Non-Invasive Method to Predict the Composition of Requeijão Cremoso Directly in Commercial Packages Using Time Domain NMR Relaxometry and Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. TD-NMR Measurements
2.3. Chemical Analysis
2.4. Multivariate Analysis
3. Results and Discussion
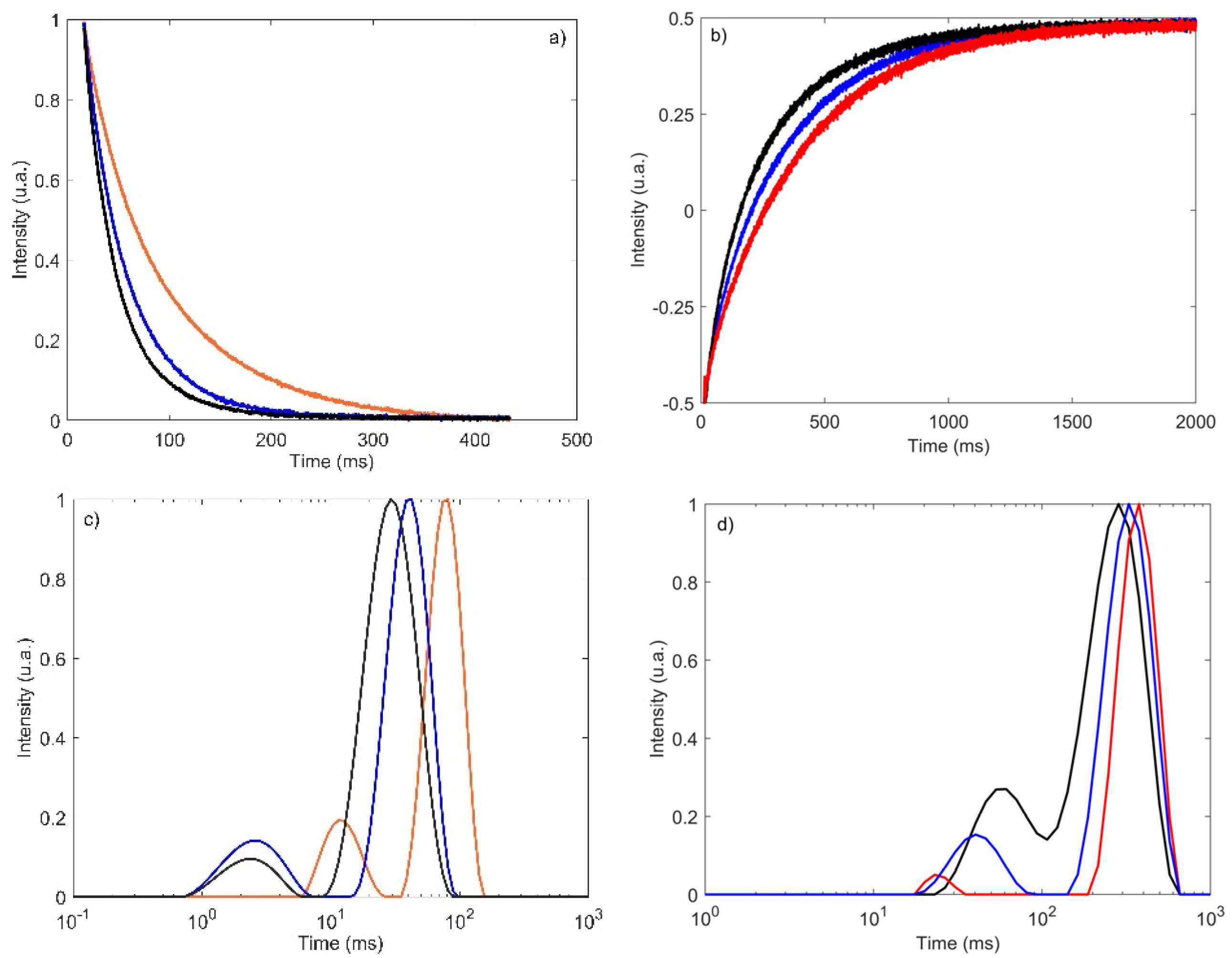
3.1. TD-NMR Decays
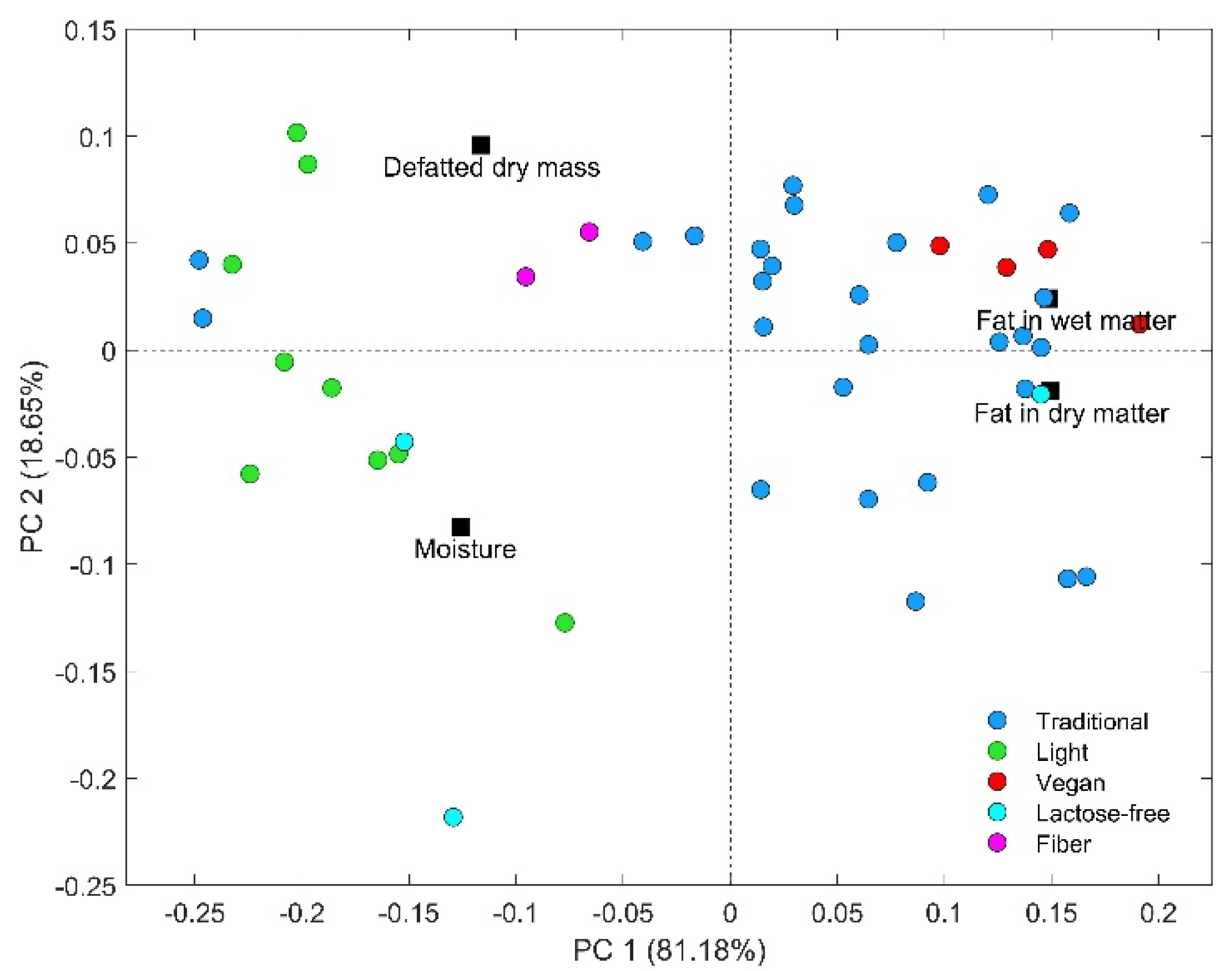
3.2. Exploratory Analysis
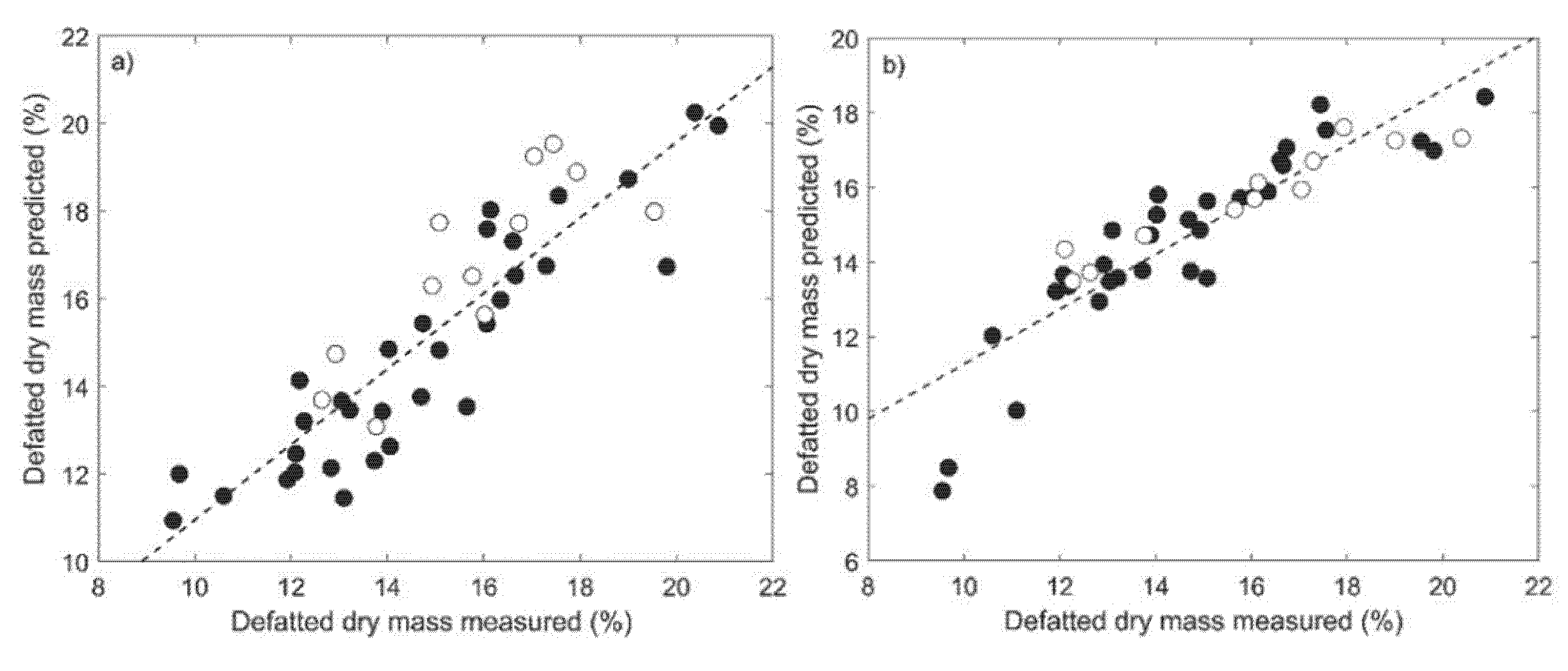
3.3. Regression Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ezeanaka, M.C.; Nsor-Atindana, J.; Zhang, M. Online Low-field Nuclear Magnetic Resonance (LF-NMR) and Magnetic Resonance Imaging (MRI) for Food Quality Optimization in Food Processing. Food Bioprocess Technol. 2019, 12, 1435–1451. [Google Scholar] [CrossRef]
- van Duynhoven, J.; Voda, A.; Witek, M.; Van As, H. Chapter 3—Time-Domain NMR Applied to Food Products. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 69, pp. 145–197. [Google Scholar]
- Kirtil, E.; Oztop, M.H. H-1 Nuclear Magnetic Resonance Relaxometry and Magnetic Resonance Imaging and Applications in Food Science and Processing. Food Eng. Rev. 2016, 8, 1–22. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.M.V.; Rebellato, A.P.; Pallone, J.A.L.; Colnago, L.A. Through-package fat determination in commercial Samples of mayonnaise and salad dressing using time-domain nuclear magnetic resonance spectroscopy and chemometrics. Food Control 2015, 48, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Santos, P.M.; Kock, F.V.C.; Santos, M.S.; Lobo, C.M.S.; Carvalho, A.S.; Colnago, L.A. Non-Invasive Detection of Adulterated Olive Oil in Full Bottles Using Time-Domain NMR Relaxometry. J. Braz. Chem. Soc. 2017, 28, 385–390. [Google Scholar] [CrossRef]
- Maher, A.D.; Rochfort, S.J. Applications of NMR in Dairy Research. Metabolites 2014, 4, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Pocan, P.; Mecit Halil, O. Chapter 10—Low-field time-domain nuclear magnetic resonance applied to dairy foods. In Dairy Foods; da Cruz, A.G., Ranadheera, C.S., Nazzaro, F., Mortazavian, A.M., Eds.; Woodhead Publishing: Duxford, UK, 2022; pp. 215–232. [Google Scholar]
