Potent Antibiotic Lemonomycin: A Glimpse of Its Discovery, Origin, and Chemical Synthesis
Abstract
1. Isolation and Structural Elucidation
2. Current Status of Biosynthesis
3. Total Synthesis of Lemonomycin
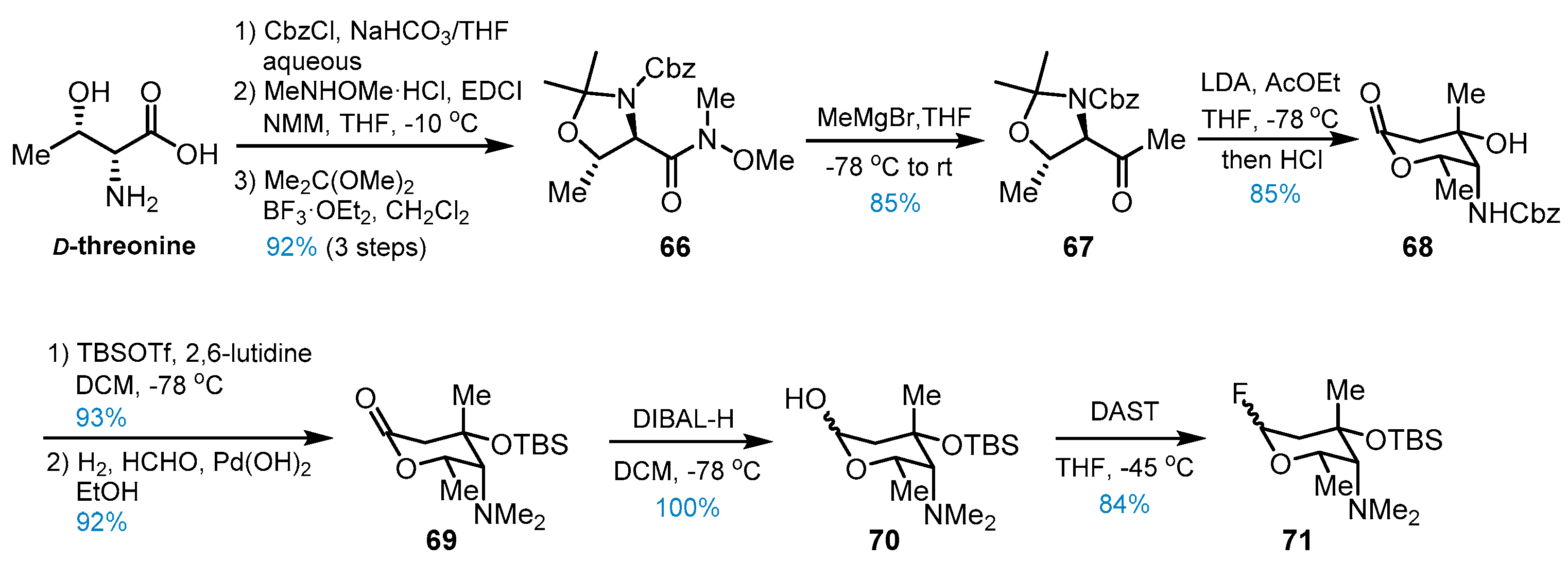
4. Other Studies toward Lemonomycin
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Butler, M.S.; Paterson, D.L. Antibiotics in the clinical pipeline in October 2019. J. Antibiot. 2020, 73, 329–364. [Google Scholar] [CrossRef]
- Whaley, H.A.; Patterson, E.L.; Dann, M.; Shay, A.J.; Porter, J.N. Isolation and characterization of Lemonomycin, a new antibiotic. Antimicrob. Agents Chemother. 1964, 8, 83–86. [Google Scholar]
- Ashley, E.; Stoltz, B. A Dipolar cycloaddition strategy for the synthesis of 3,8-diazabicyclo [3.2.1]octane tetrahydroisoquinoline antitumor antibiotics: The total synthesis of (-)-lemonomycin. Strateg. Tactics Org. Synth. 2012, 8, 351–373. [Google Scholar]
- He, H.Y.; Shen, B.; Carter, G.T. Structural elucidation of lemonomycin, a potent antibiotic from Streptomyces candidus. Tetrahedron Lett. 2000, 41, 2067–2071. [Google Scholar] [CrossRef]
- Scott, J.D.; Williams, R.M. Chemistry and biology of the tetrahydroisoquinoline antitumor amtibiotics. Chem. Rev. 2002, 102, 1669–1730. [Google Scholar] [CrossRef]
- Siengalewicz, P.; Rinner, U.; Mulzer, j. Recent progress in the total synthesis of naphthyridinomycin and lemonomycin tetrahydroisoquinoline antitumor antibiotics (TAAs). Chem. Soc. Rev. 2008, 37, 2676–2690. [Google Scholar] [CrossRef]
- Hill, G.C.; Wunz, T.P.; Remers, W.A. Computer simulation of the binding of quinocarcin to DNA. Prediction of mode of action and absolute configuration. J. Comput. Aided Mol. Des. 1988, 2, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Sygusch, J.; Brisse, F.; Hanessian, S.; Kluepfel, D. The molecular structure of naphthyridinomycin—A broad spectrum antibiotic. Tetrahedron Lett. 1974, 15, 4021–4023. [Google Scholar] [CrossRef]
- Kluepfel, D.; Baker, H.A.; Piattoni, G.; Sehgal, S.N.; Sidorowicz, A.; Singh, K.; Vézina, C. Naphthyridinomycin, a new broad-spectrum antibiotic. J. Antibiot. 1975, 28, 497–502. [Google Scholar] [CrossRef]
- Zmijewski, M.; Mikolajczak, M., Jr.; Viswanatha, V.; Hruby, V.J. Biosynthesis of the antitumor antibiotic naphthyridinomycin. J. Am. Chem. Soc. 1982, 104, 4969–4971. [Google Scholar] [CrossRef]
- Zmijewski, M.; Palaniswamy, V.A., Jr.; Gould, S.J. Naphthyridinomycin biosynthesis. The involvement of ornithine and the origin of the oxazolidine nitrogen. J. Chem. Soc. Chem. Commun. 1985, 1261–1262. [Google Scholar] [CrossRef]
