Abstract
Breast cancer is one of the most prevalent cancers in the world. Traditionally, medicinal plants have been used to cure various types of diseases and disorders. Based on a literature survey, the current study was undertaken to explore the anticancer potential of Foeniculum vulgare Mill. phytoconstituents against breast cancer target protein (PDB ID: 6CHZ) by the molecular docking technique. Molecular docking was done using Autodock/vina software. Toxicity was predicted by the Protox II server and drug likeness was predicted by Molinspiration. 100 ns MD simulation of the best protein-ligand complexes were done using the Amber 18 tool. The present molecular docking investigation has revealed that among the 40 selected phytoconstituents of F. vulgare, α-pinene and D-limonene showed best binding energy (−6 and −5.9 kcal/mol respectively) with the breast cancer target. α-Pinene and D-limonene followed all the parameters of toxicity, and 100 ns MD simulations of α-pinene and D-limonene complexes with 6CHZ were found to be stable. α-Pinene and D-limonene can be used as new therapeutic agents to cure breast cancer.
1. Introduction
Foeniculum vulgare Mill. is an important medicinal herb and one of the most widely cultivated spice plants that belongs to the family Umbelliferae. Foeniculum vulgare is a nutritionally rich reservoir of carbohydrates, proteins, monounsaturated and polyunsaturated fatty acids, short chain fatty acids (SCFA), minerals, vitamins, and energy. Previous scientific reports provide a comprehensive collection of the in vitro and in vivo pharmacological investigations which reflect the efficacy of various Foeniculum vulgare constituents in treating arthritis, cancers, conjunctivitis, digestive disorders, endocrine issues, hepatic, kidney, reproductive, and respiratory ailments due to their anti-inflammatory, anti-microbial, anti-mutagenic, anti-nociceptive, anti-oxidant, anti-spasmodic, anti-thrombotic, anti-tumor, anti-viral, apoptotic, hypoglycemic, hypolipidemic, immunomodulatory, and memory enhancing properties [1,2,3]. Phytochemical investigations have revealed the presence of several bioactive alkaloids, coumarins, flavonoids, phenolics, polyacetylenes, and terpenes with diverse functionalities and therapeutic attributes. Butyrate is a potent anti-tumor agent because of its ability to induce histone hyperacetylation which further augments cellular differentiation, cell cycle arrest and apoptosis when tested in a variety of cancer cell lines [4]. In addition to this, the anti-cancer effect of linoleic acid have been demonstrated against human breast, colon, and colorectal carcinomas and mice colon, epidermal, hepatic, mammary, prostate, and stomach carcinomas [5]. The role of oleic acid in the chemoprevention of human breast, gastric, and tongue squamous cell carcinomas is illustrated in the cell culture-based assays by Li et al. [6] and Jiang et al. [7]. Other fatty acids like myristic acid provides chemoprotection against breast cancer [8], margaric acid worked against lung cancer [9], and palmitic acid has been shown to be effective against colon and colorectal cancers [10,11]. Previous scientific reports were manually integrated to anticipate the anti-cancer potential of Foeniculum vulgare phytoconstituents against different types of cancers, and these included anisaldehyde [12], γ-asarone [13], carvone [14], chlorogenic acid [15], estragole [16], eugenol [17], fenchone [18], γ-terpinene [19], D-limonene [20], myrcene [21], α-pinene [22], quercetin-3-O-beta-D-glucuronide [23], tarns-anethole [24], α-terpineol [25], and vinylguaiacol [26], as indicated in Table 1.

Table 1.
Anti-cancer bioactive phytoconstituents of Foeniculum vulgare Mill.
Assays have demonstrated the anti-cancer potential of some important Foeniculum vulgare constituents against a wide array of cancers including human breast cancer. Using In silico approaches, we can simultaneously screen out millions of phytocompounds and drugs against any diseases. Furthermore, molecular dynamics-based screening helps to hypothesize the efficacy, stability and toxicity of the important drug candidates or pytocompounds and to design experiments for their in vivo testing. Therefore, the present study was undertaken to propose potential cytotoxic phytoconstituents of Foeniculum vulgare using in silico approaches for developing novel therapeutics for the management of human breast cancer. An important drug target candidate in the case of human breast cancer is an Estrogen Receptor α Y537S protein (PDB ID 6CHZ) which was selected on the basis of previous literature.
2. Results
2.1. Molecular Docking of Major Phytoconstituents of Foeniculum vulgare with Breast Cancer Target
Forty phytoconstituents were selected for the molecular docking studies with the Estrogen Receptor α Y537S breast cancer target protein (PDB ID 6CHZ).