- Mariette, F. NMR Relaxation of Dairy Products. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer: Dordrecht, The Netherlands, 2006; Volume 1, pp. 1697–1701. [Google Scholar]
- Pablo, T.C.; Bathazar, C.F.; Guimarães, J.T.; Coutinho, N.M.; Pimentel, T.C.; Neto, R.P.C.; Esmerino, E.A.; Freitas, M.Q.; Silva, M.C.; Tavares, M.I.B.; et al. Detection of formaldehyde in raw milk by time domain nuclear magnetic resonance and chemometrics. Food Control 2020, 110, 107006. [Google Scholar] [CrossRef]
- Santos, P.M.; Pereira-Filho, E.R.; Colnago, L.A. Detection and quantification of milk adulteration using time domain nuclear magnetic resonance (TD-NMR). Microchem. J. 2016, 124, 15–19. [Google Scholar] [CrossRef]
- Nascimento, P.A.M.; Barsanelli, P.L.; Rebellato, A.P.; Pallone, J.A.L.; Colnago, L.A.; Pereira, F.M.V. Time-Domain Nuclear Magnetic Resonance (TD-NMR) and Chemometrics for Determination of Fat Content in Commercial Products of Milk Powder. J. AOAC Int. 2017, 100, 330–334. [Google Scholar] [CrossRef]
- Castell-Palou, A.; Rossello, C.; Femenia, A.; Simal, S. Simultaneous Quantification of Fat and Water Content in Cheese by TD-NMR. Food Bioprocess Technol. 2013, 6, 2685–2694. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Wang, L.; Kathryn, L.M. Characterization of yogurts made with milk solids nonfat by rheological behavior and nuclear magnetic resonance spectroscopy. J. Food Drug Anal. 2016, 24, 804–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, T.; Wagener, M.; Barey, P.; Mariette, F. NMR assessment of mix and ice cream. Effect of formulation on liquid water and ice. Int. Dairy J. 2005, 15, 1064–1073. [Google Scholar] [CrossRef]
- Belsito, P.C.; Ferreira, M.V.S.; Cappato, L.P.; Cavalcanti, R.N.; Vidal, V.A.S.; Pimentel, T.C.; Esmerino, E.A.; Balthazar, C.F.; Neto, R.P.C.; Tavares, M.I.B.; et al. Manufacture of Requeijao cremoso processed cheese with galactooligosaccharide. Carbohydr. Polym. 2017, 174, 869–875. [Google Scholar] [CrossRef]
