Synthesis and Systematic Study on the Effect of Different PEG Units on Stability of PEGylated, Integrin-αvβ6-Specific A20FMDV2 Analogues in Rat Serum and Human Plasma
Abstract
:1. Introduction
2. Results and Discussion
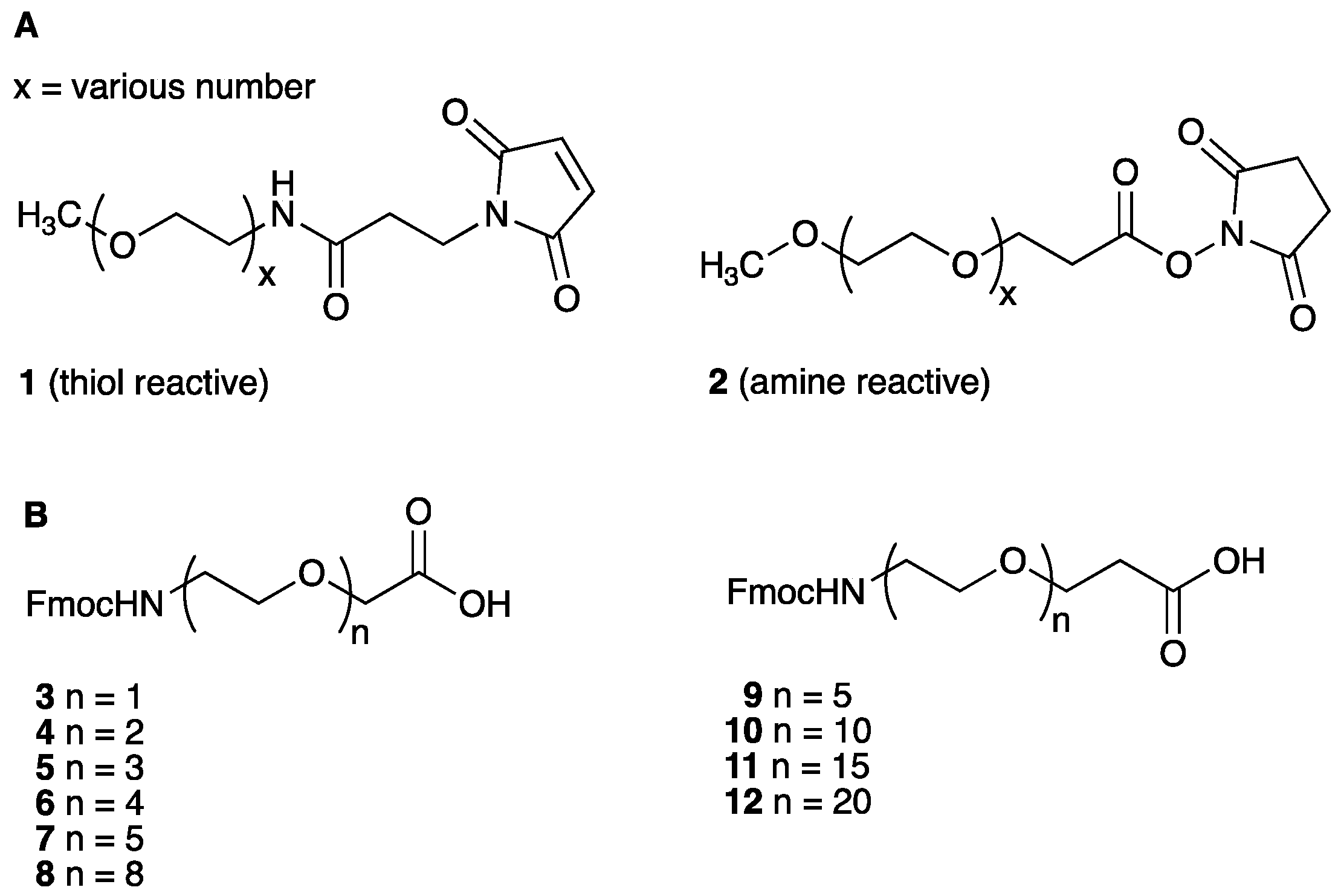
2.1. Synthesis and Stability Evaluation of A20FMDV2 (13) and PEGylated A20FMDV2 Analogues (14–23) in Rat Serum
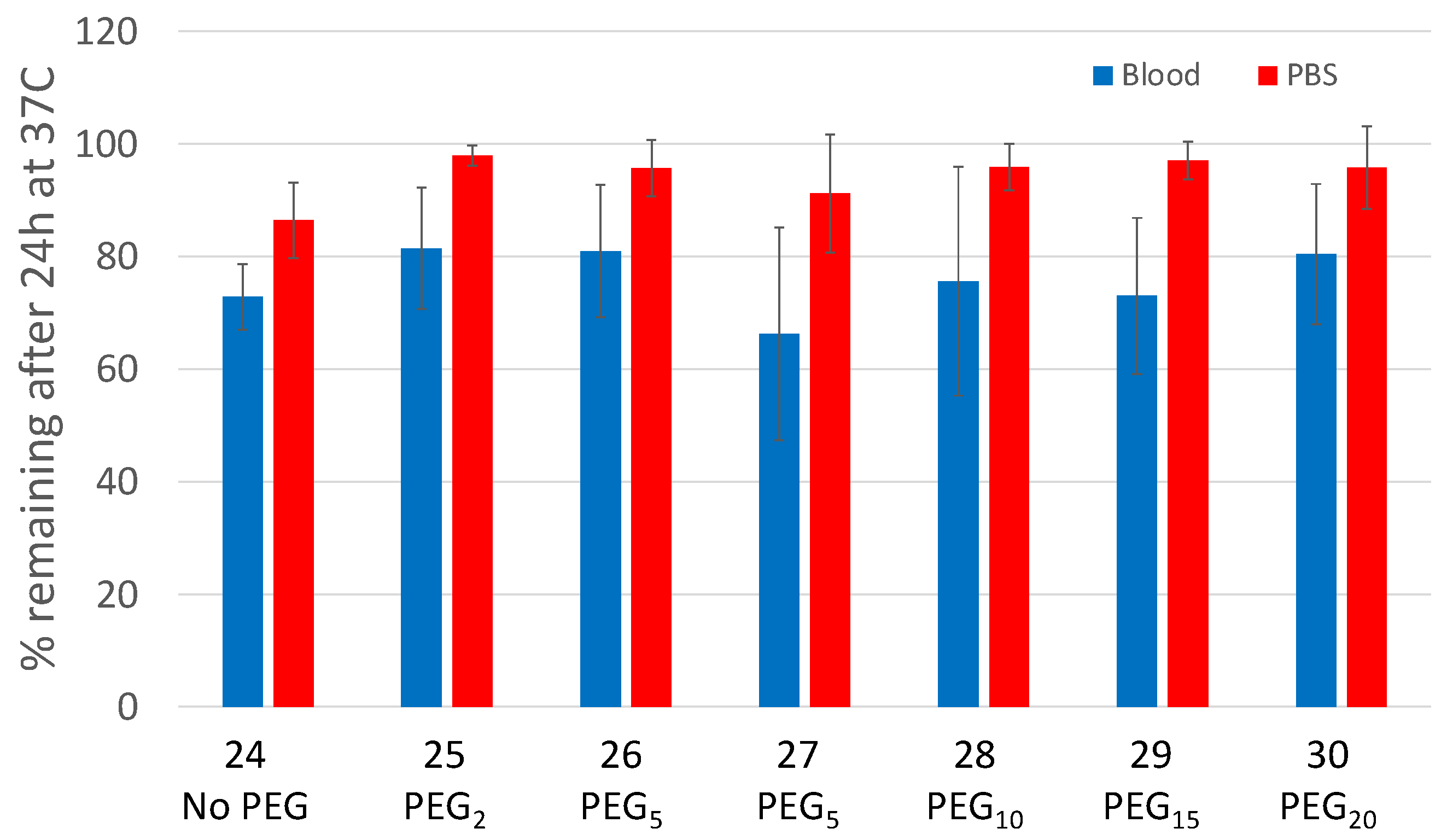
2.2. Synthesis and Human Plasma Stability of [111In]-DTPA-[2Lys(d-biotin)]A20FMDV2 (24) and PEGylated [111In]-DTPA-[2Lys(d-biotin)]A20FMDV2 Analogues (25–30)
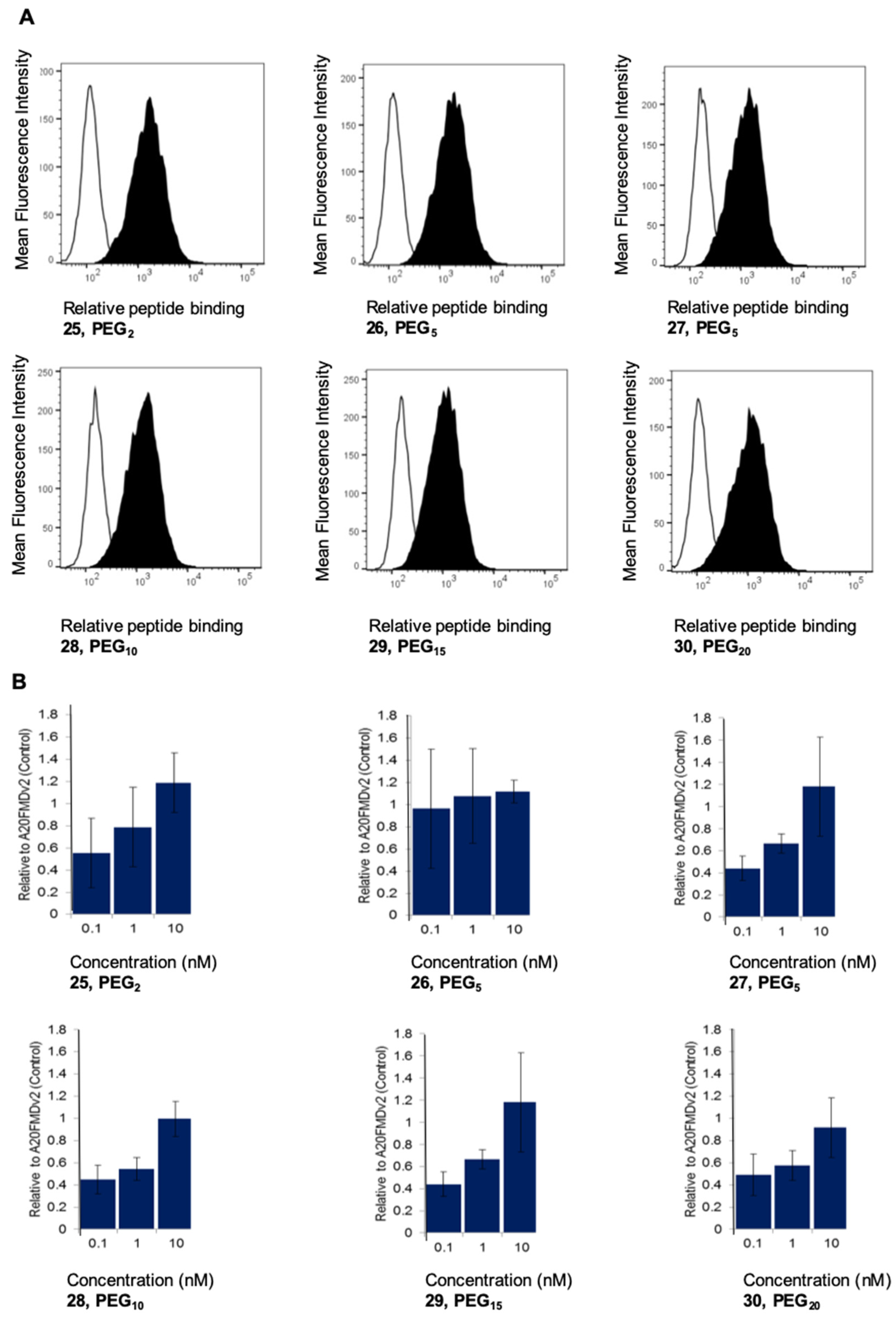
2.3. Specificity and Affinity Evaluation for A375Ppuroβ6 and A375Ppuro Cells of DTPA-[2Lys(d-biotin)]A20FMDV2 (24) and DTPA PEGylated-[2Lys(d-biotin)]A20FMDV2 Analogues (25–30) Using Flow Cytometry
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.2. General Procedure for Peptide Synthesis, Purification, and Analysis
5. Biological Studies
5.1. Cell Lines
5.2. Preparation of Peptide
5.3. Biological Assessment of PEGylated Peptides
6. Rat Serum Stability
Human Plasma Stability Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breuss, J.M.; Gillett, N.; Lu, L.; Sheppard, D.; Pytela, R. Restricted Distribution of Integrin Beta 6 MRNA in Primate Epithelial Tissues. J. Histochem. Cytochem. 1993, 41, 1521–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breuss, J.M.; Gallo, J.; DeLisser, H.M.; Klimanskaya, I.V.; Folkesson, H.G.; Pittet, J.F.; Nishimura, S.L.; Aldape, K.; Landers, D.V.; Carpenter, W. Expression of the Beta 6 Integrin Subunit in Development, Neoplasia and Tissue Repair Suggests a Role in Epithelial Remodeling. J. Cell Sci. 1995, 108, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Bates, R.C.; Bellovin, D.I.; Brown, C.; Maynard, E.; Wu, B.; Kawakatsu, H.; Sheppard, D.; Oettgen, P.; Mercurio, A.M. Transcriptional Activation of Integrin Β6 during the Epithelial-Mesenchymal Transition Defines a Novel Prognostic Indicator of Aggressive Colon Carcinoma. J. Clin. Investig. 2005, 115, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.J.; Nyström, M.L.; Marshall, J.F. αvβ6 Integrin in Wound Healing and Cancer of the Oral Cavity. J. Oral Pathol. Med. 2006, 35, 1–10. [Google Scholar] [CrossRef]