Among all of the selected phytoconstituents, α-pinene and D-limonene showed best interactions with 6CHZ with binding energies of −6.0 and −5.9 kcal/mol, respectively, followed by 1-(1-cyanocyclohexyl) pyrrolidine, α -terpineol and piperitinone (with interaction energy of −5.8 kcal/mol), eugenol, and carvone (with interaction energy of −5.6 kcal/mol), camphor, gamma-terpinene, linoleic acid, and trans-anethole (with interaction energy of −5.5 kcal/mol), α-d-glucose, and chlorogenic acid (with interaction energy of −5.3 kcal/mol), fenchone, gamma-asarone, methyl-chavicole, methyl benzaldehyde, and vinyl guaiacol (with interaction energy of −5.2 kcal/mol), quercetin−3-O-beta-D-glucuronide and ascorbic acid (with interaction energy of −5.1 kcal/mol). Other phytoconstituents such as 1,2-dithiocane, acetic acid, anisaldehyde, estragole, margaric acid, mesitol, myrcene, myristic acid, N-valeramide, N-octadecane, oleic acid, palmitic acid, pelargic acid, pentyl vinyl carbinol, petroselinic acid, stearic acid, syringol, and Z,Z-heptadeca-8,11-dien-1-yl bromide showed binding energies less than 5 kcal/mol (Table 2). Alpha-pinene showed hydrophobic interactions with Leu 346, Leu 349, Ala 350, Glu 353, Leu 384, Leu 387, Met 388, Leu 391, and Phe 404 and D-limonene with Leu 346, Leu 349, Ala 350, Glu 353, Leu 384, Leu 387, Met 388, Leu 391, Arg 394, Phe 404, Ile 424, and Leu 428 residues of the cancer drug target protein 6CHZ, as shown in Table 2. 2D interactions of the most stable protein-ligand complexes are shown in Figure 1 and 3D interactions are shown in Figure 2 and Figure 3.

Table 2.
Binding energies of important Foeniculum vulgare constituents with breast cancer target and the interacting amino acids.
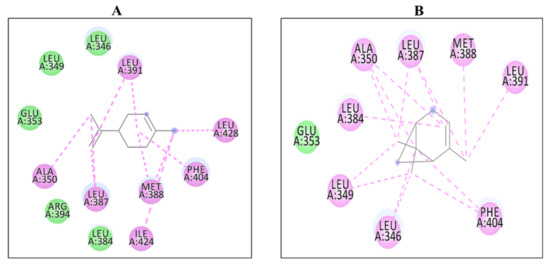
Figure 1.
2D Interaction complexes of (A) D-limonene and (B) α-pinene with 6CHZ protein.
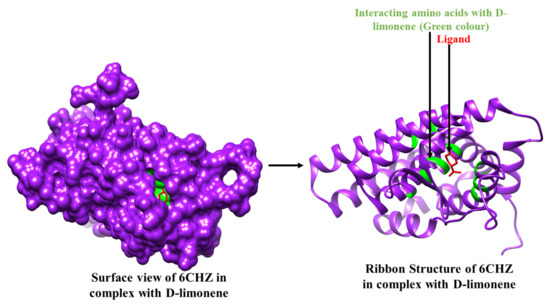
Figure 2.
3D Interaction complex of α-pinene with 6CHZ protein; where purple shows the target protein, green shows the hydrophobic interactions, and red shows the ligand molecule.
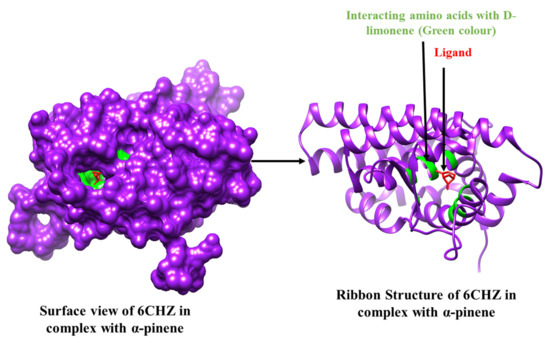
Figure 3.
3D Interaction complex of D-limonene with 6CHZ protein; purple shows the target protein, green shows the hydrophobic interactions, and red colour is shows the ligand molecule.
2.2. Drug Likeness Prediction of Foeniculum vulgare Phytoconstituents
The drug likeness parameters of all the selected phytoconstituents were assessed using Molinspiration web server. Among all the selected phytocompounds, acetic acid, anisaldehyde, ascorbic acid, gamma-asarone, trans-anethole, benzeneethanamine, camphor, carvone, 1-cyanocychexylpyrrolidin, 1-2-dithiocane, estragole, eugenol, fenchone, alpha-d-glucose, D-limonene, methylchavicole, myrcene, 1-octen-3-ol, pelargic acid, alpha-pinene, piperitinone oxide, syringol, alpha-terpeneol, gamma-terpinene, p-tolualdehyde, and N-valeramide, passed different criteria of drug likeness and can be used as future oral drugs (Table 3). On the basis of molecular docking and toxicity assessments, alpha-pinene and D-limonene both were found to be the most suitable drug candidates and hence, were selected for MD simulation studies to verify the stability of protein-ligand complexes.

Table 3.
Drug-likeness prediction of Foeniculum vulgare phytoconstituents using the Molinspiration web server.