- Ferrao, L.L.; Ferreira, M.V.S.; Cavalcanti, R.N.; Carvalho, A.F.A.; Pimentel, T.C.; Silva, H.L.A.; Silva, R.; Esmerino, E.A.; Neto, R.P.C.; Tavares, M.I.B.; et al. The xylooligosaccharide addition and sodium reduction in requeijao cremoso processed cheese. Food Res. Int. 2018, 107, 137–147. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, F.D.; Netto, A.M.; Colnago, L.A. Qualitative analysis by online nuclear magnetic resonance using Carr-Purcell-Meiboom-Gill sequence with low refocusing flip angles. Talanta 2011, 84, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.M.V.; Carvalho, A.D.; Cabeca, L.F.; Colnago, L.A. Classification of intact fresh plums according to sweetness using time-domain nuclear magnetic resonance and chemometrics. Microchem. J. 2013, 108, 14–17. [Google Scholar] [CrossRef]
- Bizzani, M.; Flores, D.W.M.; Moraes, T.B.; Colnago, L.A.; Ferreira, M.D.; Spoto, M.H.F. Non-invasive detection of internal flesh breakdown in intact Palmer mangoes using time-domain nuclear magnetic resonance relaxometry. Microchem. J. 2020, 158, 105208. [Google Scholar] [CrossRef]
- Moraes, T.B.; Colnago, L.A. Noninvasive Analyses of Food Products Using Low-field Time-domain NMR: A Review of Relaxometry Methods. Braz. J. Phys. 2022, 52. [Google Scholar] [CrossRef]
- Parker, A.J.; Zia, W.; Rehorn, C.W.G.; Blumich, B. Shimming Halbach magnets utilizing genetic algorithms to profit from material imperfections. J. Magn. Reson. 2016, 265, 83–89. [Google Scholar] [CrossRef]
- Pedersen, H.T.; Ablett, S.; Martin, D.R.; Mallett, M.J.D.; Engelsen, S.B. Application of the NMR-MOUSE to food emulsions. J. Magn. Reson. 2003, 165, 49–58. [Google Scholar] [CrossRef]
- Blumich, B.; Blumler, P.; Eidmann, G.; Guthausen, A.; Haken, R.; Schmitz, U.; Saito, K.; Zimmer, G. The NMR-mouse: Construction, excitation, and applications. Magn. Reson. Imaging 1998, 16, 479–484. [Google Scholar] [CrossRef]
- Monaretto, T.; Moraes, T.B.; Colnago, L.A. Recent 1D and 2D TD-NMR Pulse Sequences for Plant Science. Plants 2021, 10, 833. [Google Scholar] [CrossRef]