- Palaniswamy, V.A.; Gould, S.J. The incorporation of 3’-methyltyrosine and 5’-methyl DOPA into naphthyridinomycin. J. Am. Chem. Soc. 1986, 108, 5651–5652. [Google Scholar] [CrossRef]
- Peng, C.; Pu, J.Y.; Song, L.Q.; Jian, X.H.; Tang, M.C.; Tang, G.L. Hijacking a hydroxyethyl unit from a central metabolic ketose into a nonribosomal peptide assembly line. Proc. Natl. Acad. Sci. USA 2012, 109, 8540–8545. [Google Scholar] [CrossRef]
- Pu, J.Y.; Peng, C.; Tang, M.C.; Zhang, Y.; Guo, J.P.; Song, L.Q.; Hua, Q.; Tang, G.L. Naphthyridinomycin biosynthesis revealing the use of leader peptide to guide nonribosomal peptide assembly. Org. Lett. 2013, 15, 3674–3677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, W.-H.; Pu, J.-Y.; Tang, M.-C.; Zhang, L.; Peng, C.; Xu, Y.; Tang, G.-L. Extracellularly oxidative activation and inactivation of matured prodrug for cryptic self-resistance in naphthyridinomycin biosynthesis. Proc. Natl Acad. Sci. USA 2018, 115, 11232–11237. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, C.J.; Melançon, C.E., III; Liu, H.W. Natural-product sugar biosynthesis and enzymatic glycodiversification. Angew. Chem. Int. Ed. 2008, 47, 9814–9859. [Google Scholar] [CrossRef]
- Singh, S.; Phillips, G.N., Jr.; Thorson, J.S. The structural biology of enzymes involved in natural product glycosylation. Nat. Prod. Rep. 2012, 29, 1201–1237. [Google Scholar] [CrossRef][Green Version]
- Hiratsuka, T.; Koketsu, K.; Minami, A.; Kaneko, S.; Yamazaki, C.; Watanabe, K.; Oguri, H.; Oikawa, H. Core assembly mechanism of quinocarcin/SF-1739: Bimodular complex nonribosomal peptide synthetases for sequential Mannich-type reactions. Chem. Biol. 2013, 20, 1523–1535. [Google Scholar] [CrossRef]
- Yang, J.; Song, Y.; Tang, M.; Li, M.; Deng, J.; Wong, N.; Ju, J. Genome-directed discovery of tetrahydroisoquinolines from deep-sea derived Streptomyces niveus SCSIO 3406. J. Org. Chem. 2021, 86, 11107–11116. [Google Scholar] [CrossRef]
- Wen, W.-H.; Zhang, Y.; Zhang, Y.-Y.; Yu, Q.; Jiang, C.-C.; Tang, M.-C.; Pu, J.-Y.; Wu, L.; Zhao, Y.-L.; Shi, T.; et al. Reductive inactivation of the hemiaminal pharmacophore for resistance against tetrahydroisoquinoline antibiotics. Nat. Commun. 2021, 12, 7085. [Google Scholar] [CrossRef]
- Almabruk, K.H.; Dinh, L.K.; Philmus, B. Self-resistance of natural product producers: Past, present, and future focusing on self-resistant protein variants. ACS Chem. Biol. 2018, 13, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.R.; Cruz, E.G.; Stoltz, B.M. The total synthesis of (-)-lemonomycin. J. Am. Chem. Soc. 2003, 125, 15000–15001. [Google Scholar] [CrossRef] [PubMed]
