Antibacterial Activity and Components of the Methanol-Phase Extract from Rhizomes of Pharmacophagous Plant Alpinia officinarum Hance
Abstract
1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of Chloroform- and Methanol-Phase Extracts from the Rhizomes of A. officinarum Hance
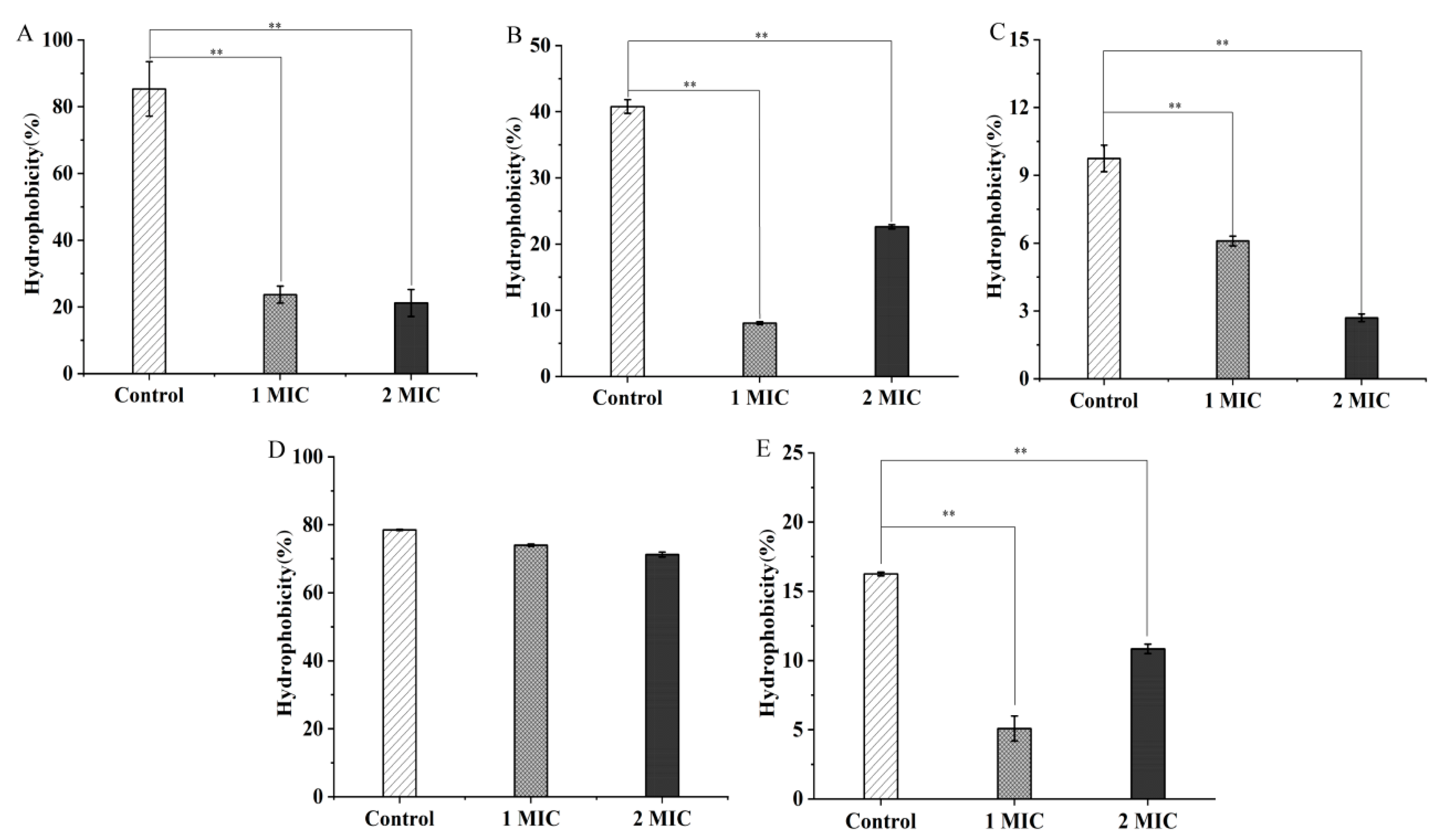
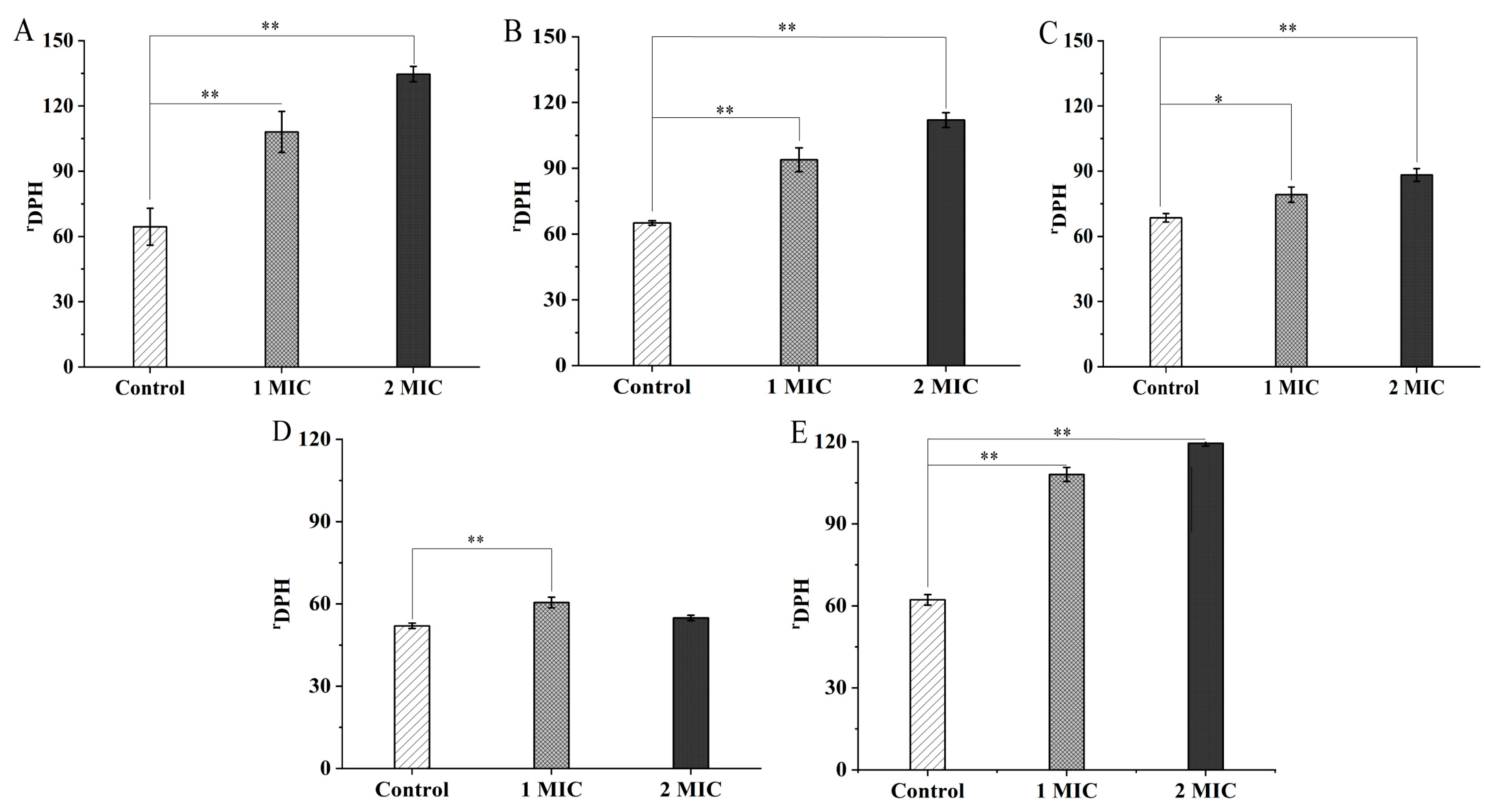
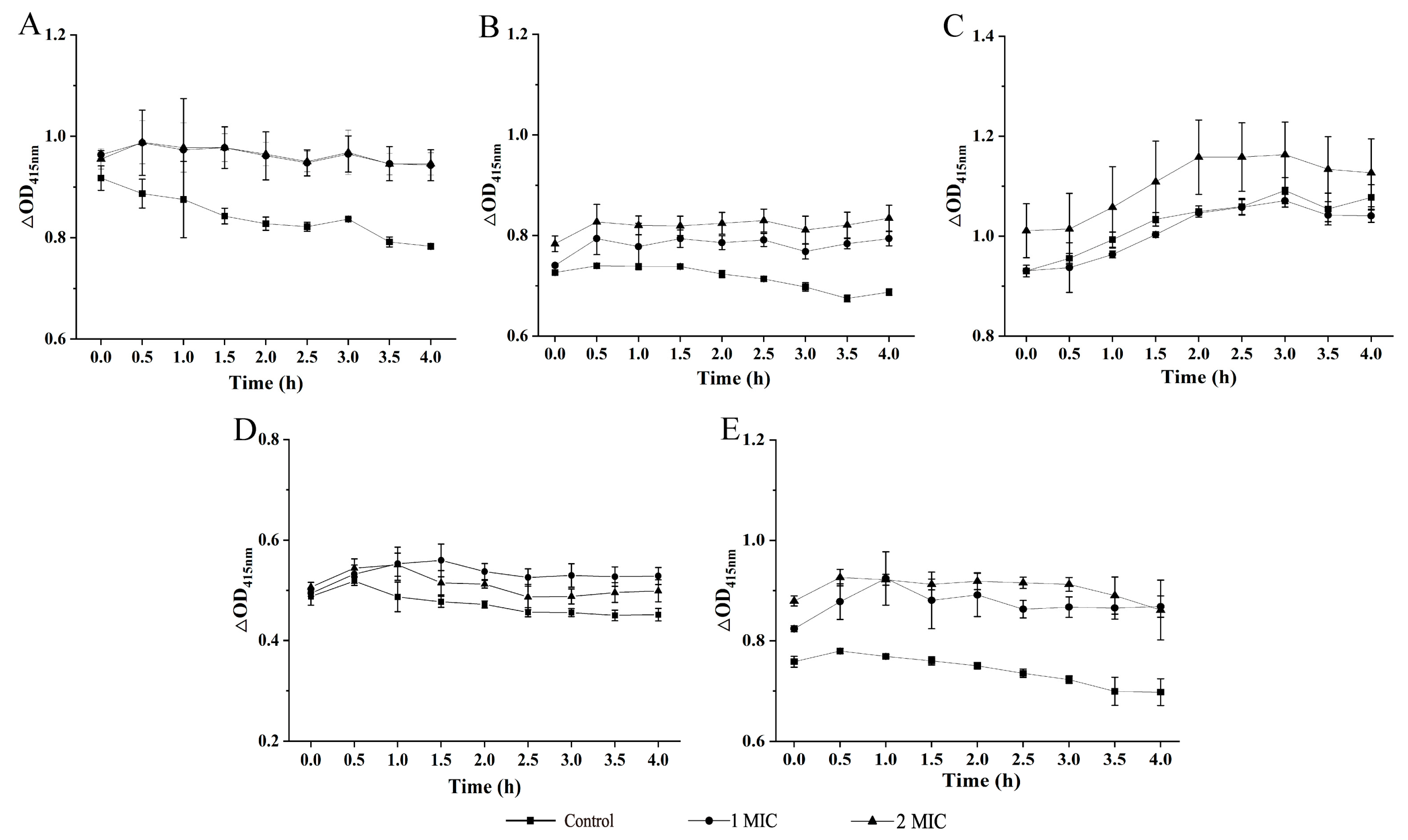
2.2. Bacterial Cell Structure Change Mediated by GMPE Treatment
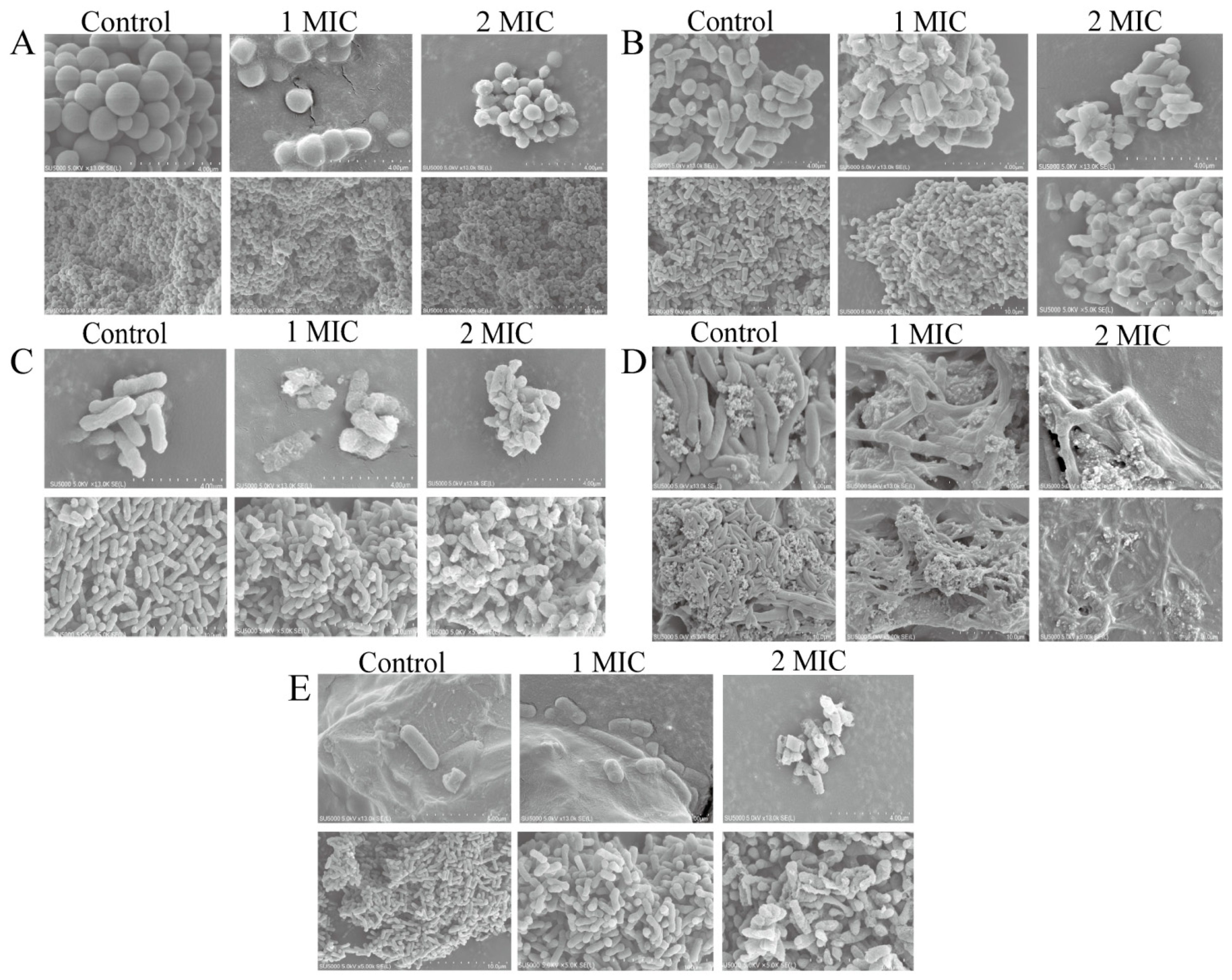
2.3. Bacterial Cell Morphological Architecture Change Mediated by GMPE Treatment
2.4. Differential Transcriptomes Mediated by GMPE Treatment
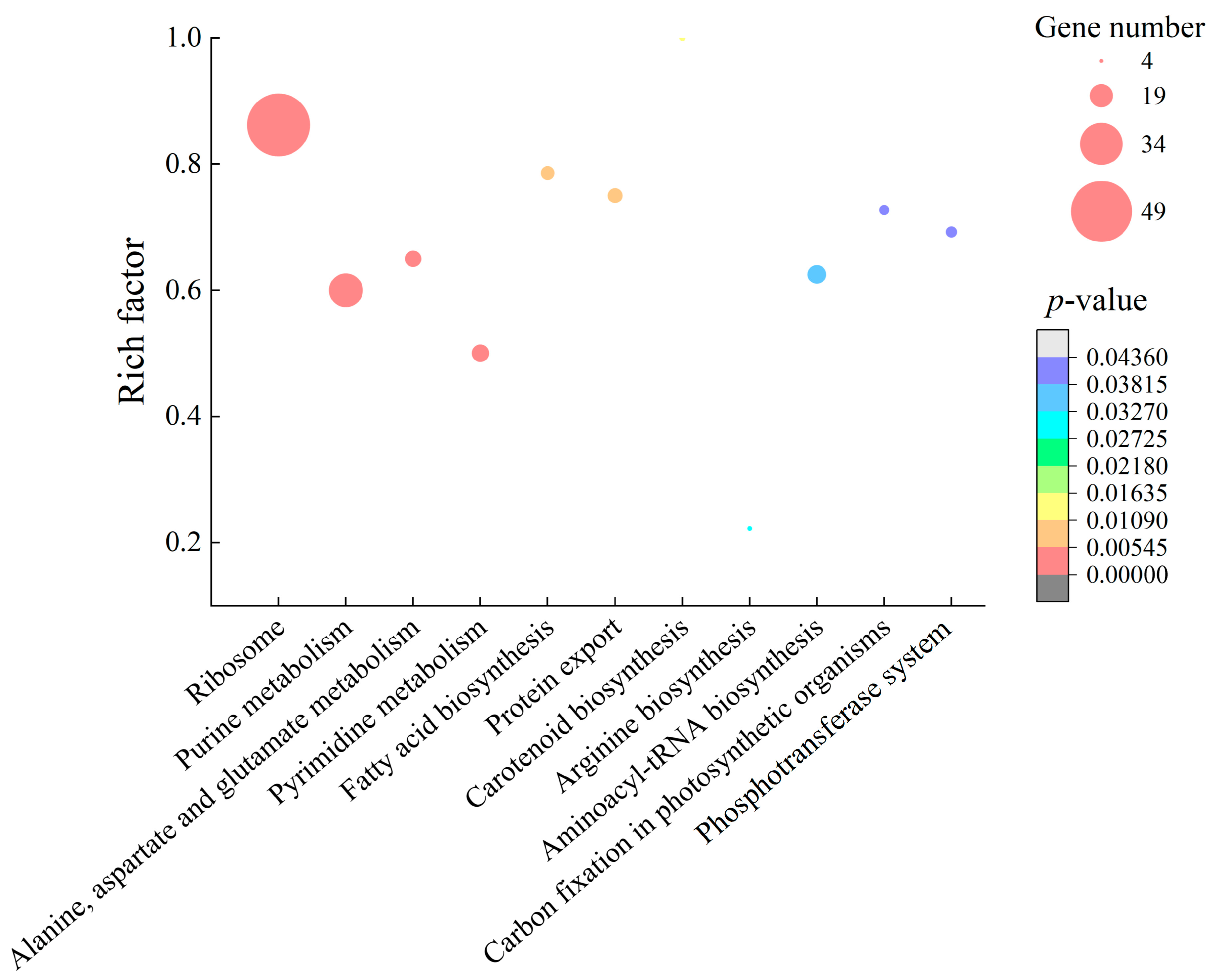
2.4.1. The Major Changed Metabolic Pathways in S. aureus ATCC8095
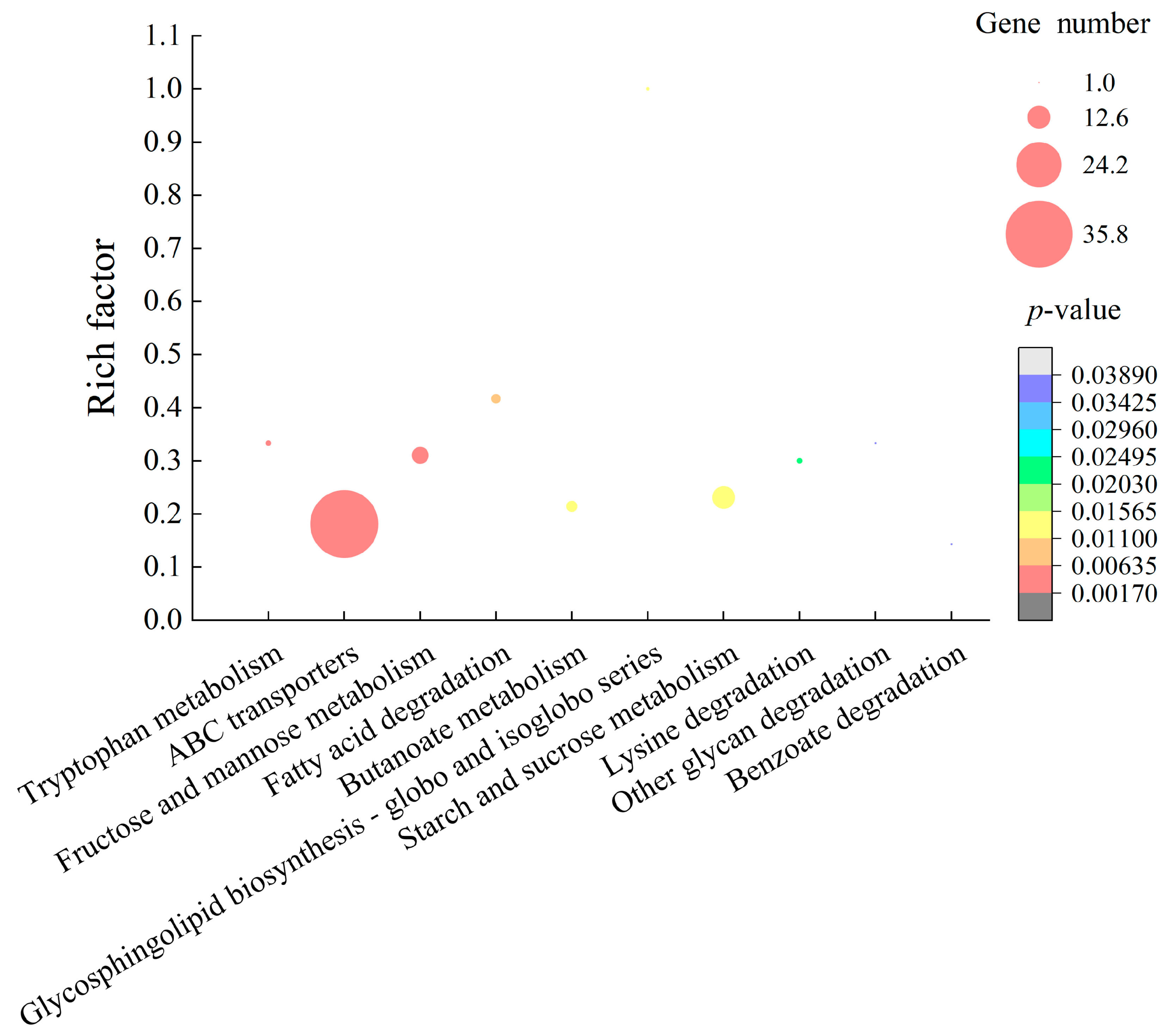
2.4.2. The Major Altered Metabolic Pathways in E. sakazakii CMCC45401
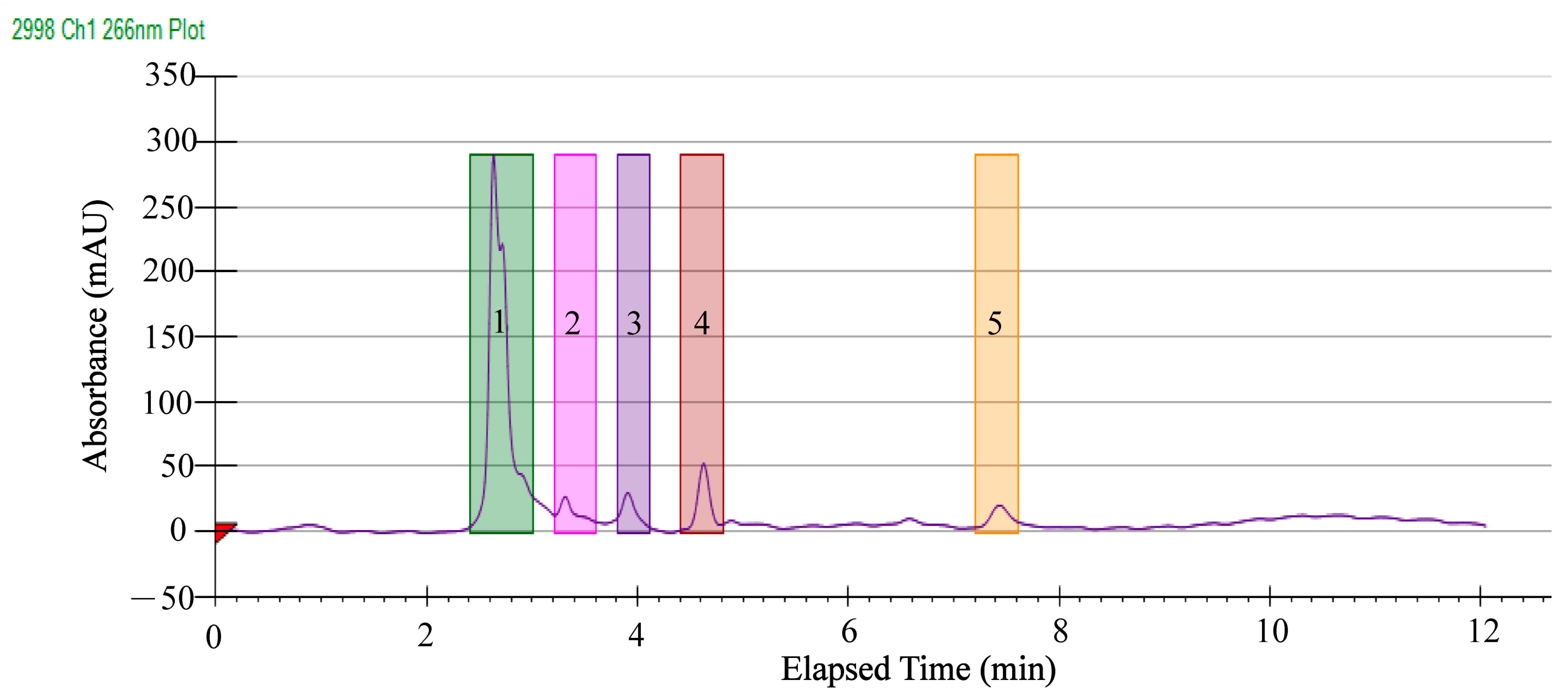
2.5. Separation of Antibacterial Components in the GMPE
2.6. Identification of Potential Antibacterial Compounds in the GMPE
3. Materials and Methods
3.1. Plant Samples and Bioactive Ingredient Extraction
3.2. Antibacterial Activity Assays
3.3. Bacterial Cell Structure Assays
3.4. Prep-HPLC Analysis
3.5. HPLC-MS Analysis
3.6. Illumina RNA Sequencing
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Alasmary, F.A.; Assirey, E.A.; El-Meligy, R.M.; Awaad, A.S.; El-Sawaf, L.A.; Allah, M.M.; Alqasoumi, S.I. Analysis of Alpina officinarum Hance, chemically and biologically. Saudi Pharm. J. 2019, 27, 1107–1112. [Google Scholar] [CrossRef]
- Abubakar, I.B.; Malami, I.; Yahaya, Y.; Sule, S.M. A review on the ethnomedicinal uses, phytochemistry and pharmacology of Alpinia officinarum Hance. J. Ethnopharmacol. 2018, 224, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Eumkeb, G.; Siriwong, S.; Phitaktim, S.; Rojtinnakorn, N.; Sakdarat, S. Synergistic activity and mode of action of flavonoids isolated from smaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli. J. Appl. Microbiol. 2012, 112, 55–64. [Google Scholar] [CrossRef]
- Basri, A.M.; Taha, H.; Ahmad, N. A review on the pharmacological activities and phytochemicals of Alpinia officinarum (galangal) extracts derived from bioassay-guided fractionation and isolation. Pharmacogn. Rev. 2017, 11, 43–56. [Google Scholar] [CrossRef]
- Honmore, V.S.; Kandhare, A.D.; Kadam, P.P.; Khedkar, V.M.; Sarkar, D.; Bodhankar, S.L.; Zanwar, A.A.; Rojatkar, S.R.; Natu, A.D. Isolates of Alpinia officinarum Hance as COX-2 inhibitors: Evidence from anti-inflammatory, antioxidant and molecular docking studies. Int. Immunopharmacol. 2016, 33, 8–17. [Google Scholar] [CrossRef]