2.3. Toxicity Prediction of Foeniculum vulgare Phytoconstituents
The toxicity of the selected forty phytoconstituents was analyzed by using the Protox II online tool. Toxicity data showed that among all the selected phytoconstituents, anisaldehyde, gamma-asarone, trans-anethole, estragole, p-tolualdehyde, and quercetin-3-O-beta-D-glucuronide are carcinogenic in nature, alpha-d-glucose and octadecenoic acid are hepatotoxic in nature, alpha-d-glucose, and quercetin-3-O-beta-D-glucuronide are immunotoxic in nature, and gamma-asarone is mutagenic in nature (Table 4). Molecular docking and toxicity data revealed that alpha-pinene and D-limonene are the best drug molecules for the management of human breast cancer. Alpha-pinene belongs to class 5 drugs with an LD50 of 3700 mg/kg body weight and D-limonene is a class 4 drug with an LD50 of 500 mg/kg body weight. The detailed in silico toxicity parameters are depicted in Table 4.

Table 4.
Toxicity assessment of Foeniculum vulgare phytoconstituents using Protox II.
2.4. Molecular Dynamic simulation of Complexes
Based on molecular docking, and toxicity investigations, alpha-pinene and D-limonene were found to be the choicest drug candidates for managing human breast cancer. Therefore, alpha-pinene and D-limonene ligand complexes with breast cancer target protein 6CHZ were selected for MD simulation studies to further verify stability of these complexes. The RMSDs of D-limonene complex with 6CHZ C-alpha was stable from the start of simulation up to 40 ns (3 Å). It showed little fluctuation between 40–50 ns (which is in the acceptable range), and afterwards it remained stable up to 100 ns at 2.9 Å (Figure 4A). Whereas, in the case of 6CHZ complex with alpha-pinene, RMSDs of C-alpha showed little fluctuation between 0–30 ns (3 Å), which remained stable between 30 to 100 ns thereafter at 2.7 Å (Figure 4B). The RMSF plots of D-limonene and alpha-pinene fitted over 6CHZ protein also showed lesser residual fluctuations in the protein’s secondary structures, such as the alpha helices and beta strands (Figure 5A).
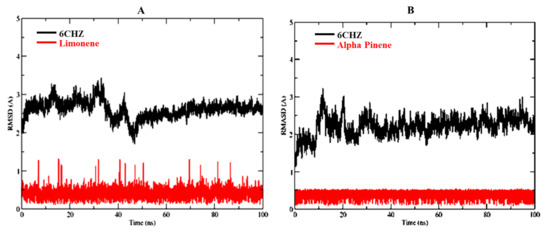
Figure 4.
RMSDs of protein-ligand complexes: (A) D-limonene with 6CHZ and (B) Alpha-pinene with 6CHZ. Red indicates ligand and black indicates C-alpha of the target protein.
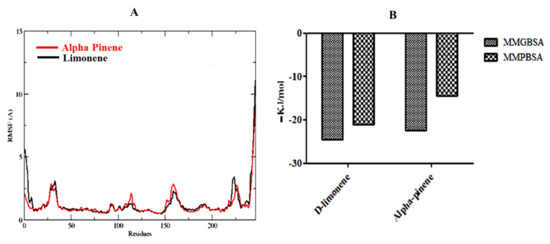
Figure 5.
(A) RMSF plots of D-limonene and alpha-pinene complexes with 6CHZ (red is indicating D-limonene and black is indicating alpha pinene) and (B) MMGBSA and MMPBSA plots of D-limonene and alpha-pinene complexes with 6CHZ.
The MMGBSA (Molecular Mechanics/Generalized Born Surface Area) of alpha-pinene is −22.39 and for D-limonene, it is −24.52 kJ/mol; whereas, the MMPBSA of alpha-pinene is −14.44 and D-limonene is −21.08 kJ/mol (Figure 5).
Radius of gyration of α-pinene and D-limonene in complex with 6CHZ is in the range of 18–19 Å and as shown in Figure 6A. The radius of gyration plots establishes the compactness of the α-pinene and D-limonene in complex with 6CHZ protein complex and confirms the stability of complexes. Solvent accessible range of alpha pinene and D-limonene complexed with 6CHZ protein is between 14,000–15,000 Å; as shown in Figure 6B.
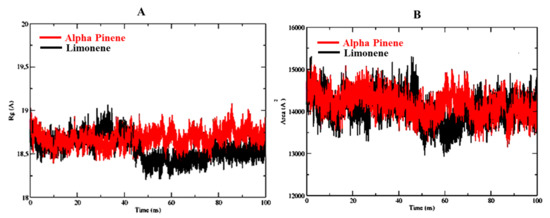
Figure 6.
(A) Radius of gyration (ROG) and (B) Solvent Accessible Surface Area of the best protein-ligand complexes. Red is indicating D-limonene and black is indicating α-pinene.
3. Discussion
Medicinal herbs always confer beneficial effects on human health when consumed in moderate quantities. Foeniculum vulgare, especially being rich in several bioactive constituents, has been used as a food condiment and ingredient throughout the world. It has been traditionally used since ancient times to cure several human diseases including arthritis, cancers, conjunctivitis, endocrine, gastric, hepatic, insomnia, kidney, reproductive, and respiratory ailments. These studies signify that F. vulgare holds a promising future, and harnessing its hidden anti-cancer potential could be an important milestone in the field of novel drug development. However, the development of potent cytotoxic agents requires investigation of the molecular mechanisms of disease prevention to substantiate the beneficial attributes as well as to authenticate the immense pharmacological importance of Foeniculum vulgare constituents.
