Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components
Abstract
:1. Introduction
2. Results
2.1. Identification and Quantification of the Phenolic Components
2.1.1. Recovery of Phenolic Compounds and Antiradical Activity
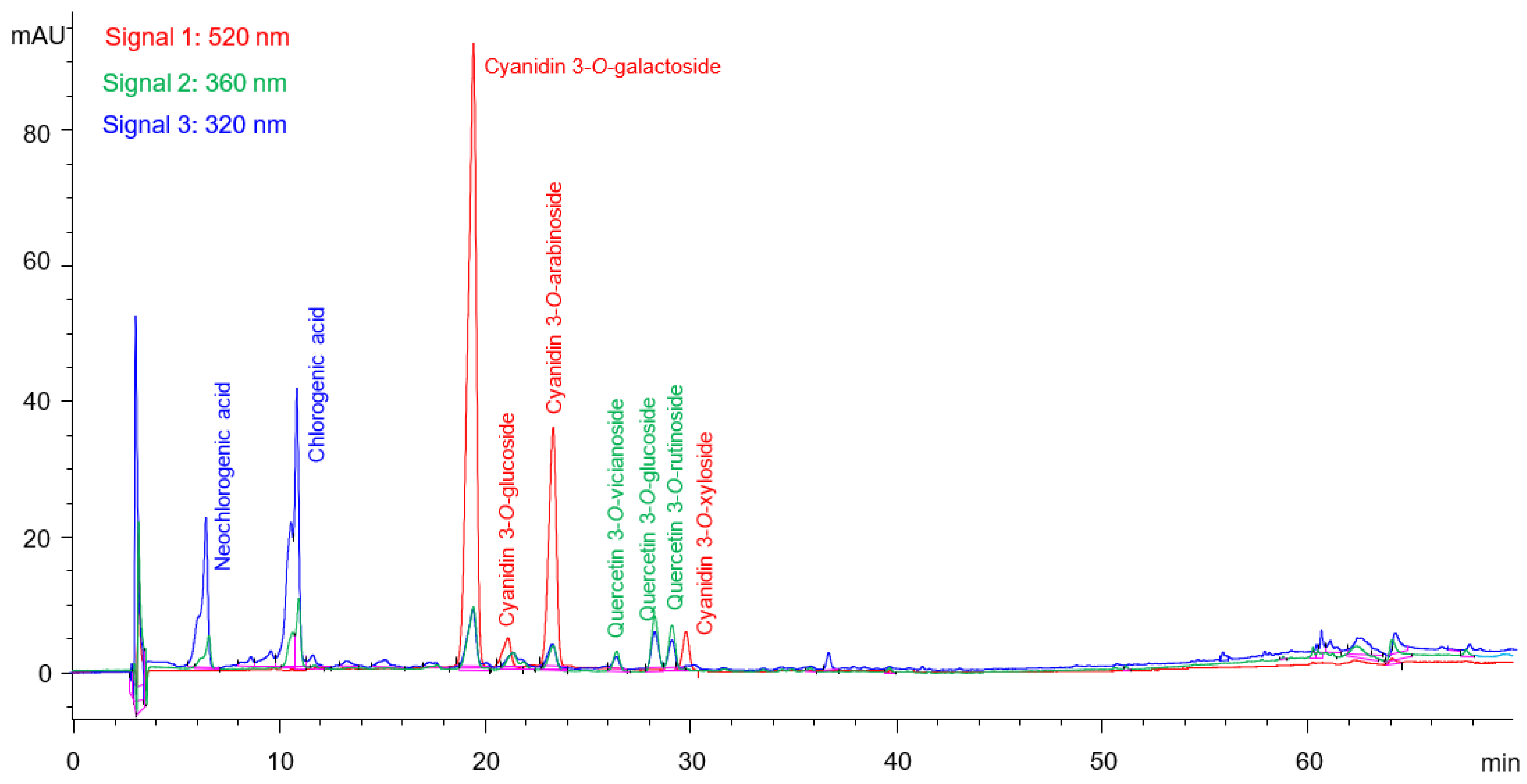
2.1.2. Phenolic Compound Analysis
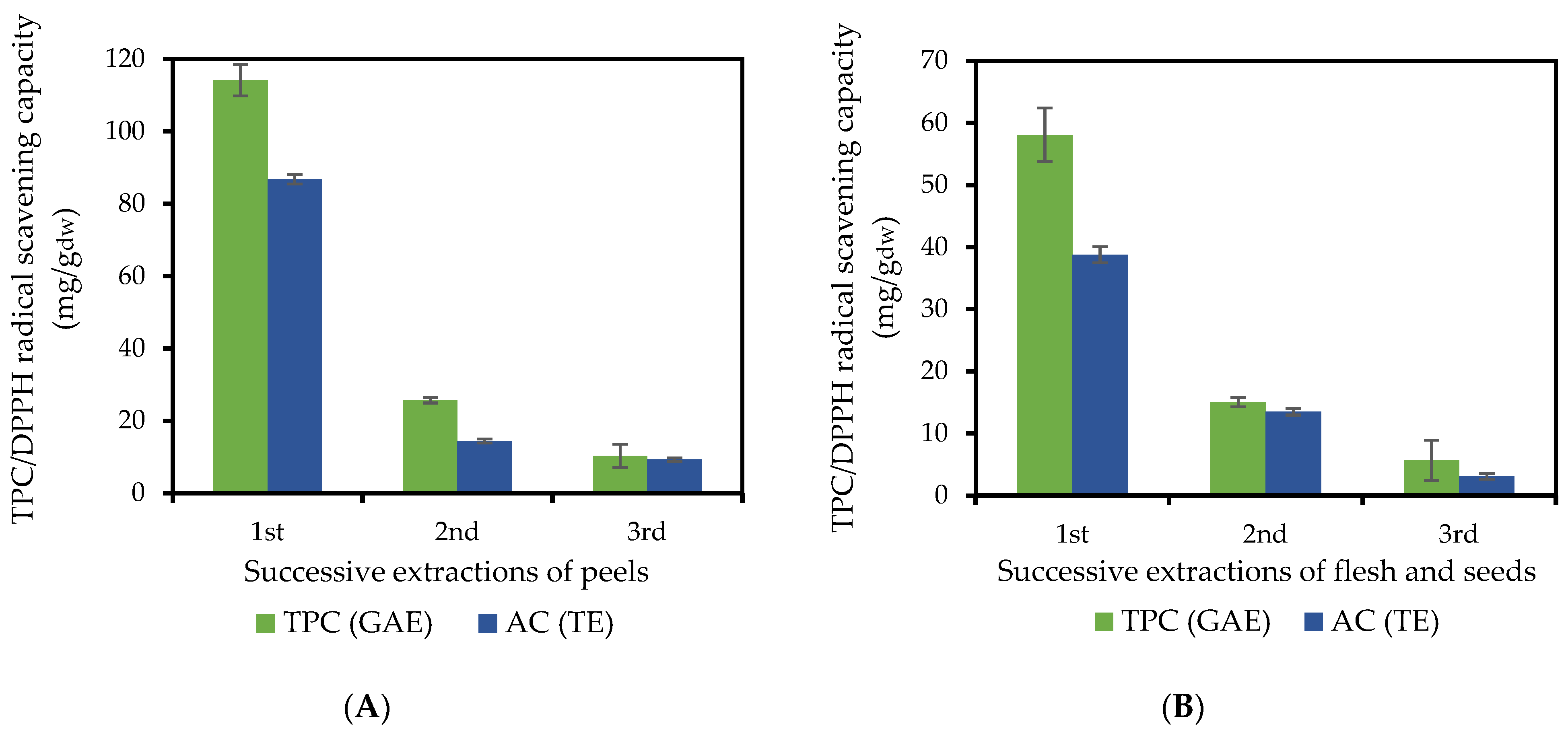
2.1.3. Phenolic Compound Partition in the Flesh and Peel of the Fruit
2.2. Juice Production and Analysis
2.3. Phenolic Compound Recovery through Pomace Extraction
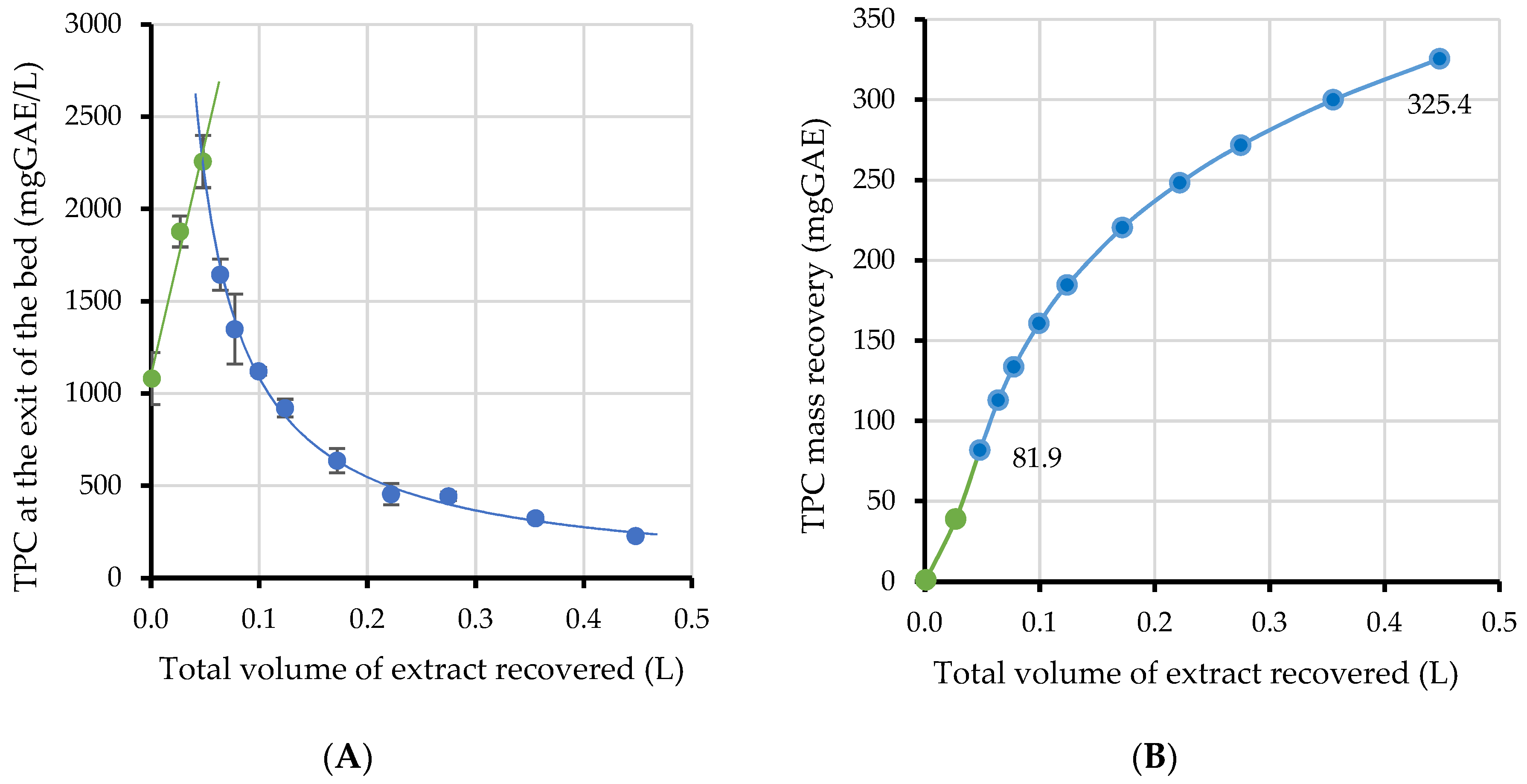
2.3.1. Extraction Kinetics
2.3.2. Extraction Yield
2.4. Encapsulation of the Phenolic Extract
3. Materials and Methods
3.1. Solvents and Reagents
3.2. Plant Material
3.3. Pretreatment Procedures
3.3.1. Peeling and Grinding
3.3.2. Pomace Drying
3.4. Extraction Procedure
3.4.1. Solid/Liquid Ultrasound Assisted Extraction (UAE)
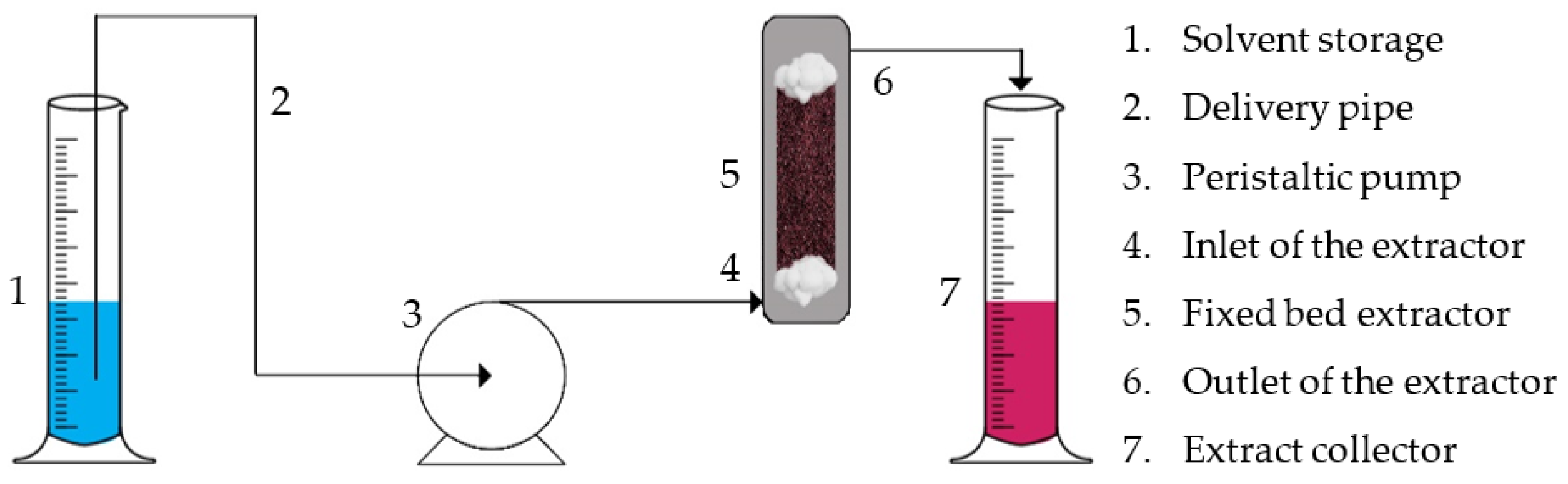
3.4.2. Fixed Bed Semi-Batch Extraction
3.5. Juicing
3.6. Spray Drying Encapsulation
3.6.1. Preparation of Feed Mixture
3.6.2. Spray Drying Encapsulation
3.7. Analytical Procedures
3.7.1. Determination of Total Phenol Content
3.7.2. Antiradical Capacity
3.7.3. HPLC-DAD Analyses
3.7.4. Encapsulation Yield and Efficiency
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak Jovanović, I. Antioxidant Activity and Polyphenols of Aronia in Comparison to other Berry Species. Agric. Conspec. Sci. 2007, 72, 301–306. [Google Scholar]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2006, 69, 164–169. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of Anthocyanins and Proanthocyanidins in Some Cultivars of Ribes, Aronia and Sambucus and Their Antioxidant Capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Cebulak, T.; Nski, J.; Kapusta, I.; Lachowicz-Wiśniewska, S. Effect of UV-C Radiation, Ultra-Sonication Electromagnetic Field and Microwaves on Changes in Polyphenolic Compounds in Chokeberry (Aronia melanocarpa). Molecules 2017, 1161. [Google Scholar] [CrossRef] [Green Version]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak Jovanović, I. Anthocyanin content and antioxidant activity of various red fruit juices. Dtsch. Lebensm. Rundsch. Z. Für Lebensm. Und Lebensm. 