Lactic Acid-Based Natural Deep Eutectic Solvents to Extract Bioactives from Marine By-Products
Abstract
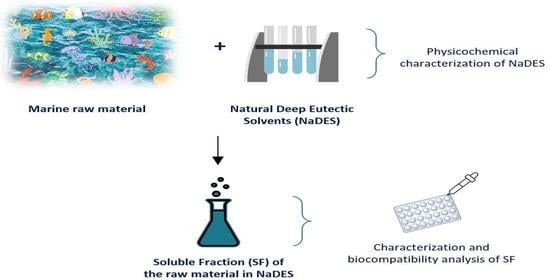
:1. Introduction
2. Results and Discussion
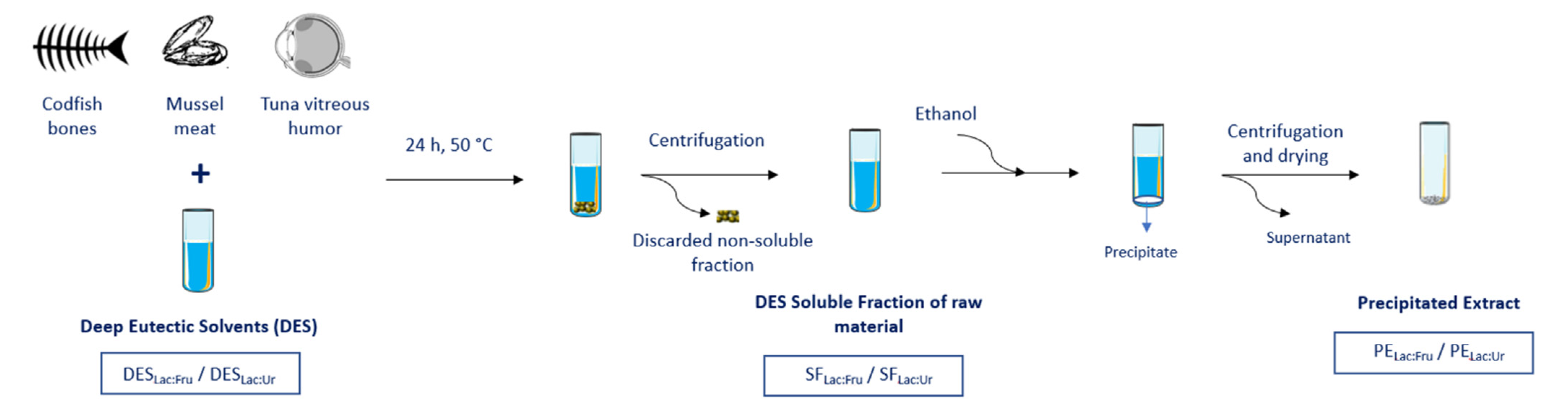
2.1. Extraction Process
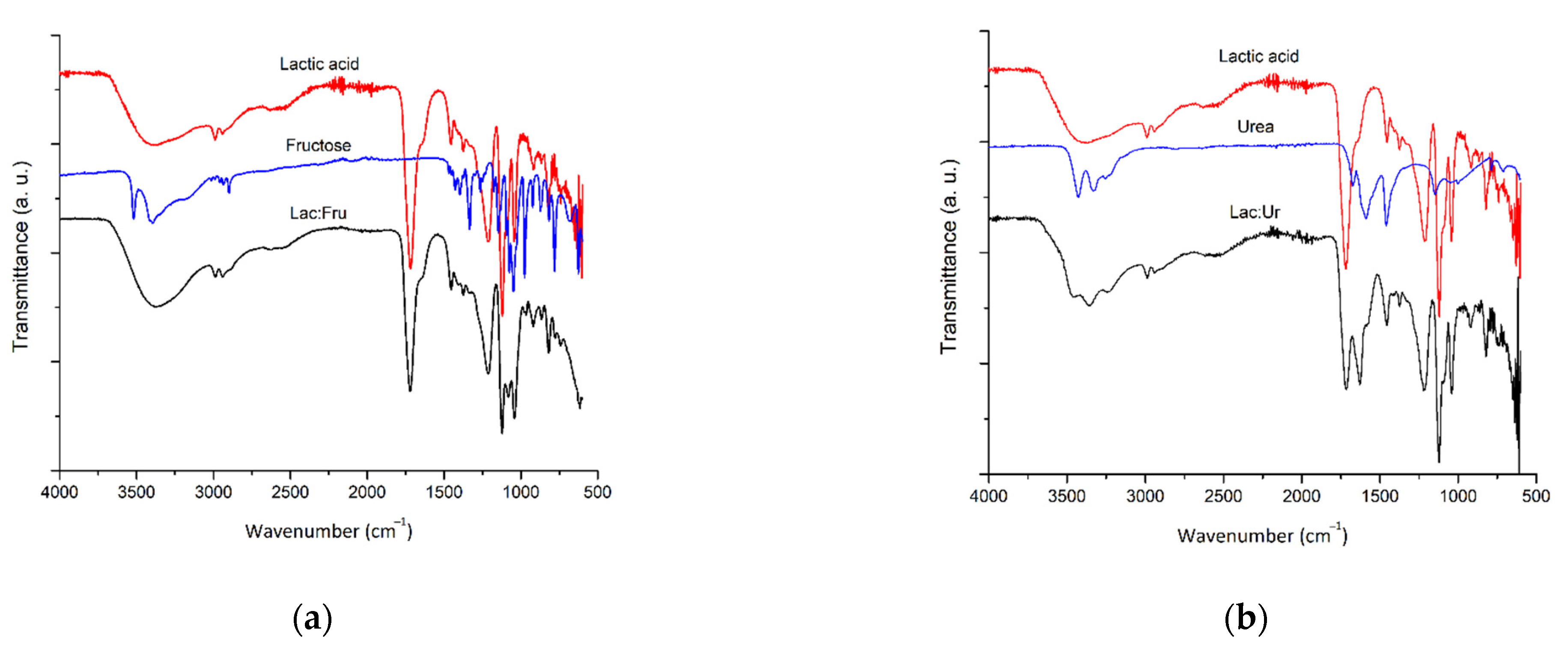
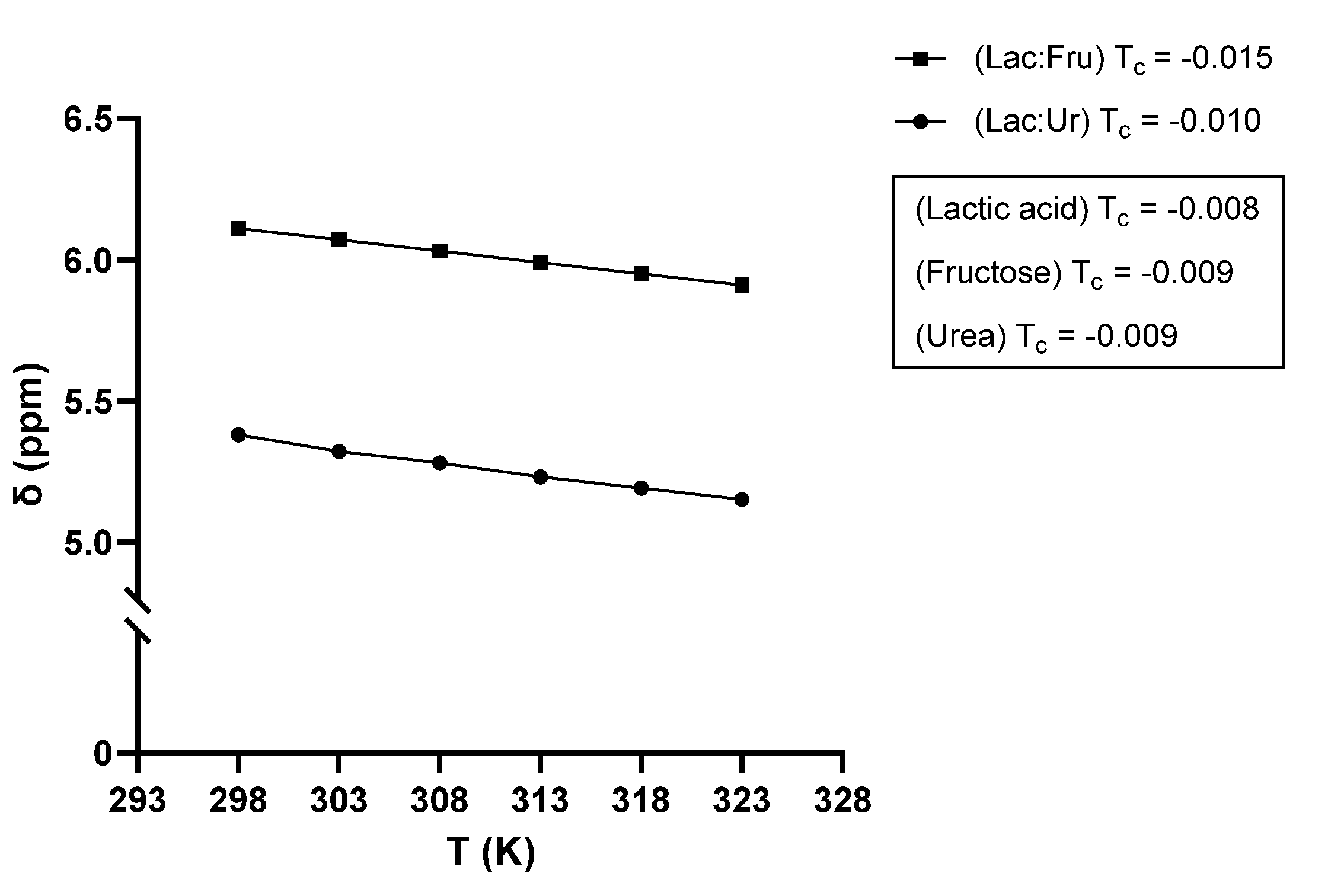
2.2. NaDES Characterization
2.2.1. FTIR Analysis
2.2.2. NMR Analysis
2.2.3. Physical Properties
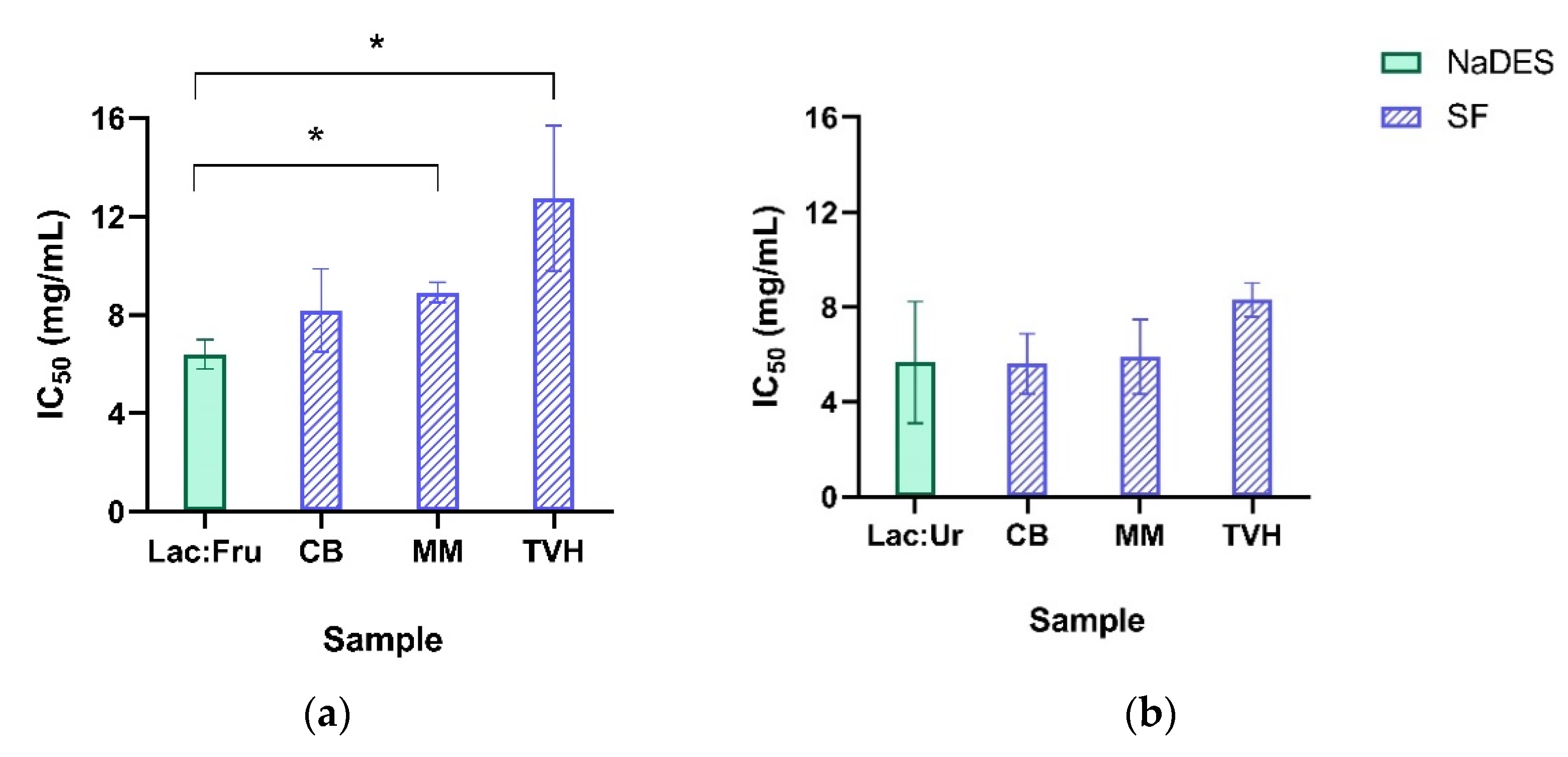
2.3. Extracts Characterization
2.4. Biocompatibility
3. Materials and Methods
3.1. Materials
3.2. NaDES Synthesis and Characterization
3.3. Extraction Process
3.4. Biomass Characterization
3.5. Extracts Characterization
3.6. Biocompatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. World Fisheries and Aquaculture. 2018. Available online: www.fao.org/publications (accessed on 27 August 2019).
- Govindharaj, M.; Roopavath, U.K.; Rath, S.N. Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J. Clean. Prod. 2019, 230, 412–419. [Google Scholar] [CrossRef]
- Jiménez, C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetics Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.A. Natural products chemistry: The emerging trends and prospective goals. Saudi Pharm. J. 2018, 26, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. International Natural Product Sciences Taskforce; Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P. Development of Plant-Based Medicines: Conservation, Efficacy and Safety; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar] [CrossRef]
- Nehete, J.Y.; Bhambar, R.S.; Narkhede, M.R.; Gawali, S.R. Natural proteins: Sources, isolation, characterization and applications. Pharmacogn. Rev. 2013, 7, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Nishizawa, K.; Yokoo, M.; Zhao, H.; Onishi, K.; Teraishi, M.; Utsumi, S.; Ishimoto, M.; Yoshikawa, M. Anti-hypertensive activity of genetically modified soybean seeds accumulating novokinin. Peptides 2008, 29, 331–337. [Google Scholar] [CrossRef]