- Srividya, A.R.; Dhanabal, S.P.; Misra, V.K.; Suja, G. Antioxidant and antimicrobial activity of Alpinia officinarum. Indian J. Pharm. Sci. 2010, 72, 145–148. [Google Scholar] [CrossRef]
- Zhang, B.B.; Dai, Y.; Liao, Z.X.; Ding, L.S. Three new antibacterial active diarylheptanoids from Alpinia officinarum. Fitoterapia 2010, 81, 948–952. [Google Scholar] [CrossRef]
- Lakshmanan, D.; Harikrishnan, A.; Jyoti, K.; Idul Ali, M.; Jeevaratnam, K. A compound isolated from Alpinia officinarum Hance inhibits swarming motility of Pseudomonas aeruginosa and down regulates virulence genes. J. Appl. Microbiol. 2020, 128, 1355–1365. [Google Scholar] [CrossRef]
- Lee, K.H.; Rhee, K.H. Anti-microbial effects of rhizome extracts of Alpinia officinarum Hance against VRE (vancomycin-resistant enterococci) and other pathogenic microorganisms. Nat. Prod. Sci. 2011, 17, 160–164. [Google Scholar] [CrossRef]
- Liu, X.; Shu, Q.; Chen, Q.; Pang, X.; Wu, Y.; Zhou, W.; Wu, Y.; Niu, J.; Zhang, X. Antibacterial efficacy and mechanism of mannosylerythritol lipids-A on Listeria monocytogenes. Molecules 2020, 25, 4857. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Liu, P.; Jin, Y.; Qin, S.; Chen, L. Identification of antibacterial components in the methanol-phase extract from edible herbaceous plant Rumex madaio Makino and their antibacterial action modes. Molecules 2022, 27, 660. [Google Scholar] [CrossRef]
- Megrian, D.; Taib, N.; Witwinowski, J.; Beloin, C.; Gribaldo, S. One or two membranes? Diderm firmicutes challenge the Gram-positive/Gram-negative divide. Mol. Microbiol. 2020, 113, 659–671. [Google Scholar] [CrossRef]
- Dos Santos, G.H.F.; Amaral, A.; da Silva, E.B. Antibacterial activity of irradiated extracts of Anacardium occidentale L. on multiresistant strains of Staphylococcus aureus. Appl. Radiat. Isot. 2018, 140, 327–332. [Google Scholar] [CrossRef]
- Jang, H.I.; Rhee, M.S. Inhibitory effect of caprylic acid and mild heat on Cronobacter spp. (Enterobacter sakazakii) in reconstituted infant formula and determination of injury by flow cytometry. Int. J. Food Microbiol. 2009, 133, 113–120. [Google Scholar] [CrossRef]
- Galinier, A.; Deutscher, J. Sophisticated regulation of transcriptional factors by the bacterial phosphoenolpyruvate: Sugar phosphotransferase system. J. Mol. Biol. 2017, 429, 773–789. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, L.; Wang, Y.; Zhu, Z.; Yan, J.; Qin, S.; Chen, L. Prophage-encoded gene VpaChn25_0734 amplifies ecological persistence of Vibrio parahaemolyticus CHN25. Curr. Genet. 2022, 68, 267–287. [Google Scholar] [CrossRef]
- Charlier, D.; Bervoets, I. Regulation of arginine biosynthesis, catabolism and transport in Escherichia coli. Amino Acids 2019, 51, 1103–1127. [Google Scholar] [CrossRef]