In the current study we found that among all forty phytocompounds, α- pinene and D-limonene showed the best binding affinity with the breast cancer target. They were also found to be non-toxic in nature. In contrast to our study, Ghasemian et al. [35] reported the anticancer activity of F. vulgare against the MCF-7 breast cancer cell line by MTT assay. They also reported that the essential oil F. vulgare increases the expression of Bax and decreases the Bcl2 gene expression. Similarly, Mohamad et al. [36] reported the anticancer activity of F. vulgare methanolic extract and essential oil against the MCF-7 breast cancer cell line and Hepg-2 liver cell line by in vivo and in vitro assays. Berrington and Lall [37] also reported the anticancer activity of F. vulgare acetone extract against the Vero African green monkey kidney cell line and the cervical cancer cell line HeLa. Batool et al. [38] also reported the anticancer activity of F. vulgare methanolic and ethanolic extract against the MCF-7 breast cancer cell line by MTT assay. Both extracts are more effective against the breast cancer cell line (methanol and ethanol—40 µg/mL). Zaahkouk et al. [39] also reported the anticancer activity of methanolic extract of Foeniculum vulgare seed against breast, colon and liver cancer. Similar to our study, Hossain [40] studied the anticancer activity of Withania somnifera phytocompounds with 6CHZ protein by molecular docking. He also reported the drug likeness and ADMET activity of phytocom pounds. There are several reports on molecular docking, MD simulation, and ADMET screening of phytoconstituents [41,42,43,44,45,46].
Phytocompounds of Rheum emodi were screened for antibacterial and antiviral properties by molecular docking, and results were validated by MD simulation [41]. Similarly, Salaria et al. [42,43] studied the antimicrobial activity of T. serpyllum compounds and the antioxidant and anti-inflammatory activities of important phytoconstituents were investigated using molecular docking and MD simulation, and the results were further validated by in vitro experiments. In addition, phytocompounds containing nanoparticles were shown to provide significant anti-cancer effects against breast cancer cell lines (MCF-7, PC-12, MDA-MB-231) [47,48,49,50,51]. Letrozole incorporated folate-conjugated polymer-lipid hybrid nanoparticles were also shown to exhibit anticancer activity against the MCF-7 and PC-12 cell lines [52]. Folic acid functionalized apoferritin is a drug delivery vehicle for equirubicin against breast cancer cells (MCF-7) [53].
4. Materials and Methods
4.1. Bioinformatics Tools
Open Babel GUI (O’Boyle et al., 2011), UCSF Chimera 1.8.1, PubChem (www.pubchem.com, accessed on 15 November 2021), RCSB PDB (http://www.rscb.org/pdb, accessed on 20 December 2021), AutoDock/vina software (https://vina.scripps.edu, accessed on 1 May 2022) [54], and Discovery Studio were used in the present investigation.
4.2. Ligand Preparation
40 major phytoconstituents found in the medicinal plant Foeniculum vulgare Mill were selected on the basis of the literature and were further selected for molecular docking studies. The three-dimensional structures of the selected phytoconstituents were downloaded from the PubChem database (www.pubchem.com, accessed on 15 November 2021) in .sdf formats. The .sdf files of the phytoconstituents were converted into PDB formats. Open Babel software was used to prepare all of the selected ligands (phytoconstituents) from the command line on an Ubuntu terminal. Chemdraw 3D version 15.0 (PerkinElmer, Waltham, MA, USA) was used to reduce the energy of all of the identified phytocompounds.
4.3. Protein Preparation
Estrogen Receptor α Y537S breast cancer target (PDB ID 6CHZ) was used for molecular docking with major phytocompounds from Foeniculum vulgare Mill. The three-dimensional 6CHZ was downloaded from the protein databank (http://www.rscb.org/pdb, accessed on 20 December 2021). It consists of a dimeric structure, and its chain A was extracted for docking using Pymol software. The active site was predicted manually by grid box analysis (grid dimensions x = 72, y = 74, z = 76 Å, center at x, y, z = −28.959, −2.617, −25.683 Å).
4.4. Molecular Docking of Major Phytoconstituents of Foeniculum vulgare with Breast Cancer Target
The selected ligands were docked to the catalytic triad of proteins using AutoDock vina, which was then saved as a pdbqt file. The population of potential ligand conformations/orientations at the binding site was estimated via docking. A vina script was used to align the ligands in the same spatial coordinates [54]. After the docking search was completed, the best conformation with the lowest docked energy was chosen. Discovery Studio (https://discover.3ds.com/d, accessed on 20 December 2021) was used to study interactions between proteins and ligands in the pdb complex preparations. A negative score (kcal/mol) was used to calculate the ligand’s binding strength. The equilibrium constant was calculated by using formula [55]:
Ki = e−ΔG/RT ΔG = Gibbs free energy; R = 1.9872 cal/mol·K; T = 298.15 °K
4.5. Drug Likeness Prediction of Foeniculum vulgare Phytoconstituents
Drug likeness prediction of F. vulgare phytoconstituents was done using the Molinspiration online server (http://www.molinspiration.com, accessed on 20 December 2021). Drug likeness is based on the Lipinski’s rule of five. According to the rule of five, the number of hydrogen acceptors should be <10, the number of hydrogen donors should be <5, the molecular weight should be <500 Da, and the partition coefficient should be >5 (estimated in terms of log P) in acceptable drug molecules. In the case of variables, only one violation is acceptable [56].
