Insights on the Modulation of SIRT5 Activity: A Challenging Balance
Abstract
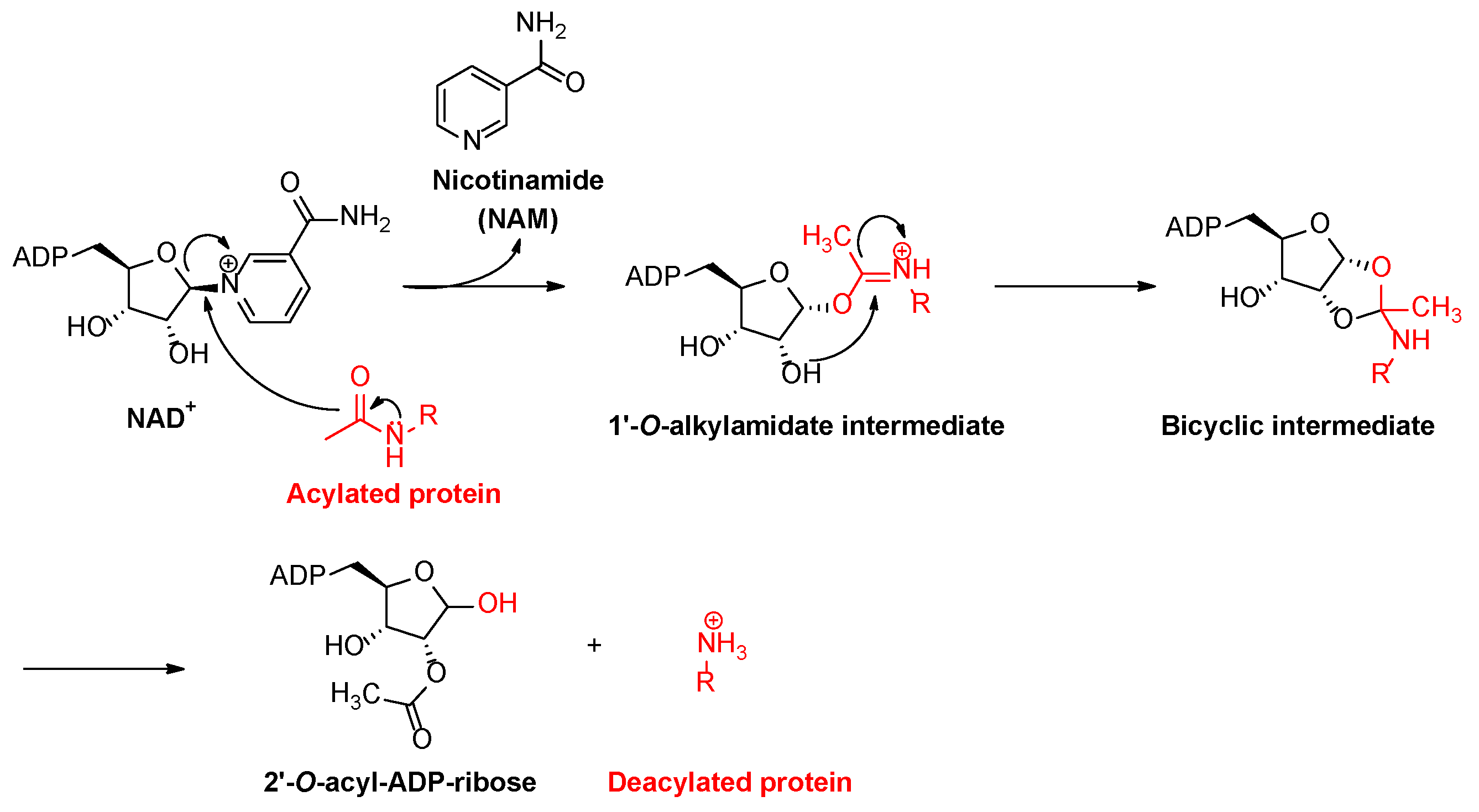
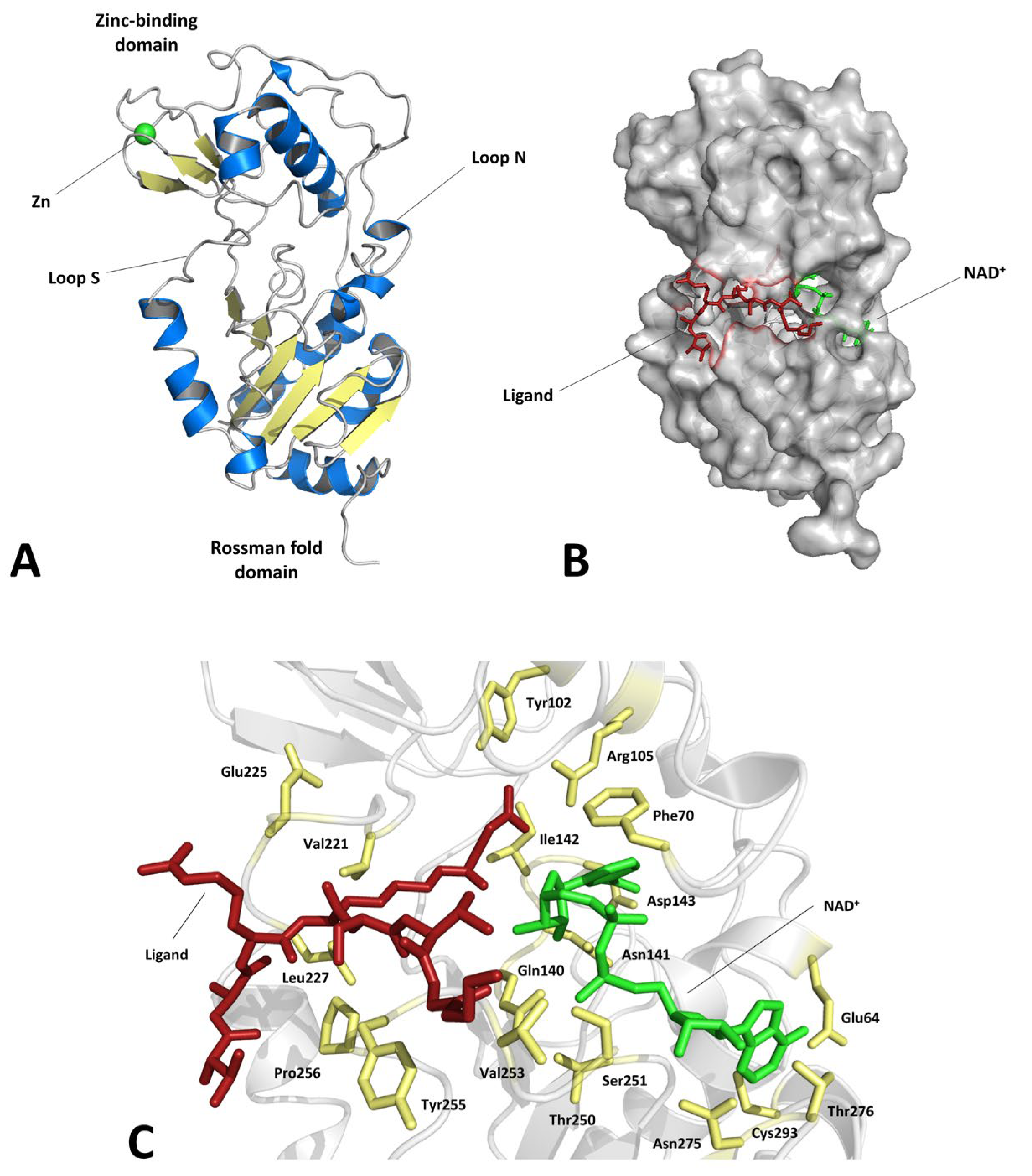
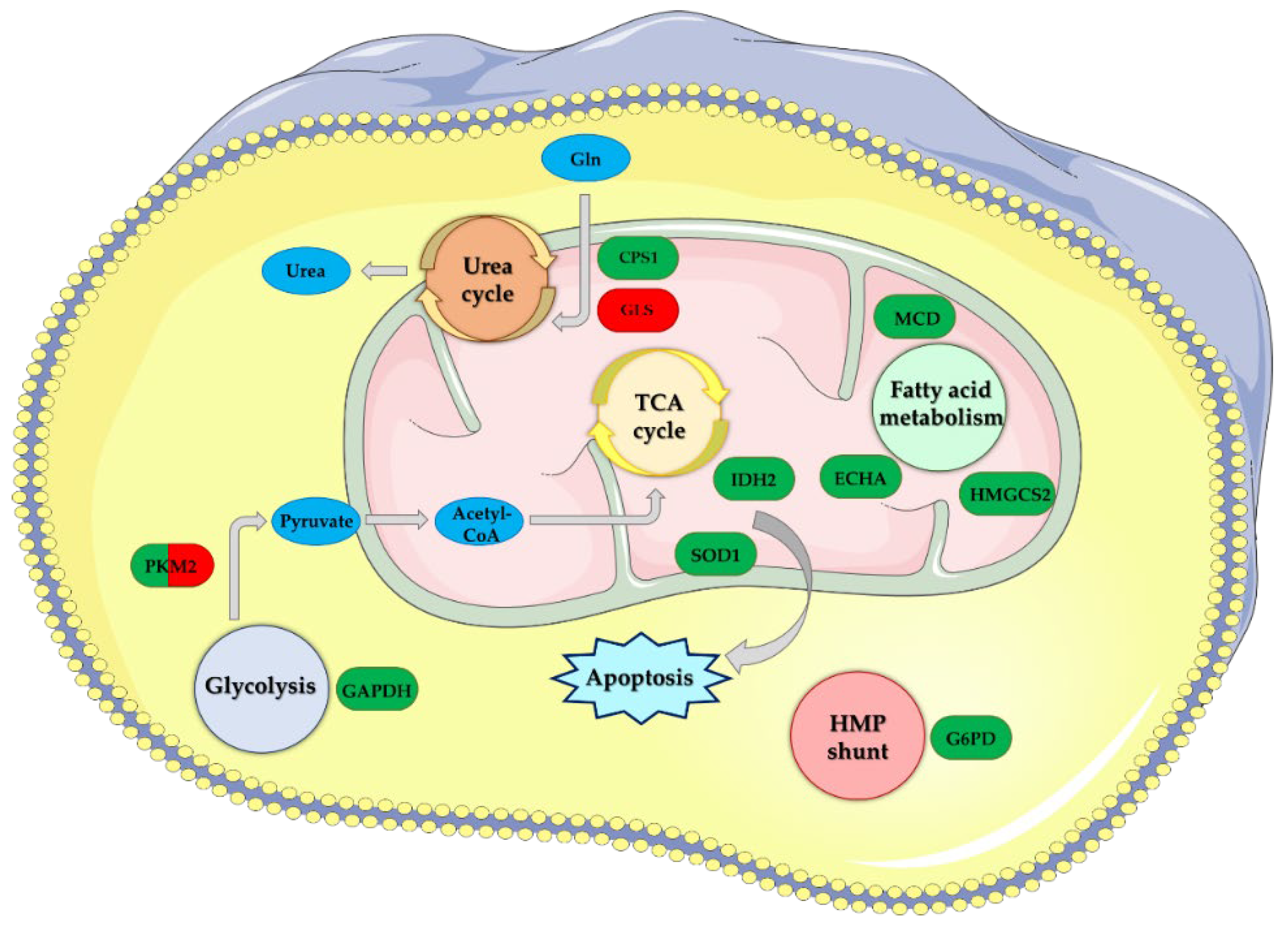
1. Introduction
2. SIRT5 Modulators
2.1. Activators
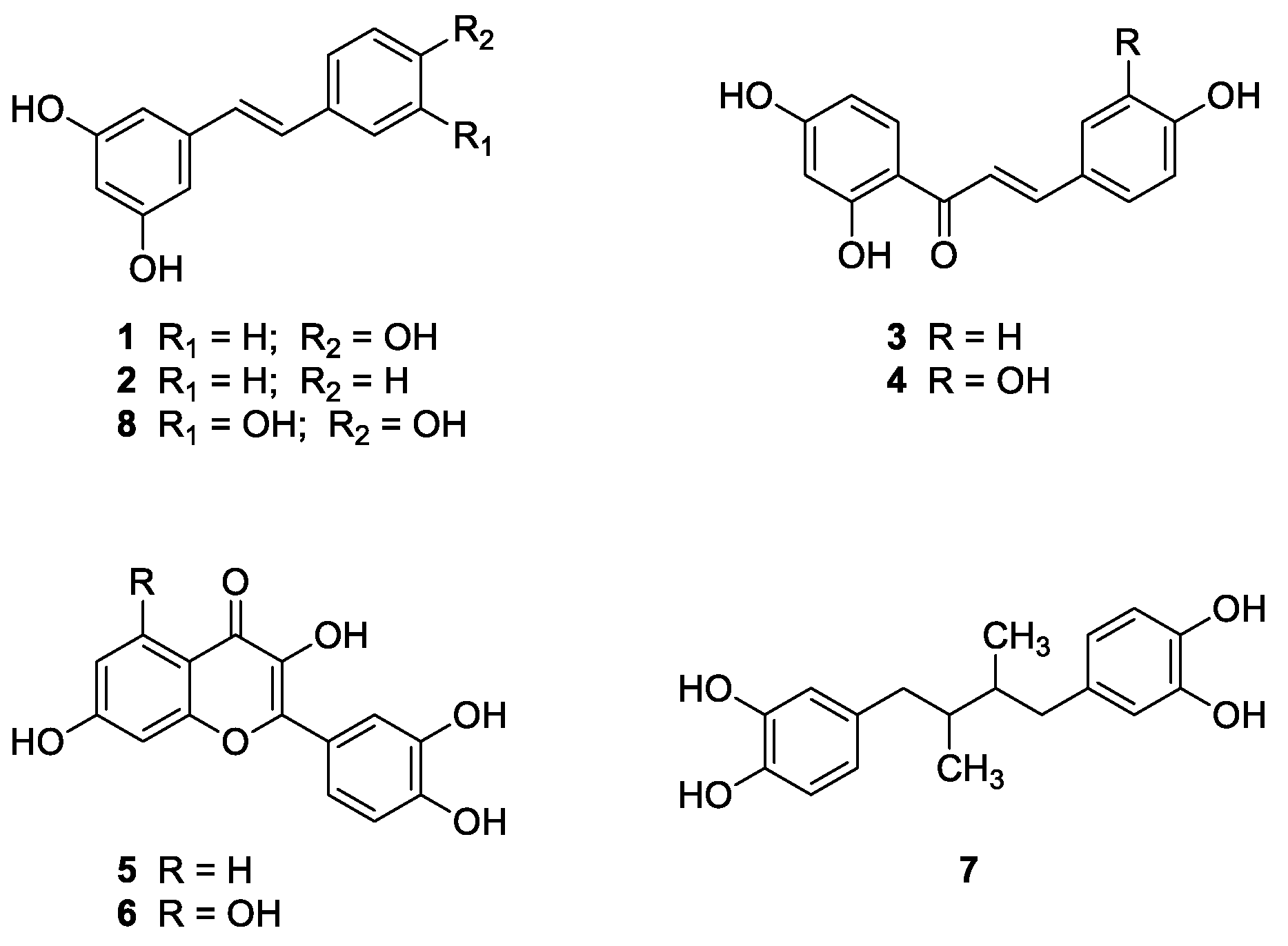
2.1.1. Natural Compounds
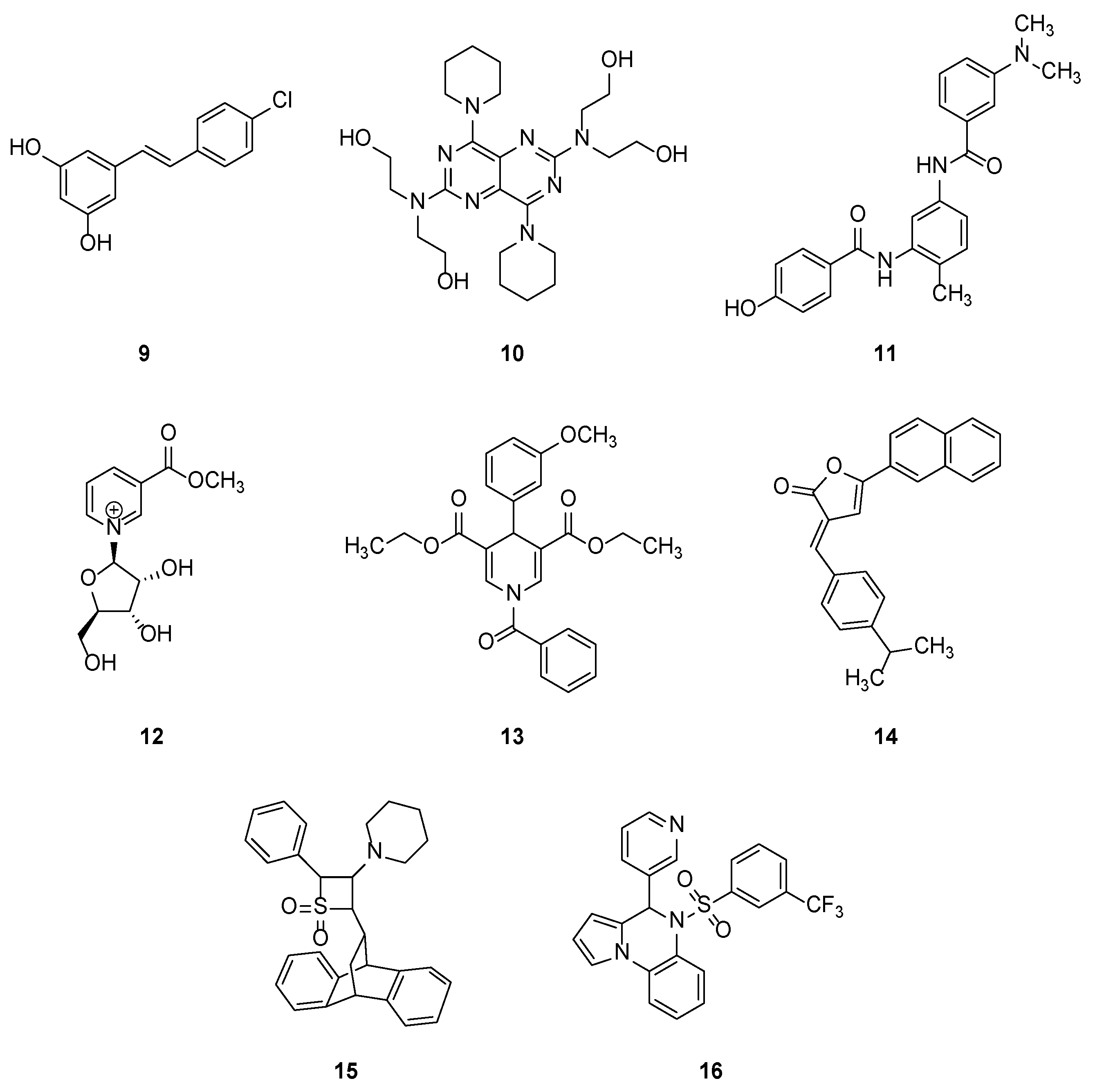
2.1.2. Synthetic Compounds
2.2. Inhibitors
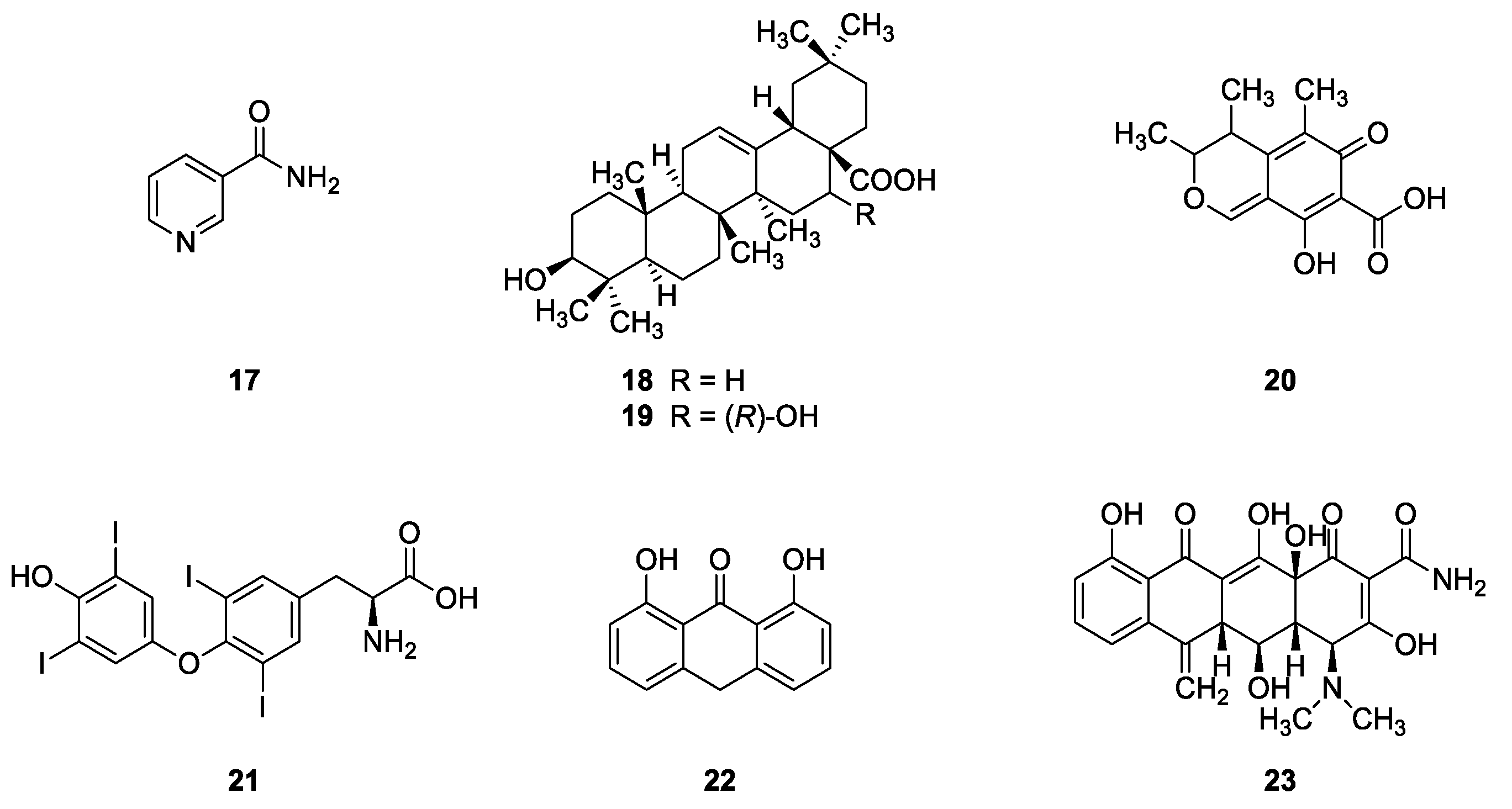
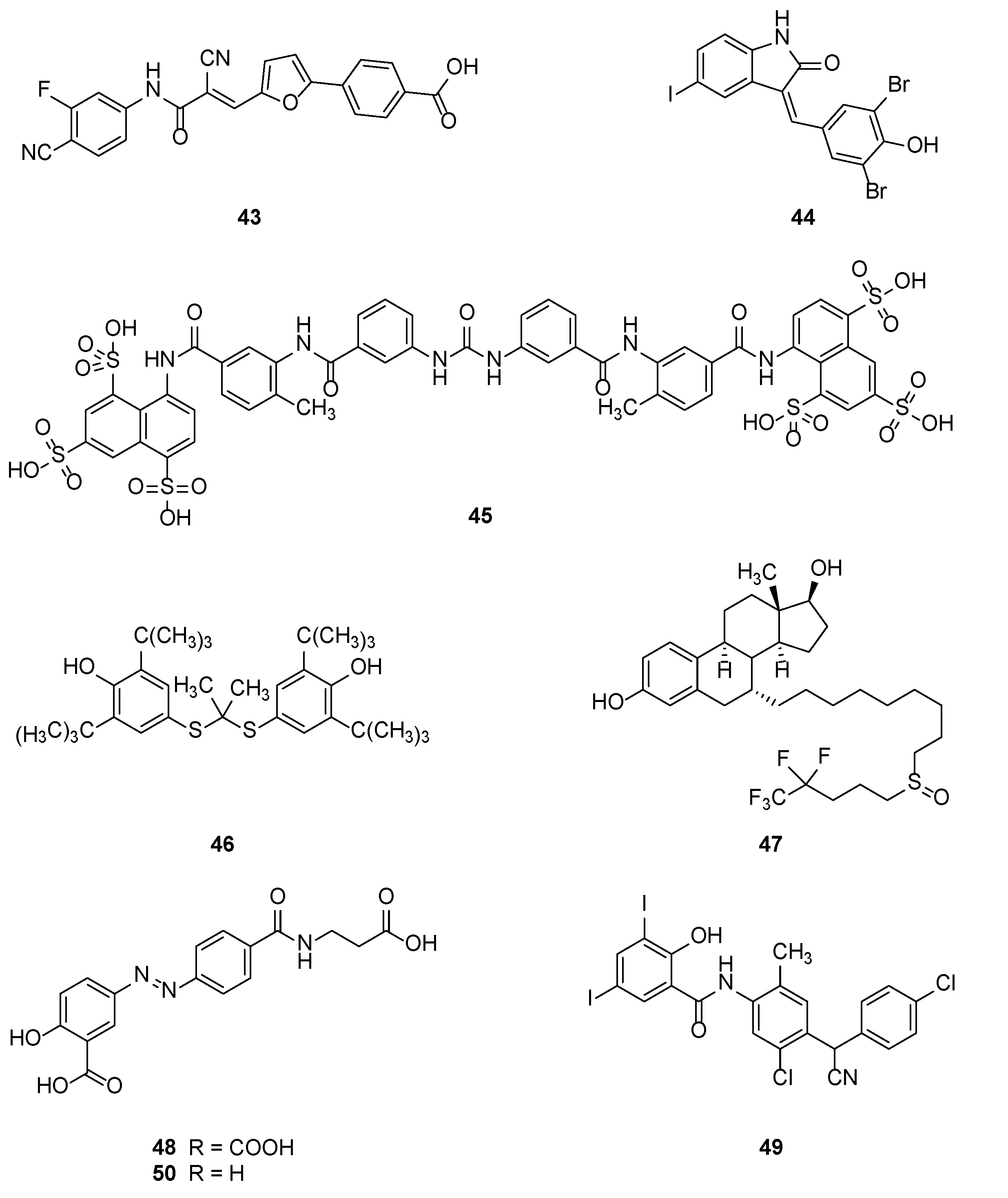
2.2.1. Natural Compounds
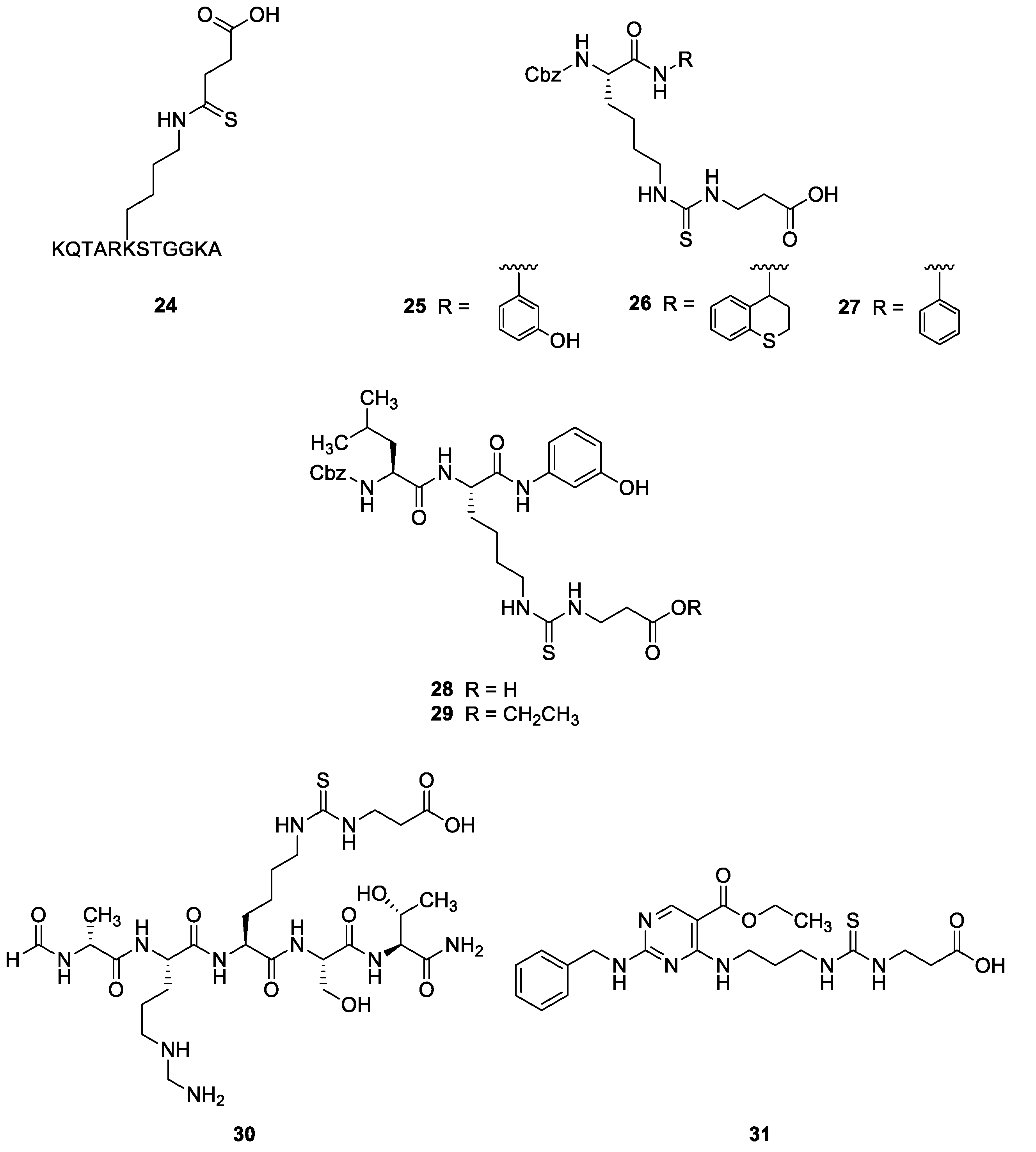
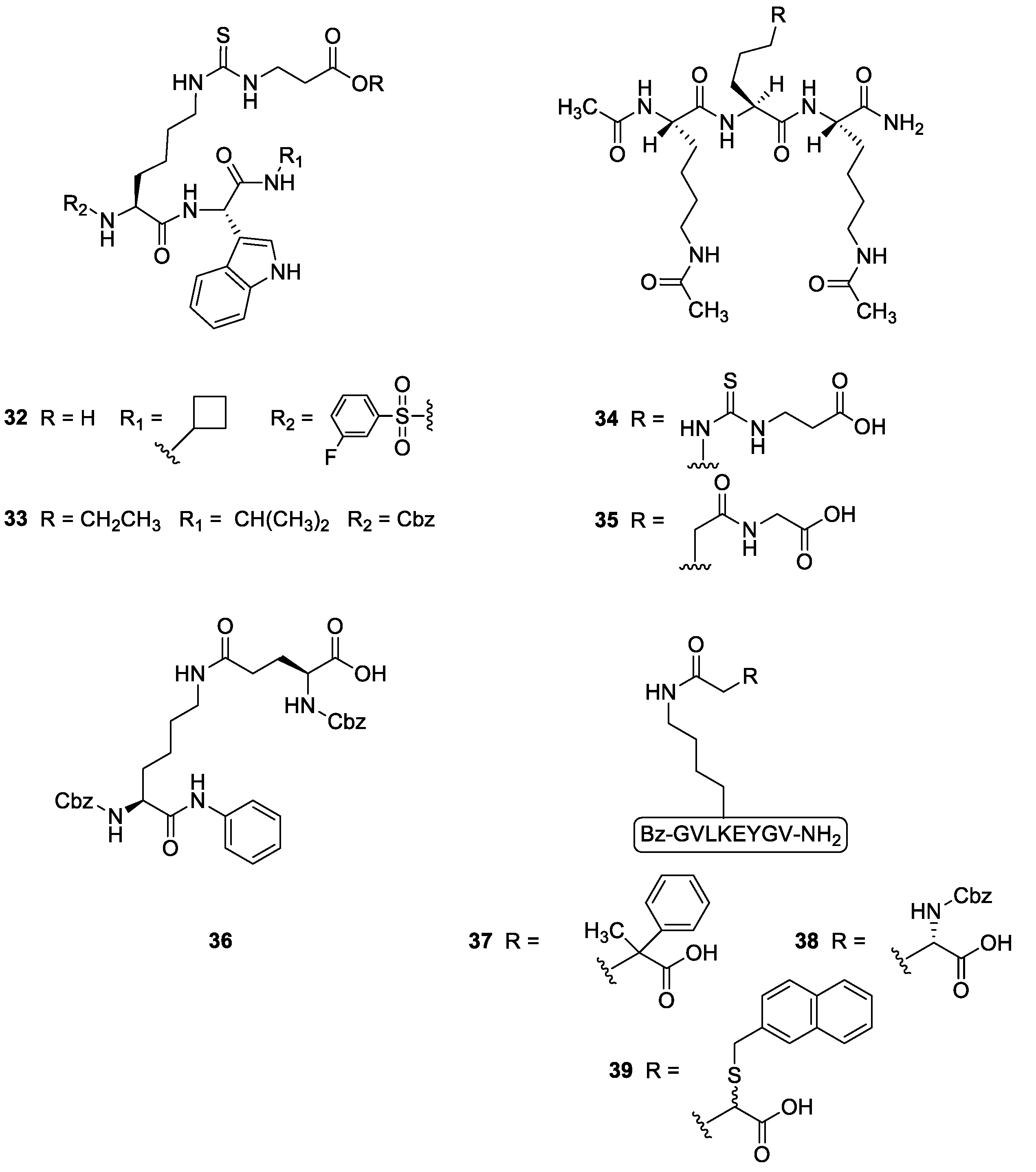
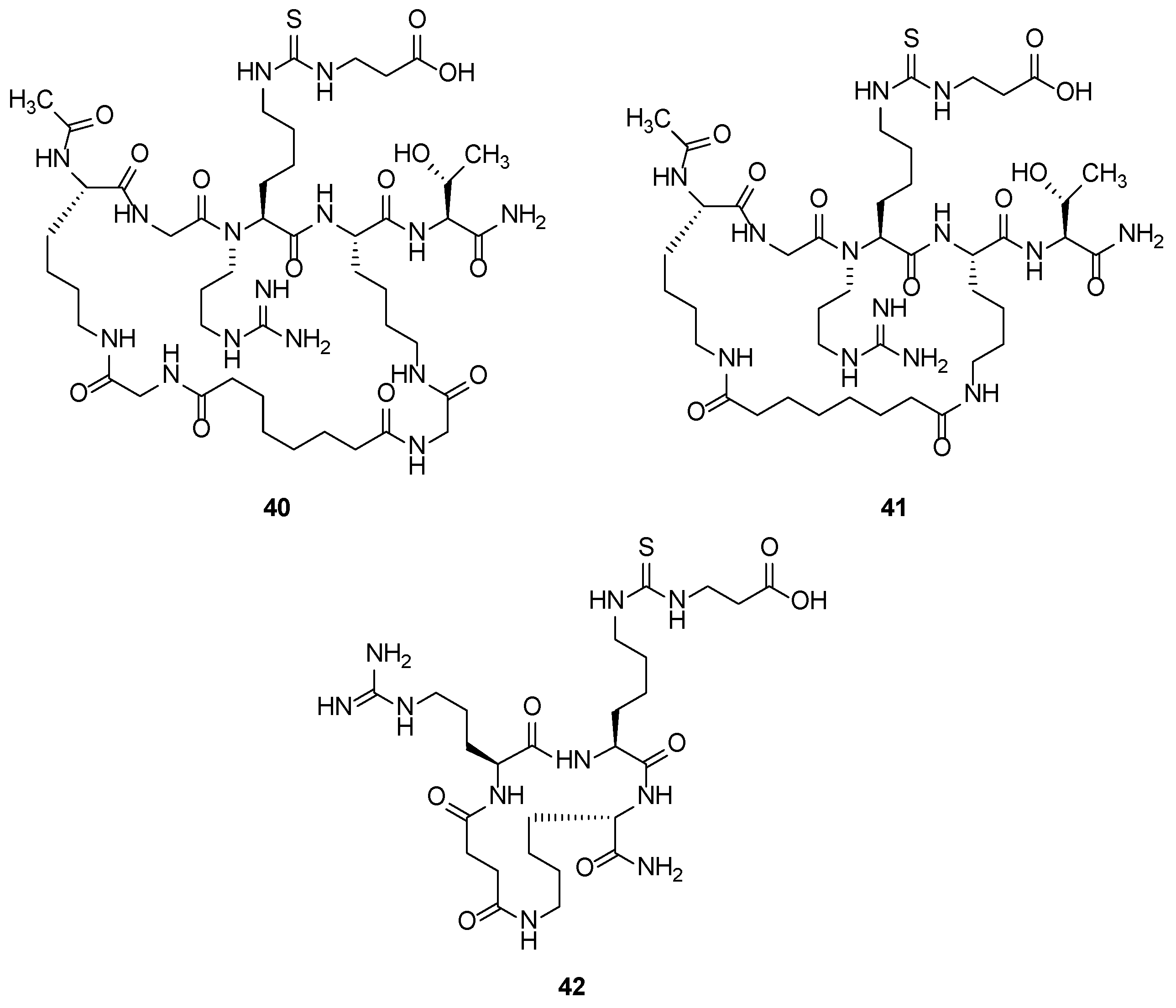
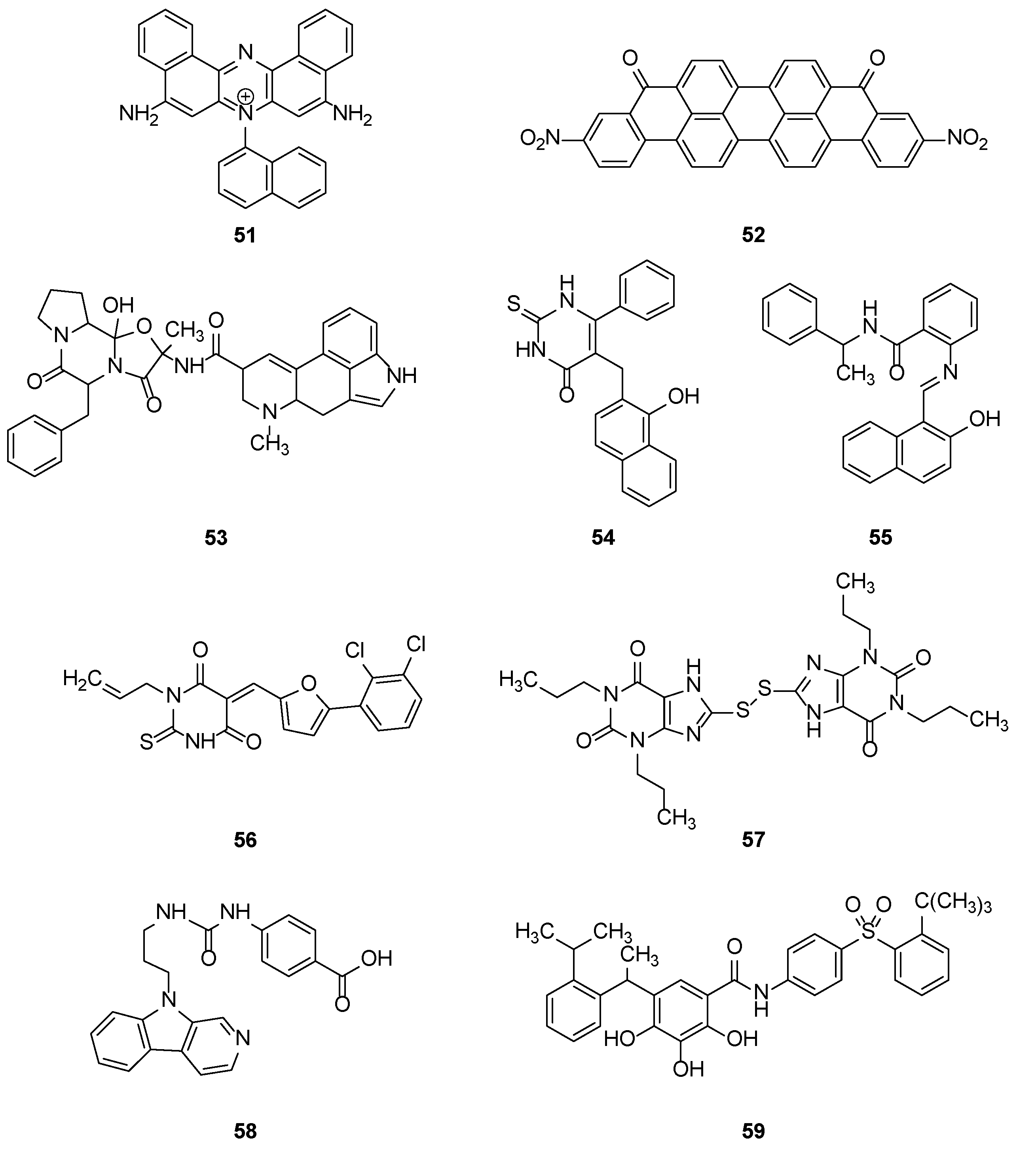
2.2.2. Synthetic Compounds
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Ma, X.; He, Y.; Yuan, C.; Chen, Q.; Li, G.; Chen, X. Sirtuin 5: A review of structure, known inhibitors and clues for developing new inhibitors. Sci. China Life Sci. 2016, 60, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.-I.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zha, X. Overview of SIRT5 as a potential therapeutic target: Structure, function and inhibitors. Eur. J. Med. Chem. 2022, 236, 114363. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.; Chen, W.; Wu, M.; Zhan, L.; Wang, C.; Jia, N.; Zhang, X.; Zang, J. Structural insights into the molecular mechanism underlying Sirt5-catalyzed desuccinylation of histone peptides. Biochem. J. 2019, 476, 211–223. [Google Scholar] [CrossRef]
- Bringman-Rodenbarger, L.R.; Guo, A.H.; Lyssiotis, C.A.; Lombard, D.B. Emerging Roles for SIRT5 in Metabolism and Cancer. Antioxid. Redox Signal. 2018, 28, 677–690. [Google Scholar] [CrossRef]
- Kumar, S.; Lombard, D.B. Functions of the sirtuin deacylase SIRT5 in normal physiology and pathobiology. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 311–334. [Google Scholar] [CrossRef]
- Nishida, Y.; Rardin, M.J.; Carrico, C.; He, W.; Sahu, A.K.; Gut, P.; Najjar, R.; Fitch, M.; Hellerstein, M.; Gibson, B.W.; et al. SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol. Cell 2015, 59, 321–332. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Li, X.; Liu, B.; Liu, M.; Liu, L.; Chen, S.; Ren, M.; Wang, Y.; Yu, M.; et al. SHMT2 Desuccinylation by SIRT5 Drives Cancer Cell Proliferation. Cancer Res. 2018, 78, 372–386. [Google Scholar] [CrossRef]