- Alvarez, A.M.R.; Rodríguez, M.L.G. Lipids in pharmaceutical and cosmetic preparations. Grasas Aceites 2000, 51, 74–96. [Google Scholar] [CrossRef] [Green Version]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2020. In brief; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Jenkin, G.R.T.; Al-Bassam, A.Z.; Harris, R.C.; Abbott, A.P.; Smith, D.J.; Holwell, D.A.; Chapman, R.J.; Stanley, C.J. The application of deep eutectic solvent ionic liquids for environmentally-friendly dissolution and recovery of precious metals. Miner. Eng. 2016, 87, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Xing, H. Separation of light hydrocarbons with ionic liquids: A review. Chinese J. Chem. Eng. 2019, 27, 1374–1382. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Ionic Liquids and Deep Eutectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S. Deep eutectic solvents as versatile media for the synthesis of noble metal nanomaterials. Nanotechnol. Rev. 2017, 6, 271–278. [Google Scholar] [CrossRef]
- Shahbaz, K.; Bagh, F.S.G.; Mjalli, F.S.; AlNashef, I.M.; Hashim, M.A. Prediction of refractive index and density of deep eutectic solvents using atomic contributions. Fluid Phase Equilib. 2013, 354, 304–311. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of Improved Deep Eutectic Solvents Using Hole Theory. ChemPhysChem 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Sumiati, T.; Suryadi, H. Potency of Deep Euteutic Solvent as an Alternative Solvent on Pretreatment Process of Lignocellulosic Biomass: Review. J. Phys. Conf. Ser. 2021, 1764, 012014. [Google Scholar] [CrossRef]
- Ünlü, A.E.; Arlkaya, A.; Takaç, S. Use of deep eutectic solvents as catalyst: A mini-review. Green Process. Synth. 2019, 8, 355–372. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, Y.; Huang, Y.; Ding, X.; Chen, J.; Xu, K. Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 2014, 139, 2565–2573. