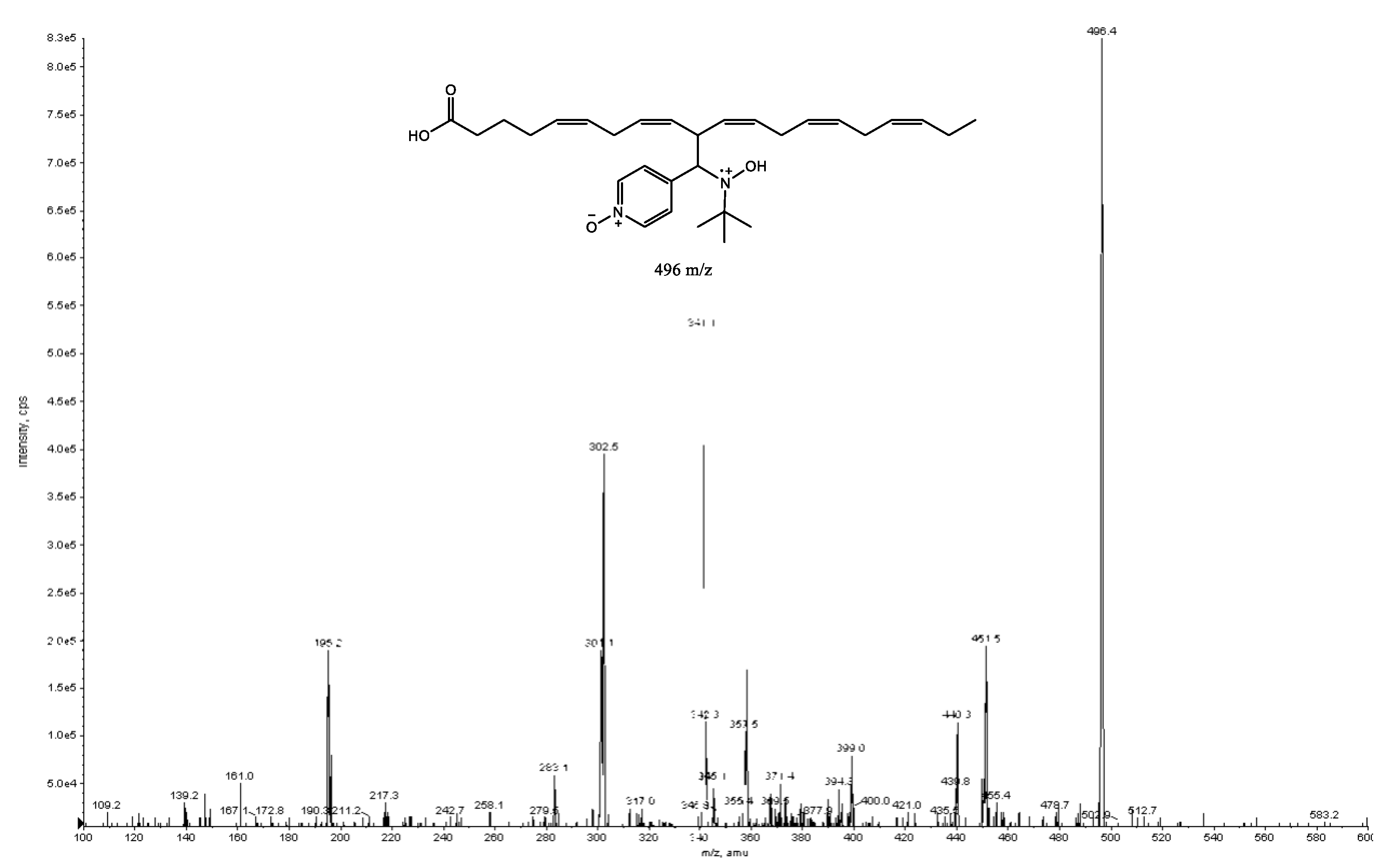
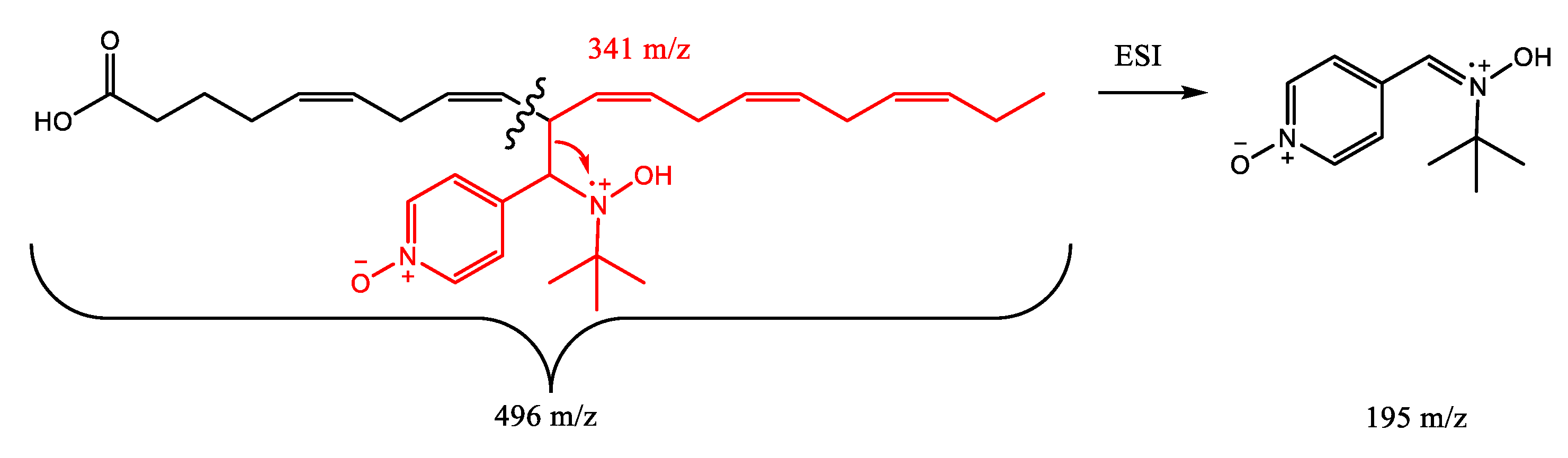
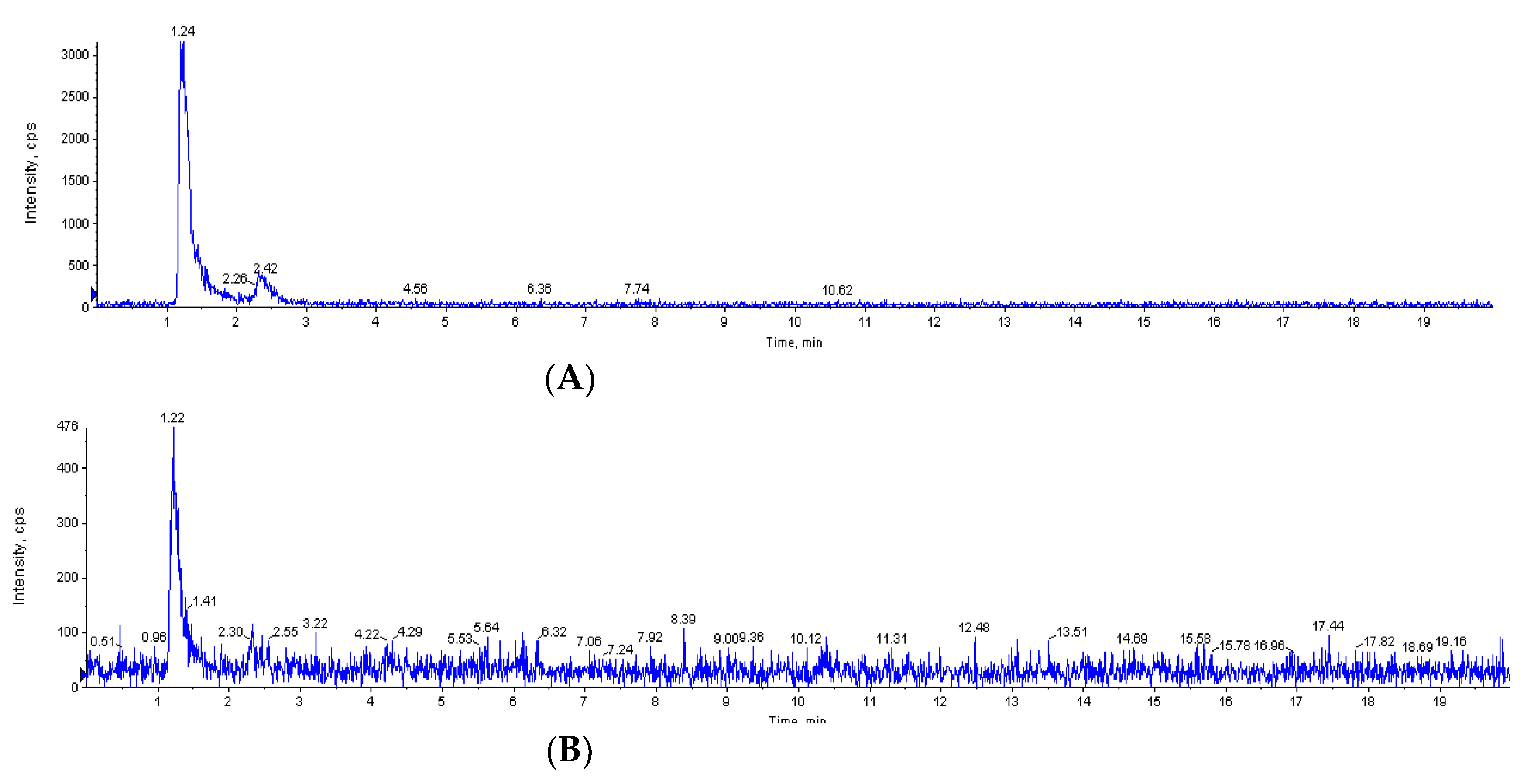
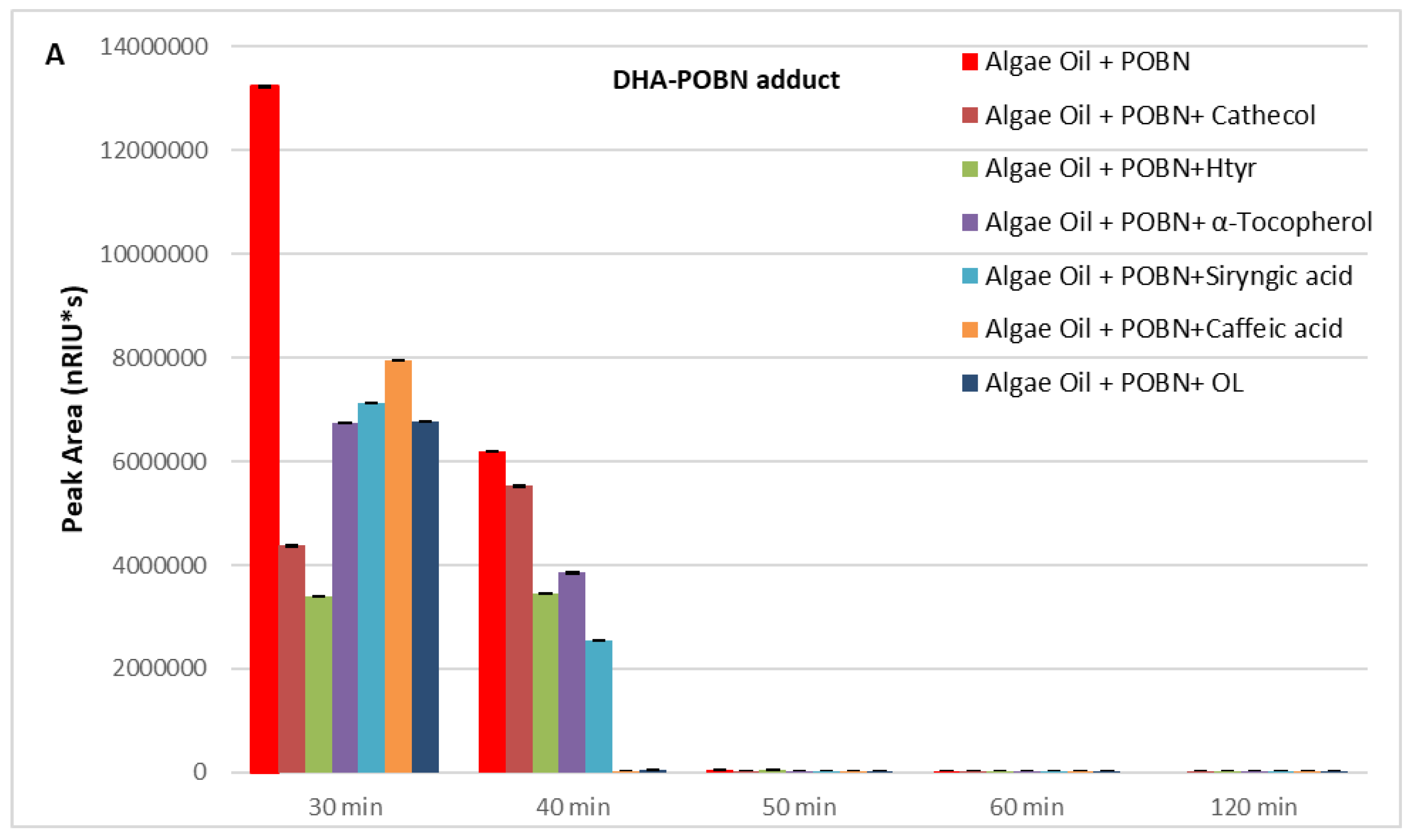
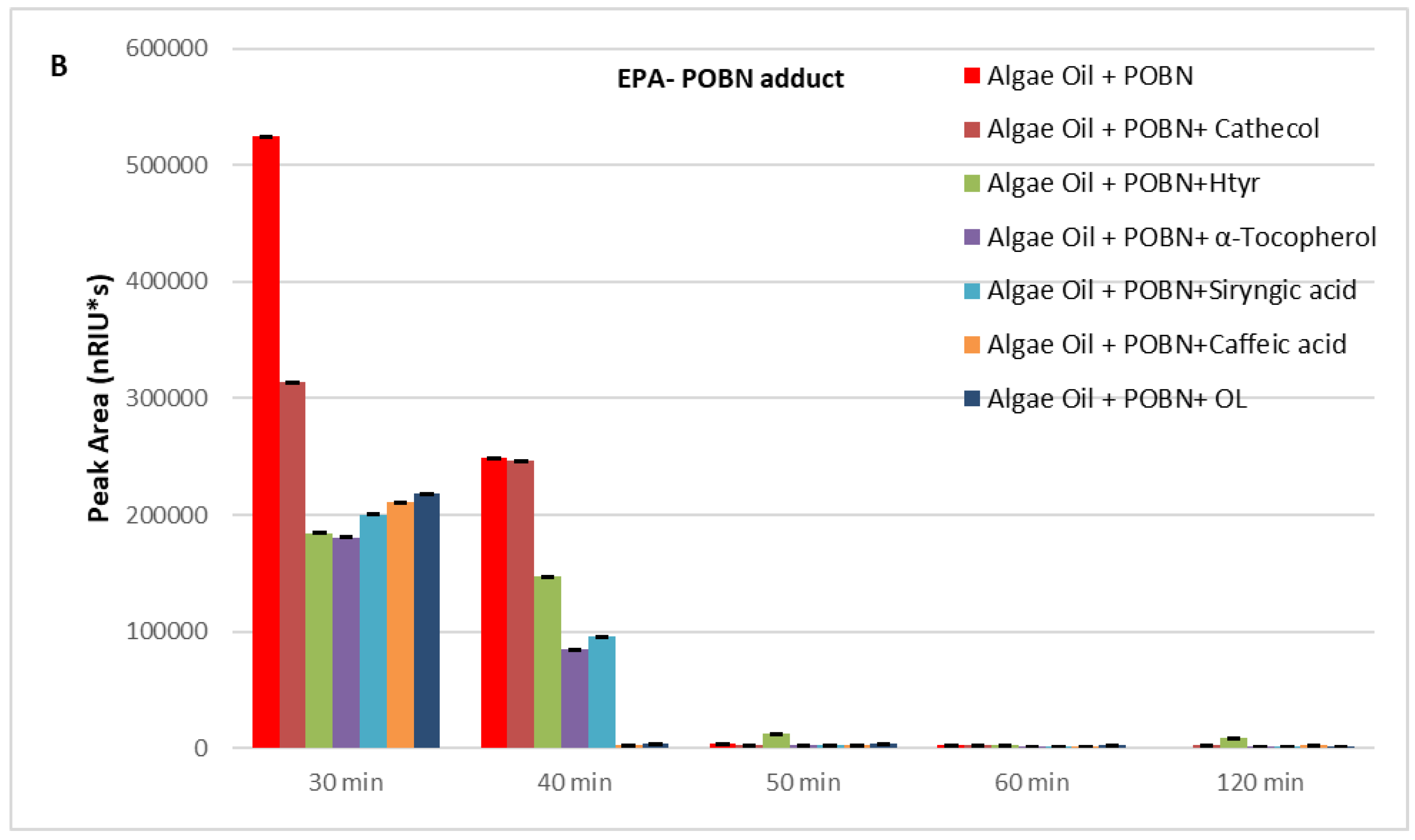
2. Results
3. Materials and Methods
3.1. Material and Reagents
3.2. Algal oil Extraction
3.3. Samples Preparation
3.4. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Calder, P.C. Immunomodulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Proc. Nutr. Soc. 1996, 55, 737–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanasundara, U.N.; Shahidi, F. Concentration of omega 3-polyunsaturated fatty acids of seal blubber oil by urea complexation: Optimization of reaction conditions. Food Chem. 1999, 65, 41–49. [Google Scholar] [CrossRef]
- Senanayake, S.P.J.N.; Shaihid, F. Concentration of docosahexaenoic acid (dha) from algal oil via urea complexation. J. Food Lipids 2000, 7, 51–61. [Google Scholar] [CrossRef]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 3, CD000376. [Google Scholar] [CrossRef]
- Flock, M.R.; Harris, W.S.; Kris-Etherton, P.M. Long-chain omega-3 fatty acids: Time to establish a dietary reference intake. Nutr. Rev. 2013, 71, 692–707. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [Green Version]
- Becker, C.C.; Claus, C.; Kyle, D.J. Developing functional foods containing algal docosahexaenoic acid. Food Technol. 1998, 52, 68–71. [Google Scholar]