- Xiangyun, Y.; Xiaomin, N.; Linping, G.; Yunhua, X.; Ziming, L.; Yongfeng, Y.; Zhiwei, C.; Shun, L.; Xiangyun, Y.; Xiaomin, N.; et al. Desuccinylation of pyruvate kinase M2 by SIRT5 contributes to antioxidant response and tumor growth. Oncotarget 2016, 8, 6984–6993. [Google Scholar] [CrossRef]
- Colak, G.; Pougovkina, O.; Dai, L.; Tan, M.; Brinke, H.T.; Huang, H.; Cheng, Z.; Park, J.; Wan, X.; Liu, X.; et al. Proteomic and Biochemical Studies of Lysine Malonylation Suggest Its Malonic Aciduria-associated Regulatory Role in Mitochondrial Function and Fatty Acid Oxidation. Mol. Cell. Proteom. 2015, 14, 3056–3071. [Google Scholar] [CrossRef]
- Lin, Z.-F.; Xu, H.-B.; Wang, J.-Y.; Lin, Q.; Ruan, Z.; Liu, F.-B.; Jin, W.; Huang, H.-H.; Chen, X. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem. Biophys. Res. Commun. 2013, 441, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Jiang, X.; Gai, J.; Sun, X.; Zhao, J.; Li, J.; Li, Y.; Cheng, M.; Du, T.; Fu, L.; et al. Sirtuin 5 regulates the proliferation, invasion and migration of prostate cancer cells through acetyl-CoA acetyltransferase 1. J. Cell. Mol. Med. 2020, 24, 14039–14049. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rong, F.; Tang, J.; Zhu, C.; Chen, X.; Jia, S.; Wang, Z.; Sun, X.; Deng, H.; Zha, H.; et al. Repression of p53 function by SIRT5-mediated desuccinylation at Lysine 120 in response to DNA damage. Cell Death Differ. 2021, 29, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Jeon, J.M.; Na, A.Y.; Kwon, O.K.; Bang, I.H.; Ha, Y.-S.; Bae, E.J.; Park, B.-H.; Lee, E.H.; Kwon, T.G.; et al. SIRT5 Directly Inhibits the PI3K/AKT Pathway in Prostate Cancer Cell Lines. Cancer Genom. Proteom. 2021, 19, 50–59. [Google Scholar] [CrossRef]
- Tang, S.-J.; Yang, J.-B. LncRNA SNHG14 aggravates invasion and migration as ceRNA via regulating miR-656-3p/SIRT5 pathway in hepatocellular carcinoma. Mol. Cell. Biochem. 2020, 473, 143–153. [Google Scholar] [CrossRef]
- Shang, B.; Xu, T.; Hu, N.; Mao, Y.; Du, X. Circ-Klhl8 overexpression increased the therapeutic effect of EPCs in diabetic wound healing via the miR-212-3p/SIRT5 axis. J. Diabetes Complicat. 2021, 35, 108020. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospecting 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Panda, S.S.; Jhanji, N. Natural Products as Potential Anti-Alzheimer Agents. Curr. Med. Chem. 2020, 27, 5887–5917. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Mori, M.; Chiarelli, L.R.; Gelain, A.; Meneghetti, F.; Villa, S. Natural products against key Mycobacterium tuberculosis enzymatic targets: Emerging opportunities for drug discovery. Eur. J. Med. Chem. 2021, 224, 113732. [Google Scholar] [CrossRef]