- Elayadi, A.N.; Samli, K.N.; Prudkin, L.; Liu, Y.-H.; Bian, A.; Xie, X.-J.; Wistuba, I.I.; Roth, J.A.; McGuire, M.J.; Brown, K.C. A Peptide Selected by Biopanning Identifies the Integrin αvβ6 as a Prognostic Biomarker for Nonsmall Cell Lung Cancer. Cancer Res. 2007, 67, 5889–5895. [Google Scholar] [CrossRef] [Green Version]
- Hazelbag, S.; Kenter, G.G.; Gorter, A.; Dreef, E.J.; Koopman, L.A.; Violette, S.M.; Weinreb, P.H.; Fleuren, G.J. Overexpression of the Alpha v Beta 6 Integrin in Cervical Squamous Cell Carcinoma Is a Prognostic Factor for Decreased Survival. J. Pathol. 2007, 212, 316–324. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Raghavan, S. Defining the Role of Integrin Alphavbeta6 in Cancer. Curr. Drug Targets 2009, 10, 645–652. [Google Scholar] [CrossRef]
- Moore, K.M.; Thomas, G.J.; Duffy, S.W.; Warwick, J.; Gabe, R.; Chou, P.; Ellis, I.O.; Green, A.R.; Haider, S.; Brouilette, K.; et al. Therapeutic Targeting of Integrin αvβ6 in Breast Cancer. J. Natl. Cancer Inst. 2014, 106, dju169. [Google Scholar] [CrossRef] [Green Version]
- Ruoslahti, E. RGD and Other Recognition Sequences for Integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Busk, M.; Pytela, R.; Sheppard, D. Characterization of the Integrin Alpha v Beta 6 as a Fibronectin-Binding Protein. J. Biol. Chem. 1992, 267, 5790–5796. [Google Scholar] [CrossRef]
- Prieto, A.L.; Edelman, G.M.; Crossin, K.L. Multiple Integrins Mediate Cell Attachment to Cytotactin/Tenascin. Proc. Natl. Acad. Sci. USA 1993, 90, 10154–10158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Griffiths, M.J.D.; Dalton, S.L.; Wu, J.; Pittet, J.-F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. A Mechanism for Regulating Pulmonary Inflammation and Fibrosis: The Integrin αvβ6 Binds and Activates Latent TGF Β1. Cell 1999, 96, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Annes, J.P.; Rifkin, D.B.; Munger, J.S. The Integrin αvβ6 Binds and Activates Latent TGFβ3. FEBS Lett. 2002, 511, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Mateu, M.G.; Valero, M.L.; Andreu, D.; Domingo, E. Systematic Replacement of Amino Acid Residues within an Arg-Gly-Asp-Containing Loop of Foot-and-Mouth Disease Virus and Effect on Cell Recognition. J. Biol. Chem. 1996, 271, 12814–12819. [Google Scholar] [CrossRef] [Green Version]