- Frankel, E.N.; Satueä-Gracia, T.; Meyer, A.S.; German, J.B. Oxidative Stability of Fish and Algae Oils Containing Long-Chain Polyunsaturated Fatty Acids in Bulk and in Oil-in-Water Emulsions. J. Agric. Food Chem. 2002, 50, 2094–2099. [Google Scholar] [CrossRef]
- Frankel, E.N. In search of better methods to evaluate natural antioxidants and oxidative stability in food lipids. Trends Food Sci. Technol. 1993, 4, 220–225. [Google Scholar] [CrossRef]
- Kontush, A.; Finckh, B.; Karten, B.; Kohlschütter, A.; Beisiegel, U. Antioxidant and prooxidant activity of alpha-tocopherol in human plasma and low density lipoprotein. J. Lipid Res. 1996, 37, 1436–1448. [Google Scholar] [CrossRef]
- Carroll, K.K. Biological effects of fish oils in relation to chronic diseases. Lipids 1986, 21, 731–732. [Google Scholar] [CrossRef]
- Kinsella, J.E. Food components with potential therapeutic benefits: The n-3 polyunsaturated fatty acids of fish oils. Food Technol. 1986, 40, 89–97. [Google Scholar]
- Kinsella, J.E. Seafoods and Fish Oils in Human Health and Disease; M. Dekker: New York, NY, USA, 1987. [Google Scholar]
- Harris, W.S. Fish oils and plasma lipid and lipoprotein metabolism in humans: A critical review. J. Lipid Res. 1989, 30, 785–807. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef]
- Moffat, C.F. Fish oil triglycerides: A wealth of variation. Lipid Technol. 1995, 7, 125–129. [Google Scholar]