- Aswad, M.; Rayan, M.; Abu-Lafi, S.; Falah, M.; Raiyn, J.; Abdallah, Z.; Rayan, A. Nature is the best source of anti-inflammatory drugs: Indexing natural products for their anti-inflammatory bioactivity. Agents Actions 2017, 67, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Zipkin, R.E. Compositions and Methods for Selectively Activating Human Sirtuins. U.S. Patent Application No. 11/166,892, 19 January 2006. [Google Scholar]
- Wu, S.; Wei, Y.; Li, J.; Bai, Y.; Yin, P.; Wang, S. SIRT5 Represses Neurotrophic Pathways and Aβ Production in Alzheimer’s Disease by Targeting Autophagy. ACS Chem. Neurosci. 2021, 12, 4428–4437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.; Law, B.Y.K.; Dias, I.D.S.R.; Qiu, C.L.; Zeng, W.; Pan, H.D.; Chen, J.Y.; Bai, Y.F.; Lv, J.; et al. Sirtuin 5 deficiency increases disease severity in rats with adjuvant-induced arthritis. Cell. Mol. Immunol. 2020, 17, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Yihan, L.; Xiaojing, W.; Ao, L.; Chuanjie, Z.; Haofei, W.; Yan, S.; Hongchao, H. SIRT5 functions as a tumor suppressor in renal cell carcinoma by reversing the Warburg effect. J. Transl. Med. 2021, 19, 521. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, H.J.; Min, H.Y.; Park, E.J.; Lee, K.M.; Ahn, Y.H.; Cho, Y.J.; Pyee, J.H. Antibacterial and antifungal activity of pinosylvin, a constituent of pine. Fitoterapia 2005, 76, 258–260. [Google Scholar] [CrossRef]
- Yerra, V.G.; Kalvala, A.K.; Kumar, A. Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J. Nutr. Biochem. 2017, 47, 41–52. [Google Scholar] [CrossRef]
- Wang, Y.; Chan, F.L.; Chen, S.; Leung, L.K. The plant polyphenol butein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase. Life Sci. 2005, 77, 39–51. [Google Scholar] [CrossRef]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [CrossRef]
- Fischer, C.; Speth, V.; Fleig-Eberenz, S.; Neuhaus, G. Induction of Zygotic Polyembryos in Wheat: Influence of Auxin Polar Transport. Plant Cell 1997, 9, 1767–1780. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Quan, M.-Y.; Wang, D.; Jia, Z.; Li, Z.-F.; Li, B.; Guo, L.; Tan, G.-J. Nordihydroguaiaretic acid can suppress progression of experimental autoimmune encephalomyelitis. IUBMB Life 2018, 70, 432–436. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat. Chem. Biol. 2020, 16, 783–790. [Google Scholar] [CrossRef]
