GO-SWCNT Buckypapers as an Enhanced Technology for Water Decontamination from Lead
Abstract
:1. Introduction
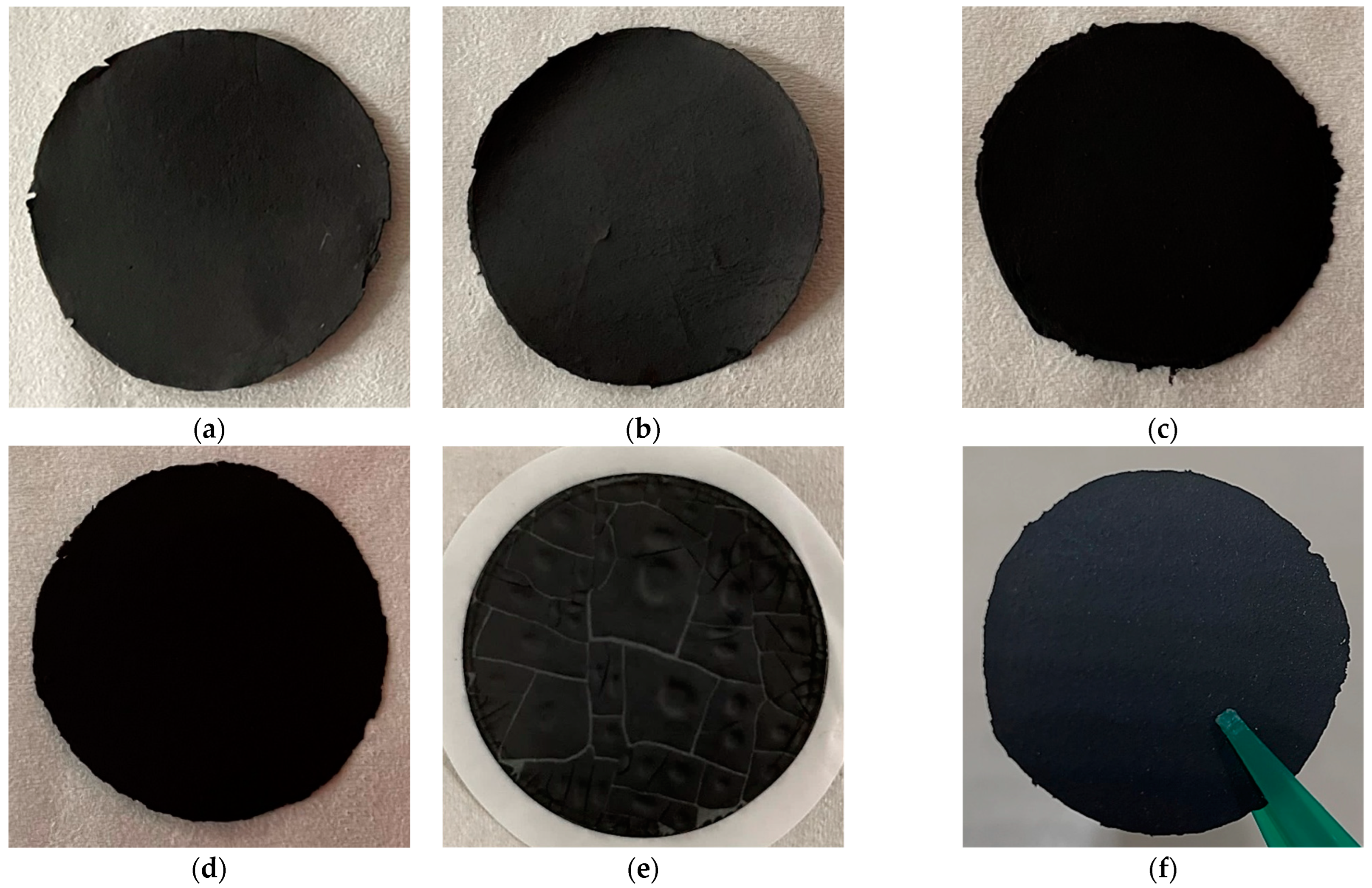
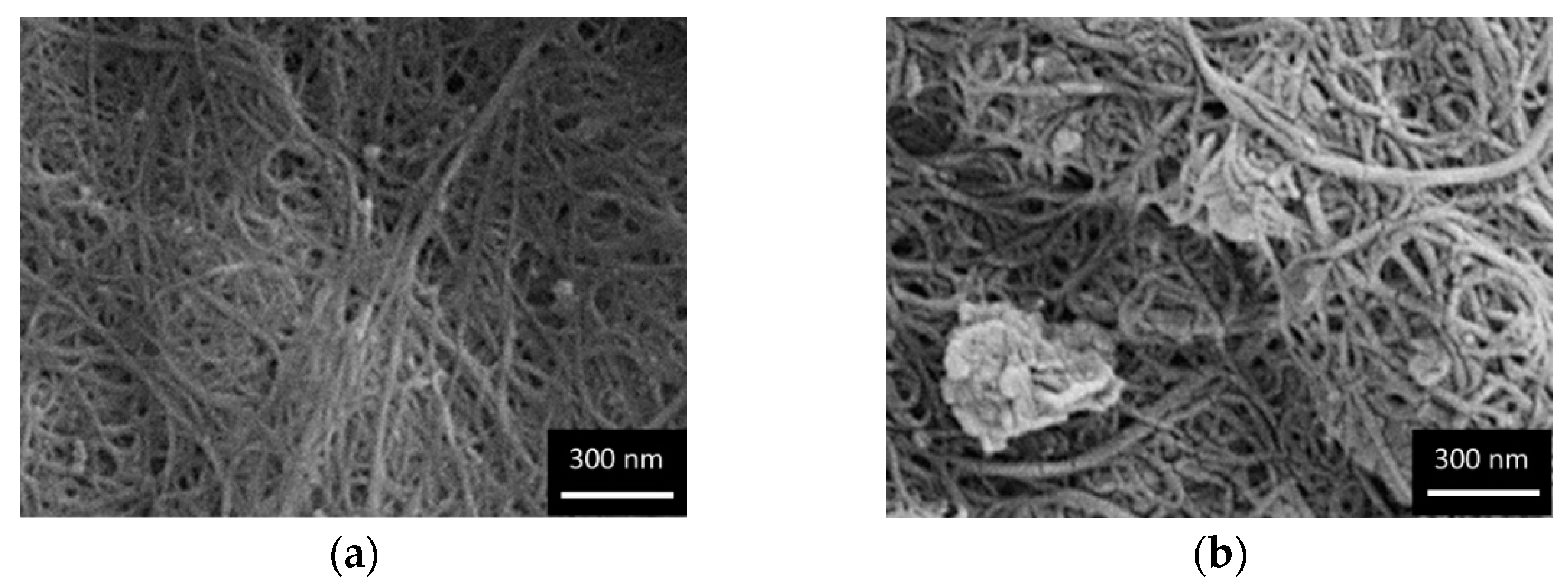
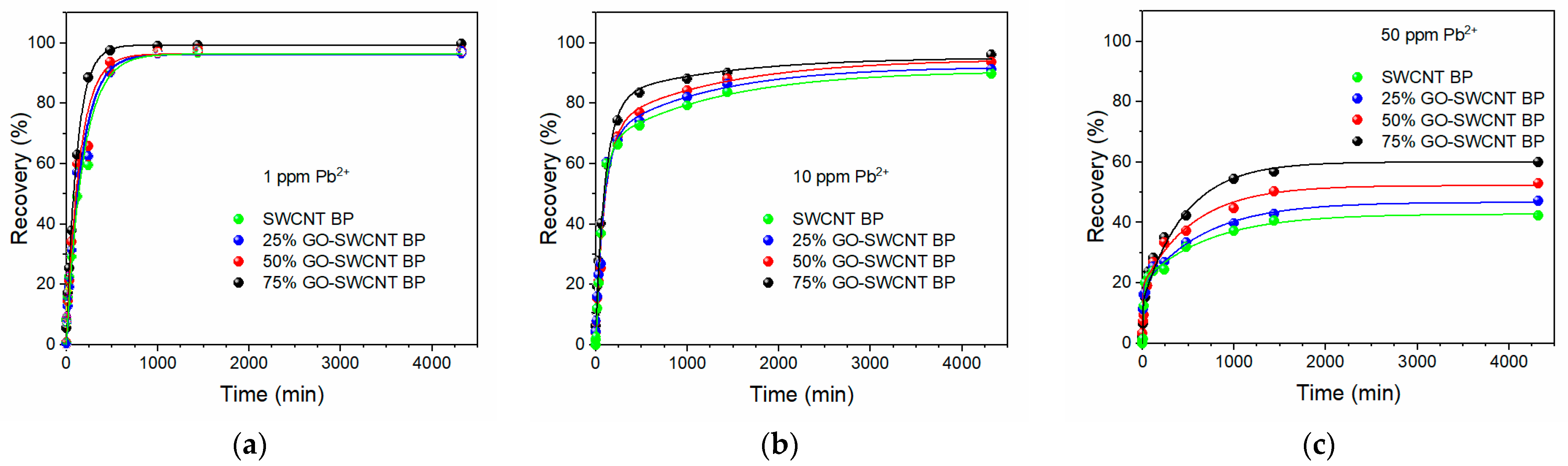
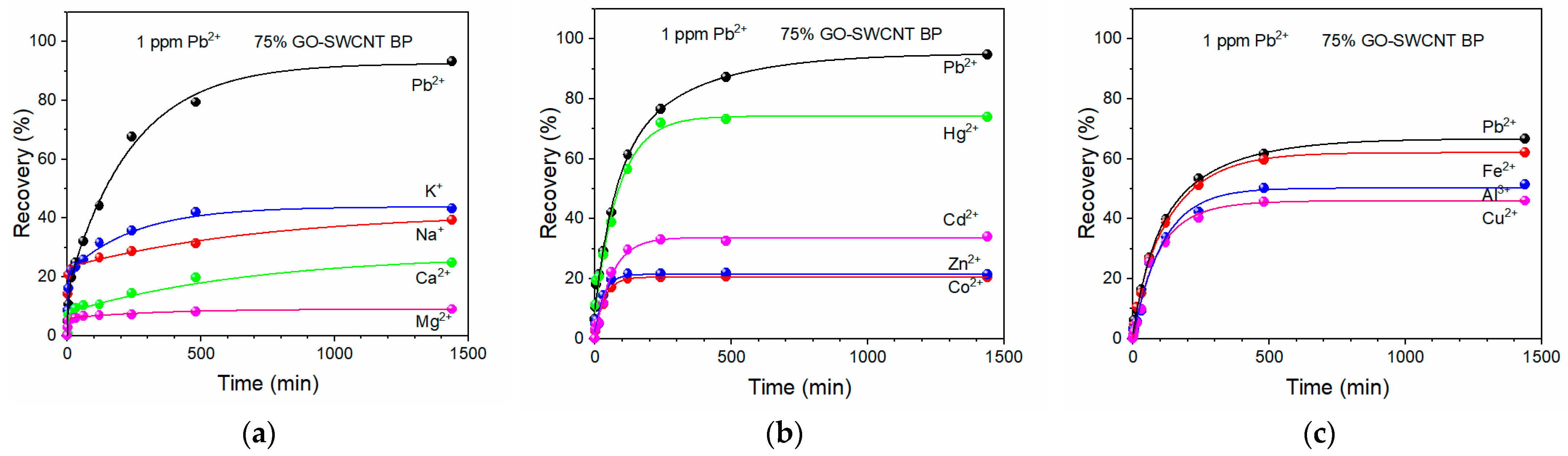
2. Results
3. Materials and Methods
3.1. Buckypaper Preparation
3.2. Characterization Procedure
3.3. Lead Adsorption by SWCNT and GO-SWCNT BPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, R.S.; Kapoor, V.; Nirola, A.; Sohi, R.; Bansal, V. Water pollution: Impact of pollutants and new promising techniques in purification process. J. Hum. Ecol. 2012, 37, 103–110. [Google Scholar] [CrossRef]
- Lim, J.Y.; Mubarak, N.M.; Abdullah, E.C.; Nizamuddin, S.; Khalid, M.; Inamuddin. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals—A review. J. Ind. Eng. Chem. 2018, 66, 29–44. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metals toxicity and the environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef] [Green Version]
- Payne, M. Lead in drinking water. Can. Med. Assoc. J. 2008, 179, 253–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahat, A.; Awual, M.R.; Khaleque, M.A.; Alam, M.Z.; Naushad, M.; Sarwaruddin Chowdhury, A.M. Large-pore diameter nano–adsorbent and its application for rapid lead (II) detection and removal from aqueous media. Chem. Eng. J. 2015, 273, 286–295. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Chen, M.; Gu, H.; Rapole, S.B.; Pallavkar, S.; Ho, T.C.; Hopper, J.; Guo, Z. Magnetic nanocomposites for environmental remediation. Adv. Powder Technol. 2013, 24, 459–467. [Google Scholar] [CrossRef]
- Water Sanitation and Health. Available online: https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/water-safety-and-quality/drinking-water-quality-guidelines (accessed on 25 May 2022).