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S. Choline chloride–based deep eutectic solvents (Ch-DESs) as promising green solvents for phenolic compounds extraction from bioresources: State-of-the-art, prospects, and challenges. Biomass Convers. Biorefinery 2020, 12, 2949–2962. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhou, Y.; Zhang, M.; Xia, Q.; Bi, W.; Chen, D.D.Y. Ecofriendly Mechanochemical Extraction of Bioactive Compounds from Plants with Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 6297–6303. [Google Scholar] [CrossRef]
- Piccirillo, C.; Silva, M.; Pullar, R.; da Cruz, I.B.; Jorge, R.; Pintado, M.; Castro, P. Extraction and characterisation of apatite- and tricalcium phosphate-based materials from cod fish bones. Mater. Sci. Eng. C 2013, 33, 103–110. [Google Scholar] [CrossRef]
- Ferraro, V.; Carvalho, A.P.; Piccirillo, C.; Santos, M.M.; Paula, P.M.E.; Pintado, M. Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues—A review. Mater. Sci. Eng. C 2013, 33, 3111–3120. [Google Scholar] [CrossRef]
- Medina Uzcátegui, L.U.; Vergara, K.; Martínez Bordes, G. Sustainable alternatives for by-products derived from industrial mussel processing: A critical review. Waste Manag. Res. J. Sustain. Circ. Econ. 2021, 40, 123–138. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; Bronze, M.d.R. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef]
- Rondon-Berrios, H.; Tandukar, S.; Mor, M.K.; Ray, E.; Bender, F.H.; Kleyman, T.R.; Weisbord, S.D. Urea for the Treatment of Hyponatremia. Clin. J. Am. Soc. Nephrol. 2018, 13, 1627–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttunen, J.K. Fructose in medicine. Postgrad. Med. J. 1971, 47, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boomsma, B.; Bikker, E.; Lansdaal, E.; Stuut, P. L-Lactic Acid-A Safe Antimicrobial for Home-and Personal Care Formulations. SOFW-J. 2015, 141, 2–5. [Google Scholar]
- Manivannan, M.; Rajendran, S. Investigation of inhibitive action of urea-Zn2+ system in the corrosion control of carbon steel in sea water. Int. J. Eng. Sci. Technol. 2011, 3, 8048–8060. [Google Scholar]