- Kraft, S.; Diefenbach, B.; Mehta, R.; Jonczyk, A.; Luckenbach, G.A.; Goodman, S.L. Definition of an Unexpected Ligand Recognition Motif for αvβ6 Integrin. J. Biol. Chem. 1999, 274, 1979–1985. [Google Scholar] [CrossRef] [Green Version]
- Logan, D.; Abu-Ghazaleh, R.; Blakemore, W.; Curry, S.; Jackson, T.; King, A.; Lea, S.; Lewis, R.; Newman, J.; Parry, N.; et al. Structure of a Major Immunogenic Site on Foot-and-Mouth Disease Virus. Nature 1993, 362, 566–568. [Google Scholar] [CrossRef]
- Jackson, T.; Sheppard, D.; Denyer, M.; Blakemore, W.; King, A.M.Q. The Epithelial Integrin αvβ6 Is a Receptor for Foot-and-Mouth Disease Virus. J. Virol. 2000, 74, 4949–4956. [Google Scholar] [CrossRef]
- DiCara, D.; Rapisarda, C.; Sutcliffe, J.L.; Violette, S.M.; Weinreb, P.H.; Hart, I.R.; Howard, M.J.; Marshall, J.F. Structure-Function Analysis of Arg-Gly-Asp Helix Motifs in αvβ6 Integrin Ligands. J. Biol. Chem. 2007, 282, 9657–9665. [Google Scholar] [CrossRef] [Green Version]
- Meecham, A.; Marshall, J. Harnessing the Power of Foot-and-Mouth-Disease Virus for Targeting Integrin Alpha-v Beta-6 for the Therapy of Cancer. Expert Opin. Drug Discov. 2021, 16, 737–744. [Google Scholar] [CrossRef]
- Saha, A.; Ellison, D.; Thomas, G.J.; Vallath, S.; Mather, S.J.; Hart, I.R.; Marshall, J.F. High-resolution in vivo Imaging of Breast Cancer by Targeting the Pro-invasive Integrin αvβ6. J. Pathol. 2010, 222, 52–63. [Google Scholar] [CrossRef]
- Dicara, D.; Burman, A.; Clark, S.; Berryman, S.; Howard, M.J.; Hart, I.R.; Marshall, J.F.; Jackson, T. Foot-and-Mouth Disease Virus Forms a Highly Stable, EDTA-Resistant Complex with Its Principal Receptor, Integrin Alphvbeta6: Implications for Infectiousness. J. Virol. 2008, 82, 1537–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firer, M.A.; Gellerman, G. Targeted Drug Delivery for Cancer Therapy: The Other Side of Antibodies. J. Hematol. Oncol. 