- Blumich, B. Introduction to compact NMR: A review of methods. Trac-Trends Anal. Chem. 2016, 83, 2–11. [Google Scholar] [CrossRef]
- Moraes, T.B.; Monaretto, T.; Colnago, L.A. Rapid and simple determination of T-1 relaxation times in time-domain NMR by Continuous Wave Free Precession sequence. J. Magn. Reson. 2016, 270, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moraes, T.B.; Monaretto, T.; Colnago, L.A. Applications of Continuous Wave Free Precession Sequences in Low-Field, Time-Domain NMR. Appl. Sci. 2019, 9, 1312. [Google Scholar] [CrossRef] [Green Version]
- Cônsolo, N.R.B.; Silva, J.; Buarque, V.L.M.; Samuelsson, L.M.; Miller, P.; Maclean, P.H.; Moraes, T.B.; Barbosa, L.C.G.S.; Higuera-Padilla, A.; Colnago, L.A.; et al. Using TD-NMR relaxometry and 1D 1H NMR spectroscopy to evaluate aging of Nellore beef. Meat Sci. 2021, 181, 108606. [Google Scholar] [CrossRef]
- Monaretto, T.; Montrazi, E.T.; Moraes, T.B.; Souza, A.A.; Rondeau-Mouro, C.; Colnago, L.A. Using T-1 as a direct detection dimension in two-dimensional time-domain NMR experiments using CWFP regime. J. Magn. Reson. 2020, 311. [Google Scholar] [CrossRef]
- Consolo, N.R.B.; Samuelsson, L.M.; Barbosa, L.; Monaretto, T.; Moraes, T.B.; Buarque, V.L.M.; Higuera-Padilla, A.R.; Colnago, L.A.; Silva, S.L.; Reis, M.M.; et al. Characterization of chicken muscle disorders through metabolomics, pathway analysis, and water relaxometry: A pilot study. Poult. Sci. 2020, 99, 6247–6257. [Google Scholar] [CrossRef] [PubMed]
- Roque, J.V.; Cardoso, W.; Peternelli, L.A.; Teofilo, R.F. Comprehensive new approaches for variable selection using ordered predictors selection. Anal. Chim. Acta 2019, 1075, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.L.; Thybo, A.K.; Bertram, H.C.; Viereck, N.; van den Erg, F.; Engelsen, S.B. Determination of Dry Matter Content in Potato Tubers by Low-Field Nuclear Magnetic Resonance (LF-NMR). J. Agric. Food Chem. 2010, 58, 10300–10304. [Google Scholar] [CrossRef]