4.6. Toxicity Prediction of F. vulgare Phytoconstituents by Protox II Server
The pharmacokinetics and toxicity of pharmacologically important phytoconstituents were predicted by using Protox II servers. Toxicity was estimated in terms of LD50 values ranging from ≤50 mg/kg (in the case of Class I compounds), between 50 to 500 mg/kg (in the case of Class II compounds), between 500 to 5000 mg/kg (in the case of Class III compounds), and >5000 mg/kg (in the case of Class IV compounds). Classes I, II, and III have less toxicity, whereas Class IV displays no toxicity [57]. Moreover, PROTOX is a rodent oral toxicity server that is used to determine LD50 values and toxicity classes of potentially cytotoxic agents [58]. Based on the molecular docking drug-likeness data and toxicity data, phytoconstituents were selected for further MD simulation analysis.
4.7. MD Simulation of Best Protein-Ligands Complexes
MD simulations of best protein-ligand complexes were done by using the Amber18 tool. MD simulations were performed to gain a better understanding of the binding interactions of the 6CHZ protein with the selected phytoconstituents, namely α-pinene and D-Limonene [51]. The ligands underwent an amber generalized force field (GAFF), while the protein was subjected to amber ff14SB [52,53]. Using Gaussian 09 software, the atomic charges of the ligands were computed using the restrained electrostatic potential (RESP) procedure at the HF/6-31G* level of theory 31, 32 [55]. Using an H++ server, proton transfer states of the ionizable residues in protein structures were investigated using the pKa method at a neutral pH. The tLeap application was used to create each system. Each system was solvated in a cubic water box with the TIP3P model after being treated with sodium ions. Each system was exposed to at least four minimizations in order to eliminate the worst conflicts. Initially, all of the sodium ions and water molecules were reduced using a steepest descent technique of 2000 steps, followed by a conjugate gradient algorithm of 3000 steps. The same approach was used to relax all of the hydrogen and water molecules in a row. Finally, the entire system was energy-minimized for 5000 steps of steepest descent and 5000 steps of conjugate gradients. The system was heated from 0 to 300 K while performing 200 ps of MD and then 300 ps of density equilibration at a fixed volume with position restrictions on the protein atoms. All protein-ligand complexes were stabilized with for 10 ns of MD without any structural restrictions at a constant pressure before the manufacturing process. By linking to a Langevin thermostat with a collision frequency of 2 cm−1, the temperature was kept at 300 K. For the unpaired electron interactions, a cut off of 10 was chosen, and the Particle Mesh Ewald (PME) [53] approach and the SHAKE algorithm was used to limit the bond lengths involving hydrogen atoms. Finally, at a temperature of 300 K, MD simulations (productions) were run for 100 ns. The computed trajectories were utilized to examine activities of all the complexes in order to determine the stability of the system. Important parameters like root mean square deviation (RMSD), root mean square fluctuation (RMSF), radius of gyration (RG), and solvent accessible surface area (SASA) were used to examine deviations of the protein and protein-ligand complexes [51,52]. Furthermore, using molecular mechanics and the Poisson–Boltzmann Surface Area (MM-PBSA) method, the total free energy of binding, the free energy of solvation (polar vs. non-polar solvation energies), and the potential energy (electrostatic and van der Waal’s interactions) of each protein-ligand complex were calculated. For the MM-PBSA computation, the last 10 ns of the MD trajectory were used [53].
5. Conclusions
Forty major phytoconstituents of Foeniculum vulgare were screened for breast cancer by molecular docking with the 6CHZ target protein. Among all of the selected phytoconstituents, D-limonene and α-pinene have the best binding affinity and follow all the parameters of toxicity. An MD simulation study validated the stability of complexes. α-pinene has a lot of potential for the treatment of breast cancer, and this hypothesis can be further validated by in vitro and in vivo experiments.
Author Contributions
Conceptualization, B.K. (Baljinder Kaur), R.R., D.S. and B.K. (Balvir Kumar); Data curation, O.A.F.; Formal analysis, B.K. (Balvir Kumar); Funding acquisition, M.B.A.A.-R.; Investigation, R.R., D.S. and B.K. (Balvir Kumar); Methodology, B.K. (Baljinder Kaur), R.R., D.S. and B.K. (Balvir Kumar); Research administration, B.K. (Baljinder Kaur); Resources, R.R., D.S.; Visualization, O.A.F., R.A.d.C., A.A. and M.B.A.A.-R.; Writing—original draft, B.K. (Baljinder Kaur), R.R. and B.K. (Balvir Kumar); Writing—review & editing, B.K. (Baljinder Kaur), D.S., O.A.F., R.A.d.C., A.A., M.B.A.A.-R., M.R. and I.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
Researcher Supporting Project (RSP-2021/378), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are thankful to Department of Biotechnology, Punjabi University Patiala, Patiala, Punjab, India and Shoolini University, Solan, Himachal Pradesh, India. The authors of this study also extend their appreciation to the Researcher Supporting Project (RSP-2021/378), King Saud University, Riyadh, Saudi Arabia, for supporting this work by paying APC charges.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Sample Availability
Yes: D-limonene is available with Rajan Rolta and Deeksha Salaria.