- Kiss, M.; Russell-Maynard, J.; Joule, A. 1,3-Dipolar cycloadditions to oxidopyraziniums. Tetrahedron Lett. 1987, 28, 2187–2190. [Google Scholar] [CrossRef]
- Allway, P.A.; Sutherland, J.K.; Joule, J.A. A synthesis of (±)-7-methoxycarbonyl-2-(3-methoxyphenylmethylidene)-8-methyl-3,8-diazabicyclo[3.2.1] Octan-4-one (1b) using dipolar cycloaddition to a 3-oxidopyrazinium. Tetrahedron Lett. 1990, 31, 4781–4782. [Google Scholar] [CrossRef]
- Yates, N.D.; Peters, D.A.; Allway, P.A.; Beddeoes, R.L.; Scopes, D.I.C.; Joule, J.A. 1,3-Dipolar cycloadditions to oxidopyraziniums. Heterocycles 1995, 40, 331–347. [Google Scholar]
- Reetz, M.T.; Schmitz, A. Stereoselective Grignard-type reaction of chiral N,N-dibenzylamino ketones. Tetrahedron Lett. 1999, 40, 2737–2740. [Google Scholar] [CrossRef]
- Reetz, M.T. Synthesis and diastereoselective reactions of N,N-dibenzylamino aldehydes and related compounds. Chem. Rev. 1999, 99, 1121–1162. [Google Scholar] [CrossRef]
- Pappo, R.; Allen, D.S., Jr.; Lemieux, R.U.; Johnson, W.S. Osmium tetroxide-catalyzed periodate oxidation of olefinic bonds. J. Org. Chem. 1956, 21, 478–479. [Google Scholar] [CrossRef]
- Thom, C.; Kocieński, P. A practical method for N-acylation of bornane-2,10-sultam and 2-oxazolidinones. Synthesis 1992, 6, 582–586. [Google Scholar] [CrossRef]
- Yoshida, A.; Akaiwa, M.; Asakawa, T.; Hamashima, Y.; Yokoshima, S.; Fukuyama, T.; Kan, T. Total synthesis of (-)-lemonomycin. Chem. Eur. J. 2012, 18, 11192–11195. [Google Scholar] [CrossRef]
- Fukuyama, T.; Nunes, J.J. Stereocontrolled total synthesis of (±)-quinocarcin. J. Am. Chem. Soc. 1988, 110, 5196–5198. [Google Scholar] [CrossRef]
- Fukuyama, T.; Yang, L.; Ajeck, K.L.; Sachleben, R.A. Total synthesis of (±)-saframycin A. J. Am. Chem. Soc. 1990, 112, 3712–3713. [Google Scholar] [CrossRef]
- Mori, K.; Rikimaru, K.; Kan, T.; Fukuyama, T. Synthetic studies on (+)-naphthyridinomycin: Stereoselective synthesis of the tetracyclic core framework. Org. Lett. 2004, 6, 3095–3097. [Google Scholar] [CrossRef] [PubMed]
- Majetich, G.; Lowery, D.; Khetani, V.; Song, J.; Hull, K.; Ringold, C. Intramolecular additions of allylsilanes to conjugated dienones. Direct stereoselective syntheses of (±)-neolemnanyl acetate and (±)-neolemnane. J. Org. Chem. 1991, 56, 3973–3988. [Google Scholar] [CrossRef]
- Gallina, C.; Liberatori, A. Condensation of 1,4-diacetylpiperazine-2,5-dione with aldehydes. Tetrahedron 1974, 30, 667–673. [Google Scholar] [CrossRef]
- Ritter, T.; Stanek, K.; Larrosa, I.; Carreira, E.M. Mild cleavage of aryl mesylates: Methanesulfonate as potent protecting group for phenols. Org. Lett. 2004, 6, 1513–1514. [Google Scholar] [CrossRef]
