Abstract
In view of that conjugated polymers (CPs) are an attractive option for constructing high-sensitive Cr2O72− sensors but suffer from lacking a general design strategy, we first proposed a rational structure design of CPs to tailor their sensing properties while validating the structure-to-performance correlation. Short side chains decorated with N and O atoms as recognition groups were instructed into fluorene to obtain monomers Fmoc-Ala-OH and Fmoc-Thr-OH. Additionally, their polymers P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) were obtained through electrochemical polymerization. P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) with high polymerization degrees have an excellent selectivity towards Cr2O72− in comparison to other cations and anions. Additionally, their limit of detection could achieve 1.98 fM and 3.72 fM, respectively. Especially, they could realize the trace detection of Cr2O72− in agricultural products (red bean, black bean, and millet). All these results indicate that short side chains decorated with N and O atoms functionalizing polyfluorene enables the ultra-trace detection of Cr2O72−. Additionally, the design strategy will spark new ideas for the construction of highly selective and sensitive Cr2O72− sensors.
1. Introduction
Environmental pollution jeopardizes people’s health even at a very low concentration due to its high toxicity, so it is receiving more and more attention [1]. If we want to formulate a reasonable and feasible treatment plan, it is necessarily to monitor these pollutions quickly, easily, and accurately. Recently, fluorescence analysis methods have been developing rapidly, and these enable high selectivity and sensitivity, fast detection, in situ analysis, and low cost [2]. Among various fluorescent materials, conjugated polymers (CPs) are comparatively dominant because they could introduce recognition groups and have a unique “molecular wire effect” [3,4,5], which greatly improves the selectivity and sensitivity of fluorescent sensors [6,7,8,9]. In the past decades, CPs-based fluorescent sensors have been the research hotpot.
Chromium (Cr) is one necessary element in industry, but the impact of hexavalent chromium (Cr(VI)) on human health should not be underestimated [10,11,12]. Once its content exceeds the standard in water (existing in the form of Cr2O72−), it will be enriched in the crops and passed to the human body through the food chain, which will cause various diseases [13,14,15,16,17,18,19]. Up to now, Cr2O72− detection materials mainly contain inorganic materials (quantum dots [20,21,22] and carbon dots [23,24,25]), organic materials (organic molecules [26,27] and CPs [28]), and organic–inorganic hybrid materials (metal-organic frameworks [29,30] and nanoclusters). Generally, inorganic materials recognize Cr2O72− by inner filtering effect (IFE) and organic materials used recognition groups through hydrogen bonds or coordination bonds, while organic–inorganic hybrid materials often rely on the synergetic effect of the aforementioned recognition mechanism of inorganic and organic materials. For Cr2O72−, which are difficult to detect at low concentrations, CPs show the advantages of trace detection. Unfortunately, few efforts have been devoted to the development of this kind