- Jepsen, S.M.; Pedersen, H.T.; Engelsen, S.B. Application of chemometrics to low-field H-1 NMR relaxation data of intact fish flesh. J. Sci. Food Agric. 1999, 79, 1793–1802. [Google Scholar] [CrossRef]
- Moraes, T.B. Transformada Inversa de Laplace para análise de sinais de Ressonância Magnética Nuclear de Baixo Campo. Química Nova 2021, 44, 7. [Google Scholar] [CrossRef]
- Monaretto, T.; Souza, A.; Moraes, T.B.; Bertucci-Neto, V.; Rondeau-Mouro, C.; Colnago, L.A. Enhancing signal-to-noise ratio and resolution in low-field NMR relaxation measurements using post-acquisition digital filters. Magn. Reson. Chem. 2019, 57, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Botelho, B.G.; Mendes, B.A.P.; Sena, M.M. Development and Analytical Validation of Robust Near-Infrared Multivariate Calibration Models for the Quality Inspection Control of Mozzarella Cheese. Food Anal. Methods 2013, 6, 881–891. [Google Scholar] [CrossRef]
- Olarewaju, O.O.; Magwaza, L.S.; Nieuwoudt, H.; Poblete-Echeverria, C.; Fawole, O.A.; Tesfay, S.Z.; Opara, U.L. Model development for non-destructive determination of rind biochemical properties of ‘Marsh’ grapefruit using visible to near-infrared spectroscopy and chemometrics. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2019, 209, 62–69. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Nieuwoudt, H.; Olivieri, A.; Aleixandre, J.L.; du Toit, W. Phenolic profiling of grapes, fermenting samples and wines using UV-Visible spectroscopy with chemometrics. Food Control 2018, 85, 11–22. [Google Scholar] [CrossRef]
- Bittante, G.; Patel, N.; Cecchinato, A.; Paolo, B. Invited review: A comprehensive review of visible and near-infrared spectroscopy for predicting the chemical composition of cheese. J. Dairy Sci. 2022, 105, 1817–1836. [Google Scholar] [CrossRef]
- Ayvaz, H.; Mortas, M.; Dogan, M.A.; Atan, M.; Tiryaki, G.Y.; Yuceer, Y.K. Near- and mid-infrared determination of some quality parameters of cheese manufactured from the mixture of different milk species. J. Food Sci. Technol. 2021, 58, 3981–3992. [Google Scholar] [CrossRef]

| CPMG | CWFP | |||
|---|---|---|---|---|
| Full | OPS | Full | OPS | |
