Co-Combination of Pregabalin and Withania coagulans-Extract-Loaded Topical Gel Alleviates Allodynia and Hyperalgesia in the Chronic Sciatic Nerve Constriction Injury for Neuropathic Pain in Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pregabalin and Withania coagulans Topical Gel
2.2.1. Obtaining Withania coagulans Extract
2.2.2. Preparation of Micro-Emulsion and Topical Gel
2.3. Characterization of Topical Gels
2.3.1. Drug–Excipient Compatibility Studies
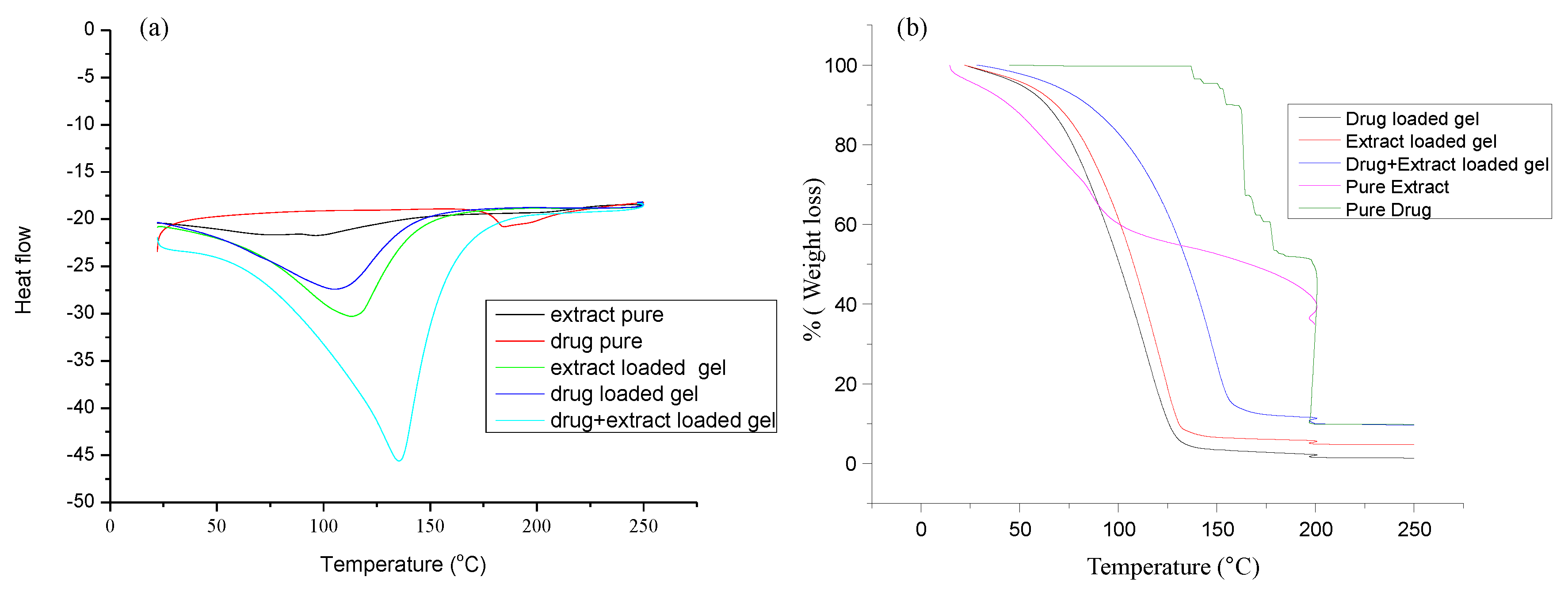
2.3.2. DSC and TG Analysis
2.3.3. X-ray Diffraction of Gels