of sensor based on CPs, which may be the difficulties of design and preparation. Our group have been committed to the design and development of new CPs and apply them in fluorescent sensing fields [12,13,14,15]. Indeed, CPs exhibit supersensitivity in detecting targets. In our previous work, we have prepared several Cr2O72− sensors based on CPs, but only two sensors could achieve ultra-trace detection. Thus, it remains a challenge to design CP-based sensors with high performance and validate a general design strategy.
In this work, we aim to rationally design the structure of CPs to tailor their sensing properties while validating the structure-to-performance correlation. Fluorene, an excellent blue emitter, was selected as the fluorophore. The steric hindrance of side chain will hinder the polymerization of fluorene and decrease the polymerization degree, which is closely related to the detection sensitivity. So, the side chains were customized with desired N and O atoms as Cr2O72− recognition group, and their chain length was kept as short as possible. Fluorene modified by two acid groups were synthesized, and their polymers (P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH)) were prepared through electrochemical polymerization. A series of experiments show that fluorescent sensors based on P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) could achieve the trace detection of Cr2O72− in agricultural products.
2. Results and Discussion
2.1. Electropolymerization of Monomers Fmoc-Ala-OH and Fmoc-Thr-OH
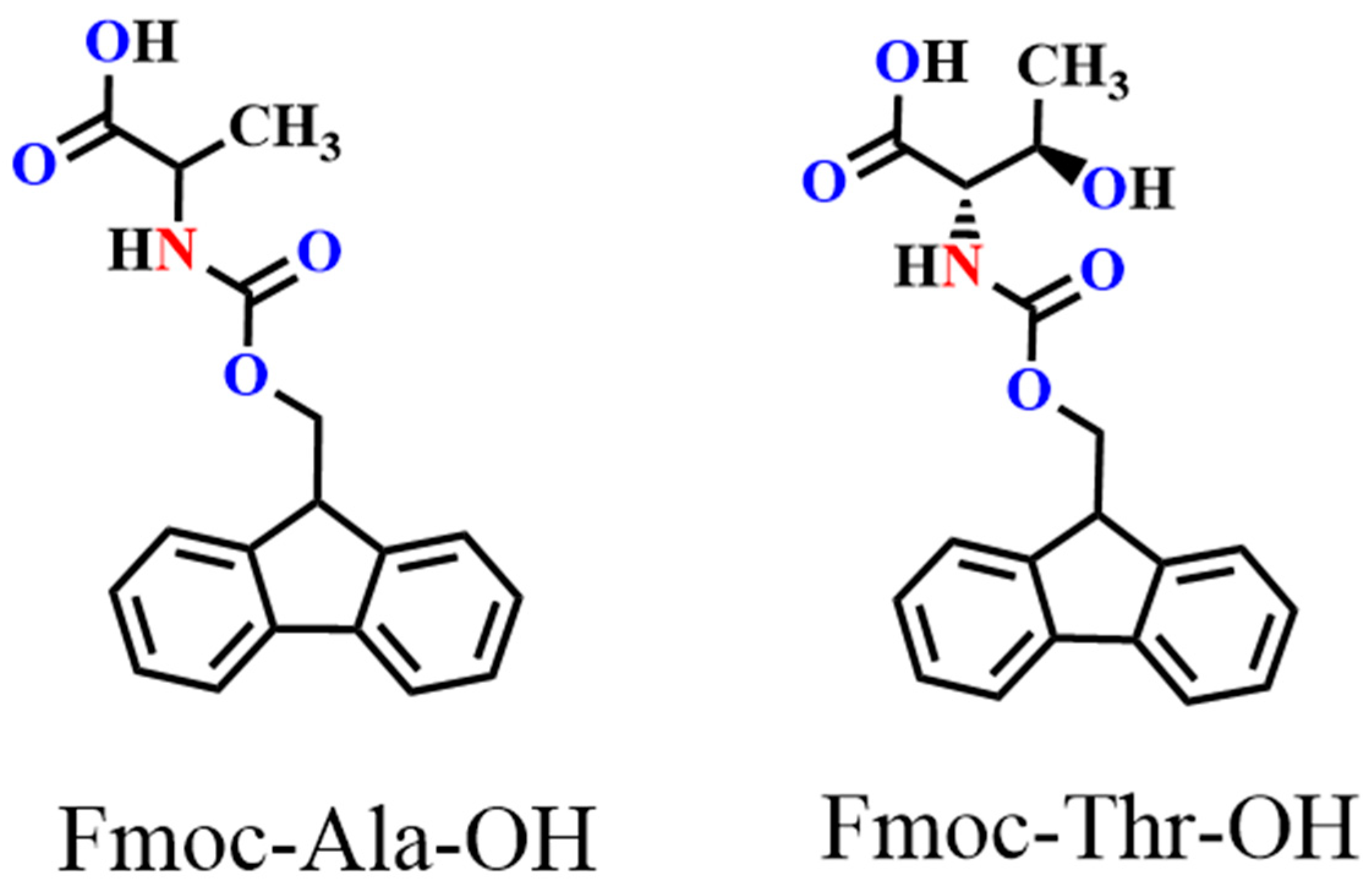
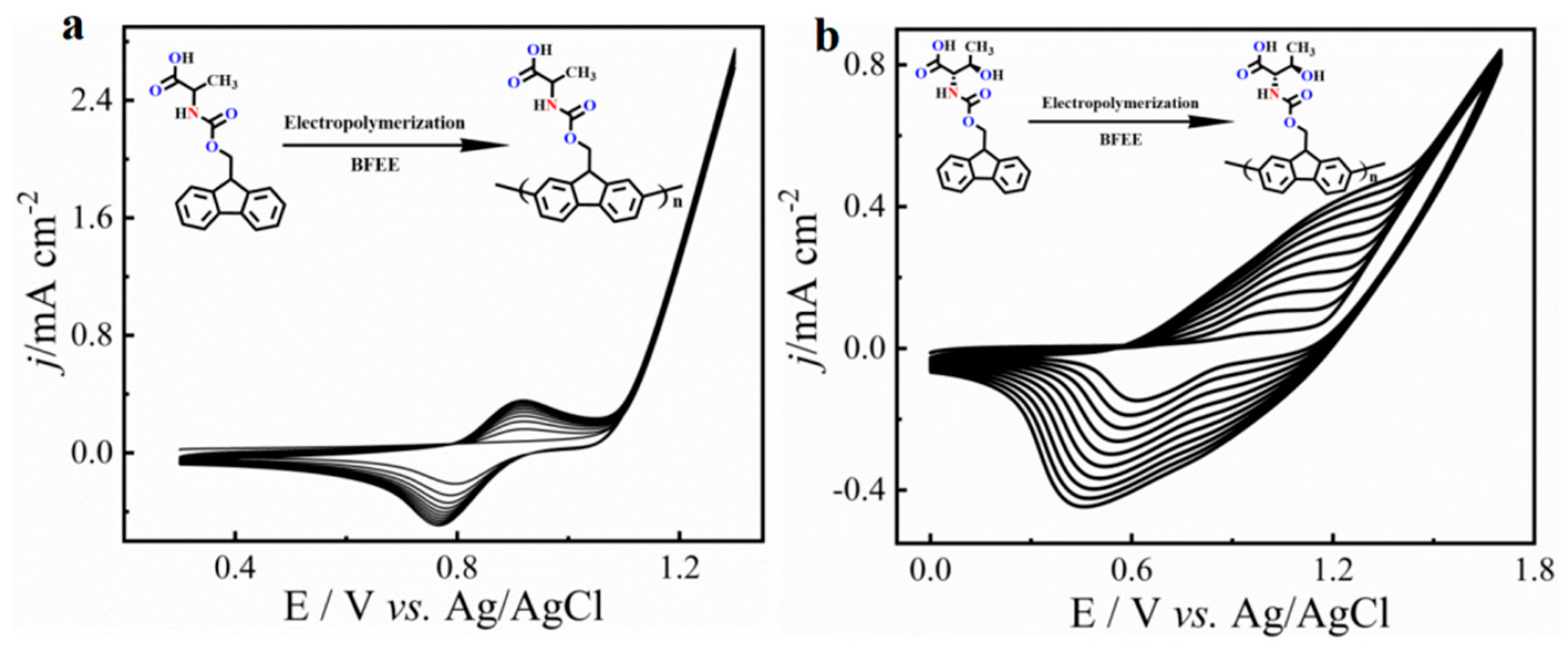
In this work, we chose an acid group decorated with N and O atoms as the functional side chains. To keep the side chain as short as possible, every side chain contained only one -NH2 and one -COOH unit, and two fluorene derivatives were obtained as monomers (Figure 1). Two monomers could easily dissolve in common solvents, such as tetrahydrofuran (THF), N,N-Dimethylformamide (DMF), and dichloromethane (DCM). However, in neutral solvents, they could not polymerize any supporting electrolytes including Bu4NPF6, Bu4NBF4, and Bu4NClO4 (Table S1). Delightfully, they could easily polymerize in a pure boron trifluoride ethyl ether (BFEE) system without adding external supporting electrolyte because BFEE can lower the polymerization potential and promote the polymerization of fused ring compounds. As shown in Figure 2, as the number of scan cycles increased, the current density of a pair of reversible redox peaks in cyclic voltammograms (CVs) increased, indicating that monomers Fmoc-Ala-OH and Fmoc-Thr-OH can be electropolymerized in BFEE. By analysing the 1H NMR spectra (Figures S1 and S2), it was found that two molecules were polymerized at the position 2,7, which was the same as other fluorene derivatives [13,14,15,31]. From GPC results, polymers P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) contained 72 and 41 repeat units, respectively. In other words, the two polymers have high polymerization degrees. Combined with other polyfluorene reported by our groups [12,13,14,15], the shorter side chain groups will reduce the polymerization hindrance of fluorene. Importantly, high polymerization degrees are beneficial to the detection sensitivity in application.
