Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells
Abstract
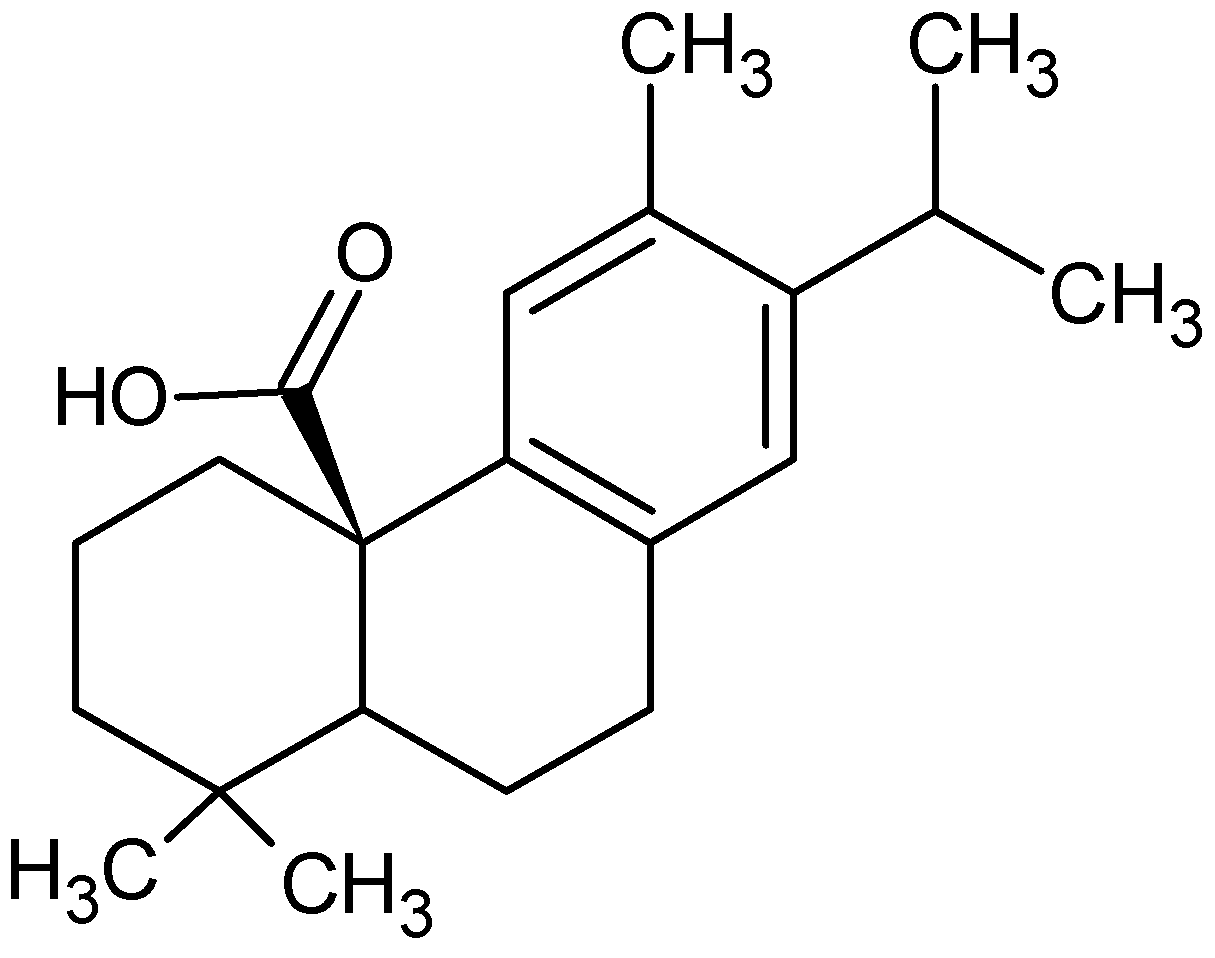
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles
2.2.1. Synthesis of CA-Loaded Albumin Nanoparticles
2.2.2. Synthesis of CA-Loaded Chitosan Nanoparticles
2.2.3. Preparation of CA-Loaded Cellulose Nanoparticles
2.2.4. Evaluation of Drug Loaded Efficiency
2.2.5. In Vitro Drug Release Study
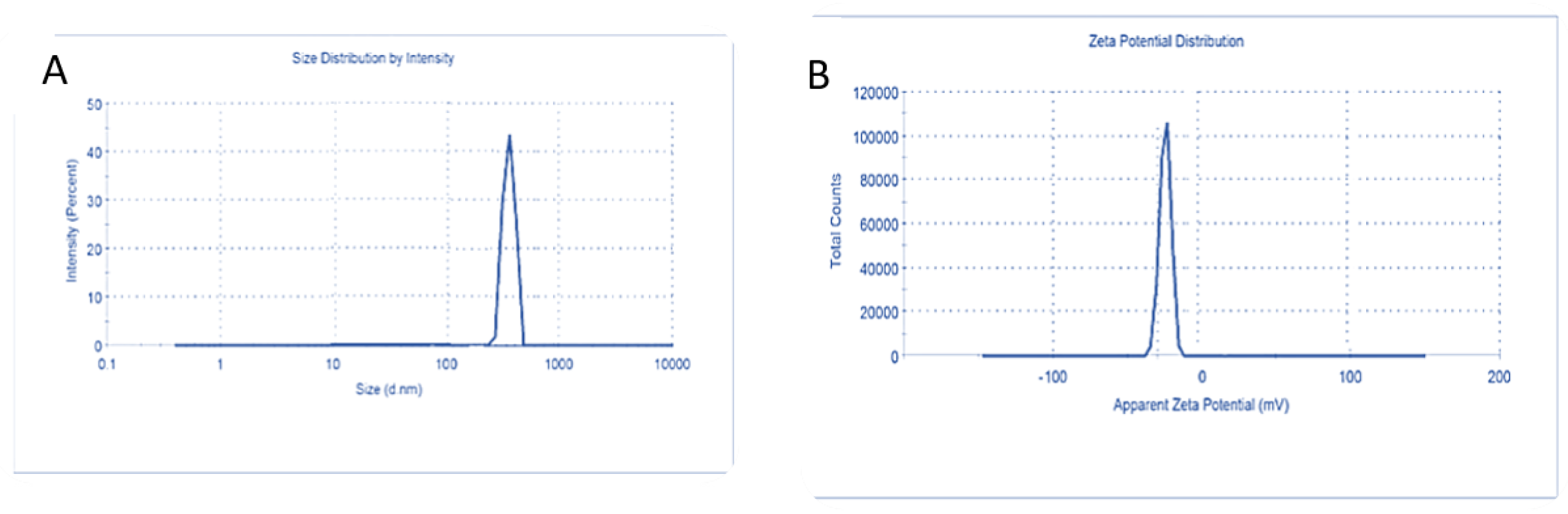
2.2.6. Nanoparticle Characterization
2.2.7. Cell Lines
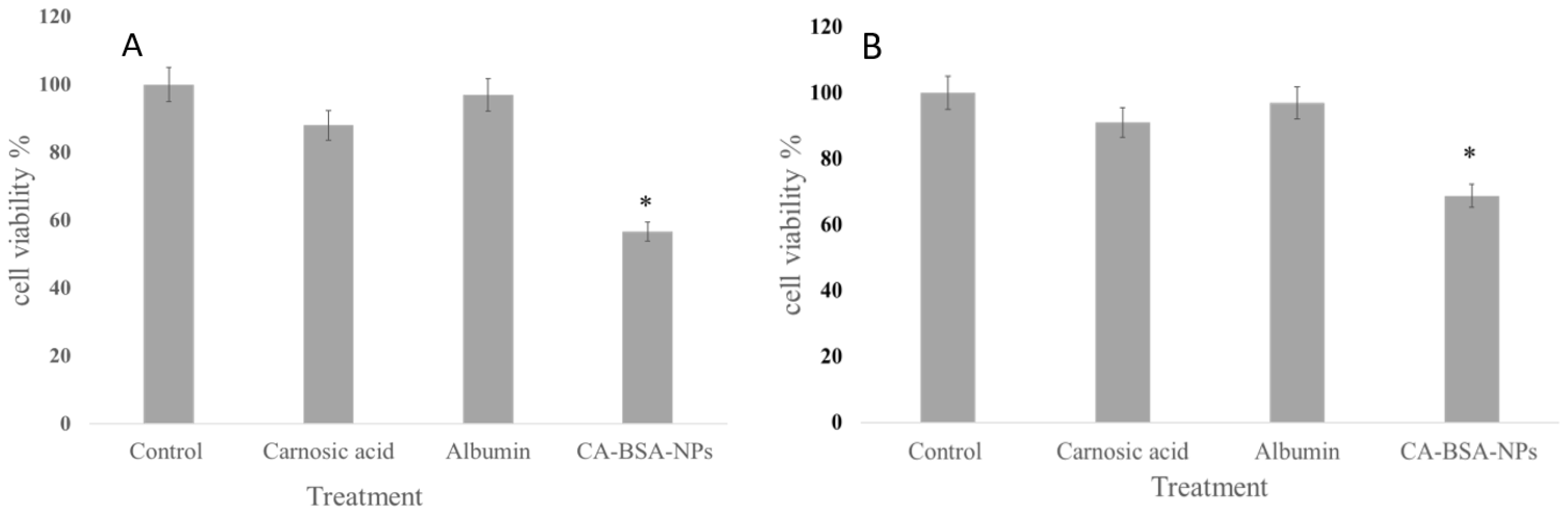
2.2.8. Cell Viability Test
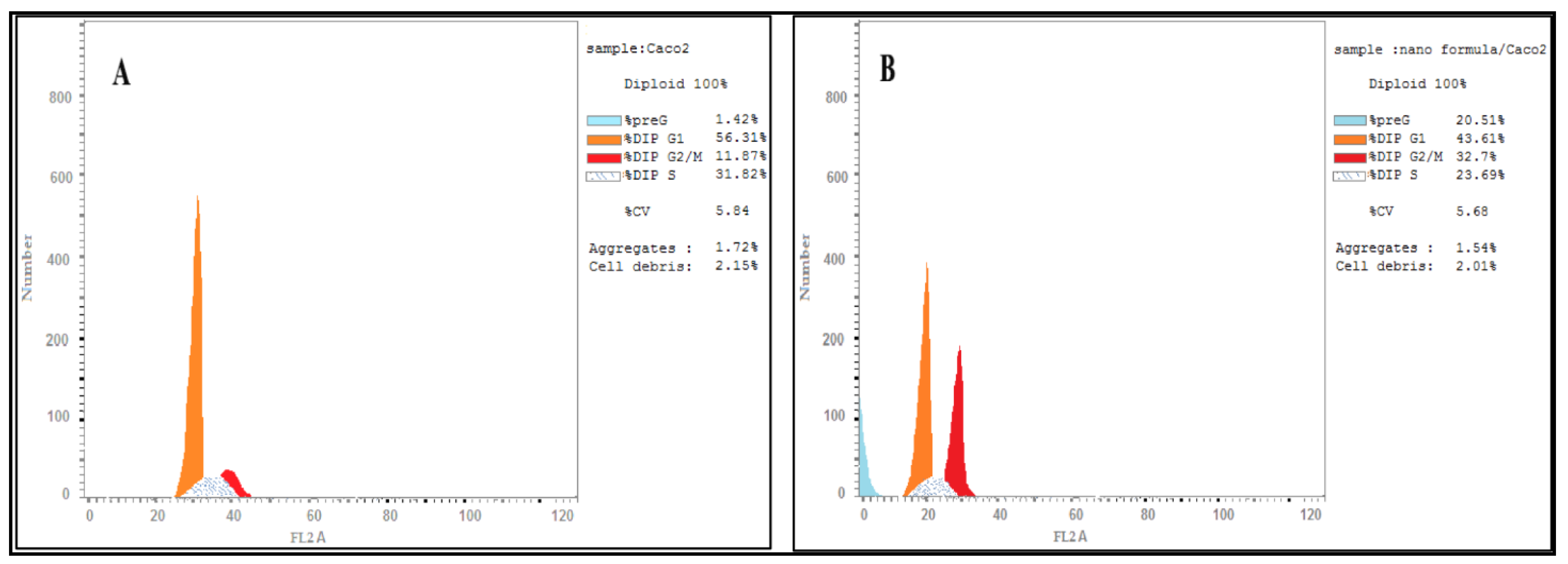
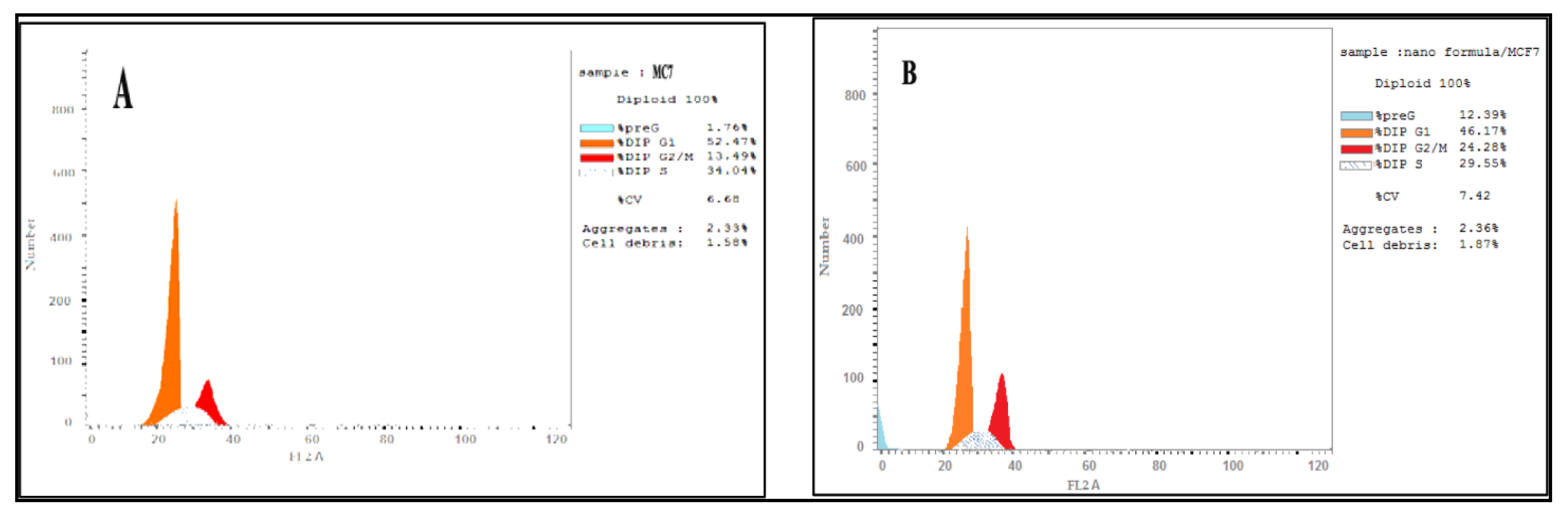
2.2.9. Cell Cycle Arrest
2.2.10. Cell Apoptosis Assay
2.2.11. Gene Expression by Real-Time PCR
3. Results
3.1. Encapsulation Efficiency and Loading Capacity
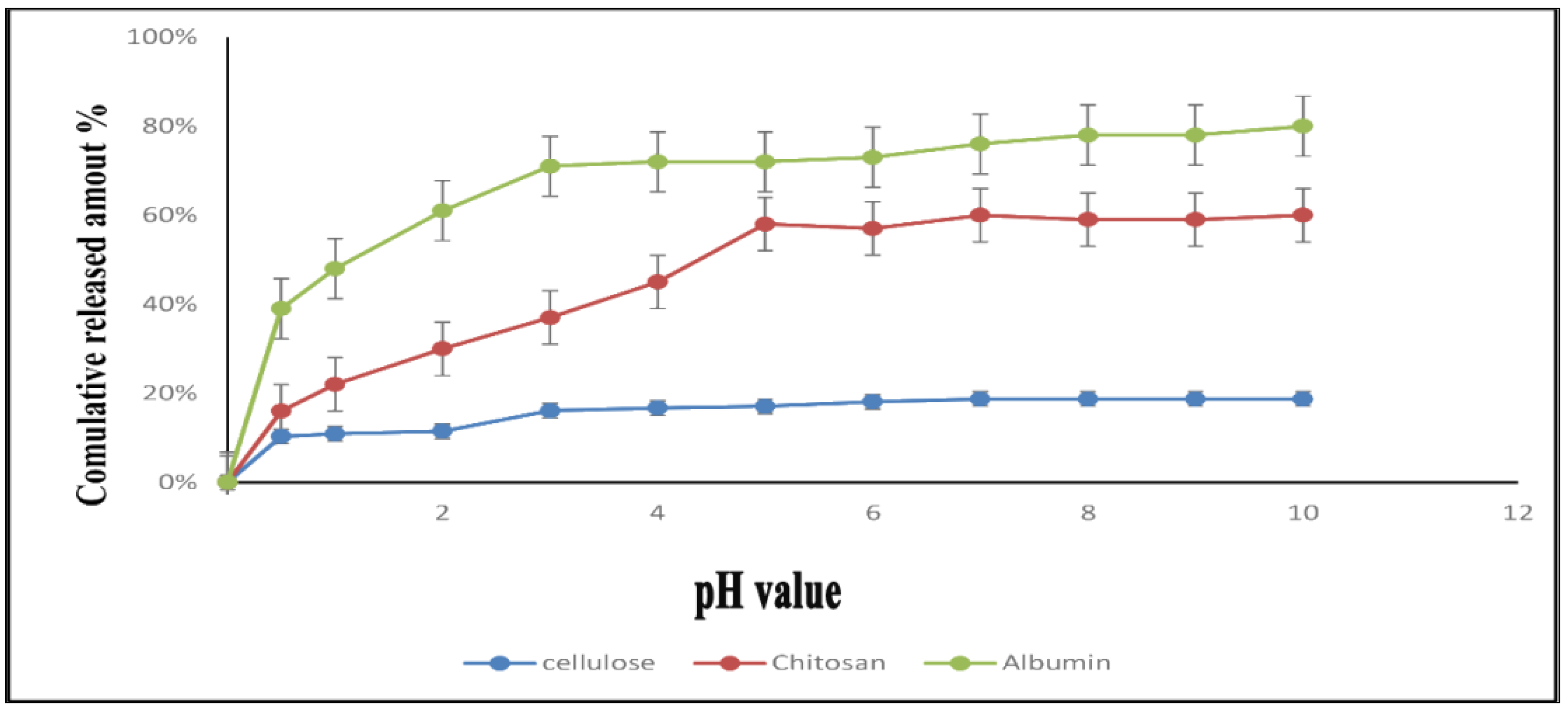
3.2. In-Vitro Drug Release Study
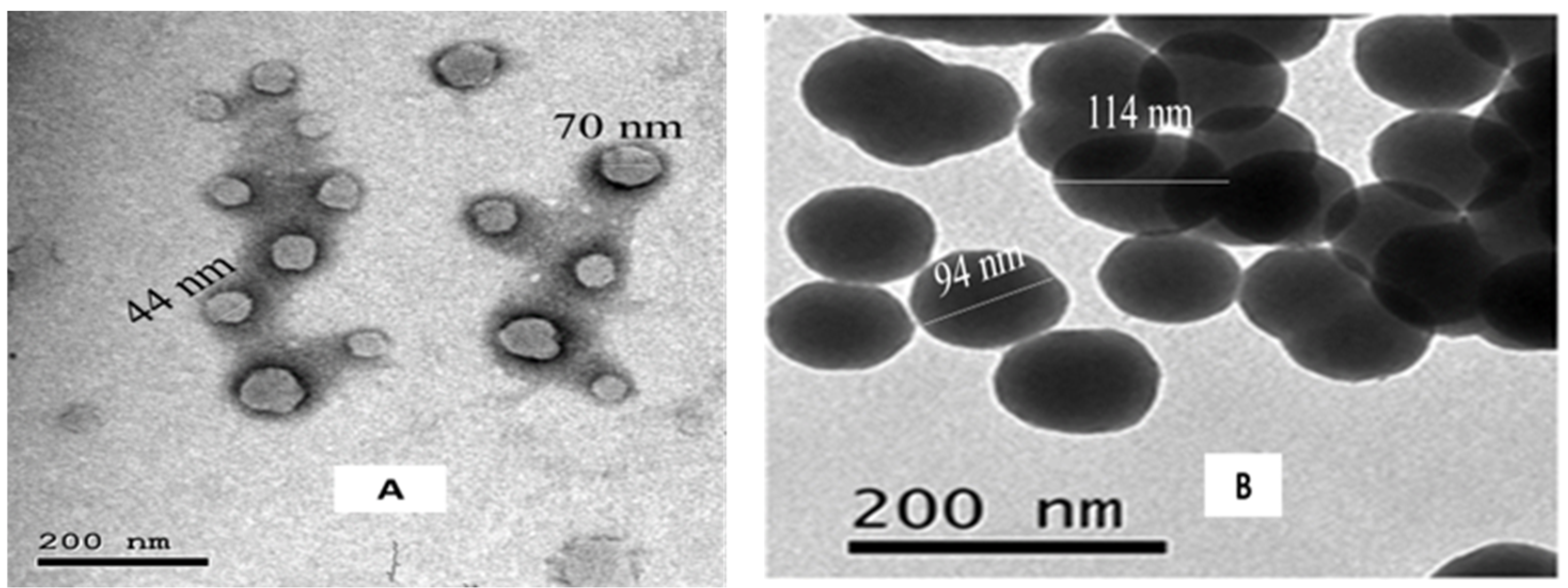
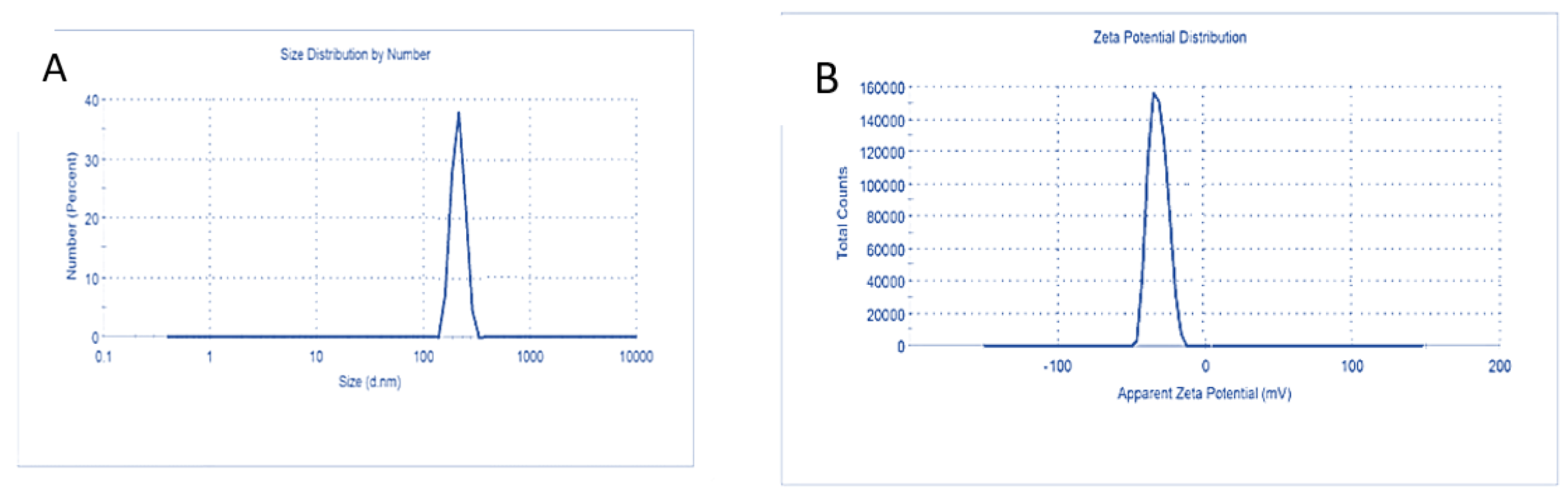
3.3. Characterization of Carnosic Acid Load on BSA-NPs
3.4. Cell Viability
3.5. DNA Content Analysis
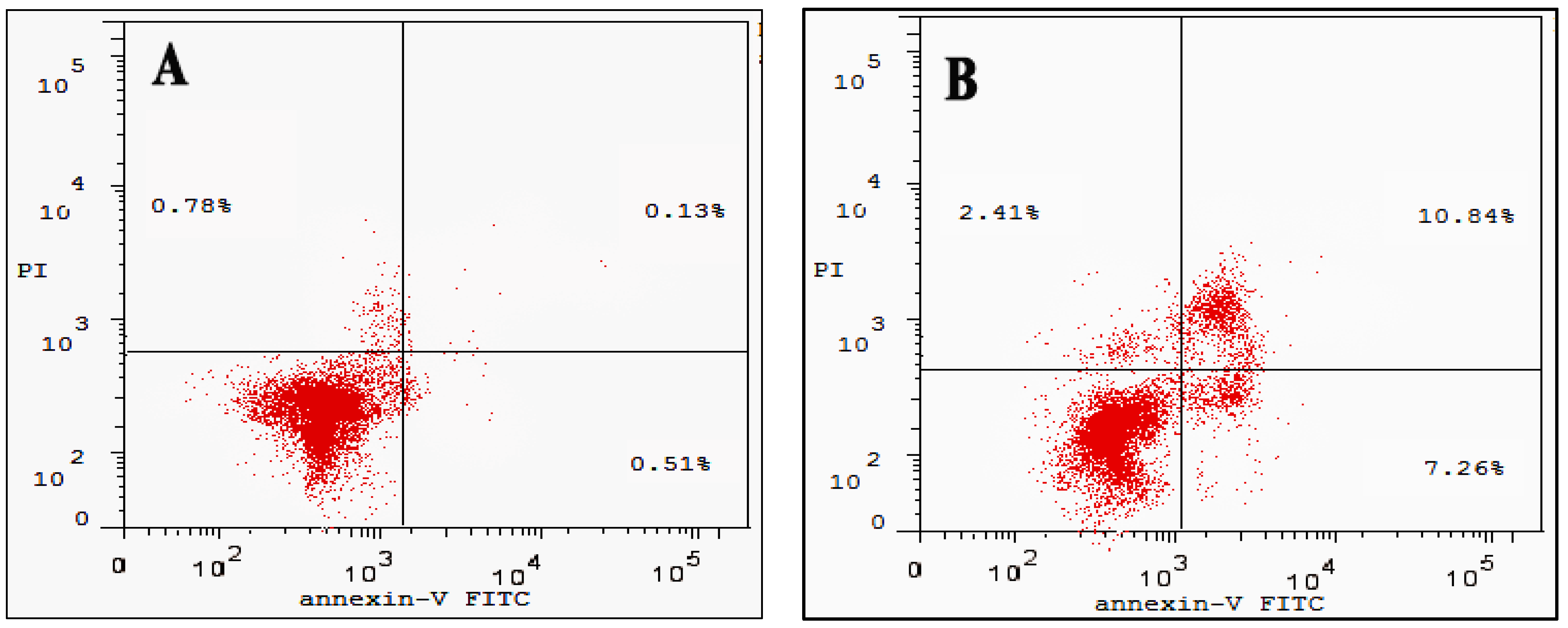
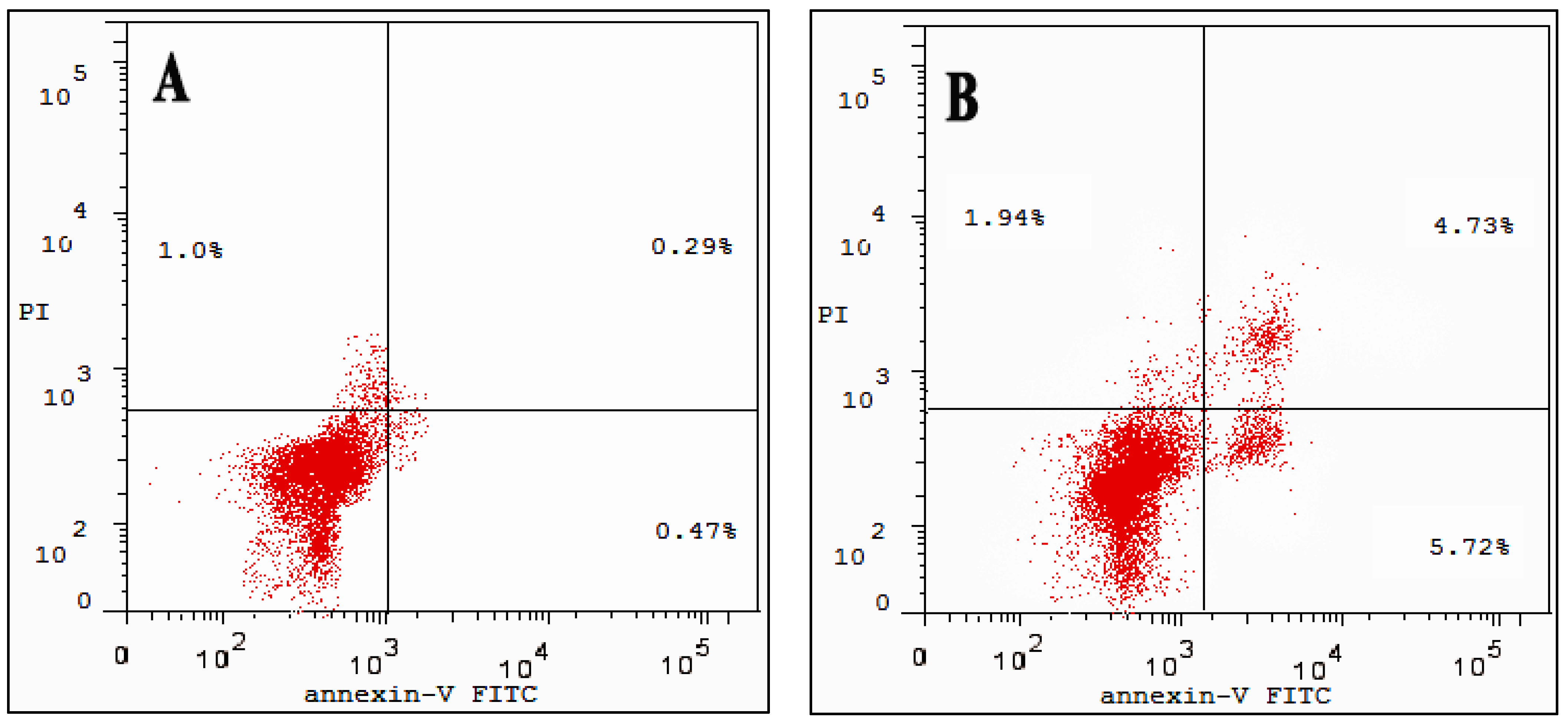
3.6. Cell Apoptosis Assay
3.7. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Qu, Y.; Kang, M.; Cheng, X.; Zhao, J. Chitosan-Coated Titanium Dioxide-Embedded Paclitaxel Nanoparticles Enhance Anti-Tumor Efficacy Against Osteosarcoma. Front. Oncol. 2020, 10, 577280. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Hong, H.-L.; Kim, G.M.; Leem, J.; Kwon, H.H. Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Molecules 2021, 26, 7589. [Google Scholar] [CrossRef] [PubMed]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourhia, M.; Laasri, F.E.; Aourik, H.; Boukhris, A.; Ullah, R.; Bari, A.; Ali, S.S.; El Mzibri, M.; Benbacer, L.; Gmouh, S. Antioxidant and Antiproliferative Activities of Bioactive Compounds Contained in Rosmarinus officinalis Used in the Mediterranean Diet. Evid.-Based Complement. Altern. Med. 2019, 2019, 7623830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lešnik, S.; Bren, U. Mechanistic Insights into Biological Activities of Polyphenolic Compounds from Rosemary Obtained by Inverse Molecular Docking. Foods 2022, 11, 67. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Cai, G.; Li, X.; Wang, D. Carnosic acid induces apoptosis of hepatocellular carcinoma cells via ROS-mediated mitochondrial pathway. Chem.-Biol. Interact. 2017, 277, 91–100. [Google Scholar] [CrossRef]
- Yan, L.; Shen, J.; Wang, J.; Yang, X.; Dong, S.; Lu, S. Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology: A publication of International Hormesis Society. Dose Response 2020, 18, 1559325820936161. [Google Scholar] [CrossRef]
- Rana, V.; Sharma, R. Chapter 5—Recent Advances in Development of Nano Drug Delivery. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 93–131. [Google Scholar] [CrossRef]
- Aminu, N.; Bello, I.; Umar, N.M.; Tanko, N.; Aminu, A.; Audu, M.M. The influence of nanoparticulate drug delivery systems in drug therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101961. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Hassan, A.; Elebeedy, D.; Matar, E.R.; El Sayed, A.F.M.; Abd El Maksoud, A.I. Investigation of Angiogenesis 392 and Wound Healing Potential Mechanisms of Zinc Oxide Nanorods. Front. Pharmacol. 2021, 12, 661217. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Ikeda, Y.; Shigeno, Y.; Konno, H.; Fujii, J. γ-Glutamylcysteine synthetase and γ-glutamyl transferase as differential enzymatic sources of γ-glutamylpeptides in mice. Amino Acids 2020, 52, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Shen, H. Application of the Nano-Drug Delivery System in Treatment of Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2020, 7, 489. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Fu, R.; Yang, J. Folic acid-modified ginsenoside Rg5-loaded bovine serum albumin nanoparticles for targeted cancer therapy in vitro and in vivo. Int. J. Nanomed. 2019, 14, 697–6988. [Google Scholar] [CrossRef] [Green Version]
- Vaka, S.R.K.; Murthy, S.N.; Repka, M.A.; Nagy, T. Upregulation of endogenous neurotrophin levels in the brain by intranasal administration of carnosic acid. J. Pharm. Sci. 2011, 100, 3139–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamoto, T.; Hattori, Y.; Takayama, K.; Maitani, Y. Novel chitosan particles and chitosan-coated emulsions inducing immune response via intranasal vaccine delivery. Pharm. Res. 2004, 21, 671–674. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef]