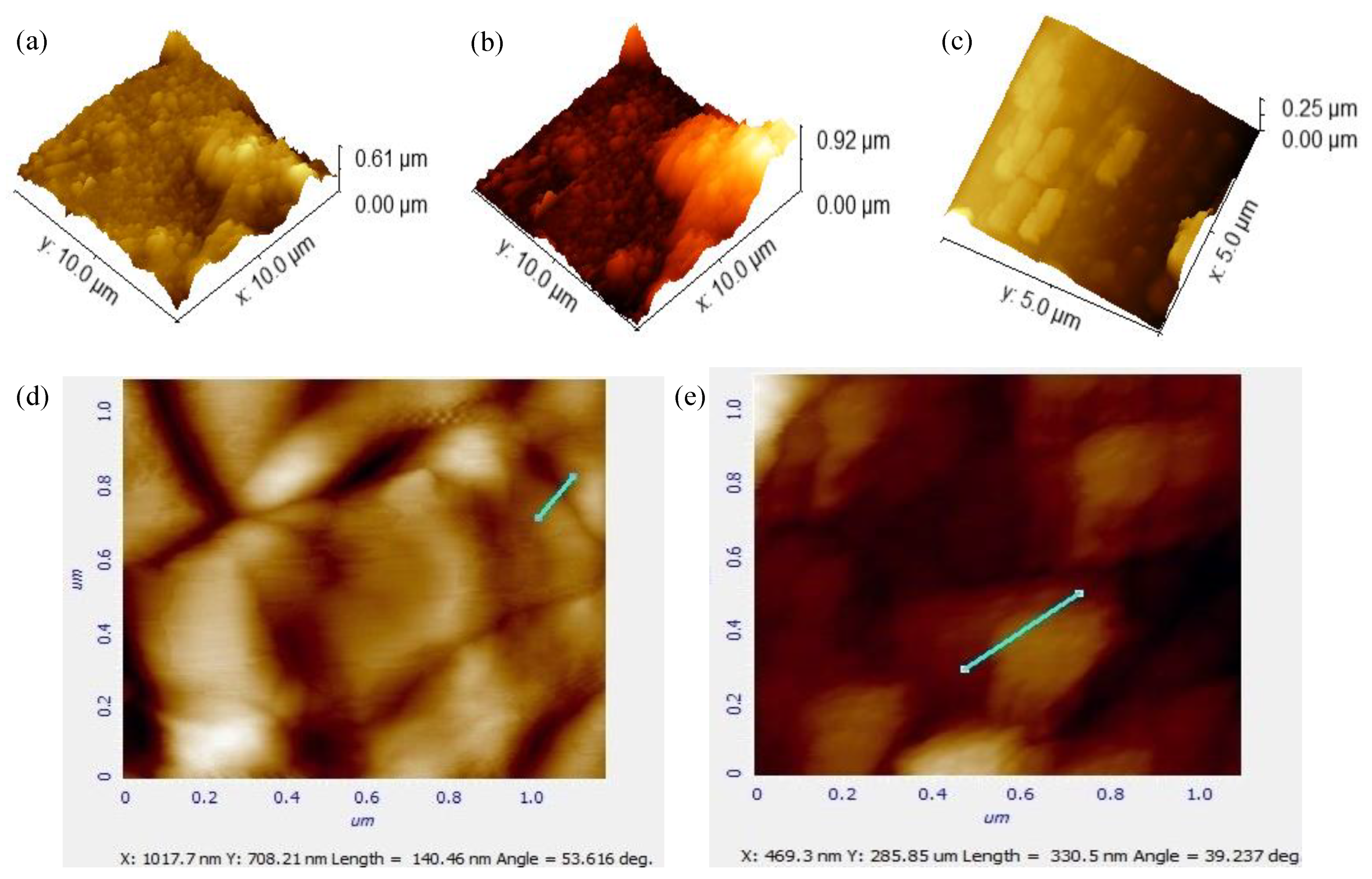
2.3.4. Atomic Force Microscopy
2.4. Ex Vivo Skin Permeability Studies
2.5. In Vitro Drug Release Studies of Topical Gels
2.6. In Vivo Studies
2.6.1. Animals
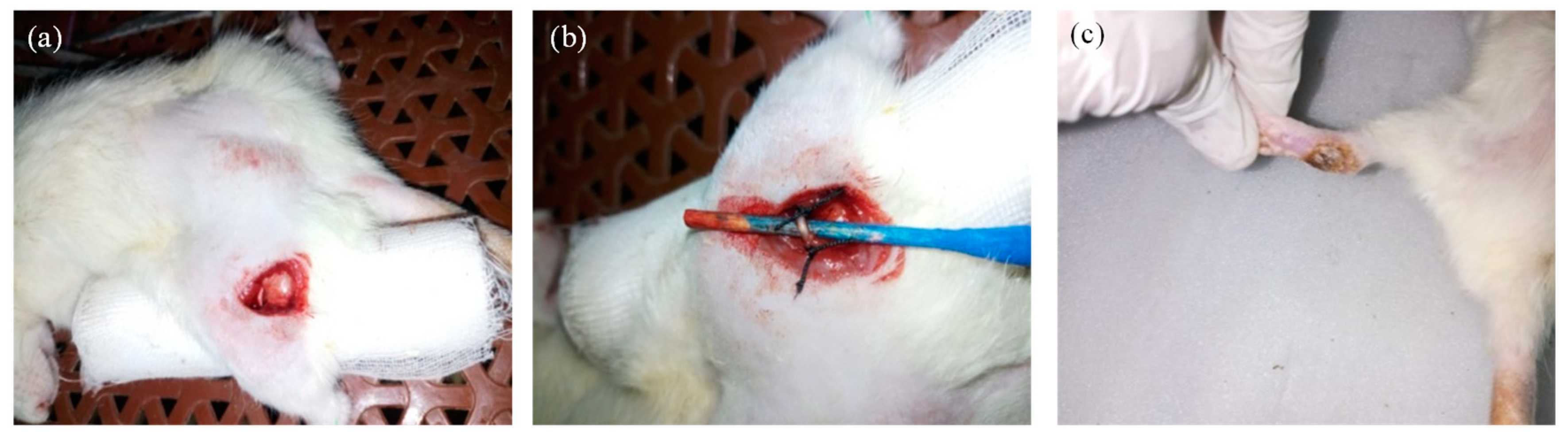
2.6.2. Induction of Neuropathic Pain Model
2.6.3. Animal Treatment Groups
- R1 = Topical application with Withania coagulans extract-loaded gel;
- R2 = Topical application of Pregabalin + Withania coagulans gel combination;
- R3 = Topical application with Pregabalin-loaded gel;
- R4 = Topical application with Pregabalin drug solution;
- R5 = No Treatment group.