References
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooti, W.; Moradi, M.; Ali-Akbari, S.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Therapeutic and pharmacological potential of Foeniculum vulgare Mill: A review. J. HerbMed Pharmacol. 2015, 4, 1–9. [Google Scholar]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef] [Green Version]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The Effects of Short-Chain Fatty Acids on Human Colon Cancer Cell Phenotype Are Associated with Histone Hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2018, 11, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhou, T.; Li, C.; Dai, Z.; Che, D.; Yao, Y.; Li, L.; Ma, J.; Yang, X.; Gao, G. High Metastaticgastric and Breast Cancer Cells Consume Oleic Acid in an AMPK Dependent Manner. PLoS ONE 2014, 9, e97330. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Matta, M.; Deubler, E.; Chajès, V.; Gunter, M.; Murphy, N.; Gaudet, M.M. Abstract LB079: Circulating plasma phospholipid fatty acid levels & breast cancer risk in the CPS-II Nutrition Cohort. Epidemiology 2021, 81, LB079. [Google Scholar] [CrossRef]
- Xu, C.; Wu, P.; Gao, J.; Zhang, L.; Ma, T.; Ma, B.; Yang, S.; Shao, G.; Yu, Y.; Huang, X.; et al. Heptadecanoic acid inhibits cell proliferation in PC-9 non-small-cell lung cancer cells with acquired gefitinib resistance. Oncol. Rep. 2019, 41, 3499–3507. [Google Scholar] [CrossRef]
- Ravi, L.; Krishnan, K. Research Article Cytotoxic Potential of N-hexadecanoic Acid Extracted from Kigelia pinnata Leaves. Asian J. Cell Biol. 2017, 12, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Bharath, B.; Perinbam, K.; Devanesan, S.; AlSalhi, M.S.; Saravanan, M. Evaluation of the anticancer potential of Hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J. Mol. Struct. 2021, 1235, 130229. [Google Scholar] [CrossRef]
- Arulvasu, C.; Shivaranjani, S.; Revati, M.; Hemavati, M. Free radical scavenging activity and cytotoxic effect of anisaldehyde on human cancer cell line. In International Conference on Advance in New Materials; Department of Inorganic Chemistry, University of Madras: Chennai, India, 2014; Volume 20. [Google Scholar]
- Haghighi, S.R.; Asadi, M.H.; Akrami, H.; Baghizadeh, A. Anti-carcinogenic and anti-angiogenic properties of the extracts of Acorus calamus on gastric cancer cells. Avicenna J. Phytomedicine 2017, 7, 145–156. [Google Scholar] [CrossRef]
- Ding, X.; Chen, H. Anticancer effects of Carvone in myeloma cells is mediated through the inhibition of p38 MAPK signalling pathway, apoptosis induction and inhibition of cell invasion. JBUON 2018, 23, 747–751. [Google Scholar]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Lashkari, A.; Najafi, F.; Kavoosi, G.; Niazi, S. Evaluating the In vitro anti-cancer potential of estragole from the essential oil of Agastache foeniculum [Pursh.] Kuntze. Biocatal. Agric. Biotechnol. 2020, 27, 101727. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef]
- Rolim, T.L.; Meireles, D.R.P.; Batista, T.M.; De Sousa, T.K.G.; Mangueira, V.M.; De Abrantes, R.A.; Pita, J.C.L.R.; Xavier, A.L.; Costa, V.C.O.; Batista, L.M.; et al. Toxicity and antitumor potential of Mesosphaerum sidifolium (Lamiaceae) oil and fenchone, its major component. BMC Complementary Altern. Med. 2017, 17, 1–12. [Google Scholar]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.-B.; Lobaccaro, J.-M.A.; Simpore, J. Chemical Composition, Antioxidant, Anti-Inflammatory and Anti-Proliferative Activities of Essential Oils of Plants from Burkina Faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef] [Green Version]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259. [Google Scholar]
- Bai, X.; Tang, J. Myrcene Exhibits Antitumor Activity Against Lung Cancer Cells by Inducing Oxidative Stress and Apoptosis Mechanisms. Nat. Prod. Commun. 2020, 15, 1934578X20961189. [Google Scholar] [CrossRef]
- Aydin, E.; Türkez, H.; Geyikoğlu, F. Antioxidative, anticancer and genotoxic properties of α-pinene on N2a neuroblastoma cells. Biologia 2013, 68, 1004–1009. [Google Scholar] [CrossRef]