- Mukaiyama, T. Explorations into new reaction chemistry. Angew. Chem. Int. Ed. 2004, 43, 5590–5614. [Google Scholar] [CrossRef]
- Magnus, P.; Matthews, K.S. Synthesis of the tetrahydroisoquinoline alkaloid (±)-renieramycin G and a (±)-lemonomycinone analogue from a common intermediate. J. Am. Chem. Soc. 2005, 127, 12476–12477. [Google Scholar] [CrossRef]
- Magnus, P.; Matthews, K.S. A divergent strategy for synthesis of the tetrahydroisoquinoline alkaloids renieramycin G and a lemonomycin analog. Tetrahedron 2012, 68, 6343–6360. [Google Scholar] [CrossRef]
- Magnus, P.; Matthews, K.S.; Lynch, V. New strategy for the synthesis of tetrahydroisoquinoline alkaloids. Org. Lett. 2003, 5, 2181–2184. [Google Scholar] [CrossRef]
- Castro, C.E.; Havlin, R.; Honwad, V.K.; Malte, A.M.; Moje, S.W. Copper(I) substitutions. Scope and mechanism of cuprous acetylide substitutions. J. Am. Chem. Soc. 1969, 91, 6464–6470. [Google Scholar] [CrossRef]
- Kaufman, T.S. Convenient one-pot synthesis of primary α-alkoxystannanes. Synlett 1997, 12, 1377–1378. [Google Scholar] [CrossRef]
- Yu, C.; Hu, L. A facile synthesis of cyclic enecarbamates using Dess–Martin periodinane. Tetrahedron Lett. 2001, 42, 5167–5170. [Google Scholar] [CrossRef]
- Pearlman, W.M. Noble metal hydroxides on carbon nonpyrophoric dry catalysts. Tetrahedron Lett. 1967, 8, 1663–1664. [Google Scholar] [CrossRef]
- Wu, Y.; Bernadat, G.; Masson, G.; Couturier, C.; Schlama, T.; Zhu, J. Synthetic studies on (-)-lemonomycin: An efficient asymmetric synthesis of lemonomycinone amide. J. Org. Chem. 2009, 74, 2046–2052. [Google Scholar] [CrossRef]
- Corey, E.J.; Xu, F.; Noe, M.C. A rational approach to catalytic enantioselective enolate alkylation using a structurally rigidified and defined chiral quaternary ammonium salt under phase transfer conditions. J. Am. Chem. Soc. 1997, 119, 12414–12415. [Google Scholar] [CrossRef]
- Lygo, B.; Wainwright, P.G. A new class of asymmetric phase-transfer catalysts derived from Cinchona alkaloids–Application in the enantioselective synthesis of α-amino acids. Tetrahedron Lett. 1997, 38, 8595–8598. [Google Scholar] [CrossRef]
- Lygo, B.; Andrews, B.I. Asymmetric phase-transfer catalysis utilizing chiral quaternary ammonium salts: Asymmetric alkylation of glycine imines. Acc. Chem. Res. 2004, 37, 518–525. [Google Scholar] [CrossRef]
- Kokotos, G.; Padrón, J.M.; Martín, T.; Gibbons, W.A.; Martin, V.S. A general approach to the asymmetric synthesis of unsaturated lipidic α-amino acids. The first synthesis of α-aminoarachidonic acid. J. Org. Chem. 1998, 63, 3741–3744. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.P. Hafnium trifluoromethanesulfonate (hafnium triflate) as a highly efficient catalyst for chemoselective thioacetalization and transthioacetalization of carbonyl compounds. J. Org. Chem. 2008, 73, 9522–9524. [Google Scholar] [CrossRef]