2.7. Pain Assessment
2.7.1. Evaluation of Thermal Hyperalgesia
Hot Plate Method
Water Bath Method
2.7.2. Evaluation of Cold Allodynia
Cold Water Method
Acetone Spray Method
2.7.3. Mechano-Hyperalgesia
Pin Prick Test
2.7.4. Dynamic Mechano-Allodynia
Cotton Bud Test
Paint Brush Method
2.7.5. Evaluation of Depressive Behavior
Tail Suspension Method
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Topical Gels
3.1.1. Drug–Excipient Compatibility Studies
3.1.2. DSC and TG Analysis
3.1.3. X-ray Diffraction of Gels
3.1.4. Atomic Force Microscopy
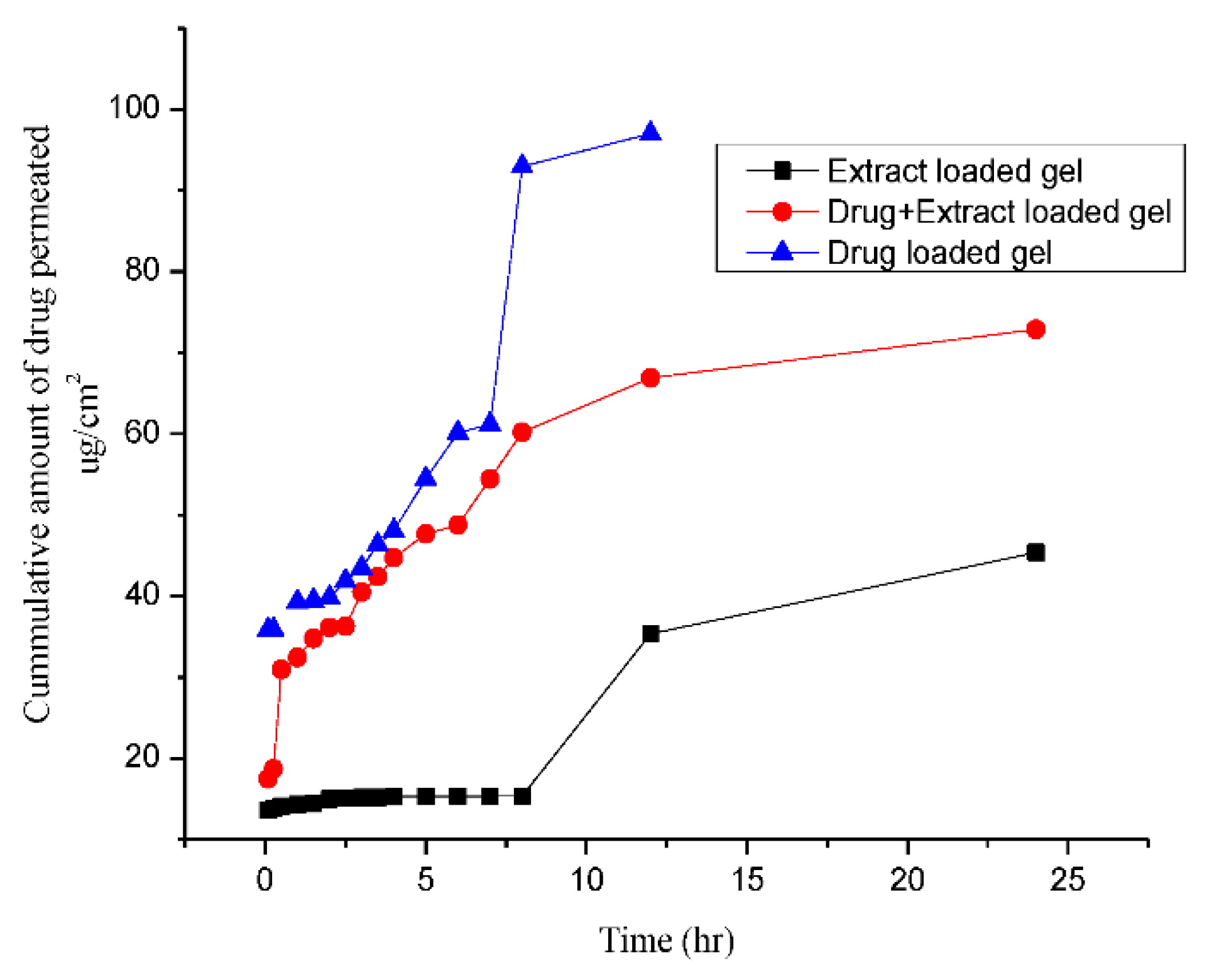
3.2. In Vitro Permeability Studies
3.3. In Vitro Drug Release and Mechanism of Drug Release
3.4. In Vivo Studies
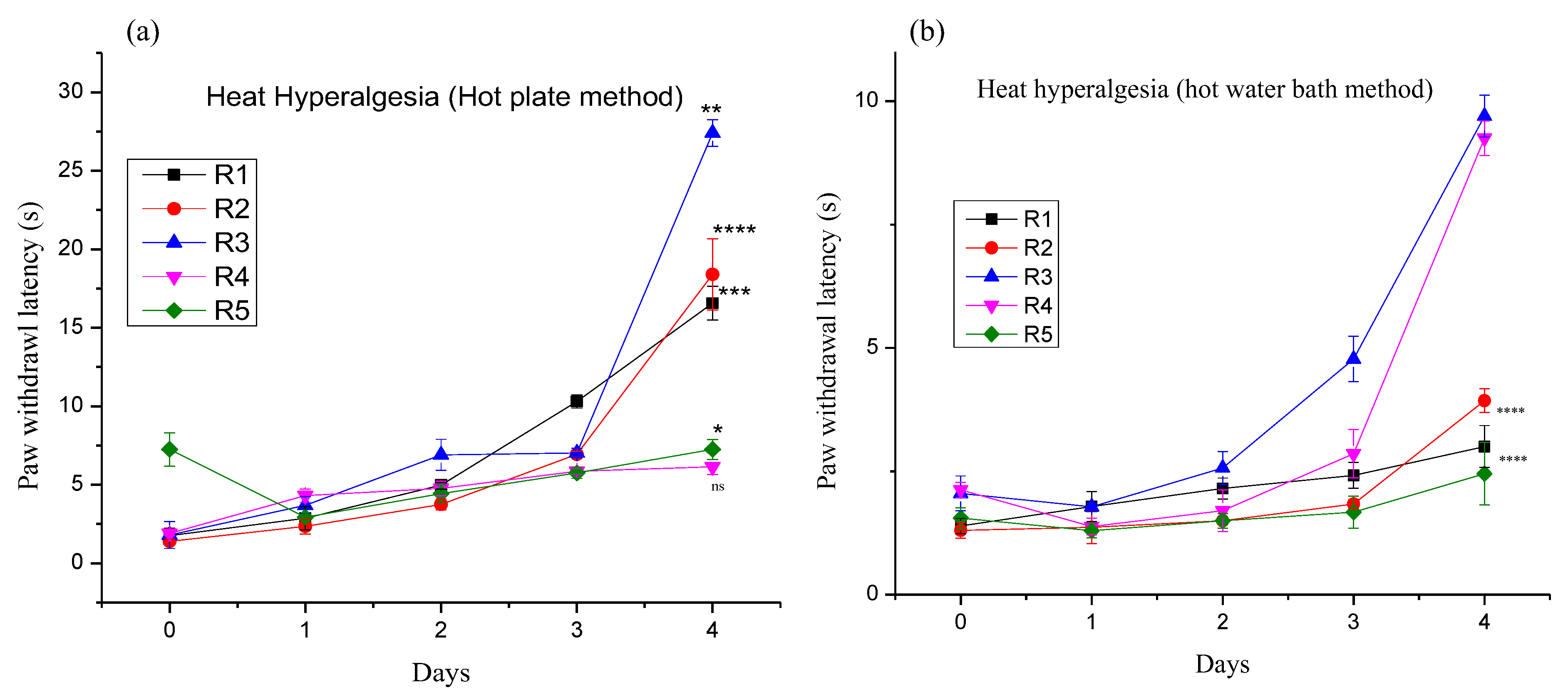
3.4.1. Effect of Different Pregabalin and Withania coagulans Combination Gels on Neuropathic Heat Hyperalgesia
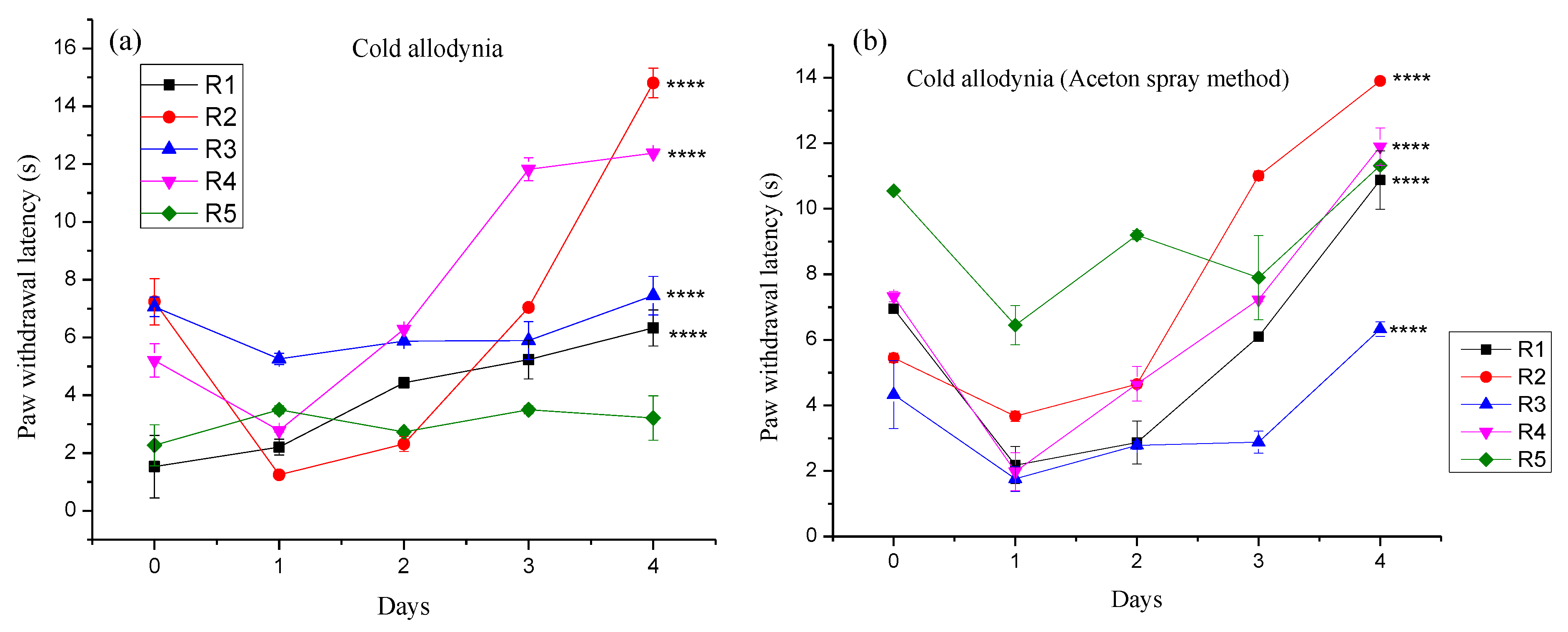
3.4.2. Effect of Different Pregabalin and Withania coagulans Combination Gels on Neuropathic Cold Allodynia
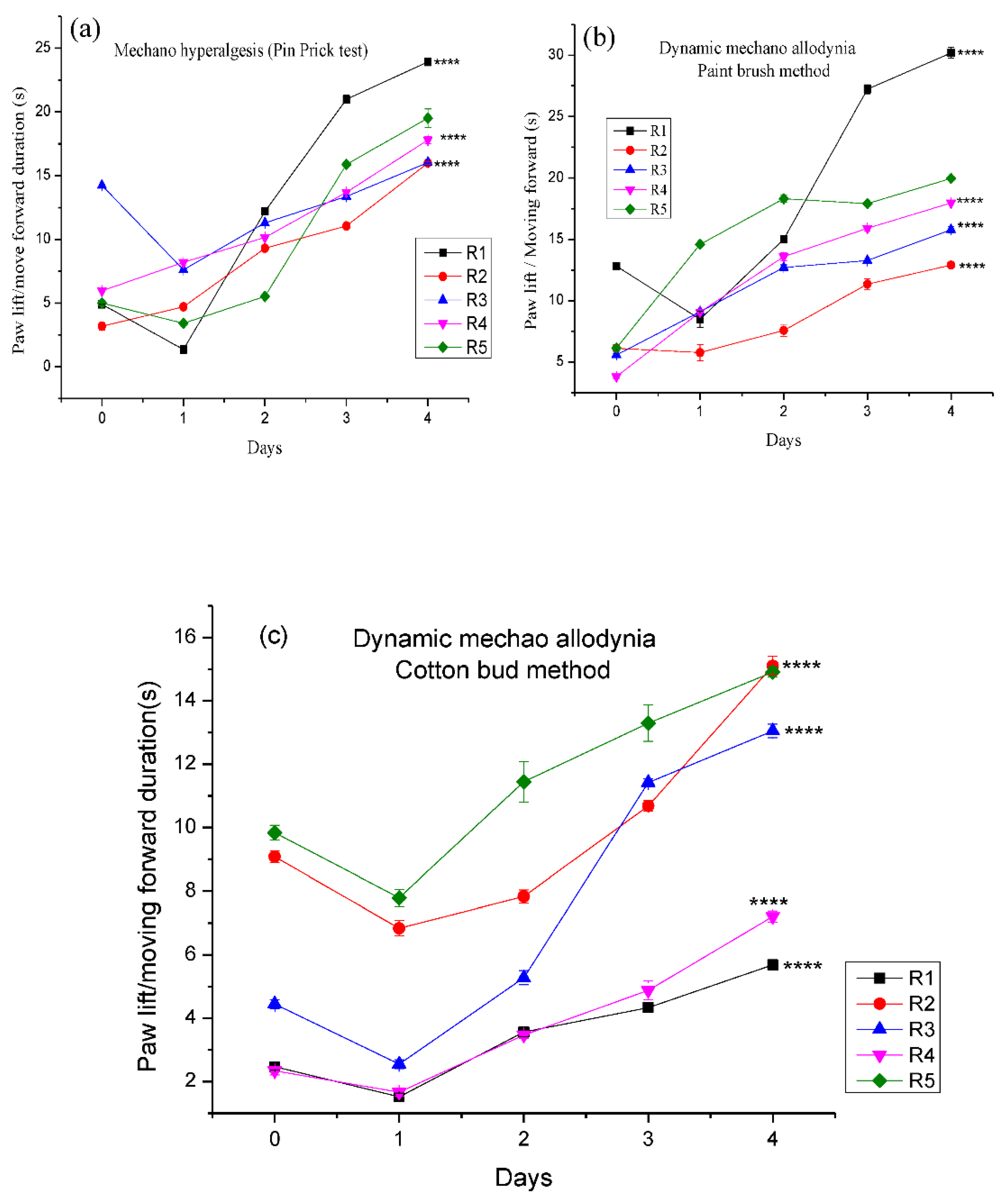
3.4.3. Effect of Different Pregabalin and Withania coagulans Combination Gels on Neuropathic Dynamic Mechano-Hyperalgesia
3.4.4. Depressive Behavior Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [Green Version]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.; Treede, R.-D. A new definition of neuropathic pain. Pain 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Shahid, M.; Subhan, F.; Ahmad, N.; Ali, G.; Akbar, S.; Fawad, K.; Sewell, R.D.E. Topical gabapentin gel alleviates allodynia and hyperalgesia in the chronic sciatic nerve constriction injury neuropathic pain model. Eur. J. Pain 2017, 21, 668–680. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Yang, M.; Liu, N.; Li, Y.-X.; Ma, H.; Ma, L.; Sun, T.; Tan, H.; Yu, J. Neuroprotective effect of anethole against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Neurochem. Res. 2018, 43, 2404–2422. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Kirkpatrick, P. Pregabalin; Nature Publishing Group: Berlin, Germany, 2005. [Google Scholar]
- Gray, P.; Kirby, J.; Smith, M.T.; Cabot, P.J.; Williams, B.; Doecke, J.; Cramond, T. Pregabalin in severe burn injury pain: A double-blind, randomised placebo-controlled trial. Pain 2011, 152, 1279–1288. [Google Scholar] [CrossRef]
- Haanpää, M.; Cruccu, G.; Nurmikko, T.; McBride, W.; Docu Axelarad, A.; Bosilkov, A.; Chambers, C.; Ernault, E.; Abdulahad, A. Capsaicin 8% patch versus oral pregabalin in patients with peripheral neuropathic pain. Eur. J. Pain 2016, 20, 316–328. [Google Scholar] [CrossRef]
- Zur, E. Topical treatment of neuropathic pain using compounded medications. Clin. J. Pain 2014, 30, 73–91. [Google Scholar] [CrossRef]