- Burns, B.P.; Hazell, S.L.; Mendz, G.L.; Kolesnikow, T.; Tillet, D.; Neilan, B.A. The Helicobacter pylori pyrB gene encoding aspartate carbamoyltransferase is essential for bacterial survival. Arch. Biochem. Biophys. 2000, 380, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, X.; Li, Z. Development of an engineered carbamoyl phosphate synthetase with released sensitivity to feedback inhibition by site-directed mutation and casting error-prone PCR. Enzyme Microb. Technol. 2019, 129, 109354. [Google Scholar] [CrossRef] [PubMed]
- Lipps, G.; Krauss, G. Adenylosuccinate synthase from Saccharomyces cerevisiae: Homologous overexpression, purification and characterization of the recombinant protein. Biochem. J. 1999, 341 Pt 3, 537–543. [Google Scholar] [CrossRef][Green Version]
- Lafita-Navarro, M.C.; Conacci-Sorrell, M. Nucleolar stress: From development to cancer. Semin. Cell Dev. Biol. 2022, 22, S1084-9521. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, R.; Xia, M.; Bai, X.; Mou, J.; Zheng, Y.; Wang, M. Effect of aspartic acid and glutamate on metabolism and acid stress resistance of Acetobacter pasteurianus. Microb. Cell Fact. 2017, 16, 109. [Google Scholar] [CrossRef]
- Xie, C.; Li, Z.M.; Bai, F.; Hu, Z.; Zhang, W.; Li, Z. Kinetic and structural insights into enzymatic mechanism of succinic semialdehyde dehydrogenase from Cyanothece sp. ATCC51142. PLoS ONE 2020, 15, e0239372. [Google Scholar] [CrossRef]
- Donoso, I.; Muñoz-Centeno, M.C.; Sànchez-Durán, M.A.; Flores, A.; Daga, R.R.; Guevara, C.M.; Bejarano, E.R. Mpg1, a fission yeast protein required for proper septum structure, is involved in cell cycle progression through cell-size checkpoint. Mol. Genet. Genom. 2005, 274, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Beis, K. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 2015, 43, 889–893. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Weerakkody, N.S.; Caffin, N.; Dykes, G.A.; Turner, M.S. Effect of antimicrobial spice and herb extract combinations on Listeria monocytogenes, Staphylococcus aureus, and spoilage microflora growth on cooked ready-to-eat vacuum-packaged shrimp. J. Food Prot. 2011, 74, 1119–1125. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Arias-Moliz, M.T.; Ferrer-Luque, C.M.; Espigares-Rodríguez, E.; Liébana-Ureña, J.; Espigares-García, M. Bactericidal activity of phosphoric acid, citric acid, and EDTA solutions against Enterococcus faecalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, e84–e89. [Google Scholar] [CrossRef] [PubMed]