2007, 103, 58–64. [Google Scholar]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compos. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [Green Version]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and Antioxidant Activity of Black Chokeberry (Aronia melanocarpa) Polyphenols: In vitro and in vivo Evidences and Possible Mechanisms of Action: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Platonova, E.Y.; Shaposhnikov, M.V.; Lee, H.Y.; Lee, J.H.; Min, K.J.; Moskalev, A. Black chokeberry (Aronia melanocarpa) extracts in terms of geroprotector criteria. Trends Food Sci. Technol. 2021, 114, 570–584. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Novak, I.; Medvidović-Kosanović, M. Flavonols, Phenolic Acids and Antioxidant Activity of Some Red Fruits. Dtsch. Lebensm. Rundsch. Z. Für Lebensm. Und Lebensm. 2007, 103, 1–10. [Google Scholar]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.; Barsett, H. Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 9574587. [Google Scholar] [CrossRef] [Green Version]
- Vagiri, M.; Jensen, M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem. 2017, 217, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Samoticha, J.; Wojdyło, A.; Lech, K. The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. LWT-Food Sci. Technol. 2016, 66, 484–489. [Google Scholar] [CrossRef]
- Bednarska, M.A.; Janiszewska-Turak, E. The influence of spray drying parameters and carrier material on the physico-chemical properties and quality of chokeberry juice powder. J. Food Sci. Technol. 2020, 57, 564–577. [Google Scholar] [CrossRef] [Green Version]
- Horszwald, A.; Julien, H.; Andlauer, W. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013, 141, 2858–2863. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Galván D’Alessandro, L.; Dimitrov, K.; Vauchel, P.; Nikov, I. Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chem. Eng. Res. Des. 2014, 92, 1818–1826. [Google Scholar] [CrossRef]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochemistry 2015, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Klisurova, D.; Petrova, I.; Ognyanov, M.; Georgiev, Y.; Kratchanova, M.; Denev, P. Co-pigmentation of black chokeberry (Aronia melanocarpa) anthocyanins with phenolic co-pigments and herbal extracts. Food Chem. 2019, 279, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Grunovaitė, L.; Pukalskienė, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J. Funct. Foods 2016, 24, 85–96. [Google Scholar] [CrossRef]
- Sójka, M.; Kołodziejczyk, K.; Milala, J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crop. Prod. 2013, 51, 77–86. [Google Scholar] [CrossRef]
- Mayer-Miebach, E.; Adamiuk, M.; Behsnilian, D. Stability of Chokeberry Bioactive Polyphenols during Juice Processing and Stabilization of a Polyphenol-Rich Material from the By-Product. Agriculture 2012, 2, 244–258. [Google Scholar] [CrossRef] [Green Version]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutilized Chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) Accessions Are Rich Sources of Anthocyanins, Flavonoids, Hydroxycinnamic Acids, and Proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef] [PubMed]
- Kapci, B.; Neradová, E.; Čížková, H.; Voldřich, M.; Rajchl, A.; Capanoglu, E. Investigating the antioxidant potential of chokeberry (Aronia melanocarpa) products. J. Food Nutr. Res. 2013, 52, 219–229. [Google Scholar]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and A. ×prunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, G.-S.; Park, S.; Kim, Y.-H.; Kim, M.-B.; Lee, W.S.; Shin, S.C. Determination of chokeberry (Aronia melanocarpa) polyphenol components using liquid chromatography-tandem mass spectrometry: Overall contribution to antioxidant activity. Food Chem. 2014, 146, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tsimogiannis, D.I.; Oreopoulou, V. The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3′, 4′-hydroxy substituted members. Innov. Food Sci. Emerg. Technol. 2006, 7, 140–146. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Oreopoulou, V. Defining the role of flavonoid structure on cottonseed oil stabilization: Study of A-and C-ring substitution. J. Am. Oil Chem. Soc. 2007, 84, 129–136. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Bimpilas, A.; Oreopoulou, V. DPPH radical scavenging and mixture effects of plant o-diphenols and essential oil constituents. Eur. J. Lipid Sci. Technol. 2017, 118, 16003473. [Google Scholar] [CrossRef]