- Li, Y.; Jia, Y.; Wang, Z.; Li, X.; Feng, W.; Deng, P.; Yuan, L. An insight into the extraction of transition metal ions by picolinamides associated with intramolecular hydrogen bonding and rotational isomerization. RSC Adv. 2014, 4, 29702–29714. [Google Scholar] [CrossRef]
- Charisiadis, P.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Siskos, M.; Gerothanassis, I.P. 1H-NMR as a structural and analytical tool of intra- and intermolecular hydrogen bonds of phenol-containing natural products and model compounds. Molecules 2014, 19, 13643–13682. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, M.M.; Müller, S.; de Castilla, A.G.; Gurikov, P.; Matias, A.; Bronze, M.; Fernández, N. Physicochemical Characterization and Simulation of the Solid–Liquid Equilibrium Phase Diagram of Terpene-Based Eutectic Solvent Systems. Molecules 2021, 26, 1801. [Google Scholar] [CrossRef] [PubMed]
- Makoś, P.; Boczkaj, G. Deep eutectic solvents based highly efficient extractive desulfurization of fuels—Eco-friendly approach. J. Mol. Liq. 2019, 296, 111916. [Google Scholar] [CrossRef]
- Chemat, F.; Anjum, H.; Shariff, A.M.; Kumar, P.; Murugesan, T. Thermal and physical properties of (Choline chloride + urea + l-arginine) deep eutectic solvents. J. Mol. Liq. 2016, 218, 301–308. [Google Scholar] [CrossRef]
- Mjalli, F.S.; Ahmed, O.U. Characteristics and intermolecular interaction of eutectic binary mixtures: Reline and Glyceline. Korean J. Chem. Eng. 2016, 33, 337–343. [Google Scholar] [CrossRef]
- Bonem, J.M. Chemical Projects Scale Up; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Leron, R.B.; Soriano, A.N.; Li, M.H. Densities and refractive indices of the deep eutectic solvents (choline chloride+ethylene glycol or glycerol) and their aqueous mixtures at the temperature ranging from 298.15 to 333.15K. J. Taiwan Inst. Chem. Eng. 2012, 43, 551–557. [Google Scholar] [CrossRef]