- Gertz, M.; Nguyen, G.T.T.; Fischer, F.; Suenkel, B.; Schlicker, C.; Fränzel, B.; Tomaschewski, J.; Aladini, F.; Becker, C.; Wolters, D.; et al. A Molecular Mechanism for Direct Sirtuin Activation by Resveratrol. PLoS ONE 2012, 7, e49761. [Google Scholar] [CrossRef] [PubMed]
- Mayack, B.K.; Sippl, W.; Ntie-Kang, F. Natural Products as Modulators of Sirtuins. Molecules 2020, 25, 3287. [Google Scholar] [CrossRef]
- Brown, D.G.; Wilkerson, E.C.; Love, W.E. A review of traditional and novel oral anticoagulant and antiplatelet therapy for dermatologists and dermatologic surgeons. J. Am. Acad. Dermatol. 2015, 72, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Van Gompel, J.J.; Kunnimalaiyaan, M.; Holen, K.; Chen, H. ZM336372, a Raf-1 activator, suppresses growth and neuroendocrine hormone levels in carcinoid tumor cells. Mol. Cancer Ther. 2005, 4, 910–917. [Google Scholar] [CrossRef][Green Version]
- Sauve, A.; Cen, Y. Activation and activators of SIRT5 2011. U.S. Patent Applicatio No. 20120329748A1, 27 December 2012. [Google Scholar]
- Hu, T.; Shukla, S.K.; Vernucci, E.; He, C.; Wang, D.; King, R.J.; Jha, K.; Siddhanta, K.; Mullen, N.J.; Attri, K.S.; et al. Metabolic Rewiring by Loss of Sirt5 Promotes Kras-Induced Pancreatic Cancer Progression. Gastroenterology 2021, 161, 1584–1600. [Google Scholar] [CrossRef]
- Schlicker, C.; Boanca, G.; Lakshminarasimhan, M.; Steegborn, C. Structure-based development of novel sirtuin inhibitors. Aging 2011, 3, 852–872. [Google Scholar] [CrossRef]
- Elkhwanky, M.-S.; Hakkola, J. Extranuclear Sirtuins and Metabolic Stress. Antioxid. Redox Signal. 2018, 28, 662–676. [Google Scholar] [CrossRef]
- Yeong, K.Y.; Berdigaliyev, N.; Chang, Y. Sirtuins and Their Implications in Neurodegenerative Diseases from a Drug Discovery Perspective. ACS Chem. Neurosci. 2020, 11, 4073–4091. [Google Scholar] [CrossRef]