- Kayesh, E.; Shangguan, L.; Korir, N.K.; Sun, X.; Bilkish, N.; Zhang, Y.; Fang, J. Fruit skin color and the role of anthocyanin. Acta Physiol. Plant. 2013, 35, 2879–2890. [Google Scholar] [CrossRef]
- Sójka, M.; Król, B. Composition of industrial seedless black currant pomace. Eur. Food Res. Technol. 2008, 228, 597–605. [Google Scholar] [CrossRef]
- Rop, O.M.; Jiri & Jurikova, T.; Valsikova, M.; Sochor, J.; Reznicek, V.; Sumczynski, D. Phenolic content, antioxidant capacity, radical oxygen species scavenging and lipid peroxidation inhibiting activities of extracts of five black chokeberry (Aronia melanocarpa (Michx.) Elliot) cultivars. J. Med. Plants Res. 2010, 4, 2431–2437. [Google Scholar]
- Ochmian, I.D.; Grajkowski, J.; Smolik, M. Comparison of Some Morphological Features, Quality and Chemical Content of Four Cultivars of Chokeberry Fruits (Aronia melanocarpa). Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 253. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, K.; Howard, L.R.; Brownmiller, C.; Prior, R.L. Changes in chokeberry (Aronia melanocarpa L.) polyphenols during juice processing and storage. J. Agric. Food Chem. 2014, 62, 4018–4025. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Ochmian, I.; Oszmiański, J.; Skupień, K. Chemical composition, phenolics, and firmness of small black fruits. J. Appl. Bot. Food Qual. 2009, 83, 64–69. [Google Scholar]
- Dudonné, S.; Dubé, P.; Anhê, F.F.; Pilon, G.; Marette, A.; Lemire, M.; Desjardins, Y. Comprehensive analysis of phenolic compounds and abscisic acid profiles of twelve native Canadian berries. J. Food Compos. Anal. 2015, 44, 214–224. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Krivak, P. The influence of interactions among phenolic compounds on the antiradical activity of chokeberries (Aronia melanocarpa). Int. J. Food Sci. Nutr. 2011, 62, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Skupien, K.; Oszmianski, J. The effect of mineral fertilization on nutritive value and biological activity of chokeberry fruit. Agric. Food Sci. 2007, 16, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Michalik, J. Transformations of phenolic compounds in an in vitro model simulating the human alimentary tract. Food Technol. Biotechnol. 2009, 47, 456–463. [Google Scholar]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Tolic, M.T.; Jurcevic, I.L.; Krbavcic, I.P.; Markovic, K.; Vahcic, N. Phenolic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D. Changes in antioxidant activity of black chokeberry juice concentrate solutions during storage. Acta Sci. Pol. 2007, 6, 49–54. [Google Scholar]
- Roda-Serrat, M.C.; Andrade, T.A.; Rindom, J.; Lund, P.B.; Norddahl, B.; Errico, M. Optimization of the Recovery of Anthocyanins from Chokeberry Juice Pomace by Homogenization in Acidified Water. Waste Biomass Valor. 2021, 12, 1815–1827. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Kanakidi, L.-D.; Tsimogiannis, D.; Kiokias, S.; Oreopoulou, V. Formulation of Rosemary Extracts through Spray-Drying Encapsulation or Emulsification. Nutraceuticals 2022, 2, 1–21. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Retention of polyphenols in encapsulated sour cherry juice in dependence of drying temperature and wall material. LWT-Food Sci. Technol. 2017, 83, 110–117. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Vidović, S.; Ramić, M.; Ambrus, R.; Vladić, J.; Szabó-Révész, P.; Gavarić, A. Aronia Berry Processing by Spray Drying: From Byproduct to High Quality Functional Powder. Food Technol. Biotechnol. 2019, 57, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Bušić, A.; Komes, D.; Belščak-Cvitanović, A.; Vojvodić Cebin, A.; Špoljarić, I.; Mršić, G.; Miao, S. The potential of combined emulsification and spray drying techniques for encapsulation of polyphenols from rosemary (Rosmarinus officinalis L.) leaves. Food Technol. Biotechnol. 2018, 56, 494–505. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Psarrou, I.; Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction Kinetics of Phenolic Antioxidants from the Hydro Distillation Residues of Rosemary and Effect of Pretreatment and Extraction Parameters. Molecules 2020, 25, 4520. [Google Scholar] [CrossRef]