- Narayani, M.; Shetty, K.V. Chromium–resistant bacteria and their environmental condition for hexavalent chromium removal: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 955–1009. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Hoch, L.B.; Mack, E.J.; Hydutsky, B.W.; Hershman, J.M.; Skluzacek, J.M.; Mallouk, T.E. Carbothermal synthesis of carbon–supported nanoscale zero–valent iron particles for the remediation of hexavalent chromium. Environ. Sci. Technol. 2008, 42, 2600–2605. [Google Scholar] [CrossRef]
- Oehmen, A.; Vergel, D.; Fradinho, J.; Reis, M.A.M.; Crespo, J.G.; Velizarov, S. Mercury removal from water streams through the ion exchange membrane bioreactor concept. J. Hazard. Mater. 2014, 264, 65–70. [Google Scholar] [CrossRef]
- Stephenson, R.J.; Duff, S.J.B. Coagulation and precipitation of a mechanical pulping effluent—I. Removal of carbon, colour and turbidity. Water Res. 1996, 30, 781–792. [Google Scholar] [CrossRef]
- Tran, T.-K.; Chiu, K.-F.; Lin, C.-Y.; Leu, H.-J. Electrochemical treatment of wastewater: Selectivity of the heavy metals removal process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, C.; Cheng, W.; Wang, X. Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron. J. Hazard. Mater. 2014, 280, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Heo, J.; Han, J.; Her, N.; Lee, S.-J.; Oh, J.; Ryu, J.; Yoon, Y. Hexavalent chromium removal by various adsorbents: Powdered activated carbon, chitosan, and single/multi-walled carbon nanotubes. Separ. Purif. Technol. 2013, 106, 63–71. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Hadi, P.; To, M.-H.; Hui, C.-W.; Lin, C.S.K.; McKay, G. Aqueous mercury adsorption by activated carbons. Water Res. 2015, 73, 37–55. [Google Scholar] [CrossRef]
- Sheng, G.; Yang, S.; Sheng, J.; Hu, J.; Tan, X.; Wang, X. Macroscopic and microscopic investigation of Ni(II) sequestration on diatomite by batch, XPS, and EXAFS techniques. Environ. Sci. Technol. 2011, 45, 7718–7726. [Google Scholar] [CrossRef]