- Gomes, P.; Leal, H.; Mendes, A.F.; Reis, F.; Cavadas, C. Dichotomous Sirtuins: Implications for Drug Discovery in Neurodegenerative and Cardiometabolic Diseases. Trends Pharmacol. Sci. 2019, 40, 1021–1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Liao, M.; Hu, M.; Li, W.; Ouyang, H.; Wang, X.; Ye, T.; Zhang, Y.; Ouyang, L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2019, 161, 48–77. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. (Ed.) Sirtuin Biology in Cancer and Metabolic Disease; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Fischer, F.; Gertz, M.; Suenkel, B.; Lakshminarasimhan, M.; Schutkowski, M.; Steegborn, C. Sirt5 Deacylation Activities Show Differential Sensitivities to Nicotinamide Inhibition. PLoS ONE 2012, 7, e45098. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-F.; You, L.; Zhu, H.; Liao, S.-G.; Li, Y.-J.; Liu, T.; Wang, A.-M.; He, B.; Lan, Y.-Y. Screening of SIRT5 inhibitors from natural products. Zhongguo Xinyao Zazhi 2015, 24, 2724–2728. [Google Scholar]
- Guetschow, E.D.; Kumar, S.; Lombard, D.B.; Kennedy, R.T. Identification of sirtuin 5 inhibitors by ultrafast microchip electrophoresis using nanoliter volume samples. Anal. Bioanal. Chem. 2015, 408, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Glas, C.; Dietschreit, J.C.B.; Wössner, N.; Urban, L.; Ghazy, E.; Sippl, W.; Jung, M.; Ochsenfeld, C.; Bracher, F. Identification of the subtype-selective Sirt5 inhibitor balsalazide through systematic SAR analysis and rationalization via theoretical investigations. Eur. J. Med. Chem. 2020, 206, 112676. [Google Scholar] [CrossRef]
- He, B.; Du, J.; Lin, H. Thiosuccinyl Peptides as Sirt5-Specific Inhibitors. J. Am. Chem. Soc. 2012, 134, 1922–1925. [Google Scholar] [CrossRef]
- Lin, H. Thiourea compounds and their use as inhibitors of SIRT2 or SIRT5 2014. U.S. Patent No. 10,556,878, 11 February 2020. [Google Scholar]
- Abril, Y.L.N.; Fernandez, I.R.; Hong, J.Y.; Chiang, Y.-L.; Kutateladze, D.A.; Zhao, Q.; Yang, M.; Hu, J.; Sadhukhan, S.; Li, B.; et al. Pharmacological and genetic perturbation establish SIRT5 as a promising target in breast cancer. Oncogene 2021, 40, 1644–1658. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Zheng, W. A Selective Cyclic Peptidic Human SIRT5 Inhibitor. Molecules 2016, 21, 1217. [Google Scholar] [CrossRef]
- Yang, F.; Su, H.; Deng, J.; Mou, L.; Wang, H.; Li, R.; Dai, Q.-Q.; Yan, Y.-H.; Qian, S.; Wang, Z.; et al. Discovery of new human Sirtuin 5 inhibitors by mimicking glutaryl-lysine substrates. Eur. J. Med. Chem. 2021, 225, 113803. [Google Scholar] [CrossRef]