- Shimizu, M.; Ikegami, T.; Akiyama, K.; Morita, E.H. A Novel Way to Express Proline-Selectively Labeled Proteins with a Wheat Germ Cell–Free Protein Synthesis System. J. Biochem. 2006, 140, 453–456. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Russo, A.; Caputo, S.; Pantusa, M.; Perri, E.; Sindona, G.; Sportelli, L. Amino acids as modulators of lipoxygenase oxidation mechanism. The identification and structural characterization of spin adducts intermediates by electron spin resonance and tandem mass spectrometry. Food Chem. 2010, 119, 533–538. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Yi, O.S.; Shin, H.K. Solubilization of vitamin C in fish oil and synergistic effect with vitamin E in retarding oxidation. J. Am. Oil Chem. Soc. 1991, 68, 740–743. [Google Scholar] [CrossRef]
- Boyd, L.C.; King, M.F.; Sheldon, B. A rapid method for determining the oxidation of n-3 fatty acids. J. Am. Oil Chem. Soc. 1992, 69, 325–330. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Kalu, C.; McNeill, G.P.; Padley, F.B.; Pierce, J.H. Effects of tocopherols, ascorbyl palmitate, and lecithin on autoxidation of fish oil. J. Am. Oil Chem. Soc. 1998, 75, 813–822. [Google Scholar] [CrossRef]
- Kulås, E.; Ackman, R.G. Properties of α-, γ-, and δ-tocopherol in purified fish oil triacylglycerols. J. Am. Oil Chem. Soc. 2001, 78, 361–367. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK–FOXO3a pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef]