2012, 5, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keat, N.; Kenny, J.; Chen, K.; Onega, M.; Garman, N.; Slack, R.J.; Parker, C.A.; Lumbers, R.T.; Hallett, W.; Saleem, A.; et al. A Microdose PET Study of the Safety, Immunogenicity, Biodistribution, and Radiation Dosimetry of 18F-FB-A20FMDV2 for Imaging the Integrin αvβ6. J. Nucl. Med. Technol. 2018, 46, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausner, S.H.; Abbey, C.K.; Bold, R.J.; Gagnon, M.K.; Marik, J.; Marshall, J.F.; Stanecki, C.E.; Sutcliffe, J.L. Targeted in vivo Imaging of Integrin αvβ6 with an Improved Radiotracer and Its Relevance in a Pancreatic Tumor Model. Cancer Res. 2009, 69, 5843–5850. [Google Scholar] [CrossRef] [Green Version]
- Hung, K.Y.; Harris, P.W.R.; Desai, A.; Marshall, J.F.; Brimble, M.A. Structure-Activity Relationship Study of the Tumour-Targeting Peptide A20FMDV2 via Modification of Lys16, Leu13, and N- and/or C-Terminal Functionality. Eur. J. Med. Chem. 2017, 136, 154–164. [Google Scholar] [CrossRef]
- Cardle, I.I.; Jensen, M.C.; Pun, S.H.; Sellers, D.L. Optimized Serum Stability and Specificity of an αvβ6 Integrin-Binding Peptide for Tumor Targeting. J. Biol. Chem. 2021, 296, 100657–100667. [Google Scholar] [CrossRef]
- Abuchowski, A.; van Es, T.; Palczuk, N.C.; Davis, F.F. Alteration of Immunological Properties of Bovine Serum Albumin by Covalent Attachment of Polyethylene Glycol. J. Biol. Chem. 1977, 252, 3578–3581. [Google Scholar] [CrossRef]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of Covalent Attachment of Polyethylene Glycol on Immunogenicity and Circulating Life of Bovine Liver Catalase. J. Biol. Chem. 1977, 252, 3582–3586. [Google Scholar] [CrossRef]
- Alconcel, S.N.S.; Baas, A.S.; Maynard, H.D. FDA-Approved Poly(Ethylene Glycol)–Protein Conjugate Drugs. Polym. Chem. 2011, 2, 1442–1448. [Google Scholar] [CrossRef]
- Gupta, V.; Bhavanasi, S.; Quadir, M.; Singh, K.; Ghosh, G.; Vasamreddy, K.; Ghosh, A.; Siahaan, T.J.; Banerjee, S.; Banerjee, S.K. Protein PEGylation for Cancer Therapy: Bench to Bedside. J. Cell Commun. Signal. 2019, 13, 319–330. [Google Scholar] [CrossRef]