- Rasool, A.; Bhat, K.M.; Sheikh, A.A.; Jan, A.; Hassan, S. Medicinal plants: Role, distribution and future. J. Pharmacogn. Phytochem. 2020, 9, 2111–2114. [Google Scholar]
- Boy, H.I.A.; Rutilla, A.J.H.; Santos, K.A.; Ty, A.M.T.; Alicia, I.Y.; Mahboob, T.; Tangpoong, J.; Nissapatorn, V. Recommended medicinal plants as source of natural products: A review. Digit. Chin. Med. 2018, 1, 131–142. [Google Scholar] [CrossRef]
- Khan, M.I.; Maqsood, M.; Saeed, R.A.; Alam, A.; Sahar, A.; Kieliszek, M.; Miecznikowski, A.; Muzammil, H.S.; Aadil, R.M. Phytochemistry, Food Application, and Therapeutic Potential of the Medicinal Plant (Withania coagulans): A Review. Molecules 2021, 26, 6881. [Google Scholar] [CrossRef]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Field, M.J.; Bramwell, S.; Hughes, J.; Singh, L. Detection of static and dynamic components of mechanical allodynia in rat models of neuropathic pain: Are they signalled by distinct primary sensory neurones? Pain 1999, 83, 303–311. [Google Scholar] [CrossRef]
- Maher, S.; Shabbir, M.; Anam, I.; Khan, N.; Iqbal, S.; Saleem, F. Anti-inflammatory and anti-oxidant activities of methanolic extract of medicinal plants from Balochistan. Int. J. Biol. Biotechnol. 2020, 15, 691–697. [Google Scholar]
- Noori, A.; Amjad, L.; Yazdani, F. Effects of Withania coagulans extract and morphine on spermatogenesis in rats. Trop. J. Pharm. Res. 2019, 18, 817–822. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Maria, D.N.; Mishra, S.R.; Guragain, D.; Wang, X.; Jablonski, M.M. Once Daily Pregabalin Eye Drops for Management of Glaucoma. ACS Nano 2019, 13, 13728–13744. [Google Scholar] [CrossRef]
- Asghar, A.; Aamir, M.N.; Shah, M.A.; Syed, S.K.; Munir, R. Development, characterization and evaluation of in vitro anti-inflammatory activity of Withania coagulans extract and extract loaded microemulsion. Pak. J. Pharm. Sci. 2021, 34, 473–479. [Google Scholar]
- Arafa, M.G.; Ayoub, B.M. DOE optimization of nano-based carrier of pregabalin as hydrogel: New therapeutic & chemometric approaches for controlled drug delivery systems. Sci. Rep. 2017, 7, 41503. [Google Scholar]
- Wang, X.-R.; Gao, S.-Q.; Niu, X.-Q.; Li, L.-J.; Ying, X.-Y.; Hu, Z.-J.; Gao, J.-Q. Capsaicin-loaded nanolipoidal carriers for topical application: Design, characterization, and in vitro/in vivo evaluation. Int. J. Nanomed. 2017, 12, 3881. [Google Scholar] [CrossRef] [Green Version]
- Atarod, M.; Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis of Pd/RGO/Fe3O4 nanocomposite using Withania coagulans leaf extract and its application as magnetically separable and reusable catalyst for the reduction of 4-nitrophenol. J. Colloid Interface Sci. 2016, 465, 249–258. [Google Scholar] [CrossRef]