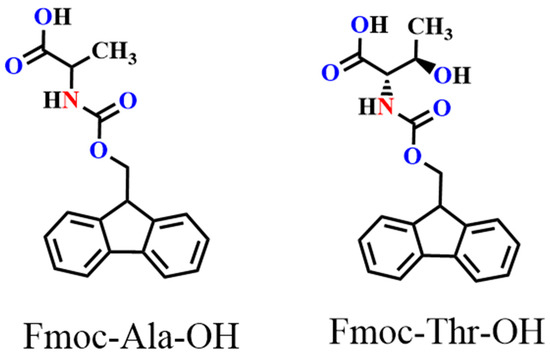
Figure 1.
Structure of monomers Fmoc-Ala-OH and Fmoc-Thr-OH.
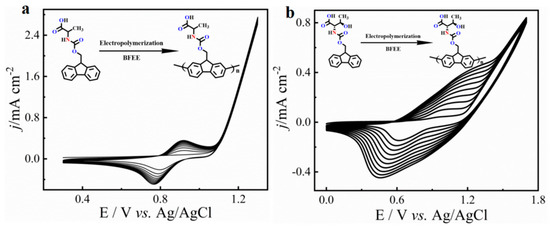
Figure 2.
Multicycle CVs of monomer Fmoc-Ala-OH (a) and Fmoc-Thr-OH (b) in the BFEE system. Potential scan rate of 100 mV s−1.
2.2. Selective and Competitive Testing of Polymers P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH)
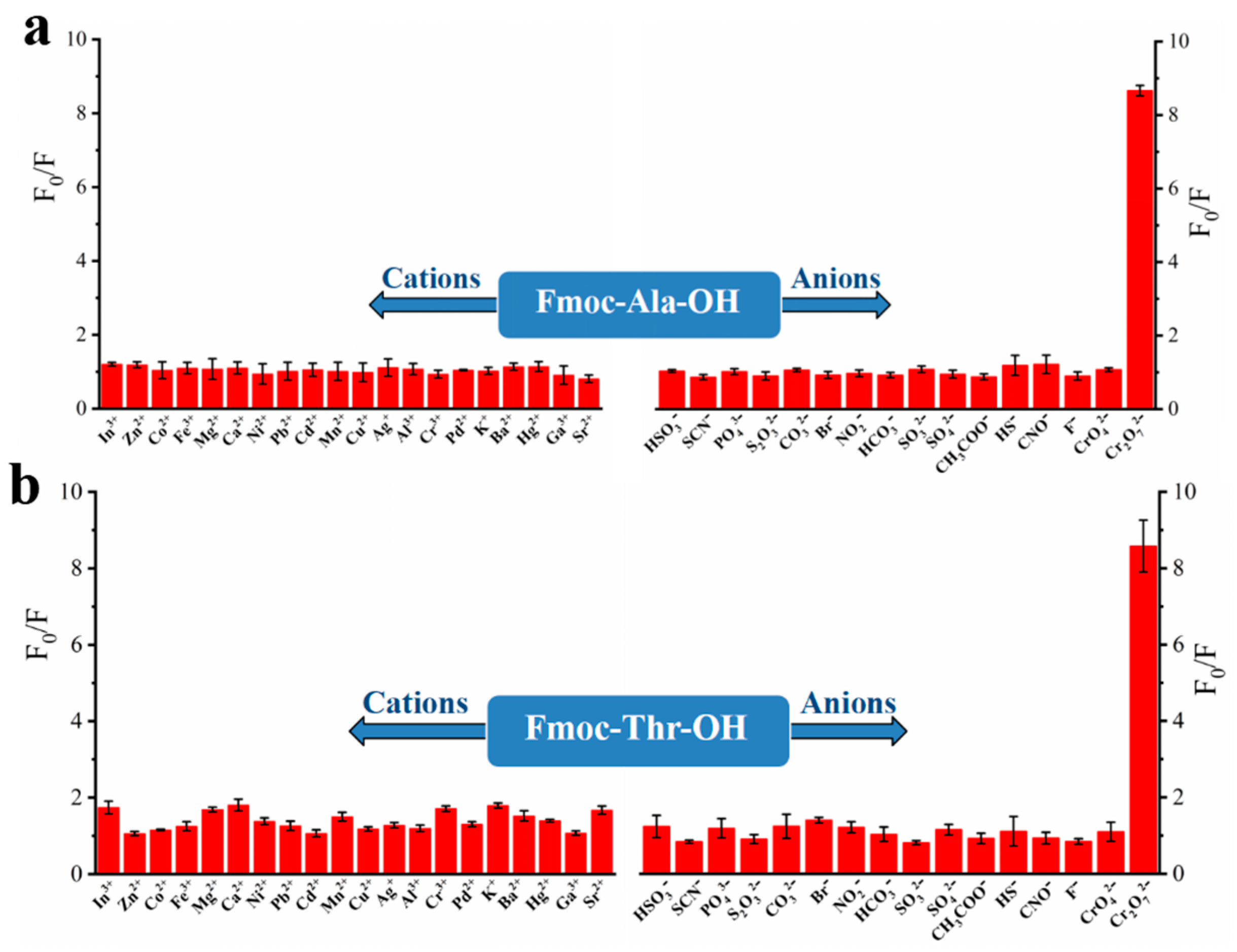
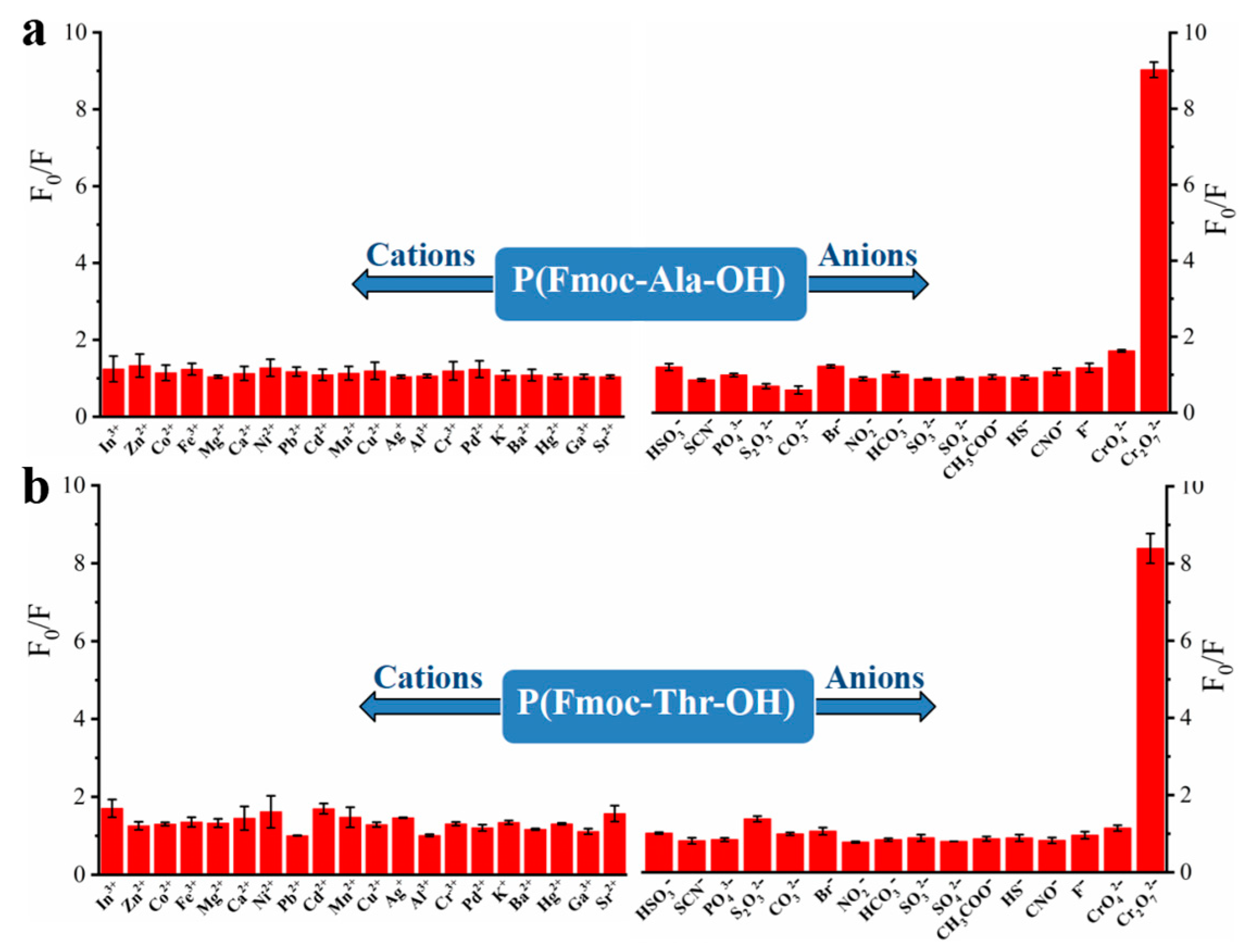
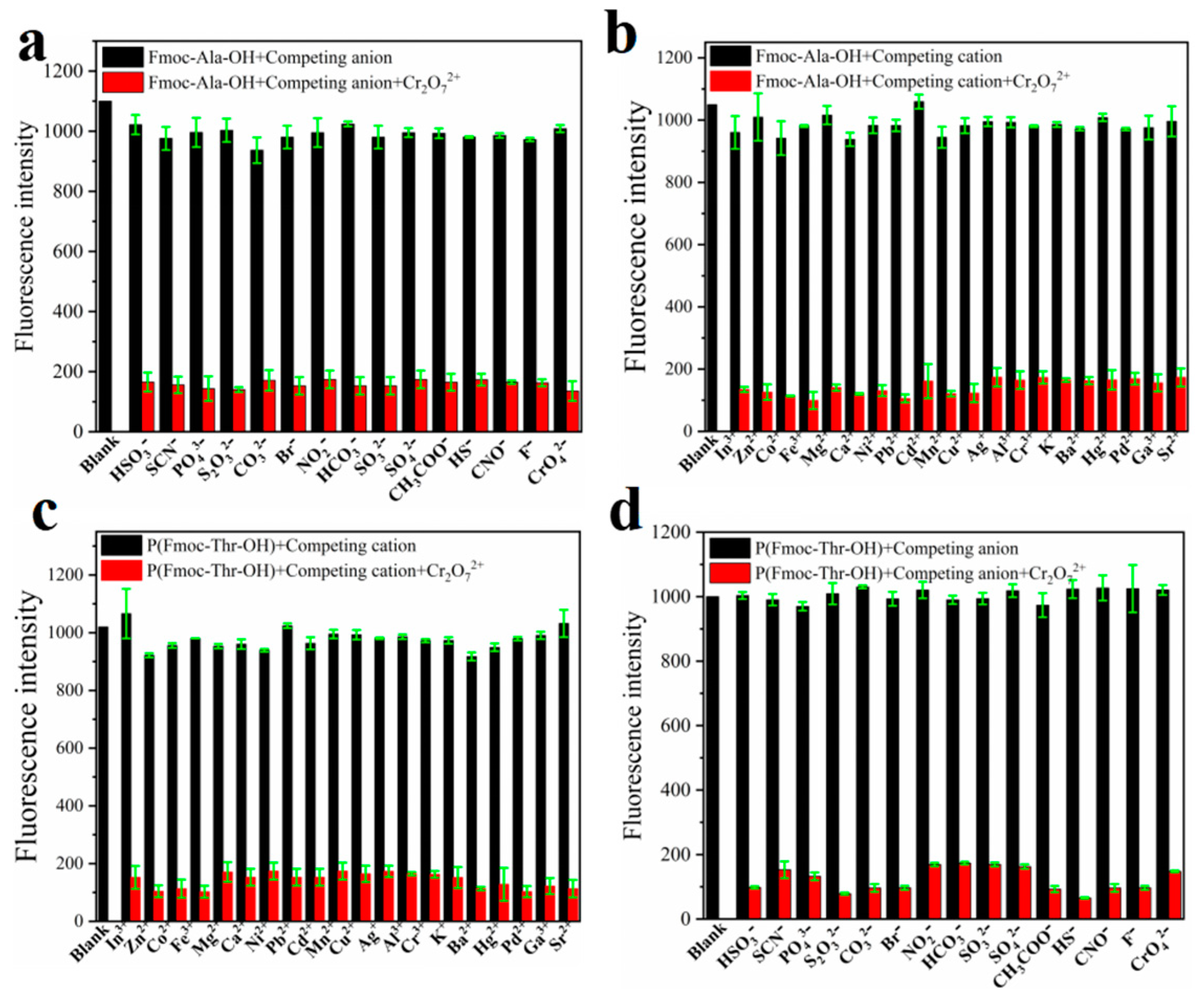
To explore the recognition of acid groups in fluorene, we first studied the selectivity of two monomers to common anions and cations. From Figure 3, we can see that only Cr2O72− could quench the fluorescence of monomers Fmoc-Ala-OH and Fmoc-Thr-OH, which indicated that they have high selectivity to Cr2O72−. We speculate that the N and O atoms may interact with Cr2O72− [32], which caused the aggregate of fluorene and resulted in fluorescence quenching [33]. Then, we studied the selectivity of their corresponding polymers. As shown in Figure 4, all their polymers P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) also exhibited the good selectivity for Cr2O72−, which were not interfered by anions and cations (Figure 5).