| Nvars * | 993 | 205 | 5965 | 75 |
| LV | 3 | 3 | 3 | 3 |
| RMSEP ** | 1.51 | 1.53 | 1.84 | 1.38 |
| R2pred | 0.49 | 0.67 | 0.82 | 0.90 |
| RPDpred | 1.37 | 1.36 | 1.46 | 1.95 |
| REPpred ** | 9.55 | 9.67 | 11.60 | 8.70 |
| CPMG | CWFP-T₁ | |||||
|---|---|---|---|---|---|---|
| Fat in Dry Matter | Fat in Wet Matter | Moisture | Fat in Dry Matter | Fat in Wet Matter | Moisture | |
| RMSEP * | 10.90 | 6.12 | 4.97 | 4.71 | 3.28 | 3.00 |
| R2pred | 0.28 | 0.23 | 0.050 | 0.92 | 0.85 | 0.70 |
| RPDpred | 1.26 | 1.24 | 1.07 | 2.92 | 2.32 | 1.77 |
| REPpred * | 20.35 | 31.13 | 7.71 | 8.79 | 16.68 | 4.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Machado, G.; Teixeira, G.G.; Garcia, R.H.d.S.; Moraes, T.B.; Bona, E.; Santos, P.M.; Colnago, L.A. Non-Invasive Method to Predict the Composition of Requeijão Cremoso Directly in Commercial Packages Using Time Domain NMR Relaxometry and Chemometrics. Molecules 2022, 27, 4434. https://doi.org/10.3390/molecules27144434
de Oliveira Machado G, Teixeira GG, Garcia RHdS, Moraes TB, Bona E, Santos PM, Colnago LA. Non-Invasive Method to Predict the Composition of Requeijão Cremoso Directly in Commercial Packages Using Time Domain NMR Relaxometry and Chemometrics. Molecules. 2022; 27(14):4434. https://doi.org/10.3390/molecules27144434
Chicago/Turabian Stylede Oliveira Machado, G., Gustavo Galastri Teixeira, Rodrigo Henrique dos Santos Garcia, Tiago Bueno Moraes, Evandro Bona, Poliana M. Santos, and Luiz Alberto Colnago. 2022. "Non-Invasive Method to Predict the Composition of Requeijão Cremoso Directly in Commercial Packages Using Time Domain NMR Relaxometry and Chemometrics" Molecules 27, no. 14: 4434. https://doi.org/10.3390/molecules27144434
APA Stylede Oliveira Machado, G., Teixeira, G. G., Garcia, R. H. d. S., Moraes, T. B., Bona, E., Santos, P. M., & Colnago, L. A. (2022). Non-Invasive Method to Predict the Composition of Requeijão Cremoso Directly in Commercial Packages Using Time Domain NMR Relaxometry and Chemometrics. Molecules, 27(14), 4434. https://doi.org/10.3390/molecules27144434