- Bernadat, G.; George, N.; Couturier, C.; Masson, G.; Schlama, T.; Zhu, J. Asymmetric synthesis of 2,4,6-trideoxy-4-(dimethylamino)-3-C-methyl-L-lyxohexopyranose (lemonose). Synlett 2011, 4, 576–578. [Google Scholar]
- Frigerio, M.; Santagostino, M.; Sputore, S.; Palmisano, G. Oxidation of alcohols with o-iodoxybenzoic acid in DMSO: A new insight into an old hypervalent iodine reagent. J. Org. Chem. 1995, 60, 7272–7276. [Google Scholar] [CrossRef]
- Rikimaru, K.; Mori, K.; Kan, T.; Fukuyama, T. Synthetic studies on (–)-lemonomycin: Stereocontrolled construction of the 3,8-diazabicyclo[3.2.1] skeleton. Chem. Commun. 2005, 3, 394–396. [Google Scholar] [CrossRef]
- Siengalewicz, P.; Brecker, L.; Mulzer, J. Stereocontrolled synthesis of the tetracyclic core framework of (-)-lemonomycin. Synlett 2008, 16, 2443–2446. [Google Scholar]
- Jiménez-Somarribas, A.; Williams, R.M. Synthetic studies on lemonomycin: Construction of the tetracyclic core. Tetrahedron 2013, 69, 7505–7512. [Google Scholar] [CrossRef][Green Version]
- Liao, X.W.; Liu, W.; Dong, W.F.; Guan, B.H.; Chen, S.Z.; Liu, Z.Z. Total synthesis of (−)-renieramycin G from l-tyrosine. Tetrahedron 2009, 65, 5709–5715. [Google Scholar] [CrossRef]
- Kren, V.; Rezanka, T. Sweet antibiotics-the role of glycosidic residues in antibiotic and antitumor activity and their randomization. FEMS Microbiol. Rev. 2008, 32, 858–889. [Google Scholar] [CrossRef] [PubMed]
- Constantine, K.L.; Mueller, L.; Huang, S.; Abid, S.; Lam, K.S.; Li, W.; Leet, J.E. Conformation and absolute configuration of nocathiacin I determined by NMR spectroscopy and chiral capillary electrophoresis. J. Am. Chem. Soc. 2002, 124, 7284–7285. [Google Scholar] [CrossRef]
- Zhang, C.; Herath, K.; Jayasuriya, H.; Ondeyka, J.G.; Zink, D.L.; Occi, J.; Birdsall, G.; Venugopal, J.; Ushio, M.; Burgess, B.; et al. Thiazomycins, thiazolyl peptide antibiotics from Amycolatopsis fastidiosa. J. Nat. Prod. 2009, 72, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Northcote, P.T.; Siegel, M.; Borders, D.B.; Lee, M.D. Glycothiohexide α, a novel antibiotic produced by “Sebekia” sp., LL-14E605. III. Structural elucidation. J. Antibiot. 1994, 47, 901–908. [Google Scholar] [CrossRef]
- Kang, H.S.; Brady, S.F. Arimetamycin A: Improving clinically relevant families of natural products through sequence-guided screening of soil metagenomes. Angew. Chem. Int. Ed. 2013, 52, 11063–11067. [Google Scholar] [CrossRef]