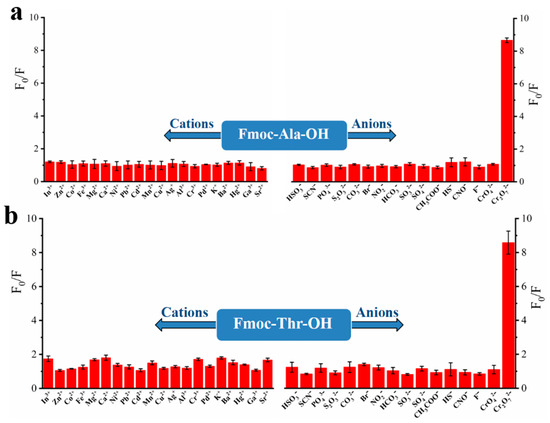
Figure 3.
Fluorescence quenching of monomer Fmoc-Ala-OH (a) and Fmoc-Thr-OH (b) to various cations/anions.
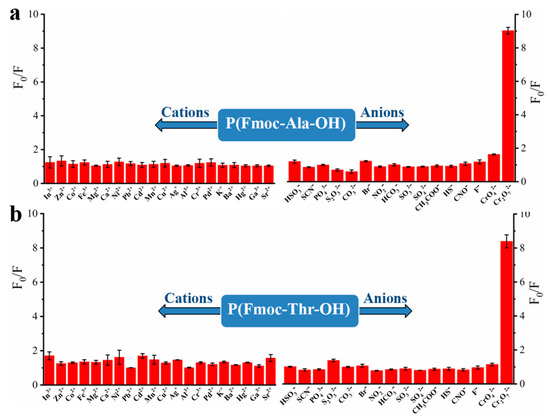
Figure 4.
Fluorescence quenching of P(Fmoc-Ala-OH) (a) and P(Fmoc-Thr-OH) (b) to various cations/anions.
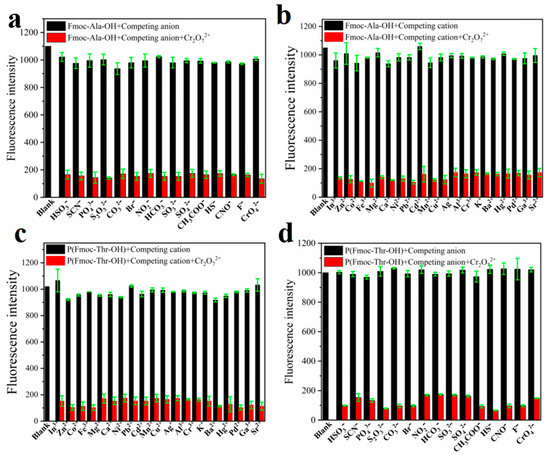
Figure 5.
Fluorescence intensity of P(Fmoc-Ala-OH) (a,b) and P(Fmoc-Thr-OH) (c,d) in the mixed DMSO/EtOH (v/v = 1:800) containing various ions. The black bars represent the addition of the competing ions to a solution of P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH). The red bars represent the change of the emission that occurs upon the subsequent addition of Cr2O72− to the above solution.
2.3. Sensitivity Test of P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH)
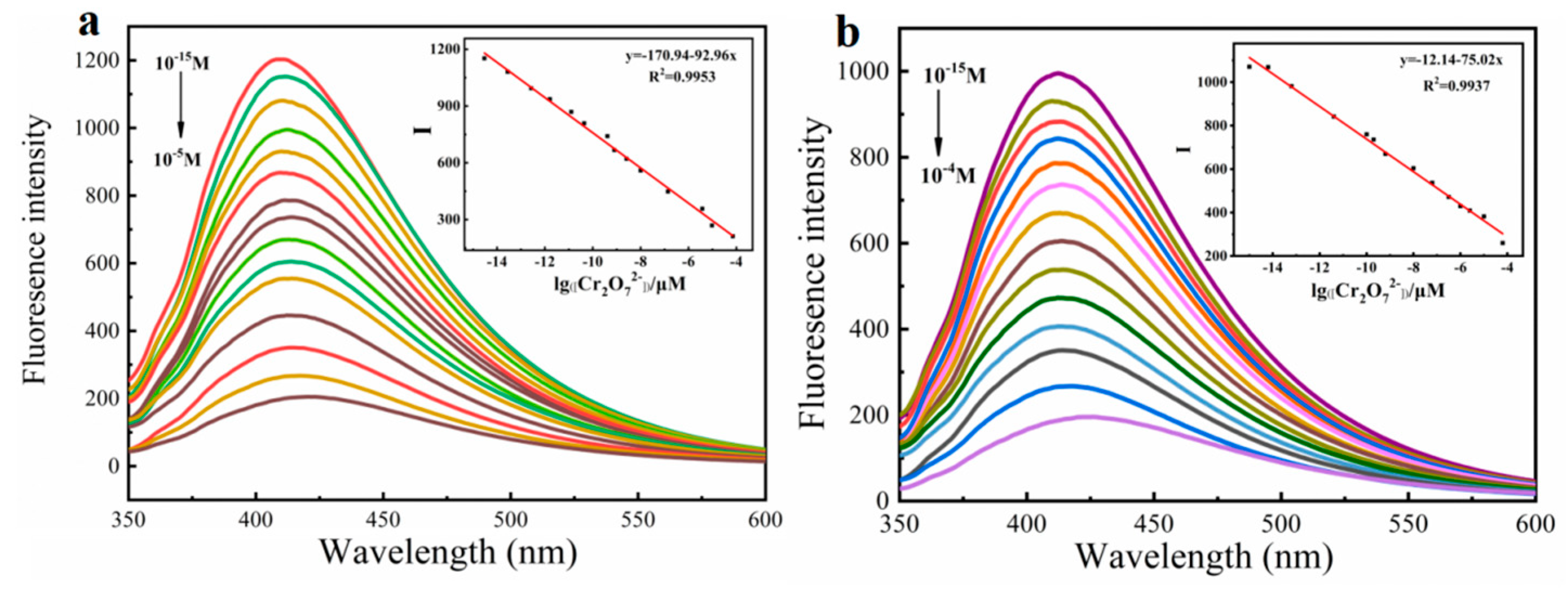
Based on the above results, both monomers and polymers showed specific recognition to Cr2O72−, so we further explore their sensitivity. Additionally, the linear relationship between fluorescence intensity and Cr2O72− concentration was studied. As shown in Figure S1, monomers Fmoc-Ala-OH and Fmoc-Thr-OH have sensitivity to Cr2O72− in nM level, while their limits of detection (LODs) were 0.11 nM and 0.27 nM, respectively. When they were prepared into polymers, their detection sensitivity was greatly improved and reached upto fM, and their LODs achieved 1.98 fM and 3.72 fM, respectively (Figure 6). This verifies that the molecular wire effect of polymer can greatly improve the sensitivity of the detection of Cr2O72−, which further indicates that this type of acid-functionalized polyfluorene fluorescence material has the ability of ultra-trace detection of Cr2O72−. Compared with other Cr2O72− sensors reported, P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) showed the lowest LOD, which is owed to the delicate design of side chains and the preparation of high-quality polymers.