- Rajabi, N.; Auth, M.; Troelsen, K.R.; Pannek, M.; Bhatt, D.P.; Fontenas, M.; Hirschey, M.D.; Steegborn, C.; Madsen, A.S.; Olsen, C.A. Mechanism-Based Inhibitors of the Human Sirtuin 5 Deacylase: Structure-Activity Relationship, Biostructural, and Kinetic Insight. Angew. Chem. Int. Ed. 2017, 56, 14836–14841. [Google Scholar] [CrossRef]
- Rajabi, N.; Hansen, T.N.; Nielsen, A.L.; Nguyen, H.T.; Bæk, M.; Bolding, J.E.; Bahlke, O.; Petersen, S.E.G.; Bartling, C.R.O.; Strømgaard, K.; et al. Investigation of Carboxylic Acid Isosteres and Prodrugs for Inhibition of the Human SIRT5 Lysine Deacylase Enzyme. Angew. Chem. Int. Ed. 2022, 61, e202115805. [Google Scholar] [CrossRef] [PubMed]
- Bolding, J.E.; Martín-Gago, P.; Rajabi, N.; Gamon, L.F.; Hansen, T.N.; Davies, M.J.; Olsen, C.A. Aryl Fluorosulfate-Based Inhibitors that Covalently Target the SIRT5 Lysine Deacylase. bioRxiv 2022. [Google Scholar] [CrossRef]
- Yan, D.; Franzini, A.; Pomicter, A.D.; Halverson, B.J.; Antelope, O.; Mason, C.C.; Ahmann, J.M.; Senina, A.V.; Vellore, N.A.; Jones, C.L.; et al. SIRT5 Is a Druggable Metabolic Vulnerability in Acute Myeloid Leukemia. Blood Cancer Discov. 2021, 2, 266–287. [Google Scholar] [CrossRef] [PubMed]
- Zang, W.; Hao, Y.; Wang, Z.; Zheng, W. Novel thiourea-based sirtuin inhibitory warheads. Bioorganic Med. Chem. Lett. 2015, 25, 3319–3324. [Google Scholar] [CrossRef]
- He, Y.; Yan, L.; Zang, W.; Zheng, W. Novel sirtuin inhibitory warheads derived from the Nε-acetyl-lysine analog l-2-amino-7-carboxamidoheptanoic acid. Org. Biomol. Chem. 2015, 13, 10442–10450. [Google Scholar] [CrossRef]
- Polletta, L.; Vernucci, E.; Carnevale, I.; Arcangeli, T.; Rotili, D.; Palmerio, S.; Steegborn, C.; Nowak, T.; Schutkowski, M.; Pellegrini, L.; et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 2015, 11, 253–270. [Google Scholar] [CrossRef]
- Roessler, C.; Nowak, T.; Pannek, M.; Gertz, M.; Nguyen, G.T.T.; Scharfe, M.; Born, I.; Sippl, W.; Steegborn, C.; Schutkowski, M. Chemical Probing of the Human Sirtuin 5 Active Site Reveals Its Substrate Acyl Specificity and Peptide-Based Inhibitors. Angew. Chem. Int. Ed. 2014, 53, 10728–10732. [Google Scholar] [CrossRef]