- Mabhiza, D.; Chitemerere, T.; Mukanganyama, S. Antibacterial Properties of Alkaloid Extracts from Callistemon citrinus and Vernonia adoensis against Staphylococcus aureus and Pseudomonas aeruginosa. Int. J. Med. Chem. 2016, 2016, 6304163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2020, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bouattour, E.; Fakhfakh, J.; Dammak, D.F.; Jabou, K.; Damak, M.; Jarraya, R.M. Hexane extract of Echinops spinosissimus Turra subsp. spinosus from Tunisia: A potential source of acetylated sterols—Investigation of its biological activities. Chem. Biodivers. 2016, 13, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Forsythe, S.J.; El-Nezami, H. Probiotics interaction with foodborne pathogens: A potential alternative to antibiotics and future challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 3320–3333. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Yuk, H.G. Antimicrobial efficacy of Syzygium antisepticum plant extract against Staphylococcus aureus and methicillin-resistant S. aureus and its application potential with cooked chicken. Food Microbiol. 2018, 72, 176–184. [Google Scholar] [CrossRef]
- Xu, L.; Kim, J.K.; Bai, Q.; Zhang, X.; Kakiyama, G.; Min, H.K.; Sanyal, A.J.; Pandak, W.M.; Ren, S. 5-cholesten-3β,25-diol 3-sulfate decreases lipid accumulation in diet-induced nonalcoholic fatty liver disease mouse model. Mol. Pharmacol. 2013, 83, 648–658. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Pandak, W.M.; Heuman, D.; Hylemon, P.B.; Ren, S. High glucose induces lipid accumulation via 25-Hydroxycholesterol DNA-CpG methylation. iScience 2020, 23, 101102. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Hamamoto, H.; Sekimizu, K.; Okonogi, S. Biological activities and antibacterial biomarker of Sesbania grandiflora bark extract. Drug Discov. Ther. 2017, 11, 70–77. [Google Scholar] [CrossRef][Green Version]
- Yan, F.; Dang, Q.; Liu, C.; Yan, J.; Wang, T.; Fan, B.; Cha, D.; Li, X.; Liang, S.; Zhang, Z. 3,6-O-[N-(2-Aminoethyl)-acetamide-yl]-chitosan exerts antibacterial activity by a membrane damage mechanism. Carbohydr. Polym. 2016, 149, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Voss, D.; Montville, T.J. 1,6-Diphenyl-1,3,5-hexatrine as a reporter of inner spore membrane fluidity in Bacillus subtilis and Alicyclobacillus acidoterrestris. J. Microbiol. Methods 2014, 96, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Sugimoto, Y.; Aoki, T. Ovotransferrin antimicrobial peptide (OTAP-92) kills bacteria through a membrane damage mechanism. Biochim. Biophys. Acta 2000, 1523, 196–205. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sun, B.; Liu, T.; Zheng, H.; Gu, W.; He, W.; Sun, F.; Wang, Y.; Yang, M.; Bei, W.; et al. Genomic and transcriptomic analyses reveal distinct biological functions for cold shock proteins (VpaCspA and VpaCspD) in Vibrio parahaemolyticus CHN25 during low-temperature survival. BMC Genom. 2017, 18, 436. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Yu, P.; Ren, S.; Zhu, Z.; Jin, Y.; Yan, J.; Peng, X.; Chen, L. Prophage-related gene VpaChn25_0724 contributes to cell membrane integrity and growth of Vibrio parahaemolyticus CHN25. Front. Cell. Infect. Microbiol. 2020, 10, 595709. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
| Bacterial Strain | DIZ (Diameter, mm) | |
|---|---|---|
| GMPE | GCPE | |
| Aeromonas hydrophila | 16.03 ± 0.71 a | 12.03 ± 0.01 a |
| Aeromonas hydrophila ATCC35654 | — | — |
| Enterobacter sakazakii CMCC45401 | 11.54 ± 0.71 a | — |
| Escherichia coli K12 | — | 10.5 ± 0.71 a |
| Enterobacter cloacae ATCC13047 | — | — |
| Escherichia coli ATCC8739 | — | — |
| Escherichia coli ATCC25922 | — | — |
| Enterobacter cloacae | — | — |
| Listeria monocytogenes ATCC19115 | — | 7.25 ± 0.35 a |
| Pseudomonas aeruginosa ATCC9027 | — | — |
| Pseudomonas aeruginosa ATCC27853 | — | — |
| Staphylococcus aureus GIM1.160 | — | 12.75 ± 1.06 a |
| Staphylococcus aureus ATCC8095 | 12.32 ± 0.35 a | — |
| Staphylococcus aureus ATCC29213 | 12.00 ± 1.41 a | — |
| Staphylococcus aureus GIM1.441 | 12.52 ± 0.71 a | — |
| Staphylococcus aureus ATCC25923 | — | — |
| Staphylococcus aureus ATCC6538 | — | — |
| Salmonella paratyphi-A CMCC50093 | — | — |
| Salmonella enterica subsp. enterica (ex Kauffmann and Edwards) Le Minor and Popoff serovar Choleraesuis ATCC13312 | — | — |
| Salmonellaenterica subsp. enterica (ex Kauffmann and Edwards) Le Minor and Popoff serovar Vellore ATCC15611 | — | 8.50 ± 0.05 a |
| Shigella dysenteriae CMCC51252 | — | — |
| Shigella flexneri CMCC51572 | — | — |
| Shigella flexneri ATCC12022 | — | — |
| Shigella flexneri ATCC51574 | — | — |
| Shigella sonnei ATCC25931 | — | — |
| Shigella sonnei CMCC51592 | — | — |
| Vibrio alginolyticus ATCC17749 | — | — |
| Vibrio alginolyticus ATCC33787 | — | — |
| Vibrio alginolyticus | — | — |
| Vibrio harvey ATCC BAA-1117 | — | — |
| Vibrio harveyi ATCC33842 | — | — |
| Vibrio parahaemolyticus B3-13 | — | — |
| Vibrio parahaemolyticus B4-10 | — | — |
| Vibrio parahaemolyticus B5-29 | — | — |
| Vibrio parahaemolyticus B9-35 | — | — |
| Vibrio parahaemolyticus ATCC17802 | 11.03 ± 1.40 a | — |
| Vibrio vulnificus ATCC27562 | — | — |
| Vibrio vulnificus | — | 7.75 ± 0.35 a |
| Vibrio fluvialis ATCC33809 | — | 7.02 ± 0.01 a |
| Vibrio metschnikovii ATCC700040 | 9.05 ± 0.01 a | 11.25 ± 0.35 a |
| Vibrio mimicus bio-56759 | — | 8.25 ± 0.35 a |