- Huseman, E.D.; Byl, J.A.W.; Chapp, S.M.; Schley, N.D.; Osheroff, N.; Townsend, S.D. Synthesis and cytotoxic evaluation of arimetamycin A and its daunorubicin and doxorubicin hybrids. ACS Cent. Sci. 2021, 7, 1327–1337. [Google Scholar] [CrossRef]
- Hegde, V.R.; Patel, M.G.; Das, P.R.; Pramanik, B.; Puar, M.S. A family of novel macrocyclic lactones, the saccharocarcins produced by Saccharothrix aerocolonigenes subsp. antihiotica. II. Physico-chemical properties and structure determination. J. Antibiot. 1997, 50, 126–134. [Google Scholar] [CrossRef]
- Pfrengle, F.H.-U. Reissig, Amino sugars and their mimetics via 1,2-oxazines. Chem. Soc. Rev. 2010, 39, 549–557. [Google Scholar] [CrossRef]
- Ding, F.; Cai, S.; William, R.; Liu, X.-W. Pathways leading to 3-amino- and 3-nitro-2,3-dideoxy sugars: Strategies and synthesis. RSC Adv. 2013, 3, 13594. [Google Scholar] [CrossRef]
- Skarbek, K.; Milewska, M.J. Biosynthetic and synthetic access to amino sugars. Carbohydr. Res. 2016, 434, 44–71. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.; Kulkarni, S.S. Chemical synthesis of rare, deoxy-amino sugars containing bacterial glycoconjugates as potential vaccine candidates. Molecules 2018, 23, 1997. [Google Scholar] [CrossRef]
- Huseman, E.D.; Townsend, S.D. De novo synthesis of an L-lemonose thioglycoside donor from D-Threonine. Tetrahedron Lett. 2021, 73, 153097. [Google Scholar] [CrossRef]
- Gloster, T.M. Exploitation of carbohydrate processing enzymes in biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, H.U.; Chae, T.U.; Cho, J.S.; Kim, J.W.; Shin, J.H.; Kim, D.I.; Ko, Y.-S.; Jang, W.D.; Jang, Y.-S. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019, 2, 18–33. [Google Scholar] [CrossRef]























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, S.; Wang, Y.; Hong, R.; Huang, S.-H. Potent Antibiotic Lemonomycin: A Glimpse of Its Discovery, Origin, and Chemical Synthesis. Molecules 2022, 27, 4324. https://doi.org/10.3390/molecules27134324
Tao S, Wang Y, Hong R, Huang S-H. Potent Antibiotic Lemonomycin: A Glimpse of Its Discovery, Origin, and Chemical Synthesis. Molecules. 2022; 27(13):4324. https://doi.org/10.3390/molecules27134324
Chicago/Turabian StyleTao, Shunan, Yang Wang, Ran Hong, and Sha-Hua Huang. 2022. "Potent Antibiotic Lemonomycin: A Glimpse of Its Discovery, Origin, and Chemical Synthesis" Molecules 27, no. 13: 4324. https://doi.org/10.3390/molecules27134324
APA StyleTao, S., Wang, Y., Hong, R., & Huang, S.-H. (2022). Potent Antibiotic Lemonomycin: A Glimpse of Its Discovery, Origin, and Chemical Synthesis. Molecules, 27(13), 4324. https://doi.org/10.3390/molecules27134324