- Hiyama, N.; Ando, T.; Maemura, K.; Sakatani, T.; Amano, Y.; Watanabe, K.; Kage, H.; Yatomi, Y.; Nagase, T.; Nakajima, J.; et al. Glutamate-cysteine ligase catalytic subunit is associated with cisplatin resistance in lung adenocarcinoma. Jpn. J. Clin. Oncol. 2018, 48, 303–307. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, C.; Ma, Q.; Chen, W.; Atyah, M.; Yin, Y.; Fu, P.; Liu, S.; Hu, B.; Ren, N.; et al. High GCLC level in tumor tissues is associated with poor prognosis of hepatocellular carcinoma after curative resection. J. Cancer 2019, 10, 3333–3343. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Singh, S.G.; Naik, H.; Jain, D.; Bisla, S. Herbal excipients in novel drug delivery system. Int. J. Compr. Pharm. 2011, 2, 1–7. [Google Scholar]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the War against Inflammation with Natural Products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, M.; Sotgia, F.; Sisci, D.; Cappello, A.R.; Lisanti, M.P. Mitochondrial “power” drives tamoxifen resistance: NQO1 and GCLC are new therapeutic targets in breast cancer. Oncotarget 2017, 8, 20309–20327. [Google Scholar] [CrossRef] [Green Version]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenita, J.J.L.; Tibrewal, R.; Rathore, S.S.; Manjula, D.; Barnabas, W.; Mahesh, A.R. Formulation and optimization of albumin nanoparticles loaded ivabradine hydrochloride using response surface design. J. Drug Deliv. Sci. Technol. 2021, 63, 102461. [Google Scholar] [CrossRef]
- Abdel-Hakeem, M.A.; Mongy, S.; Hassan, B.; Tantawi, O.I.; Badawy, I. Curcumin Loaded Chitosan-Protamine Nanoparticles Revealed Antitumor Activity Via Suppression of NF-κB, Proinflammatory Cytokines and Bcl-2 Gene Expression in the Breast Cancer Cells. J. Pharm. Sci. 2021, 110, 3298–3305. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Abd El Maksoud, A.I.; Wageed, M.; Alotaibi, A.S.; Elebeedy, D.; Khalil, H.; Hassan, A.; Abdella, A. Characterization and Anticancer Activity of Biosynthesized Au/Cellulose Nanocomposite from Chlorella vulgaris. Polymers 2021, 13, 3340. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Dobberpuhl, M.R.; Maher, D.M.; Jaggi, M.; Chauhan, S.C. Design of curcumin loaded cellulose nanoparticles for prostate cancer. Curr. Drug Metab. 2012, 13, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Hakeem, M.A.; Abdel-Haseb, O.M.; Abdel-Ghany, S.E.; Cevik, E.; Sabit, H. Doxorubicin loaded on chitosan-protamine nanoparticles triggers apoptosis via downregulating Bcl-2 in breast cancer cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101423. [Google Scholar] [CrossRef]
- Rasul, A.; Imran Khan, M.; Ur Rehman, M.; Abbas, G.; Aslam, N.; Ahmad, S.; Abbas, K.; Akhtar Shah, P.; Iqbal, M.; Ahmed Al Subari, A.M.; et al. In vitro Characterization and Release Studies of Combined Nonionic Surfactant-Based Vesicles for the Prolonged Delivery of an Immunosuppressant Model Drug. Int. J. Nanomed. 2020, 15, 7937–7949. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.; Raslan, S.; Tombuloglu, H.; Shamseddin, A.; Cevik, E.; Said, O.A.; Madyan, E.F.; Senel, M.; Bozkurt, A.; Rehman, S.; et al. Vorinostat-loaded titanium oxide nanoparticles (anatase) induce G2/M cell cycle arrest in breast cancer cells via PALB2 upregulation. 3 Biotech 2020, 10, 407. [Google Scholar] [CrossRef]