| Bacterial Strain | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| A. hydrophila | 1.95 | 3.90 |
| E. sakazakii CMCC45401 | 3.90 | 7.81 |
| S. aureus ATCC8095 | 3.90 | 7.81 |
| V. metschnikovii ATCC700040 | 7.81 | 15.62 |
| V. parahaemolyticus ATCC17802 | 3.90 | 7.81 |
| No | Compound | Classification | RT (min) | Formula | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | o-Methoxy cinnamaldehyde | Phenols | 11.6 | C10H10O2 | 40.12 |
| 2 | Phosphoric acid | Organic acids | 0.65 | H3O4P | 6.90 |
| 3 | Indole | Alkaloids | 3.82 | C8H7N | 2.30 |
| 4 | Acetamide | Alkaloids | 13.95 | C2H5NO | 2.20 |
| 5 | L-Pipecolic acid | Amino acid and derivatives | 1.47 | C6H11NO2 | 1.95 |
| 6 | 12,13-DHOME | Fatty acyls | 11.88 | C18H34O4 | 1.91 |
| 7 | Kojibiose | Fatty acyls | 0.72 | C12H22O11 | 1.73 |
| 8 | β-D-Fructose 2-phosphate | Organooxygen compounds | 0.75 | C6H13O9P | 1.73 |
| 9 | L-Asparagine | Amino acids and derivatives | 0.64 | C4H8N2O3 | 1.64 |
| 10 | 3α,6β-Ditigloyloxytropan-7β-ol | Alkaloids | 13.21 | C18H27NO5 | 0.92 |
| 11 | D-α-Aminobutyric acid | Amino acids and derivatives | 0.65 | C4H9NO2 | 0.81 |
| 12 | Proline; L-Proline | Amino acids and derivatives | 0.73 | C5H9NO2 | 0.66 |
| 13 | D-Proline | Amino acids and derivatives | 0.76 | C5H9NO2 | 0.66 |
| 14 | L-Aspartic acid | Amino acids and derivatives | 0.63 | C4H7NO4 | 0.64 |
| 15 | Maltol | Phenols | 0.9 | C6H6O3 | 0.54 |
| 16 | cis-Aconitic acid | Organic acids and derivatives | 1.46 | C6H6O6 | 0.54 |
| 17 | L-Glutamic acid | Amino acids and derivatives | 0.66 | C5H9NO4 | 0.47 |
| 18 | DL-Alanine; L-Alanine | Amino acids and derivatives | 0.64 | C3H7NO2 | 0.39 |
| 19 | Epicatechin; (+)-Epicatechin | Flavonoids | 5.08 | C15H14O6 | 0.38 |
| 20 | L-Ornithine | Amino acids and derivatives | 0.55 | C5H12N2O2 | 0.35 |
| 21 | L-Arginine | Amino acids and derivatives | 0.6 | C6H14N4O2 | 0.35 |
| 22 | Sucrose | Carbohydrates | 0.89 | C12H22O11 | 0.35 |
| 23 | Erucic acid | Fatty acyls | 13.28 | C22H42O2 | 0.31 |
| 24 | O-Acetyl ethanolamine | Alkaloids | 0.67 | C4H9NO2 | 0.31 |
| 25 | Linamarin | Organooxygen compounds | 0.71 | C10H17NO6 | 0.30 |
| 26 | Ethyl caproate | Esters | 0.74 | C8H16O2 | 0.29 |
| 27 | Lubiprostone | Fatty acyls | 12.75 | C20H32F2O5 | 0.28 |
| 28 | Trimethoprim | Pyrimidines | 5.08 | C14H18N4O3 | 0.25 |
| 29 | L-Pipecolic acid | Amino acids and derivatives | 0.69 | C6H11NO2 | 0.23 |
| 30 | Pyrrolidonecarboxylic acid | Carboxylic acids and derivatives | 0.67 | C5H7NO3 | 0.23 |
| 31 | L-Carnitine | Vitamins | 0.69 | C7H15NO3 | 0.23 |
| 32 | Phosphorylcholine | Choline | 0.67 | C5H14NO4P | 0.22 |
| 33 | 8,9-DiHETrE | Fatty acyls | 13.03 | C20H34O4 | 0.21 |