- Derayea, S.M.; Attia, T.Z.; Elnady, M. Development of spectrofluorimetric method for determination of certain antiepileptic drugs through condensation with ninhydrin and phenyl acetaldehyde. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 48–54. [Google Scholar] [CrossRef]
- Gupta, C.; Puri, R.; Hussain, A.; Jain, S.K. Development and Validation of Ninhydrin Based Colorimetric Spectrophotometric Assay for Determination of Pregabalin in Different Dissolution Mediums. Eurasian J. Anal. Chem. 2012, 8, 90–98. [Google Scholar]
- Bennett, G.J.; Xie, Y.-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Scheich, B.; Vincze, P.; Szőke, É.; Borbély, É.; Hunyady, Á.; Szolcsányi, J.; Dénes, Á.; Környei, Z.; Gaszner, B.; Helyes, Z. Chronic stress-induced mechanical hyperalgesia is controlled by capsaicin-sensitive neurones in the mouse. Eur. J. Pain 2017, 21, 1417–1431. [Google Scholar] [CrossRef]
- Bölcskei, K.; Horváth, D.; Szolcsanyi, J.; Pethő, G. Heat injury-induced drop of the noxious heat threshold measured with an increasing-temperature water bath: A novel rat thermal hyperalgesia model. Eur. J. Pharmacol. 2007, 564, 80–87. [Google Scholar] [CrossRef]
- Widyadharma, I.P.E.; Purwata, T.E.; Suprapta, D.N.; Sudewi, A.R. Anthocyanin derived from purple sweet potato water extracts ameliorated oxidative stress, inflammation, mechanical Allodynia, and cold Allodynia among chronic constriction injury-induced neuropathic pain in rats. Open Access Maced. J. Med. Sci. 2020, 8, 529–536. [Google Scholar] [CrossRef]
- Jiang, R.; Wei, H. Beneficial effects of octreotide in alcohol-induced neuropathic pain. Role of H 2S, BDNF, TNF-α and Nrf2. Acta Cirúrgica Bras. 2021, 36, e360408. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.M.; Drescher, C.; Englbrecht, J.S.; Klein, T.; Magerl, W.; Zahn, P.K. Progesterone relates to enhanced incisional acute pain and pinprick hyperalgesia in the luteal phase of female volunteers. Pain 2019, 160, 1781–1793. [Google Scholar] [CrossRef]
- Hong, D.G.; Hwang, S.-M.; Park, J.-M. Efficacy of ganglion impar block on vulvodynia: Case series and results of mid-and long-term follow-up. Medicine 2021, 100, e26799. [Google Scholar] [CrossRef]
- Silva, M.A.; Trevisan, G.; Klafke, J.Z.; Rossato, M.F.; Walker, C.I.B.; Oliveira, S.M.; Silva, C.R.; Boligon, A.A.; Flores, F.C.; de Bona Silva, C. Antinociceptive and anti-inflammatory effects of Aloe saponaria Haw on thermal injury in rats. J. Ethnopharmacol. 2013, 146, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, P.; de Freitas, R.L.; Boccella, S.; Iannotta, M.; Belardo, C.; Mazzitelli, M.; Romano, R.; De Gregorio, D.; Coimbra, N.C.; Palazzo, E. Characterization of the sensory, affective, cognitive, biochemical, and neuronal alterations in a modified chronic constriction injury model of neuropathic pain in mice. J. Neurosci. Res. 2020, 98, 338–352. [Google Scholar] [CrossRef]