- Nowosielski, B.; Jamrógiewicz, M.; Łuczak, J.; Śmiechowski, M.; Warmińska, D. Experimental and predicted physicochemical properties of monopropanolamine-based deep eutectic solvents. J. Mol. Liq. 2020, 309, 113110. [Google Scholar] [CrossRef]
- Abbott, A.P.; Barron, J.C.; Ryder, K.; Wilson, D. Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chem. Eur. J. 2007, 13, 6495–6501. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Shariff, A.M.; Lal, B. The study on temperature dependence of viscosity and surface tension of several Phosphonium-based deep eutectic solvents. J. Mol. Liq. 2017, 241, 500–510. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Fu, L.; Yang, Y.; Wang, Y.; Hu, X.; Wang, F.; Mu, T. Surface Tension of 50 Deep Eutectic Solvents: Effect of Hydrogen-Bonding Donors, Hydrogen-Bonding Acceptors, Other Solvents, and Temperature. Ind. Eng. Chem. Res. 2019, 58, 12741–12750. [Google Scholar] [CrossRef]
- McFerrin, A.R.; Davison, R.R. The effect of surface phenomena on a solvent extraction process. AIChE J. 1971, 17, 1021–1027. [Google Scholar] [CrossRef]
- Freire, M.G.; Carvalho, P.J.; Fernandes, A.M.; Marrucho, I.M.; Queimada, A.J.; Coutinho, J.A.P. Surface tensions of imidazolium based ionic liquids: Anion, cation, temperature and water effect. J. Colloid Interface Sci. 2007, 314, 621–630. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, W.; Zhang, X.-D.; Dai Z., Y.; Zhang, Y.Q. Steam Explosion-Assisted Extraction of Protein from Fish Backbones and Effect of Enzymatic Hydrolysis on the Extracts. Foods 2021, 10, 1942. [Google Scholar] [CrossRef]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef]
- Fernández, A.; Grienke, U.; Soler-Vila, A.; Guihéneuf, F.; Stengel, D.B.; Tasdemir, D. Seasonal and geographical variations in the biochemical composition of the blue mussel (Mytilus edulis L.) from Ireland. Food Chem. 2015, 177, 43–52. [Google Scholar] [CrossRef]