- van Witteloostuijn, S.B.; Pedersen, S.L.; Jensen, K.J. Half-Life Extension of Biopharmaceuticals Using Chemical Methods: Alternatives to PEGylation. ChemMedChem 2016, 11, 2474–2495. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Yuan, Z.; Hung, H.-C.; Ma, J.; Jain, P.; Tsao, C.; Xie, J.; Zhang, P.; Lin, X.; Wu, K.; et al. Revealing the Immunogenic Risk of Polymers. Angew. Chem. Int. Ed. 2018, 57, 13873–13876. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Yokoyama, M. Toxicity and Immunogenicity Concerns Related to PEGylated-Micelle Carrier Systems: A Review. Sci. Technol. Adv. Mater. 2019, 20, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.M.; Chess, R.B. Effect of PEGylation on Pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Swierczewska, M.; Lee, K.C.; Lee, S. What Is the Future of PEGylated Therapies? Expert Opin. Emerg. Drugs 2015, 20, 531–536. [Google Scholar] [CrossRef]
- Hausner, S.H.; Kukis, D.L.; Gagnon, M.K.J.; Stanecki, C.E.; Ferdani, R.; Marshall, J.F.; Anderson, C.J.; Sutcliffe, J.L. Evaluation of [64Cu]Cu-DOTA and [64Cu]Cu-CB-TE2A Chelates for Targeted Positron Emission Tomography with an αvβ6-Specific Peptide. Mol. Imaging 2009, 8, 111–121. [Google Scholar] [CrossRef]
- Hu, L.Y.; Bauer, N.; Knight, L.M.; Li, Z.; Liu, S.; Anderson, C.J.; Conti, P.S.; Sutcliffe, J.L. Characterization and Evaluation of 64Cu-Labeled A20FMDV2 Conjugates for Imaging the Integrin αvβ6. Mol. Imaging Biol. 2014, 16, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Hausner, S.H.; Bauer, N.; Hu, L.Y.; Knight, L.M.; Sutcliffe, J.L. The Effect of Bi-Terminal PEGylation of an Integrin αvβ6–Targeted 18F Peptide on Pharmacokinetics and Tumor Uptake. J. Nucl. Med. 2015, 56, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Satpati, D.; Bauer, N.; Hausner, S.H.; Sutcliffe, J.L. Synthesis of [64Cu]DOTA-ADIBON3-Ala-PEG28-A20FMDV2 via Copper-Free Click Chemistry for PET Imaging of Integrin αvβ6. J. Radioanal. Nucl. Chem. 2014, 302, 765–771. [Google Scholar] [CrossRef]