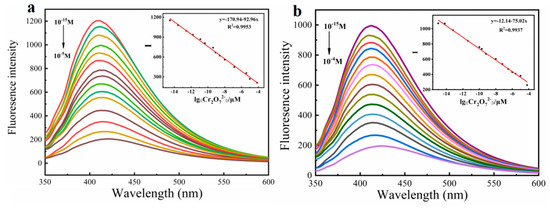
Figure 6.
Fluorescence emission spectra of P(Fmoc-Ala-OH) (a) 2.5 μM and P(Fmoc-Thr-OH) (b) 4.2 μM toward Cr2O72− with different concentrations in DMSO-EtOH, respectively. Inset: Linear plots of their fluorescence intensity against Cr2O72− concentration. (Ex = 335 nm, 350 nm).
2.4. Application
To explore the feasibility of sensors P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) applied to real samples, we detected Cr2O72− in red bean, black bean, and millet samples by standard addition method [34]. As shown in Table 1 and Table 2, P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) were not significantly quenched by each agricultural products sample, indicating that these agricultural product samples may not contain Cr2O72−. We then added the standard concentration of Cr2O72− to three agricultural products samples containing P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) and found that the fluorescence intensity of the samples was quenched. In actual samples, two fluorescence sensors have good detection results, and the recovery rates are (94.0–103.0%) and (91.0–101.5%), respectively, indicating that the two fluorescent sensors P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) are able to detect Cr2O72− in agricultural product samples. Based on the above results, we believe that this type of amino-acid-functionalized polyfluorene fluorescent sensor can be applied to the detection of Cr2O72− in real agricultural products.

Table 1.
Determination of Cr2O72− in agricultural products samples solution using P(Fmoc-Ala-OH).

Table 2.
Determination of Cr2O72− in agricultural product samples solution using P(Fmoc-Thr-OH).
3. Materials and Methods
3.1. Materials and Instruments
The materials and instruments used in this work, and the corresponding characterization of monomer Fmoc-Ala-OH and Fmoc-Thr-OH, are listed in the Supplementary Information.
Fmoc-Ala-OH (98%, Aladdin, Shanghai, China), Fmoc-Thr-OH (98%, Aladdin), tetrahydrofuran (THF, 99%, Aladdin), N,N-Dimethylformamide (DMF, 99%, Aladdin), dichloromethane (DCM, 99.99%, Aladdin), boron trifluoride diethyl etherate (BFEE, 98%, Aladdin) were used directly. Twice distilled water was used throughout all experiments. The aqueous solution of Sn2+ was prepared from its chloride salt; the aqueous solution of Ag+ was prepared from its perchloric acid salt; aqueous solutions of Sr2+, Ga3+, Pd2+, Hg2+, Ba2+, K+, Cr3+, Al3+, Cu2+, Mn2+, Cd2+, Pb2+, Ni2+, Ca2+, Mg2+, Fe2+, Fe3+, Co2+, Zn2+, and In3+ were prepared from their nitrate salts. The aqueous solution of Cr2O72− was prepared from its kalium salt; aqueous solutions of F−, CNO−, HS−, CH3COO−, SO42−, SO32−, HCO32−, NO2−, Br−, CO32−, S2O32−, PO43−, SCN−, and HSO3− were prepared from their sodium salts. Sodium dihydrogen phosphate (NaH2PO4), disodium hydrogen phosphate (Na2HPO4), hydrochloric acid, and aqueous ammonia were purchased from Tianjin Damao Chemical Plant (Tianjin, China).
Electrochemical polymerization was performed using a Versa Stat 3 electrochemical workstation (EG&G Princeton Applied Research, Shanghai, China) under computer control. The GPC determination of P1 and P2 was carried out using American Waters 1525 gel chromatography (Waters, MA, USA; chromatographic column: Agilent PLgel 5um MIXED-C, manufactured by GB, Palo Alto, CA, USA) and the mobile phase was DMF. Absorption spectra were obtained from an Agilent 8454 UV-vis spectrophotometer (Agilent, Palo Alto, CA, USA). All fluorescent experiments were studied on a Hitachi F-4600 fluorospectrophotometer (Hitachi, Tokyo, Japan) with excitation/emission slit set at 5 nm.
3.2. Electrosynthesis of P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH)
P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) were prepared in boron trifluoride ethyl ether (BFEE) system by direct anodization of monomer Fmoc-Ala-OH and Fmoc-Thr-OH, respectively. Electrochemical polymerization was accomplished using a classic one-chamber three-electrode system. The reference electrode was Ag/AgCl, and the working and counter electrodes were ITO. The electrochemical polymerization was carried out in the BFEE system, and the polymers P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) were obtained at voltages of 1.2 V and 1.38 V, respectively. The obtained polymer films were first rinsed by anhydrous ether and then dried under vacuum at 65 °C for 24 h. Molecular weight tests of P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) were tested used Gel Permeation Chromatography (GPC) and DMF was as mobile phase. P(Fmoc-Ala-OH): Mw = 45,202, Mn = 22,431, PDI = 2.01; P(Fmoc-Thr-OH): Mw = 22,920, Mn = 13,820, PDI = 1.65.