- Iwamura, M.; Inamoto, N. Novel formation of nitroxide radicals by radical addition to nitrones. Bull. Chem. Soc. Jpn. 1967, 40, 703. [Google Scholar] [CrossRef] [Green Version]
- Janzen, E.G.; Blackburn, B.J. Detection and identification of short-lived free radicals by electron spin resonance trapping techniques (spin trapping). Photolysis of organolead,-tin, and-mercury compounds. J. Am. Chem. Soc. 1969, 91, 4481–4490. [Google Scholar] [CrossRef]
- Mottley, C.; Mason, R.P. Nitroxide radical adducts in biology: Chemistry, applications, and pitfalls. In Spin Labeling; Springer: Boston, MA, USA, 1989; pp. 489–546. [Google Scholar]
- Rosselin, M.; Poeggeler, B.; Durand, G. Nitrone Derivatives as Therapeutics: From Chemical Modification to Specific-Targeting. Curr. Top. Med. Chem. 2017, 17, 2006–2022. [Google Scholar] [CrossRef]
- Mazziotti, A.; Mazzotti, F.; Pantusa, M.; Sportelli, L.; Sindona, G. Pro-oxidant activity of oleuropein determined in vitro by electron spin resonance spin-trapping methodology. J. Agric. Food Chem. 2006, 54, 7444–7449. [Google Scholar] [CrossRef]
- Perri, E.; Mazzotti, F.; Raffaelli, A.; Sindona, G. High-throughput screening of tocopherols in natural extracts. J. Mass Spectrom. 2000, 35, 1360–1361. [Google Scholar] [CrossRef]