- Trilaksani, W.; Riyanto, B.; Wahyuningsih, T. Characterization and Antioxidant Activity of Hyaluronan from Vitreous Humor of Yellowfin Tuna Eye (Thunnus albacares). J. Pengolah. Has. Perikan. Indones. 2019, 22. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Leonardo, I.C.; Krstić, L.; Enríquez-de-Salamanca, A.; Diebold, Y.; González-García, M.J.; Gaspar, F.B.; Matias, A.A.; Bronze, M.R.; Fernández, N. Potential Ophthalmological Application of Extracts Obtained from Tuna Vitreous Humor Using Lactic Acid-Based Deep Eutectic Systems. Foods. 2022, 11, 342. [Google Scholar] [CrossRef]
- Manda, G.; Mocanu, M.A.; Marin, D.E.; Taranu, I. Dual Effects Exerted in Vitro by Micromolar Concentrations of Deoxynivalenol on Undifferentiated Caco-2 Cells. Toxins 2015, 7, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Cano-Sancho, G.; González-Arias, C.A.; Ramos, A.J.; Sanchis, V.; Fernández-Cruz, M.L. Cytotoxicity of the mycotoxins deoxynivalenol and ochratoxin A on Caco-2 cell line in presence of resveratrol. Toxicol. In Vitro 2015, 29, 1639–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bony, S.; Carcelen, M.; Olivier, L.; Devaux, A. Genotoxicity assessment of deoxynivalenol in the Caco-2 cell line model using the Comet assay. Toxicol. Lett. 2006, 166, 67–76. [Google Scholar] [CrossRef] [PubMed]
- González, C.G.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents for the “green”extraction of vanillin from vanilla pods. Flavour Fragr. J. 2018, 33, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef] [Green Version]
- Galland-Irmouli, A.V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional value of proteins from edible seaweed Palmaria palmata (dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Sambruy, Y.; Ferruzza, S.; Ranaldi, G.; De Angelis, I. Intestinal Cell Culture Models: Applications in Toxicology and Pharmacology. Cell Biol. Toxicol. 2001, 17, 301–317. [Google Scholar] [CrossRef]
- Serra, A.T.; Seabra, I.J.; Braga, M.E.M.; Bronze, M.R.; De Sousa, H.C.; Duarte, C.M.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 1: Recovery of extract fractions rich in bioactive compounds. J. Supercrit. Fluids 2010, 55, 184–191. [Google Scholar] [CrossRef]