- Jiménez-Castañeda, M.E.; Medina, D.I. Use of surfactant-modified zeolites and clays for the removal of heavy metals from water. Water 2017, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Nayak, A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 2012, 180, 81–90. [Google Scholar] [CrossRef]
- Kolluru, S.S.; Agarwal, S.; Sireesha, S.; Sreedhar, I.; Kale, S.R. Heavy metal removal from wastewater using nanomaterials-process and engineering aspects. Process Saf. Environ. Prot. 2021, 150, 323–355. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Memon, S.; Memon, N. Dichromate extraction by calix[4]arene appended amberlite XAD–4 resin. Separ. Sci. Technol. 2014, 49, 664–672. [Google Scholar] [CrossRef]
- Dave, P.N.; Chopda, L.V. Application of iron oxide nanomaterials for the removal of heavy metals. J. Nanotechnol. 2014, 2014, 398569. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef]
- Renu, M.A.; Singh, K.; Upadhyaya, S.; Dohare, R.K. Removal of heavy metals from wastewater using modified agricultural adsorbents. Mater. Today Proc. 2017, 4, 10534–10538. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental remediation and application of nanoscale zero–valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ. Sci. Pollut. Res. 2012, 19, 1224–1228. [Google Scholar] [CrossRef]
- Kabbashi, N.A.; Atieh, M.A.; Al-Mamun, A.; Mirghami, M.E.S.; Alam, M.D.Z.; Yahya, N. Kinetic adsorption of application of carbon nanotubes for Pb(II) removal from aqueous solution. J. Environ. Sci. 2009, 21, 539–544. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Huang, H.; Zeng, G.; Liu, Y.; Wang, X.; Lin, N.; Qi, Y. Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl. Surf. Sci. 2013, 279, 432–440. [Google Scholar] [CrossRef]