- Mazzotti, F.; Di Donna, L.; Taverna, D.; Nardi, M.; Aiello, D.; Napoli, A.; Sindona, G. Evaluation of dialdehydic anti-inflammatory active principles in extra-virgin olive oil by reactive paper spray mass spectrometry. Int. J. Mass Spectrom. 2013, 352, 87–91. [Google Scholar] [CrossRef]
- Di Donna, L.; Benabdelkamel, H.; Mazzotti, F.; Napoli, A.; Nardi, M.; Sindona, G. High-throughput assay of oleopentanedialdheydes in extra virgin olive oil by the UHPLC− ESI-MS/MS and isotope dilution methods. Anal. Chem. 2011, 83, 1990–1995. [Google Scholar] [CrossRef]
- Procopio, A.; Alcaro, S.; Nardi, M.; Oliverio, M.; Ortuso, F.; Sacchetta, P.; Pieragostino, D.; Sindona, G. Synthesis, biological evaluation, and molecular modeling of oleuropein and its semisynthetic derivatives as cyclooxygenase inhibitors. J. Agric. Food Chem. 2009, 57, 11161–11167. [Google Scholar] [CrossRef]
- Procopio, A.; Celia, C.; Nardi, M.; Oliverio, M.; Paolino, D.; Sindona, G. Lipophilic hydroxytyrosol esters: Fatty acid conjugates for potential topical administration Procopio, A. J. Nat. Prod. 2011, 74, 2377–2381. [Google Scholar] [CrossRef]
- Sindona, G.; Caruso, A.; Cozza, A.; Fiorentini, S.; Lorusso, B.; Marini, E.; Nardi, M.; Procopio, A.; Zicari, S. Anti-inflammatory effect of 3,4-DHPEA-EDA [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl) hex-4-enoate] on primary human vascular endothelial cells. Curr. Med. Chem. 2012, 19, 4006–4013. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; De Luca, G.; Maiuolo, J.; Oliverio, M.; Sindona, G.; Procopio, A. Biomimetic synthesis and antioxidant evaluation of 3,4-DHPEA-EDA [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate]. Food Chem. 2014, 162, 89–93. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives. Food Funct. 2017, 8, 4684–4692. [Google Scholar] [CrossRef]
- Juli, G.; Oliverio, M.; Bellizzi, D.; Cantafio, M.E.G.; Grillone, K.; Passarino, G.; Colica, C.; Nardi, M.; Rossi, M.; Procopio, A.; et al. Anti-tumor activity and epigenetic impact of the polyphenol oleacein in multiple myeloma. Cancers 2019, 11, 990. [Google Scholar] [CrossRef] [Green Version]
- Benincasa, C.; Santoro, I.; Nardi, M.; Cassano, A.; Sindona, G. Eco-friendly extraction and characterisation of nutraceuticals from olive leaves. Molecules 2019, 24, 3481. [Google Scholar] [CrossRef] [Green Version]
- Bonacci, S.; Di Gioia, M.L.; Costanzo, P.; Maiuolo, L.; Tallarico, S.; Nardi, M. Natural Deep Eutectic Solvent as Extraction Media for the Main Phenolic Compounds from Olive Oil Processing Wastes. Antioxidants 2020, 9, 513. [Google Scholar] [CrossRef]
- Topare, N.S.; Raut, S.J.; Renge, V.C.; Khedkar, S.V.; Chavanand, Y.P.; Bhagat, S.L. Extraction of oil from algae by solvent extraction and oil expeller method. Int. J. Chem. Sci. 2011, 9, 1746–1750. [Google Scholar]