| 34 | Procyanidin B2 | Flavonoids | 4.78 | C30H26O12 | 0.20 |
| 35 | 2-Picolinic acid | Organic acids | 1.33 | C6H5NO2 | 0.19 |
| 36 | 8-Geranyloxypsoralen | Coumarins | 13.29 | C21H22O4 | 0.17 |
| 37 | Alpha-D-Glucose; D-Tagatose | Carbohydrates; organooxygen compounds | 0.76 | C6H12O6 | 0.17 |
| 38 | Safrole | Benzodioxols | 12.26 | C10H10O2 | 0.12 |
| 39 | Thiamine | Vitamins | 0.70 | C12H16N4OS | 0.12 |
| 40 | Caryophyllene oxide | Sesquiterpenes | 11.66 | C15H24O | 0.11 |
| 41 | α-Tocopherol | Phenols | 13.37 | C29H50O2 | 0.11 |
| 42 | L-Lysine | Amino acids and derivatives | 0.64 | C6H14N2O2 | 0.11 |
| 43 | Sarracine | Alkaloids | 13.14 | C18H27NO5 | 0.08 |
| 44 | Palmitoylethanolamide | Fatty acid amides | 12.61 | C18H37NO2 | 0.08 |
| 45 | 2-Hydroxyethanesulfonate | Organic acids | 0.76 | C2H6O4S | 0.05 |
| 46 | Demethoxyencecalin | Phenols | 11.80 | C13H14O2 | 0.01 |
| No | Compound | Classification | RT (min) | Formula | Peak Area (%) |
|---|---|---|---|---|---|
| 1 | p-Octopamine | Phenols | 3.84 | C8H11NO2 | 62.64 |
| 2 | Acetamide | Alkaloids | 13.95 | C2H5NO | 14.30 |
| 3 | Indole | Alkaloids | 3.82 | C8H7N | 4.90 |
| 4 | 12,13-DiHOME | Fatty acyls | 11.88 | C18H34O4 | 2.85 |
| 5 | Phosphoric acid | Organic acids | 0.65 | H3O4P | 2.64 |
| 6 | 3α,6β-ditigloyloxytropan-7β-ol | Alkaloids | 13.21 | C18H27NO5 | 1.71 |
| 7 | Sarracine | Alkaloids | 13.14 | C18H27NO5 | 1.71 |
| 8 | Lubiprostone | Fatty acyls | 12.75 | C20H32F2O5 | 1.36 |
| 9 | o-Methoxycinnamaldehyde | Phenols | 11.6 | C10H10O2 | 1.35 |
| 10 | Epicatechin; (+)-epicatechin | Flavonoids | 5.08 | C15H14O6 | 0.85 |
| 11 | Erucic acid | Fatty acyls | 13.28 | C22H42O2 | 0.75 |
| 12 | Trimethoprim | Pyrimidines | 5.08 | C14H18N4O3 | 0.64 |
| 13 | 8,9-DiHETrE | Fatty acyls | 13.03 | C20H34O4 | 0.46 |
| 14 | 8-Geranyloxypsoralen | Coumarins | 13.29 | C21H22O4 | 0.42 |
| 15 | 4-Hydroxyphenylacetylglutamic acid | Others | 12.99 | C13H15NO6 | 0.35 |
| 16 | L-Pipecolic acid; pipecolic acid; (2E)-decanoyl-ACP | Amino acids and derivatives; Carboxylic acids and derivatives | 1.47 | C6H11NO2 | 0.34 |
| 17 | D-α-aminobutyric acid | Carboxylic acids and derivatives | 0.65 | C4H9NO2 | 0.31 |
| 18 | Uracil | Nucleotides and its derivates | 1.91 | C4H4N2O2 | 0.31 |
| 19 | Caryophyllene oxide | Sesquiterpenes | 11.66 | C15H24O | 0.27 |
| 20 | L-epicatechin | Flavonoids | 5.08 | C15H14O6 | 0.26 |
| 21 | Palmitoylethanolamide | Fatty acid amides | 12.61 | C18H37NO2 | 0.21 |
| 22 | Safrole | Benzodioxoles | 12.26 | C10H10O2 | 0.18 |
| 23 | Oleic acid; vaccenic acid; petroselinic acid | Fatty acyls | 13.03 | C18H34O2 | 0.18 |