- Popa, G.; Dragostin, O.; Buzia, O.D.; Tartau, L.M.; Profire, L.; Gafitanu, C. Studies on Obtaining and Characterization a Pregabalin-cyclodextrin Complex for Taste Masking Purpose. Rev. Chim. 2017, 68, 337–380. [Google Scholar] [CrossRef]
- Tripathi, D.; Modi, A.; Narayan, G.; Rai, S.P. Green and cost effective synthesis of silver nanoparticles from endangered medicinal plant Withania coagulans and their potential biomedical properties. Mater. Sci. Eng. C 2019, 100, 152–164. [Google Scholar] [CrossRef]
- Peerzade, N.; Sayed, N.; Das, N. Antimicrobial and phytochemical screening of methanolic fruit extract of Withania coagulans L. Dunal for evaluating the antidiabetic activity. Pharma Innov. J. 2018, 7, 197–204. [Google Scholar]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Salimi, A.; Hedayatipour, N.; Moghimipour, E. The effect of various vehicles on the naproxen permeability through rat skin: A mechanistic study by DSC and FT-IR techniques. Adv. Pharm. Bull. 2016, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Dave, V.; Sharma, S.; Yadav, R.B.; Agarwal, U. Herbal liposome for the topical delivery of ketoconazole for the effective treatment of seborrheic dermatitis. Appl. Nanosci. 2017, 7, 973–987. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.S.G.; Toshniwal, S.S. Formulation Taste Masked Orodispersible Tablet of Pregabalin. Int. J. Drug Deliv. 2013, 5, 56. [Google Scholar]
- Ghumman, S.A.; Bashir, S.; Noreen, S.; Khan, A.M.; Malik, M.Z. Taro-corms mucilage-alginate microspheres for the sustained release of pregabalin: In vitro & in vivo evaluation. Int. J. Biol. Macromol. 2019, 139, 1191–1202. [Google Scholar]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Plant-mediated green synthesis of nanostructures: Mechanisms, characterization, and applications. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 199–322. [Google Scholar]
- Lamichhane, S.; Park, J.-B.; Sohn, D.H.; Lee, S. Customized novel design of 3D printed pregabalin tablets for intra-gastric floating and controlled release using fused deposition modeling. Pharmaceutics 2019, 11, 564. [Google Scholar] [CrossRef] [Green Version]
- Pajic, N.B.; Nikolic, I.; Mitsou, E.; Papadimitriou, V.; Xenakis, A.; Randjelovic, D.; Dobricic, V.; Smitran, A.; Cekic, N.; Calija, B. Biocompatible microemulsions for improved dermal delivery of sertaconazole nitrate: Phase behavior study and microstructure influence on drug biopharamaceutical properties. J. Mol. Liq. 2018, 272, 746–758. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Yee, N.J.; Kaur Ambar Jeet Singh, B.J.; Panneerselvam, J.; Madheswaran, T.; Chellian, J.; Satija, S.; Mehta, M.; Gulati, M.; Gupta, G. Formulation and characterization of glibenclamide and quercetin-loaded chitosan nanogels targeting skin permeation. Ther. Deliv. 2019, 10, 281–293. [Google Scholar] [CrossRef]