- Kalbas, D.; Liebscher, S.; Nowak, T.; Meleshin, M.; Pannek, M.; Popp, C.; Alhalabi, Z.; Bordusa, F.; Sippl, W.; Steegborn, C.; et al. Potent and Selective Inhibitors of Human Sirtuin 5. J. Med. Chem. 2018, 61, 2460–2471. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, W. Cyclic peptide-based potent human SIRT6 inhibitors. Org. Biomol. Chem. 2016, 14, 5928–5935. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Yan, L.; Zheng, W. Simple N ε-thioacetyl-lysine-containing cyclic peptides exhibiting highly potent sirtuin inhibition. Bioorganic Med. Chem. Lett. 2016, 26, 1612–1617. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, W. Cyclic Tripeptide-based Potent and Selective Human SIRT5 Inhibitors. Med. Chem. 2020, 16, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ji, S.; Yu, Z.-J.; Wang, H.-L.; Cheng, X.; Li, W.-J.; Jing, L.; Yu, Y.; Chen, Q.; Yang, L.-L.; et al. Structure-based discovery of new selective small-molecule sirtuin 5 inhibitors. Chem. Biol. Drug Des. 2017, 91, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Suenkel, B.; Fischer, F.; Steegborn, C. Inhibition of the human deacylase Sirtuin 5 by the indole GW5074. Bioorganic Med. Chem. Lett. 2013, 23, 143–146. [Google Scholar] [CrossRef]
- Schuetz, A.; Min, J.; Antoshenko, T.; Wang, C.-L.; Allali-Hassani, A.; Dong, A.; Loppnau, P.; Vedadi, M.; Bochkarev, A.; Sternglanz, R.; et al. Structural Basis of Inhibition of the Human NAD+-Dependent Deacetylase SIRT5 by Suramin. Structure 2007, 15, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.; Rumpf, T.; Scharfe, M.; Stolfa, D.A.; Schmitt, M.L.; He, W.; Verdin, E.; Sippl, W.; Jung, M. Inhibitors of the NAD+-Dependent Protein Desuccinylase and Demalonylase Sirt5. ACS Med. Chem. Lett. 2012, 3, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Ciarlo, E.; Santos, A.; Grandmaison, G.; Dos Santos, I.; Le Roy, D.; Roger, T. The sirtuin inhibitor cambinol impairs MAPK signaling, inhibits inflammatory and innate immune responses and protects from septic shock. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 1498–1510. [Google Scholar] [CrossRef]
- Orecchia, A.; Scarponi, C.; Di Felice, F.; Cesarini, E.; Avitabile, S.; Mai, A.; Mauro, M.L.; Sirri, V.; Zambruno, G.; Albanesi, C.; et al. Sirtinol Treatment Reduces Inflammation in Human Dermal Microvascular Endothelial Cells. PLoS ONE 2011, 6, e24307. [Google Scholar] [CrossRef]
- Han, H.; Li, C.; Li, M.; Yang, L.; Zhao, S.; Wang, Z.; Liu, H.; Liu, D. Design, Synthesis, and Biological Evaluation of 8-Mercapto-3,7-Dihydro-1H-Purine-2,6-Diones as Potent Inhibitors of SIRT1, SIRT2, SIRT3, and SIRT5. Molecules 2020, 25, 2755. [Google Scholar] [CrossRef]