- Yamazaki, S.; Miyoshi, N.; Kawabata, K.; Yasuda, M.; Shimoi, K. Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking β2-adrenergic signaling. Arch. Biochem. Biophys. 2014, 557, 18–27. [Google Scholar] [CrossRef]
- Contant, C.; Rouabhia, M.; Loubaki, L.; Chandad, F.; Semlali, A. Anethole induces anti-oral cancer activity by triggering apoptosis, autophagy and oxidative stress and by modulation of multiple signaling pathways. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Hassan, S.B.; Gali-Muhtasib, H.; Göransson, H.; Larsson, R. Alpha terpineol: A potential anticancer agent which acts through suppressing NF-κB signalling. Anticancer Res. 2010, 30, 1911–1919. [Google Scholar]
- Kim, D.-H.; Han, S.-I.; Go, B.; Oh, U.H.; Kim, C.-S.; Jung, Y.-H.; Lee, J.; Kim, J.-H. 2-Methoxy-4-vinylphenol Attenuates Migration of Human Pancreatic Cancer Cells via Blockade of FAK and AKT Signaling. Anticancer Res. 2019, 39, 6685–6691. [Google Scholar] [CrossRef]
- Terasaki, M.; Ito, H.; Kurokawa, H.; Tamura, M.; Okabe, S.; Matsui, H.; Hyodo, I. Acetic acid is an oxidative stressor in gastric cancer cells. J. Clin. Biochem. Nutr. 2018, 63, 36–41. [Google Scholar] [CrossRef] [Green Version]
- A Head, K. Ascorbic acid in the prevention and treatment of cancer. Altern. Med. Rev. 1998, 3, 174–186. [Google Scholar]
- Moayedi, Y.; Greenberg, S.A.; Jenkins, B.A.; Marshall, K.L.; Dimitrov, L.V.; Nelson, A.M.; Owens, D.M.; Lumpkin, E.A. Camphor white oil induces tumor regression through cytotoxic T cell-dependent mechanisms. Mol. Carcinog. 2018, 58, 722–734. [Google Scholar] [CrossRef]
- Al Bratty, M.; Makeen, H.A.; Alhazmi, H.A.; Syame, S.M.; Abdalla, A.N.; Homeida, H.E.; Sultana, S.; Ahsan, W.; Khalid, A. Phytochemical, Cytotoxic, and Antimicrobial Evaluation of the Fruits of Miswak Plant, Salvadora persica L. J. Chem. 2020, 2020, 4521951. [Google Scholar] [CrossRef]
- El-Garawani, I.; El Nabi, S.H.; Nafie, E.; Almeldin, S. Foeniculum vulgare and Pelargonium graveolens Essential Oil Mixture Triggers the Cell Cycle Arrest and Apoptosis in MCF-7 Cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. -Anti-Cancer Agents) 2019, 19, 1103–1113. [Google Scholar] [CrossRef]
- Chiarenza, A.; Scarselli, M.; Novi, F.; Lempereur, L.; Bernardini, R.; Corsini, G.U.; Maggio, R. Apomorphine, dopamine and phenylethylamine reduce the proportion of phosphorylated insulin receptor substrate 1. Eur. J. Pharmacol. 2001, 433, 47–54. [Google Scholar] [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Jabeen, M.; Chauhan, A.; Abdelhameed, A. Design, synthesis and in vitro anticancer evaluation of a stearic acid-based ester conjugate. Anticancer Res. 2013, 33, 2517–2524. [Google Scholar] [PubMed]
- Shahbazian, S.; Akbarzadeh, A.; Torabi, S.; Omidi, M. Anti-cancer activity of pegylated liposomal trans-anethole on breast cancer cell lines MCF-7 and T47D. Biotechnol. Lett. 2015, 37, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, A.; Al-Marzoqi, A.-H.; Mostafavi, S.K.S.; Alghanimi, Y.K.; Teimouri, M. Chemical Composition and Antimicrobial and Cytotoxic Activities of Foeniculum vulgare Mill Essential Oils. J. Gastrointest. Cancer 2019, 51, 260–266. [Google Scholar] [CrossRef]
- Mohamad, R.H.; El-Bastawesy, A.M.; Abdel-Monem, M.G.; Noor, A.M.; Al-Mehdar, H.A.R.; Sharawy, S.M.; El-Merzabani, M.M. Antioxidant and Anticarcinogenic Effects of Methanolic Extract and Volatile Oil of Fennel Seeds (Foeniculum vulgare). J. Med. Food 2011, 14, 986–1001. [Google Scholar] [CrossRef]
- Berrington, D.; Lall, N. Anticancer Activity of Certain Herbs and Spices on the Cervical Epithelial Carcinoma (HeLa) Cell Line. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Batool, R.; Salahuddin, H.; Mahmood, T.; Ismail, M. Study of anticancer and antibacterial activities of Foeniculum vulgare, Justicia adhatoda and Urtica dioica as natural curatives. Cell. Mol. Biol. 2017, 63, 109–114. [Google Scholar] [CrossRef]
- Zaahkouk, S.M.; Aboul-Ela, E.L.; Ramadan, M.A.; Bakry, S.; Mhany, B.M. Anti-carcinogenic activity of methanolic extract of Foeniculum vulgare seeds (Foeniculum vulgare) against breast, colon, and liver cancer cells. Int. J. Adv. Res. 2015, 3, 1525–1537. [Google Scholar]