- Alqosaibi, A.I.; Abdel-Ghany, S.; Al-Mulhim, F.; Sabit, H. Vorinostat enhances the therapeutic potential of Erlotinib via MAPK in lung cancer cells. Cancer Treat. Res. Commun. 2022, 30, 100509. [Google Scholar] [CrossRef] [PubMed]
- Alqosaibi, A.I.; Abdel-Ghany, S.; Sabit, H. Temozolomide modulates the expression of miRNAs in colorectal cancer. Cancer Treat. Res. Commun. 2021, 27, 100308. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Song, H.; Sung, M.K.; Kang, Y.H.; Lee, K.W.; Park, J.H. Carnosic acid inhibits the epithelial-mesenchymal transition in B16F10 melanoma cells: A possible mechanism for the inhibition of cell migration. Int. J. Mol. Sci. 2014, 15, 12698–12713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Huang, M.; Liu, D.; Li, Y.; Luo, Q.; Pham, K.; Wang, M.; Zhang, J.; Zhang, R.; Peng, Z.; et al. Absorption and Transport Characteristics and Mechanisms of Carnosic Acid. Biology 2021, 10, 1278. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, R.; Rezayat, S.M.; Faridi-Majidi, R.; Sadri, K.; Jaafari, M.R. Optimization of Docetaxel Loading Conditions in Liposomes: Proposing potential products for metastatic breast carcinoma chemotherapy. Sci. Rep. 2020, 10, 5569. [Google Scholar] [CrossRef] [Green Version]
- Zeb, A.; Rana, I.; Choi, H.-I.; Lee, C.-H.; Baek, S.-W.; Lim, C.-W.; Khan, N.; Arif, S.T.; Sahar, N.u.; Alvi, A.M.; et al. Potential and Applications of Nanocarriers for Efficient Delivery of Biopharmaceuticals. Pharmaceutics 2020, 12, 1184. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, Q.; Xu, H.; Yan, Z. Effects of nanoparticle size on antitumor activity of 10-hydroxycamptothecin-conjugated gold nanoparticles: In vitro and in vivo studies. Int. J. Nanomed. 2016, 11, 929–940. [Google Scholar] [CrossRef] [Green Version]