| NaDES | Viscosity at 25 °C (mPa/s) | Viscosity at 50 °C (mPa/s) | Density (g/mL) | Surface Tension (mN/m) | Refractive Index |
|---|---|---|---|---|---|
| Lac:Fru | 746.70 ± 16.37 | 113.40 ± 5.76 | 1.25 ± 0.23 | 44.62 ± 0.07 | 1.4590 ± 0.0004 |
| Lac:Ur | 732.91 ± 10.22 | 187.93 ± 9.61 | 1.24 ± 0.45 | 43.96 ± 0.03 | 1.4368 ± 0.0006 |
| Raw Material | Lac:Fru | Lac:Ur |
|---|---|---|
| CB | 9.3 ± 1.3 | 10.7 ± 2.1 |
| MM | 19.3 ± 1.8 | 22.5 ± 1.9 |
| TVH | 13.1 ± 1.7 | 15.3 ± 1.6 |
| Raw Material | Composition | Lac:Fru | Lac:Ur |
|---|---|---|---|
| CB | Lipids | 1.54 | 1.86 |
| Protein | 53.12 | 57.63 | |
| Ash | 19.84 | 22.74 | |
| MM | Lipids | 4.52 | 5.07 |
| Protein | 66.11 | 75.20 | |
| Ash | 6.40 | 6.94 | |
| TVH | Lipids | 4.97 | 5.71 |
| Protein | 49.66 | 50.66 | |
| Ash | 27.98 | 29.03 |
| NaDES | Designation | Molar Ratio |
|---|---|---|
| Lactic acid:Fructose | Lac:Fru | 5:1 |
| Lactic acid:Urea | Lac:Ur | 4:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, M.M.; Cardeira, M.; Matias, A.A.; Bronze, M.R.; Fernández, N. Lactic Acid-Based Natural Deep Eutectic Solvents to Extract Bioactives from Marine By-Products. Molecules 2022, 27, 4356. https://doi.org/10.3390/molecules27144356
Abdallah MM, Cardeira M, Matias AA, Bronze MR, Fernández N. Lactic Acid-Based Natural Deep Eutectic Solvents to Extract Bioactives from Marine By-Products. Molecules. 2022; 27(14):4356. https://doi.org/10.3390/molecules27144356
Chicago/Turabian StyleAbdallah, Maha M., Martim Cardeira, Ana A. Matias, Maria Rosário Bronze, and Naiara Fernández. 2022. "Lactic Acid-Based Natural Deep Eutectic Solvents to Extract Bioactives from Marine By-Products" Molecules 27, no. 14: 4356. https://doi.org/10.3390/molecules27144356
APA StyleAbdallah, M. M., Cardeira, M., Matias, A. A., Bronze, M. R., & Fernández, N. (2022). Lactic Acid-Based Natural Deep Eutectic Solvents to Extract Bioactives from Marine By-Products. Molecules, 27(14), 4356. https://doi.org/10.3390/molecules27144356