3.3. Detection of Cr2O72−
P(Fmoc-Ala-OH) (2.5 × 10−3 M) solution and P(Fmoc-Thr-OH) (4.2 × 10−3 M) solution were prepared by dimethyl sulfoxide (DMSO). Selective, competitive, and sensitive experiments of two polymers were performed in DMSO-EtOH (v/v = 1:800) system.
3.4. Preparation of Agricultural Products Samples
The red bean, black bean, and millet were purchased from supermarkets. To evaluate the applicability of P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) in real samples, we carried out experiments using standard addition methods. After adding different concentrations of Cr2O72− to real samples, P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) were used to detect these samples with or without Cr2O72−.
4. Conclusions
In conclusion, we proposed a design strategy to construct Cr2O72− sensors with high selectivity and sensitivity. Two fluorene derivatives with N and O atoms in short side chains were easily polymerize in BFEE system. Thanks to the N and O atoms, two monomers (Fmoc-Ala-OH and Fmoc-Thr-OH) and their polymers (P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH)) could specific recognize Cr2O72− without avoiding the interference of common cations and anions. Additionally, two polymers showed high sensitivity to Cr2O72− and their LODs achieved fM. In particular, P(Fmoc-Ala-OH) and P(Fmoc-Thr-OH) realized the trace detection of Cr2O72− in agricultural products. All these results showed that short side chains with N and O atoms functionalized conjugated polymer chains could enable the ultra-trace detection of Cr2O72−.
Supplementary Materials
The following are available online https://www.mdpi.com/article/10.3390/molecules27134294/s1, Figure S1: 1H NMR spectra of Fmoc-Ala-OH (a) and P(Fmoc-Ala-OH) (b), Figure S2: 1H NMR spectra of Fmoc-Thr-OH (a) and P(Fmoc-Thr-OH) (b), Figure S3: Fluorescence spectra of Fmoc-Ala-OH (a) and Fmoc-Thr-OH (b) toward Cr2O72− with different concentrations in DMSO/EtOH solution. Inset: linear plots of their fluorescence intensity against the Cr2O72− concentration, Table S1: Polymerization of monomers Fmoc-Ala-OH and Fmoc-Thr-OH in different solvents.
Author Contributions
Conceptualization, H.L. and F.L.(Fei Li); methodology, H.L. and X.C.; software, H.L., F.L. (Fang Liu) and W.X.; validation, H.L. and J.X.; formal analysis, F.L. (Fei Li), L.S. and G.Z.; investigation, H.L. and F.L. (Fei Li); resources, H.L., X.C. and R.Y.; data curation, H.L.; Writing—original draft preparation, H.L. and F.L. (Fei Li); Writing—review and editing, J.X., G.Z. and R.Y.; visualization, G.Z.; supervision, G.Z. and J.X.; project administration, G.Z. and J.X.; funding acquisition, G.Z. and J.X.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation (52073128, 51863009, 5210031010), Natural Science Foundation of Jiangxi Province (20212BAB203012), Scientific Research Foundation for Doctors in Jiangxi Science and Technology Normal University (2017BSQD006), Projects for Postgraduate Innovation in Jiangxi (YC2021-X02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interests.
Sample Availability
Not available.
References
- Yu, J.; Zhang, C. Fluorescent sensing for amines with a low detection limit based on conjugated porous polymers. J. Mater. Chem. C 2020, 8, 16463–16469. [Google Scholar] [CrossRef]
- Wang, T.S.; Zhang, N.; Bar, W.; Bao, Y.Y. Fluorescent chemosensors based on conjugated polymers with N-heterocyclic moieties: Two decades of progress. Polym. Chem. 2020, 11, 3095–3114. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, B.Y.; Wen, Y.P.; Lu, L.M.; Xu, J.K. Facile fabrication of a cost-effective, water-soluble, and electrosynthesized poly(9-aminofluorene) fluorescent sensor for the selective and sensitive detection of Fe(III) and inorganic phosphates. Sens. Actuat. B Chem. 2012, 171, 786–794. [Google Scholar] [CrossRef]
- Zhang, G.; Wen, Y.P.; Guo, C.Q.; Xu, J.K.; Lu, B.Y.; Duan, X.M.; He, H.H.; Yang, J. A cost-effective and practical polybenzanthrone-based fluorescent sensor for efficient determination of palladium (II) ion and its application in agricultural crops and environment. Anal. Chim. Acta. 2013, 805, 87–94. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Zhang, J.; Ding, W.C.; Xu, J.K.; Wen, Y.P. Highly selective fluorescent sensor based on electrosynthesized oligo(1-pyreneboronic acid) enables ultra-trace analysis of Cu2+ in environment and agro-product samples. Sens. Actuat. B Chem. 2017, 253, 224–230. [Google Scholar] [CrossRef]
- Pak, Y.L.; Wang, Y.T.; Xu, Q.L. Conjugated polymer based fluorescent probes for metal ions. Coordin. Chem. Rev. 2021, 433, 213745. [Google Scholar] [CrossRef]
- Wang, C.H.; Nesterov, E.E. Amplifying fluorescent conjugated polymer sensor for singlet oxygen detection. Chem. Commun. 2019, 55, 8955–8958. [Google Scholar] [CrossRef]
- Meng, B.; Liu, J.; Wang, L.X. Oligo(ethylene glycol) as side chains of conjugated polymers for optoelectronic applications. Polym. Chem. 2020, 11, 1261–1270. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.Y.; Zhu, S.X.; Yao, C.; Ban, D.D.; Liu, R.H.; Li, L.D.; Wang, S. Controllable Targeted Accumulation of Fluorescent Conjugated Polymers on Bacteria Mediated by a Saccharide Bridge. Chem. Mater. 2020, 32, 438–447. [Google Scholar] [CrossRef]