- Hossain, A. Molecular Docking, Drug-Likeness and ADMET Analysis, Application of Density Functional Theory (DFT) and Molecular Dynamics (MD) Simulation to the Phytochemicals from Withania Somnifera as Potential Antagonists of Estrogen Receptor Alpha (ER-α). Curr. Comput.-Aided Drug Des. 2021, 17, 797–805. [Google Scholar] [CrossRef]
- Rolta, R.; Salaria, D.; Kumar, V.; Patel, C.N.; Sourirajan, A.; Baumler, D.J.; Dev, K. Molecular docking studies of phytocompounds of Rheum emodi Wall with proteins responsible for antibiotic resistance in bacterial and fungal pathogens: in silico approach to enhance the bio-availability of antibiotics. J. Biomol. Struct. Dyn. 2020, 40, 3789–3803. [Google Scholar] [CrossRef]
- Salaria, D.; Rolta, R.; Patel, C.N.; Dev, K.; Sourirajan, A.; Kumar, V. In vitro and in silico analysis of Thymus serpyllum essential oil as bioactivity enhancer of antibacterial and antifungal agents. J. Biomol. Struct. Dyn. 2021, 16, 1–20. [Google Scholar] [CrossRef]
- Salaria, D.; Rolta, R.; Sharma, N.; Patel, C.N.; Ghosh, A.; Dev, K.; Sourirajan, A.; Kumar, V. In vitro and in silico antioxidant and anti-inflammatory potential of essential oil of Cymbopogon citratus (DC.) Stapf. of North-Western Himalaya. J. Biomol. Struct. Dyn. 2021, 1–15. [Google Scholar] [CrossRef]
- Rolta, R.; Yadav, R.; Salaria, D.; Trivedi, S.; Imran, M.; Sourirajan, A.; Baumler, D.J.; Dev, K. In silico screening of hundred phytocompounds of ten medicinal plants as potential inhibitors of nucleocapsid phosphoprotein of COVID-19: An approach to prevent virus assembly. J. Biomol. Struct. Dyn. 2020, 39, 7017–7034. [Google Scholar] [CrossRef]
- Mehta, J.; Rolta, R.; Salaria, D.; Awofisayo, O.; Fadare, O.A.; Sharma, P.P.; Rathi, B.; Chopra, A.; Kaushik, N.; Choi, E.H.; et al. Phytocompounds from Himalayan Medicinal Plants as Potential Drugs to Treat Multidrug-Resistant Salmonella typhimurium: An In Silico Approach. Biomedicines 2021, 9, 1402. [Google Scholar] [CrossRef]
- Salaria, D.; Rolta, R.; Mehta, J.; Awofisayo, O.; Fadare, O.A.; Kaur, B.; Kumar, B.; da Costa, R.A.; Chandel, S.R.; Kaushik, N.; et al. Phytoconstituents of traditional Himalayan Herbs as potential inhibitors of Human Papillomavirus (HPV-18) for cervical cancer treatment: An In silico Approach. PLoS ONE 2022, 17, e0265420. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A. Poor aqueous solubility—an industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Yang, H.; Du, Z.; Lv, W.-J.; Zhang, X.-Y.; Zhai, H.-L. In silico toxicity evaluation of dioxins using structure–activity relationship (SAR) and two-dimensional quantitative structure–activity relationship (2D-QSAR). Arch. Toxicol. 2019, 93, 3207–3218. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-S.; Cerutti, D.S.; Mermelstein, D.; Lin, C.; Legrand, S.; Giese, T.J.; E Roitberg, A.; Case, D.A.; Walker, R.C.; York, D.M. GPU-Accelerated Molecular Dynamics and Free Energy Methods in Amber18: Performance Enhancements and New Features. J. Chem. Inf. Model. 2018, 58, 2043–2050. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Song, L.F.; Lee, T.S.; Zhu, C.; York, D.M.; Merz, K.M., Jr. Using AMBER18 for relative free energy calculations. J. Chem. Inf. Modeling 2019, 59, 3128–3135. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- E Frisch, R.; Snow, R.C.; A Johnson, L.; Gerard, B.; Barbieri, R.; Rosen, B. Magnetic resonance imaging of overall and regional body fat, estrogen metabolism, and ovulation of athletes compared to controls. J. Clin. Endocrinol. Metab. 1993, 77, 471–477. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Duan, L.; Chen, F.; Liu, H.; Wang, Z.; Pan, P.; Zhu, F.; Zhang, J.Z.H.; Hou, T. Assessing the performance of MM/PBSA and MM/GBSA methods. 7. Entropy effects on the performance of end-point binding free energy calculation approaches. Phys. Chem. Chem. Phys. 2018, 20, 14450–14460. [Google Scholar] [CrossRef]
- Anza, M.; Endale, M.; Cardona, L.; Cortes, D.; Eswaramoorthy, R.; Zueco, J.; Rico, H.; Trelis, M.; Abarca, B. Antimicrobial Activity, in silico Molecular Docking, ADMET and DFT Analysis of Secondary Metabolites from Roots of Three Ethiopian Medicinal Plants. Adv. Applic. Bioinform. Chem. AABC. 2021, 14, 117. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