- Zhao, G.; Ren, X.; Gao, X.; Tan, X.; Li, J.; Chen, C.; Huang, Y.; Wang, X. Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans. 2011, 40, 10945–10952. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Majumder, M.; Alemany, L.B.; Narayanan, T.N.; Ibarra, M.A.; Pradhan, B.K.; Ajayan, P.M. Engineered graphite oxide materials for application in water purification. ACS Appl. Mater. Interfaces 2011, 3, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Wang, K.; Xia, Y.; Li, Z. Novel melamine modified metal-organic frameworks for remarkably high removal of heavy metal Pb(II). Desalination 2018, 430, 120–127. [Google Scholar] [CrossRef]
- Baratta, M.; Mastropietro, T.F.; Bruno, R.; Tursi, A.; Negro, C.; Ferrando-Soria, J.; Mashin, A.I.; Nezhdanov, A.; Nicoletta, F.P.; De Filpo, G.; et al. Multivariate metal–organic framework/single–walled carbon nanotube buckypaper for selective lead decontamination. ACS Appl. Nano Mater. 2022, 5, 5223–5233. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon nanomaterials for the treatment of heavy metal-contaminated water and environmental remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Khalid, M.; Karri, R.R.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.; Mazari, S.A. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for removal of heavy metals and dyes. J. Hazard. Mater. 2021, 413, 125375. [Google Scholar] [CrossRef]
- Alshahrani, A.; Alharbi, A.; Alnasser, S.; Almihdar, M.; Alsuhybani, M.; AlOtaibi, B. Enhanced heavy metals removal by a novel carbon nanotubes buckypaper membrane containing a mixture of two biopolymers: Chitosan and i-carrageenan. Separ. Purif. Technol. 2021, 276, 119300. [Google Scholar] [CrossRef]
- De Filpo, G.; Pantuso, E.; Mashin, A.I.; Baratta, M.; Nicoletta, F.P. WO3/buckypaper membranes for advanced oxidation processes. Membranes 2020, 10, 157. [Google Scholar] [CrossRef]