- Zheng, Y.; Ouyang, W.-Q.; Wei, Y.-P.; Syed, S.F.; Hao, C.-S.; Wang, B.-Z.; Shang, Y.-H. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int. J. Nanomed. 2016, 11, 5971. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wu, C.; Fan, G.; Li, T.; Gong, H.; Cao, F. Ginkgo biloba extracts-loaded starch nano-spheres: Preparation, characterization, and in vitro release kinetics. Int. J. Biol. Macromol. 2018, 106, 148–157. [Google Scholar] [CrossRef]
- Cojocaru, V.; Ranetti, A.E.; Hinescu, L.G.; Ionescu, M.; Cosmescu, C.; Poștoarcă, A.G.; Cinteză, L.O. Formulation and evaluation of in vitro release kinetics of Na3CaDTPA decorporation agent embedded in microemulsion-based gel formulation for topical delivery. Farmacia 2015, 63, 656–664. [Google Scholar]
- Plaza-Villegas, F.; Heir, G.; Markman, S.; Khan, J.; Noma, N.; Benoliel, R.; Patel, J.; Eliav, E. Topical pregabalin and diclofenac for the treatment of neuropathic orofacial pain in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 449–456. [Google Scholar] [CrossRef]
- Lorenzoni, R.; Contri, R.V.; de Lima, C.K.F.; Barreto, F.; de Araujo, B.V.; Pohlmann, A.R.; de Miranda, A.L.P.; Dalla Costa, T.; Guterres, S.S. Dermatopharmacokinetic and pharmacodynamic evaluation of a novel nanostructured formulation containing capsaicinoids for treating neuropathic pain. Int. J. Pharm. 2021, 596, 120294. [Google Scholar] [CrossRef]
- Nigam, K.; Ruba, P.H.; Kapoor, P.; Gabrani, R.; Dang, S. Nano-carriers for natural therapeutics in management of neuropathic pain. In Nanoformulations in Human Health; Springer: Berlin/Heidelberg, Germany, 2020; pp. 361–376. [Google Scholar]
- Forouzanfar, F.; Hosseinzadeh, H.; Khorrami, M.B.; Asgharzade, S.; Rakhshandeh, H. Attenuating effect of Portulaca oleracea extract on chronic constriction injury induced neuropathic pain in rats: An evidence of anti-oxidative and anti-inflammatory effects. CNS Neurol. Disord. Drug Targets Former. Curr. Drug Targets CNS Neurol. Disord. 2019, 18, 342–349. [Google Scholar] [CrossRef]
- Ullah, R.; Badshah, W.; Ali, G.; Ullah, A.; Khan, S.U.; Ahmad, N.; Shahid, M.; Naveed, M.; Ullah, S.; Bangash, S.A. Cassia artemisiodes attenuates nociceptive and diabetes-induced neuropathic pain modalities apropos antioxidant and anti-inflammatory mechanisms. Biomed. Pharmacother. 2022, 149, 112834. [Google Scholar] [CrossRef]
- García-Alonso, G.; Atzori, M.; Salgado, R.; Báez, A.; Miranda, M.; Rangel, A.; Guevara, E.; Cuevas, R.; Vega-Riquer, J.M.; Avila-Acevedo, J.G. Antidepressant Effect of Buddleja cordata Methanolic Extract in Chronic Stress Mouse Model. Pharmacogn. Mag. 2021, 17, 780. [Google Scholar]