| Peel (Fresh Vasis) | Peel (Dry Basis) | Flesh (Fresh Basis) | Flesh (Dry Basis) | Fruit (Fresh Basis) | Fruit (Dry Basis) | |
|---|---|---|---|---|---|---|
| Total phenolic content | 49 ± 2 | 150 ± 5 | 17.6 ± 0.3 | 79 ± 3 | 21.9 ± 0.4 | 94 ± 2 |
| Antiradical capacity | 35 ± 1 | 111 ± 1 | 12.3 ± 0.1 | 55 ± 1 | 15.6 ± 0.1 | 67.1 ± 0.4 |
| Anthocyanins | ||||||
| Cyanidin 3-O-galactoside | 21.1 ± 1.0 | 65 ± 3 | 1.3 ± 0.1 | 5.7 ± 0.2 | 4.0 ± 0.1 | 17.4 ± 0.6 |
| Cyanidin 3-O-arabinoside * | 8.0 ± 0.3 | 25 ± 1 | 0.47 ± 0.02 | 2.1 ± 0.1 | 1.52 ± 0.05 | 6.5 ± 0.2 |
| Cyanidin 3-O-glucoside * | 1.8 ± 0.0 | 5.5 ± 0.1 | 0.08 ± 0.00 | 0.37 ± 0.01 | 0.31 ± 0.00 | 1.36 ± 0.02 |
| Cyanidin 3-O-xyloside * | 1.2 ± 0.1 | 3.8 ± 0.3 | 0.08 ± 0.00 | 0.38 ± 0.01 | 0.23 ± 0.02 | 1.05 ± 0.06 |
| Total | 32.1 ± 1.4 | 99 ± 4 | 1.9 ± 0.1 | 8.5 ± 0.3 | 6.1 ± 0.2 | 26.3 ± 0.7 |
| Hydroxycinnamic acids | ||||||
| Chlorogenic acid | 0.80 ± 0.08 | 2.4 ± 0.2 | 0.45 ± 0.01 | 2.03 ± 0.06 | 0.50 ± 0.01 | 2.14 ± 0.04 |
| Neochlorogenic acid * | 0.40 ± 0.01 | 1.2 ± 0.0 | 0.24 ± 0.01 | 1.09 ± 0.03 | 0.27 ± 0.01 | 1.13 ± 0.02 |
| Total | 1.20 ± 0.07 | 3.7 ± 0.2 | 0.69 ± 0.02 | 3.12 ± 0.04 | 0.77 ± 0.13 | 3.27 ± 0.05 |
| Flavonols | ||||||
| quercetin 3-O-vicianoside * | 0.22 ± 0.02 | 0.69 ± 0.05 | 0.068 ± 0.001 | 0.307 ± 0.001 | 0.09 ± 0.00 | 0.39 ± 0.01 |
| quercetin 3-O-glucoside * | 0.80 ± 0.00 | 2.48 ± 0.00 | 0.147 ± 0.002 | 0.659 ± 0.012 | 0.24 ± 0.00 | 1.02 ± 0.01 |
| quercetin 3-O-rutinoside | 0.76 ± 0.05 | 2.35 ± 0.14 | 0.130 ± 0.002 | 0.584 ± 0.008 | 0.22 ± 0.01 | 0.94 ± 0.03 |
| Total | 1.79 ± 0.03 | 5.5 ± 0.1 | 0.35 ± 0.00 | 1.55 ± 0.00 | 0.55 ± 0.01 | 2.32 ± 0.00 |
| Concentration (mg/g) | Reference | |
|---|---|---|
| Total phenolic content | 13.3, 10.79–19.21, 18.45–23.40, 7.78–12.85 (FW) | [27,28,37,38] |
| 78.49, 80.08, {19.6–27.8} (DW) | [3,16,19] | |
| Antiradical capacity | 11.00 (FW) | [27] |
| Total anthocyanins | 2.52–4.47, 6.19 * (FW) | [28,39] |
| 39.17, {0.26–0.52} * (DW) | [16,29] | |