- Rashid, M.H.-O.; Ralph, S.F. Carbon nanotube membranes: Synthesis, properties, and future filtration applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Patole, S.P.; Arif, M.F.; Susantyoko, R.A.; Almheiri, S.; Kumar, S. A wet-filtration-zipping approach for fabricating highly electroconductive and auxetic graphene/carbon nanotube hybrid buckypaper. Sci. Rep. 2018, 8, 12188. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Z.; Liu, Y.; Leng, J. Buckypaper and its composites for aeronautic applications. Compos. B Eng. 2020, 199, 108231. [Google Scholar] [CrossRef]
- Tursi, A.; Mastropietro, T.F.; Bruno, R.; Baratta, M.; Ferrando-Soria, J.; Mashin, A.I.; Nicoletta, F.P.; Pardo, E.; De Filpo, G.; Armentano, D. Synthesis and enhanced capture properties of a new BioMOF@SWCNT-BP: Recovery of the endangered rare-earth elements from aqueous systems. ACS Adv. Mater. Interfaces 2021, 8, 2100730. [Google Scholar] [CrossRef]
- Huang, J.; Her, S.-C.; Yang, X.; Zhi, M. Synthesis and characterization of multi-walled carbon nanotube/graphene nanoplatelet hybrid film for flexible strain sensors. Nanomaterials 2018, 8, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-Q.; Xu, Z.-L.; Abouali, S.; Garakani, M.A.; Kim, J.-K. Porous graphene oxide/carbon nanotube hybrid films as interlayer for lithium-sulfur batteries. Carbon 2016, 99, 624–632. [Google Scholar] [CrossRef]
- Yuan, G.-J.; Xie, J.-F.; Li, H.-H.; Shan, B.; Zhang, X.-X.; Liu, J.; Li, L.; Tian, Y.-Z. Thermally reduced graphene oxide/carbon nanotube composite films for thermal packaging applications. Materials 2020, 13, 317. [Google Scholar] [CrossRef] [Green Version]
- Minett, A.; Fràysse, J.; Gang, G.; Kim, G.-T.; Roth, S. Nanotube actuators for nanomechanics. Curr. Appl. Phys. 2002, 2, 61–64. [Google Scholar] [CrossRef]