- Vaka, S.R.K.; Shivakumar, H.N.; Repka, M.A.; Murthy, S.N. Formulation and evaluation of carnosic acid nanoparticulate system for upregulation of neurotrophins in the brain upon intranasal administration. J. Drug Target. 2013, 21, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Dong, S.; Dong, M.; Li, Y.; Sun, Z.; Zhang, X.; Wang, Y.; Teng, L.; Wang, D. Transferrin-conjugated liposomes loaded with carnosic acid inhibit liver cancer growth by inducing mitochondria-mediated apoptosis. Int. J. Pharm. 2021, 607, 121034. [Google Scholar] [CrossRef]
- Abás, E.; Bellés, A.; Rodríguez-Diéguez, A.; Laguna, M.; Grasa, L. Selective cytotoxicity of cyclometalated gold(III) complexes on Caco-2 cells is mediated by G2/M cell cycle arrest. Metallomics 2021, 13, mfab034. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, K.W.; Chae, I.G.; Kundu, J.; Kim, E.H.; Kundu, J.K.; Chun, K.S. Carnosic acid inhibits STAT3 signaling and induces apoptosis through generation of ROS in human colon cancer HCT116 cells. Mol. Carcinog. 2016, 55, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhao, Y.; Zhang, X. Knockdown of Glutamate Cysteine Ligase Catalytic Subunit by siRNA Causes the Gold Nanoparticles-Induced Cytotoxicity in Lung Cancer Cells. PLoS ONE 2015, 10, e0118870. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, L.; Zheng, S. Global Reprogramming of Apoptosis-Related Genes during Brain Development. Cells 2021, 10, 2901. [Google Scholar] [CrossRef] [PubMed]