- Santoro, I.; Nardi, M.; Benincasa, C.; Costanzo, P.; Giordano, G.; Procopio, A.; Sindona, G. Sustainable and selective extraction of lipids and bioactive compounds from microalgae. Molecules 2019, 24, 4347. [Google Scholar] [CrossRef] [Green Version]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef]
- Bettina, P.; Mihalas, B.P.; De Iuliis, N.J.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Sci. Rep. 2017, 7, 6247. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, I.; Russo, A.; Perri, E.; Sindona, G.; Nardi, M. Lipid Peroxidation in Algae Oil: Antagonist Effects of Natural Antioxidants. Molecules 2022, 27, 4453. https://doi.org/10.3390/molecules27144453
Santoro I, Russo A, Perri E, Sindona G, Nardi M. Lipid Peroxidation in Algae Oil: Antagonist Effects of Natural Antioxidants. Molecules. 2022; 27(14):4453. https://doi.org/10.3390/molecules27144453
Chicago/Turabian StyleSantoro, Ilaria, Anna Russo, Enzo Perri, Giovanni Sindona, and Monica Nardi. 2022. "Lipid Peroxidation in Algae Oil: Antagonist Effects of Natural Antioxidants" Molecules 27, no. 14: 4453. https://doi.org/10.3390/molecules27144453
APA StyleSantoro, I., Russo, A., Perri, E., Sindona, G., & Nardi, M. (2022). Lipid Peroxidation in Algae Oil: Antagonist Effects of Natural Antioxidants. Molecules, 27(14), 4453. https://doi.org/10.3390/molecules27144453