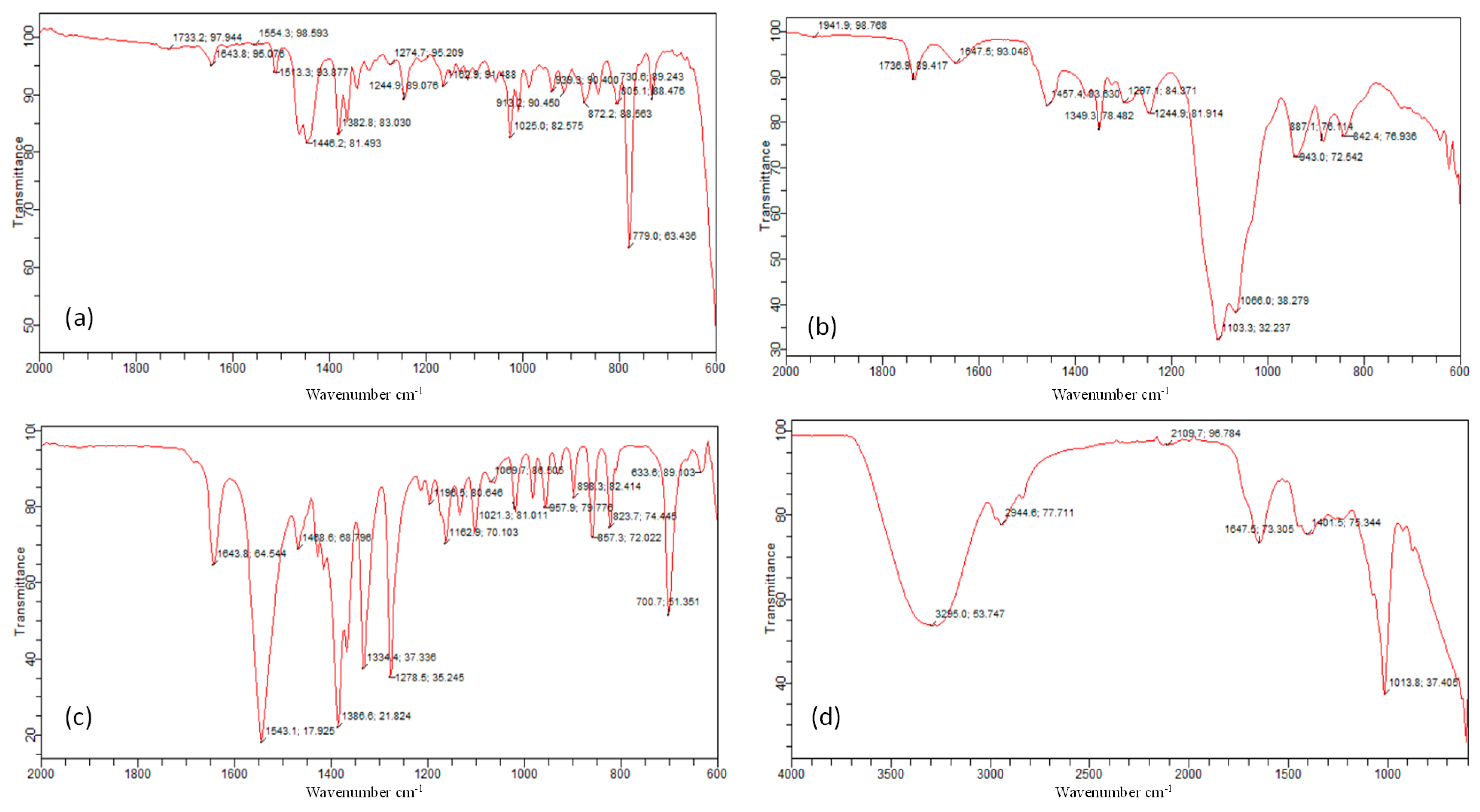




| Ingredients | N-H Bend | N-O Stretch | C-H Bend | C-O Stretch | O-H Bend | CH2-CH3 |
|---|---|---|---|---|---|---|
| Pregabalin | 1643 cm−1 | 1543 cm−1 | 1468 cm−1 | 1279 cm−1 | 857–933 cm−1 [33]. | 633 cm−1 |
| Extract | 1013 cm−1 | 2944 cm−1 | ||||
| Frankincense oil | 1643 cm−1 | 1554 cm−1 | 1446 cm−1 | 1274 cm−1 | 939 cm−1 | |
| Smix | 1647 cm−1 | 1457 cm−1 | 1297 cm−1 | 943 cm−1 | ||
| Drug loaded gel | 1640 cm−1 | 1457 cm−1 | 618 cm−1 | |||
| Extract loaded gel | 1636 cm−1 | 1457 cm−1 | ||||
| Drug + extract loaded gel | 1640 cm−1 | 1461 cm−1 | 622 cm−1 |
| Sr. no | Zero Order | First Order | Krosmeyer Pappas | Higuchi | Hixson Crowell | Result | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ko | R2 | K1 | R2 | KKP | R2 | n | KH | R2 | KHC | R2 | ||
| E | 0.256 | 0.909 | 0.124 | 0.627 | 0.124 | 0.627 | 0.124 | 5.035 | 0.952 | 0.003 | 0.9325 | Higuchi + fickian |
| D | 0.199 | 0.862 | 0.004 | 0.783 | 18.519 | 0.742 | 0.206 | 3.68 | 0.802 | 0.001 | 0.8049 | Zero order + non fickian |
| E + D | 0.173 | 0.947 | 0.003 | 0.950 | 10.688 | 0.947 | 0.282 | 3.24 | 0.958 | 0.001 | 0.001 | Higuchi + fickian |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, A.; Aamir, M.N.; Sheikh, F.A.; Ahmad, N.; Elsherif, M.A.; Abbas Bukhari, S.N. Co-Combination of Pregabalin and Withania coagulans-Extract-Loaded Topical Gel Alleviates Allodynia and Hyperalgesia in the Chronic Sciatic Nerve Constriction Injury for Neuropathic Pain in Animal Model. Molecules 2022, 27, 4433. https://doi.org/10.3390/molecules27144433
Asghar A, Aamir MN, Sheikh FA, Ahmad N, Elsherif MA, Abbas Bukhari SN. Co-Combination of Pregabalin and Withania coagulans-Extract-Loaded Topical Gel Alleviates Allodynia and Hyperalgesia in the Chronic Sciatic Nerve Constriction Injury for Neuropathic Pain in Animal Model. Molecules. 2022; 27(14):4433. https://doi.org/10.3390/molecules27144433
Chicago/Turabian StyleAsghar, Anam, Muhammad Naeem Aamir, Fatima Akbar Sheikh, Naveed Ahmad, Mervat A. Elsherif, and Syed Nasir Abbas Bukhari. 2022. "Co-Combination of Pregabalin and Withania coagulans-Extract-Loaded Topical Gel Alleviates Allodynia and Hyperalgesia in the Chronic Sciatic Nerve Constriction Injury for Neuropathic Pain in Animal Model" Molecules 27, no. 14: 4433. https://doi.org/10.3390/molecules27144433
APA StyleAsghar, A., Aamir, M. N., Sheikh, F. A., Ahmad, N., Elsherif, M. A., & Abbas Bukhari, S. N. (2022). Co-Combination of Pregabalin and Withania coagulans-Extract-Loaded Topical Gel Alleviates Allodynia and Hyperalgesia in the Chronic Sciatic Nerve Constriction Injury for Neuropathic Pain in Animal Model. Molecules, 27(14), 4433. https://doi.org/10.3390/molecules27144433