- Huynh, T.T.; Sreekumar, S.; Mpoy, C.; Rogers, B.E. A Comparison of 64 Cu-Labeled Bi-Terminally PEGylated A20FMDV2 Peptides Targeting Integrin αvβ6. Oncotarget 2022, 13, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.; Sreekumar, S.; Mpoy, C.; Rogers, B.E. Therapeutic Efficacy of 177Lu-Labeled A20FMDV2 Peptides Targeting αvβ6. Pharmaceuticals 2022, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.-U. The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef] [Green Version]
- Hausner, S.H.; Carpenter, R.D.; Bauer, N.; Sutcliffe, J.L. Evaluation of an Integrin αvβ6-Specific Peptide Labeled with [18F]Fluorine by Copper-Free, Strain-Promoted Click Chemistry. Nucl. Med. Biol. 2013, 40, 233–239. [Google Scholar] [CrossRef]
- Hausner, S.H.; Bold, R.J.; Cheuy, L.Y.; Chew, H.K.; Daly, M.E.; Davis, R.A.; Foster, C.C.; Kim, E.J.; Sutcliffe, J.L. Preclinical Development and First-in-Human Imaging of the Integrin αvβ6 with [18F]αvβ6-Binding Peptide in Metastatic Carcinoma. Clin. Cancer Res. 2019, 25, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.R.; Kent, S.B.H.; Engelhard, M.; Merrifield, R.B. A New Synthetic Route to Tert-Butyloxycarbonylaminoacyl-4-(Oxymethyl)Phenylacetamidomethyl-Resin, an Improved Support for Solid-Phase Peptide Synthesis. J. Org. Chem. 1978, 43, 2845–2852. [Google Scholar] [CrossRef]
- Harris, P.W.R.; Yang, S.H.; Brimble, M.A. An Improved Procedure for the Preparation of Aminomethyl Polystyrene Resin and Its Use in Solid Phase (Peptide) Synthesis. Tetrahedron Lett. 2011, 52, 6024–6026. [Google Scholar] [CrossRef]
- Hausner, S.H.; DiCara, D.; Marik, J.; Marshall, J.F.; Sutcliffe, J.L. Use of a Peptide Derived from Foot-and-Mouth Disease Virus for the Noninvasive Imaging of Human Cancer: Generation and Evaluation of 4-[18F]Fluorobenzoyl A20FMDV2 for in vivo Imaging of Integrin αvβ6 Expression with Positron Emission Tomography. Cancer Res. 2007, 67, 7833–7840. [Google Scholar] [CrossRef] [Green Version]
- John, A.E.; Luckett, J.C.; Tatler, A.L.; Awais, R.O.; Desai, A.; Habgood, A.; Ludbrook, S.; Blanchard, A.D.; Perkins, A.C.; Jenkins, R.G.; et al. Preclinical SPECT/CT Imaging of αvβ6 Integrins for Molecular Stratification of Idiopathic Pulmonary Fibrosis. J. Nucl. Med. 2013, 54, 2146–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arano, Y.; Uezono, T.; Akizawa, H.; Ono, M.; Wakisaka, K.; Nakayama, M.; Sakahara, H.; Konishi, J.; Yokoyama, A. Reassessment of Diethylenetriaminepentaacetic Acid (DTPA) as a Chelating Agent for Indium-111 Labeling of Polypeptides Using a Newly Synthesized Monoreactive DTPA Derivative. J. Med. Chem. 1996, 39, 3451–3460. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.; Neidle, A.; Marks, N. Significant Differences in the Degradation of Pro-Leu-Gly-NH2 by Human Serum and That of Other Species. Proc. Soc. Exp. Biol. Med. 1975, 148, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Benuck, M.; Marks, N. Differences in the Degradation of Hypothalamic Releasing Factors by Rat and Human Serum. Life Sci. 1976, 19, 1271–1276. [Google Scholar] [CrossRef]
- Witter, A.; Scholtens, H.; Verhoef, J. H-Pro-[3H]Leu-Gly-NH2: Metabolism in Human and Rat Plasma Investigated by High-Pressure Liquid Chromatography. Neuroendocrinology 1980, 30, 377–381. [Google Scholar] [CrossRef]
- McDermott, J.R.; Smith, A.I.; Biggins, J.A.; Hardy, J.A.; Dodd, P.R.; Edwardson, J.A. Degradation of Luteinizing Hormone-Releasing Hormone by Serum and Plasma in vitro. Regul. Pept. 1981, 2, 69–79. [Google Scholar] [CrossRef]
- Powell, M.F.; Grey, H.; Gaeta, F.; Sette, A.; Colón, S. Peptide Stability in Drug Development: A Comparison of Peptide Reactivity in Different Biological Media. J. Pharm. Sci. 1992, 81, 731–735. [Google Scholar] [CrossRef]
- Jenssen, H.; Aspmo, S.I. Serum Stability of Peptides. In Peptide-Based Drug Design; Otvos, L., Ed.; Methods in Molecular BiologyTM.; Humana Press: Totowa, NJ, USA, 2008; pp. 177–186. [Google Scholar] [CrossRef]
- Kogelberg, H.; Tolner, B.; Thomas, G.J.; Di Cara, D.; Minogue, S.; Ramesh, B.; Sodha, S.; Marsh, D.; Lowdell, M.W.; Meyer, T.; et al. Engineering a Single-Chain Fv Antibody to αvβ6 Integrin Using the Specificity-Determining Loop of a Foot-and-Mouth Disease Virus. J. Mol. Biol. 2008, 382, 385–401. [Google Scholar] [CrossRef] [Green Version]
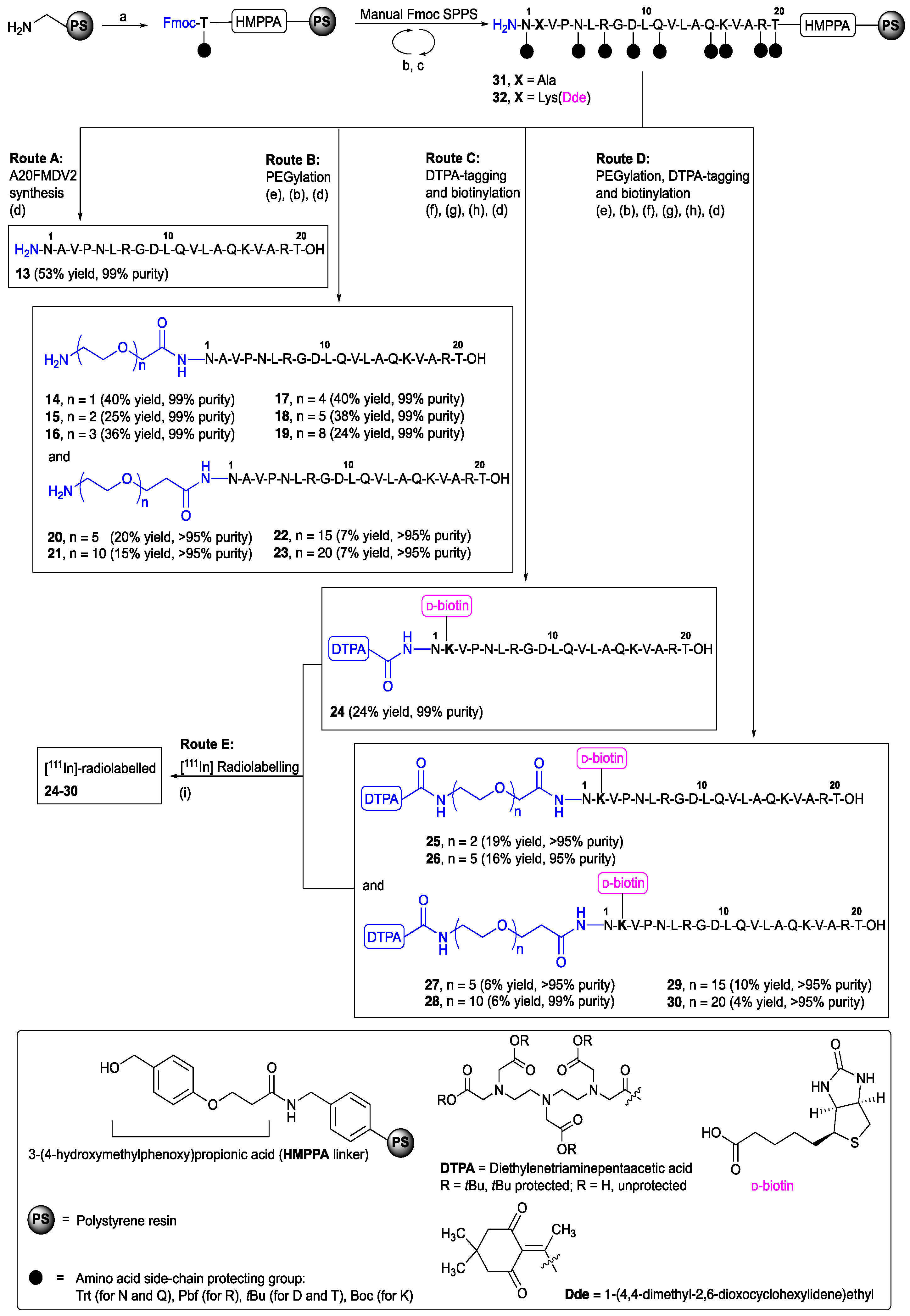

| Compound | N-term. | X1 | X2 |
|---|---|---|---|
| 13 (A20FMDV2) | NH2 | - | Ala |
| 14 | NH2 | -CH2-CH2-O-CH2-CO- | Ala |
| 15 | NH2 | -(CH2-CH2-O)2-CH2-CO- | Ala |