| Primer’s Name | Sequences of Primers |
|---|---|
| COX-2 forward COX-2 reverse | 5′-GAATGGGGTGATGAGCAGTT-3′ 5′-CAGAAGGGCAGGATACAGC-3′ |
| BCL-2 forward BCL-2 reverse | 5′-CCTGTGGATGACTGAGTACC-3′ 5′-GAGACAGCCAGGAGAAATCA-3′ |
| GCLC forward GCLC reverse | 5′-GGCACAAGGACGTTCTCAAGT-3′ 5′-CAAAGGGTAGGATGGTTTGGG-3′ |
| β-actin forward β-actin reverse | 5′-GTGACATCCACACCCAGAGG-3′ 5′-ACAGGATGTCAAAACTGCCC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khella, K.F.; Abd El Maksoud, A.I.; Hassan, A.; Abdel-Ghany, S.E.; Elsanhoty, R.M.; Aladhadh, M.A.; Abdel-Hakeem, M.A. Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells. Molecules 2022, 27, 4102. https://doi.org/10.3390/molecules27134102
Khella KF, Abd El Maksoud AI, Hassan A, Abdel-Ghany SE, Elsanhoty RM, Aladhadh MA, Abdel-Hakeem MA. Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells. Molecules. 2022; 27(13):4102. https://doi.org/10.3390/molecules27134102
Chicago/Turabian StyleKhella, Katren F., Ahmed I. Abd El Maksoud, Amr Hassan, Shaimaa E. Abdel-Ghany, Rafaat M. Elsanhoty, Mohammed Abdullah Aladhadh, and Mohamed A. Abdel-Hakeem. 2022. "Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells" Molecules 27, no. 13: 4102. https://doi.org/10.3390/molecules27134102
APA StyleKhella, K. F., Abd El Maksoud, A. I., Hassan, A., Abdel-Ghany, S. E., Elsanhoty, R. M., Aladhadh, M. A., & Abdel-Hakeem, M. A. (2022). Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells. Molecules, 27(13), 4102. https://doi.org/10.3390/molecules27134102