| Cyanidin 3-O-galactoside | 2.92, 4.10–4.40, 1.01–1.20, 4.24 * (FW) | [25,27,37,39] |
| 12.82, 2.21–14.50, {0.19–0.34} *, {0.40–0.85} (DW) | [3,19,26,29] | |
| Cyanidin 3-O-araboniside | 1.35, 1.90–2.10, 1.54 * (FW) | [25,27,39] |
| 5.81, 1.05–2.16, {0.07–0.12} *, {0.14–0.32} ** (DW) | [3,19,26,29] | |
| Cyanidin 3-O-glucoside | 0.078–0.27 *, 0.20 *, 0.19 * (FW) | [38,39,40] |
| 0.42, 0.049–0.191, {0.03–0.14} *, {0.07–0.14} * (DW) | [3,19,26,29] | |
| Cyanidin 3-O-xyloside | 0.13–0.14, 0.20 * (FW) | [25,39] |
| 0.52, ND, ND–0.150 (DW) | [3,16,26] | |
| Total hydroxycinnamic acids | 1.21, 0.63, 1.16 (FW) | [39,41,42] |
| {2.87–3.93} (DW) | [29] | |
| Chlorogenic acid | 0.72–0.96, 0.69–0.74, 0.70 (FW) | [25,38,39] |
| 3.02, 3.32–6.42, {1.17–1.51} (DW) | [3,26,29] | |
| Neochlorogenic acid | 0.59–0.79, 0.56, 0.46 (FW) | [38,39,41] |
| 2.9, 2.16–6.54, {1.10–1.75} (DW) | [3,26,29] | |
| Total Flavonols | 0.71, 0.19–0.41, 0.34 (FW) | [7,39,43] |
| {0.13–0.25} (DW) | [29] | |
| Quercetin 3-O-galactoside | 0.09–0.14, 0.28 ***, 0.11 (FW) | [39,44,45] |
| 0.36, {0.17–0.27} *** (DW) | [3,19] | |
| Quercetin 3-O-glucoside | 0.07–0.08, 0.043 ***, 0.07 (FW) | [39,44,46] |
| 0.21, {0.10–0.15} **** (DW) | [3,19] | |
| Quercetin 3-O-rutinoside | 0.05–0.06, 0.042 ***, 0.04 (FW) | [38,39,46] |
| 0.15, {0.31–0.42} (DW) | [3,19] | |
| Quercetin derivatives unindentified | 0.27 (DW) | [3] |
| Laboratory Product | Commercial Product | Previous Research | References | |
|---|---|---|---|---|
| Total solid content | 197 ± 1 | 178.4 ± 0.1 | 13.42–21.54 ** | [47] |
| Reducing sugars | 53.0 ± 0.5 | 56.8 | ||
| Total phenolic content | 6231 ± 159 | 2550 ± 83 | 9154, 3002–6639 | [6,47] |
| Antiradical capacity | 4265 ± 89 | 2257 | 3026–10,059 | [47] |
| Anthocyanins | ||||
| Cyanidin 3-O-galactoside | 987 ± 12 | 47.5 | 1816 *, 787 | [3,6] |
| Cyanidin 3-O-arabinoside | 308 ± 8 | 8.6 | 647 *, 324 | [3,6] |
| Cyanidin 3-O-glucoside | 54 ± 5 | 2.1 | 74.3 *, 28.1 | [3,6] |
| Cyanidin 3-O-xyloside | 45 ± 2 | ND | 99.8 *, 33.6 | [3,6] |
| Total | 1395 ± 17 | 58.2 | 2637 *, 154–1228 * | [6,47] |
| Hydroxycinnamic acids | ||||