| 16 | NH2 | -(CH2-CH2-O)3-CH2-CO- | Ala |
| 17 | NH2 | -(CH2-CH2-O)4-CH2-CO- | Ala |
| 18 | NH2 | -(CH2-CH2-O)5-CH2-CO- | Ala |
| 19 | NH2 | -(CH2-CH2-O)8-CH2-CO- | Ala |
| 20 | NH2 | -(CH2-CH2-O)5-CH2-CH2-CO- | Ala |
| 21 | NH2 | -(CH2-CH2-O)10-CH2-CH2-CO- | Ala |
| 22 | NH2 | -(CH2-CH2-O)15-CH2-CH2-CO- | Ala |
| 23 | NH2 | -(CH2-CH2-O)20-CH2-CH2-CO- | Ala |
| 24 | DTPA-NH | - | Lys(d-biotin) |
| 25 | DTPA-NH | -(CH2-CH2-O)2-CH2-CO- | Lys(d-biotin) |
| 26 | DTPA-NH | -(CH2-CH2-O)5-CH2-CO- | Lys(d-biotin) |
| 27 | DTPA-NH | -(CH2-CH2-O)5-CH2-CH2-CO- | Lys(d-biotin) |
| 28 | DTPA-NH | -(CH2-CH2-O)10-CH2-CH2-CO- | Lys(d-biotin) |
| 29 | DTPA-NH | -(CH2-CH2-O)15-CH2-CH2-CO- | Lys(d-biotin) |
| 30 | DTPA-NH | -(CH2-CH2-O)20-CH2-CH2-CO- | Lys(d-biotin) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, K.-y.; Kowalczyk, R.; Desai, A.; Brimble, M.A.; Marshall, J.F.; Harris, P.W.R. Synthesis and Systematic Study on the Effect of Different PEG Units on Stability of PEGylated, Integrin-αvβ6-Specific A20FMDV2 Analogues in Rat Serum and Human Plasma. Molecules 2022, 27, 4331. https://doi.org/10.3390/molecules27144331
Hung K-y, Kowalczyk R, Desai A, Brimble MA, Marshall JF, Harris PWR. Synthesis and Systematic Study on the Effect of Different PEG Units on Stability of PEGylated, Integrin-αvβ6-Specific A20FMDV2 Analogues in Rat Serum and Human Plasma. Molecules. 2022; 27(14):4331. https://doi.org/10.3390/molecules27144331
Chicago/Turabian StyleHung, Kuo-yuan, Renata Kowalczyk, Ami Desai, Margaret A. Brimble, John F. Marshall, and Paul W. R. Harris. 2022. "Synthesis and Systematic Study on the Effect of Different PEG Units on Stability of PEGylated, Integrin-αvβ6-Specific A20FMDV2 Analogues in Rat Serum and Human Plasma" Molecules 27, no. 14: 4331. https://doi.org/10.3390/molecules27144331
APA StyleHung, K.-y., Kowalczyk, R., Desai, A., Brimble, M. A., Marshall, J. F., & Harris, P. W. R. (2022). Synthesis and Systematic Study on the Effect of Different PEG Units on Stability of PEGylated, Integrin-αvβ6-Specific A20FMDV2 Analogues in Rat Serum and Human Plasma. Molecules, 27(14), 4331. https://doi.org/10.3390/molecules27144331