- Yang, L.-L.; He, Y.-Y.; Chen, Q.-L.; Qian, S.; Wang, Z.-Y. Design and Synthesis of New 9-Substituted Norharmane Derivatives as Potential Sirt5 Inhibitors. J. Heterocycl. Chem. 2016, 54, 1457–1466. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Qian, S.; He, Y.; Chen, Q. 9-Substituted Pyrido [3,4-b]Indole Derivative, ITS preparing Method, and its Application as SIRT Inhibitor, UNIVXIHUA. China Patent Application No. CN105884767, 11 January 2016. [Google Scholar]
- Yang, L.-L.; Wang, H.-L.; Yan, Y.-H.; Liu, S.; Yu, Z.-J.; Huang, M.-Y.; Luo, Y.; Zheng, X.; Yu, Y.; Li, G.-B. Sensitive fluorogenic substrates for sirtuin deacylase inhibitor discovery. Eur. J. Med. Chem. 2020, 192, 112201. [Google Scholar] [CrossRef]
- Walter, M.; Chen, I.P.; Vallejo-Gracia, A.; Kim, I.-J.; Bielska, O.; Lam, V.L.; Hayashi, J.M.; Cruz, A.; Shah, S.; Gross, J.D.; et al. SIRT5 is a proviral factor that interacts with SARS-CoV-2 Nsp14 protein. bioRxiv 2022. [Google Scholar] [CrossRef]
- Yang, L.; Peltier, R.; Zhang, M.; Song, D.; Huang, H.; Chen, G.; Chen, Y.; Zhou, F.; Hao, Q.; Bian, L.; et al. Desuccinylation-Triggered Peptide Self-Assembly: Live Cell Imaging of SIRT5 Activity and Mitochondrial Activity Modulation. J. Am. Chem. Soc. 2020, 142, 18150–18159. [Google Scholar] [CrossRef]
- Carafa, V.; Altucci, L.; Nebbioso, A. Dual Tumor Suppressor and Tumor Promoter Action of Sirtuins in Determining Malignant Phenotype. Front. Pharmacol. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, M.; Cazzaniga, G.; Meneghetti, F.; Villa, S.; Gelain, A. Insights on the Modulation of SIRT5 Activity: A Challenging Balance. Molecules 2022, 27, 4449. https://doi.org/10.3390/molecules27144449
Mori M, Cazzaniga G, Meneghetti F, Villa S, Gelain A. Insights on the Modulation of SIRT5 Activity: A Challenging Balance. Molecules. 2022; 27(14):4449. https://doi.org/10.3390/molecules27144449
Chicago/Turabian StyleMori, Matteo, Giulia Cazzaniga, Fiorella Meneghetti, Stefania Villa, and Arianna Gelain. 2022. "Insights on the Modulation of SIRT5 Activity: A Challenging Balance" Molecules 27, no. 14: 4449. https://doi.org/10.3390/molecules27144449
APA StyleMori, M., Cazzaniga, G., Meneghetti, F., Villa, S., & Gelain, A. (2022). Insights on the Modulation of SIRT5 Activity: A Challenging Balance. Molecules, 27(14), 4449. https://doi.org/10.3390/molecules27144449