| 24 | Aristolindiquinone | Quinones | 11.14 | C12H10O4 | 0.18 |
| 25 | Cholesterol | Steroids and steroid derivatives | 11.86 | C27H46O | 0.16 |
| 26 | Cinchonine | Alkaloids | 11.99 | C19H22N2O | 0.15 |
| 27 | L-glutamic acid | Amino acids and derivatives | 0.66 | C5H9NO4 | 0.15 |
| 28 | L-threonine | Amino acids and derivatives | 0.64 | C4H9NO3 | 0.15 |
| 29 | L-homoserine | Amino acid and derivatives | 0.67 | C4H9NO3 | 0.13 |
| 30 | AICAR | Imidazole ribonucleosides and ribonucleotides | 13.28 | C9H15N4O8P | 0.13 |
| 31 | α-cyperone | Sesquiterpenoids | 12.2 | C15H22O | 0.13 |
| 32 | Vidarabine | Purine nucleosides | 2.28 | C10H13N5O4 | 0.13 |
| 33 | Procyanidin B2 | Flavonoids | 4.78 | C30H26O12 | 0.12 |
| 34 | Valerenic acid | Sesquiterpenoids | 11.24 | C15H22O2 | 0.12 |
| 35 | L-asparagine | Amino acids and derivatives | 0.64 | C4H8N2O3 | 0.12 |
| 36 | Proline; L-proline | Amino acids and derivatives | 0.73 | C5H9NO2 | 0.12 |
| 37 | D-proline | Carboxylic acids and derivatives | 0.76 | C5H9NO2 | 0.12 |
| 38 | Bisabolol oxide A | Sesquiterpenoids | 11.5 | C15H26O2 | 0.11 |
| 39 | β-Sitosterol; β-sitosterol | Steroids and steroid derivatives | 12.93 | C29H50O | 0.10 |
| 40 | Kirenol | Diterpenoids | 13.16 | C20H34O4 | 0.10 |
| 41 | Trans-caryophyllene | Sesquiterpenes | 12.12 | C15H24 | 0.09 |
| 42 | Styrene oxide | Benzene and substituted derivatives | 5.94 | C8H8O | 0.09 |
| 43 | Levamisole | Imidazothiazoles | 12.04 | C11H12N2S | 0.08 |
| 44 | Betulin | Triterpenoids | 12.32 | C30H50O2 | 0.08 |
| 45 | 2,5-Dihydroxybenzaldehyde | Phenols | 5.09 | C7H6O3 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Wang, Y.; Sun, M.; Xu, Y.; Chen, L. Antibacterial Activity and Components of the Methanol-Phase Extract from Rhizomes of Pharmacophagous Plant Alpinia officinarum Hance. Molecules 2022, 27, 4308. https://doi.org/10.3390/molecules27134308
Fu J, Wang Y, Sun M, Xu Y, Chen L. Antibacterial Activity and Components of the Methanol-Phase Extract from Rhizomes of Pharmacophagous Plant Alpinia officinarum Hance. Molecules. 2022; 27(13):4308. https://doi.org/10.3390/molecules27134308
Chicago/Turabian StyleFu, Junfeng, Yaping Wang, Meng Sun, Yingwei Xu, and Lanming Chen. 2022. "Antibacterial Activity and Components of the Methanol-Phase Extract from Rhizomes of Pharmacophagous Plant Alpinia officinarum Hance" Molecules 27, no. 13: 4308. https://doi.org/10.3390/molecules27134308
APA StyleFu, J., Wang, Y., Sun, M., Xu, Y., & Chen, L. (2022). Antibacterial Activity and Components of the Methanol-Phase Extract from Rhizomes of Pharmacophagous Plant Alpinia officinarum Hance. Molecules, 27(13), 4308. https://doi.org/10.3390/molecules27134308