| Chlorogenic acid | 628 ± 38 | 127.1 | 415.9 | [3] |
| Neochlorogenic acid | 438 ± 5 | 161.0 | 290.81 | [3] |
| Total | 1116 ± 45 | 654 | ||
| Flavonols | ||||
| quercetin 3-O-vicianoside | 38.8 ± 1.1 | 27.5 | [3] | |
| quercetin 3-O-glucoside | 77.7 ± 0.2 | 31.2 | [3] | |
| quercetin 3-O-rutinoside | 89.7 ± 3.1 | 49.7 | [3] | |
| Flavonols unindentified | 62.8 ± 4 | 46.9 | [3] | |
| Total | 269 ± 12 | 27.9 ± 4 |
| Dried Aronia Pomace | Fresh Aronia Pomace | |
|---|---|---|
| Total phenolic content | 22.5 ± 0.06 | 33.6 ± 0.9 |
| Anthocyanins | 5.7 ± 0.2 | 12.21 |
| Hydroxycinnamic acids | 1.63 ± 0.03 | 1.69 |
| Flavonols | 1.51 ± 0.09 | 1.72 |
| Content in Berries | Recovery in Juice | Recovery in Dry Pomace Extraction | |||
|---|---|---|---|---|---|
| (mg/g Dry Fruit) | (mg/g Dry Fruit) | % | (mg/g Dry Fruit) | % | |
| Total phenolic content | 94 ± 2 | 12.3 ± 0.3 | 13.1 | 16.41 ± 0.04 | 17.5 |
| Antiradical activity | 67.1 ± 0.4 | 8.29 ± 0.17 | 12.4 | 11.12 ± 0.17 | 16.6 |
| Anthocyanins | 26.3 ± 0.7 | 2.73 ± 0.03 | 10.4 | 4.15 ± 0.15 | 15.9 |
| Hydroxycinnamic acids | 3.27 ± 0.05 | 2.15 ± 0.09 | 66.1 | 1.18 ± 0.06 | 35.1 |
| Flavonols | 2.32 ± 0.00 | 0.52 ± 0.02 | 22.5 | 1.10 ± 0.06 | 47.0 |
| Yield (%) | Efficiency (%) | |
|---|---|---|
| Total phenolic content | ||
| Maltodextrin | 92.2 ± 2.6 | 99 |
| Maltodextrin-gum arabic | 96.6 ± 2.4 | 100 |
| Anthocyanins | ||
| Maltodextrin | 91.4 ± 1.6 | 99 |
| Maltodextrin-gum arabic | 88.5 ± 2.0 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules 2022, 27, 4375. https://doi.org/10.3390/molecules27144375
Kaloudi T, Tsimogiannis D, Oreopoulou V. Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules. 2022; 27(14):4375. https://doi.org/10.3390/molecules27144375
Chicago/Turabian StyleKaloudi, Theodora, Dimitrios Tsimogiannis, and Vassiliki Oreopoulou. 2022. "Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components" Molecules 27, no. 14: 4375. https://doi.org/10.3390/molecules27144375
APA StyleKaloudi, T., Tsimogiannis, D., & Oreopoulou, V. (2022). Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules, 27(14), 4375. https://doi.org/10.3390/molecules27144375