- Zhou, H.L.; Li, X.D.; Wang, L.H.; Liang, Y.F.; Jialading, A.; Wang, Z.S.; Zhang, J.G. Application of SERS quantitative analysis method in food safety detection. Rev. Anal. Chem. 2021, 40, 173–186. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wen, Y.P.; Zhang, H.; Chai, J.D.; Duan, X.M.; Shen, L.; Zhang, G.; Xu, J.K. Highly sensitive fluorescent sensor based on electrosynthesized poly(FmocL-serine) enables ultra-trace analysis of Cr2O72− in water and agro-product samples. Sens. Actuat. B Chem. 2018, 277, 394–400. [Google Scholar] [CrossRef]
- Li, F.; Duan, X.M.; Li, H.; Zou, L.; Liu, G.Q.; Liu, F.; Zhang, G.; Xu, J.K. Dual effect of aminobutyric acid group and “molecular wire effect” of conjugated polymer enables ultra-trace detection of Cr2O72− in fruits. Microchem. J. 2022, 178, 107426. [Google Scholar] [CrossRef]
- Li, F.; Duan, X.M.; Hu, S.X.; Zhang, L.; Shen, L.; Liu, F.; Li, H.; Zhang, G.; Xu, J.K. Ultra-sensitive detection of Cr2O72− in farmland achieved by an electrosynthesized fluorescent poly(Fmoc-succinimide). Dyes Pigment. 2021, 193, 109568. [Google Scholar] [CrossRef]
- Li, F.; Zhang, G.; Zou, L.; Zhang, X.X.; Liu, F.; Li, H.; Xu, J.K.; Duan, X.M. Amino Acid Groups Enable Electrosynthesized Polyfluorenes to Specifically Recognize Cr2O72−. ACS Appl. Polym. Mater. 2022, 4, 815–821. [Google Scholar] [CrossRef]
- Iyengar, V.; Elmadfa, I. Food Safety Security: A new Concept for Enhancing Food Safety Measures. Int. J. Vitam. Nutr. Res. 2012, 82, 216–222. [Google Scholar] [CrossRef]
- Garcia, S.N.; Osburn, B.I.; Cullor, J.S. A one health perspective on dairy production and dairy food safety. One Health 2019, 7, 100086. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Ferey, G.; Morris, R.E.; Serre, C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Li, C.J.; Zhu, H.M.; Li, C.Y.; Qian, H.; Yao, W.R.; Guo, Y.H. The present situation of pesticide residues in China and their removal and transformation during food processing. Food Chem. 2021, 354, 129552. [Google Scholar] [CrossRef]
- Tiwari, S.; Deb, M.K.; Sen, B.K. Cloud point extraction and diffuse reflectance-Fourier transform infrared spectroscopic determination of chromium(VI): A probe to adulteration in food stuffs. Food Chem. 2017, 221, 47–53. [Google Scholar] [CrossRef]
- Wu, N.T.; Li, Y.; Zeng, M.; Gao, J.W.; Tang, Y.P.; Zeng, Z.; Zheng, Y.H. Design of chalcopyrite-type CuFeSe2 nanocrystals: Microstructure, magnetism, photoluminescence and sensing performances. J. Solid State Chem. 2019, 271, 292–297. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Yang, Z.L.; Chen, X.Y.; Hu, D.; Hong, Y.P. Facile gradient oxidation synthesizing of highly-fluorescent MoO3 quantum dots for Cr2O72− trace sensing. Inorg. Chem. Commun. 2020, 118, 108001. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Dextran modified magnetic nanoparticles based solid phase extraction coupled with linear sweep voltammetry for the speciation of Cr(VI) and Cr (III) in tea, coffee, and mineral water samples. Food Chem. 2019, 292, 151–159. [Google Scholar] [CrossRef]
- Qiao, G.X.; Lu, D.; Tang, Y.P.; Gao, J.W.; Wang, Q.M. Smart choice of carbon dots as a dual-mode onsite nanoplatform for the trace level detection of Cr2O72. Dyes Pigment. 2019, 163, 102–110. [Google Scholar] [CrossRef]
- Su, Y.; Xie, Z.G.; Zheng, M. Carbon dots with concentration-modulated fluorescence: Aggregation-induced multicolor emission. J. Colloid Interf. Sci. 2020, 573, 241–249. [Google Scholar] [CrossRef]
- Li, X.X.; Xu, H.Y.; Kong, F.Z.; Wang, R.H. A Cationic Metal-Organic Framework Consisting of Nanoscale Cages: Capture, Separation, and Luminescent Probing of Cr2O72− through a Single-Crystal to Single-Crystal Process. Angew. Chem. Int. Edit. 2013, 52, 13769–13773. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Das, K.R.; Antony, M.J.; Varghese, S. Highly bluish-white light emissive and redox active conjugated poly-N-phenyl anthranilic acid polymer fluoroprobe for analytical sensing. Polymer 2019, 181, 121747. [Google Scholar] [CrossRef]
- Wu, X.X.; Fu, H.R.; Han, M.L.; Zhou, Z.; Ma, L.F. Tetraphenylethylene Immobilized Metal-Organic Frameworks: Highly Sensitive Fluorescent Sensor for the Detection of Cr2O72− and Nitroaromatic Explosives. Cryst. Growth Des. 2017, 17, 6041–6048. [Google Scholar] [CrossRef]
- Miao, C.L. A bi-functional 3D Pb-II-organic framework for Knoevenagel condensation reaction and highly selective luminescent sensing of Cr2O72−. Inorg. Chem. Commun. 2019, 105, 86–92. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Y.M.; He, X.Q.; Zhang, W.J.; Tian, M.G.; Feng, R.Q.; Zhang, R.Y.; Li, X.C.; Guo, L.F.; Yu, X.Q.; et al. Red-Emitting Mitochondrial Probe with Ultrahigh Signal-to-Noise Ratio Enables High-Fidelity Fluorescent Images in Two-Photon Microscopy. Anal. Chem. 2015, 87, 12088–12095. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Niu, H.Y.; He, S.J.; Cai, Y.Q. One-step fabrication of high quantum yield sulfur- and nitrogen-doped carbon dots for sensitive and selective detection of Cr(VI). RSC Adv. 2016, 6, 107717–107722. [Google Scholar] [CrossRef]
- Zhang, M.X.; Chen, J.C.; Wang, M.L.; Yuan, M.J.; Li, R.; Feng, X.X.; He, Y.L.; Mao, X.Z.; Li, Y.L.; Xiong, Z.; et al. Pyrene-Based Nonwoven Fabric with Tunable Fluorescence Properties by Employing the Aggregation-Caused Quenching Effect. ACS Appl. Mater. Inter. 2021, 13, 9036–9042. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z.; Wu, X.L.; Kuang, H.; Zhu, J.P.; Liu, L.Q. Development of a fluorescent quantification strip assay for the detection of lead. Food Agr. Immunol. 2020, 31, 642–652. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).