- Kausar, A.; Ilyas, H.; Siddiq, M. Current research status and application of polymer/carbon nanofiller buckypaper: A review. Polym. Plast. Technol. Eng. 2017, 56, 1780–1800. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Al-Zoubi, H.; Nghiem, L.D.; in het Panhuis, M. Synthesis and characterisation of MWNT/chitosan and MWNT/chitosan-crosslinked buckypaper membranes for desalination. Desalination 2017, 418, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Musielak, M.; Gagor, A.; Zawisza, B.; Talik, E.; Sitko, R. Graphene oxide/carbon nanotube membranes for highly efficient removal of metal ions from water. ACS Appl. Mater. Interfaces 2019, 11, 28582–28590. [Google Scholar] [CrossRef]
- Cooper, S.M.; Chuang, H.F.; Cinke, M.; Cruden, B.A.; Meyyappan, M. Gas permeability of a buckypaper membrane. Nano Lett. 2003, 2, 189–192. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Z.Y.; Wang, B.; Zhang, C.; Kramer, L. Processing and property investigation of single-walled carbon nanotube (SWNT) buckypaper/epoxy resin matrix nanocomposites. Compos. Appl. Sci. Manuf. 2004, 35, 1225–1232. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, S.; Kim, Y.; Her, N.; Heo, J.; Han, J.; Jang, M.; Park, C.M.; Yoon, Y. Comprehensive evaluation on removal of lead by graphene oxide and metal organic framework. Chemosphere 2019, 231, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Agrawal, S.; Sinha, A.; Rao, T.R.; Balakrishnan, J.; Thakur, A.D. Low-cost non-explosive synthesis of graphene oxide for scalable applications. Sci. Rep. 2018, 8, 12007. [Google Scholar] [CrossRef]



| Sample | Tensile Strength (MPa) | Fracture Strain (%) | Water Contact-Angle (°) | Porosity (%) |
|---|---|---|---|---|
| SWCNT BP | 13.8 ± 0.9 | 2.2 ± 0.5 | 71.3 ± 0.5 | 74 ± 5 |
| 25% GO-SWCNT BP | 11.3 ± 0.8 | 1.9 ± 05 | 62.6 ± 0.5 | 70 ± 5 |
| 50% GO-SWCNT BP | 7.2 ± 0.6 | 1.5 ± 0.4 | 51.2 ± 0.5 | 65 ± 5 |
| 75% GO-SWCNT BP | 4.7 ± 0.6 | 1.3 ± 0.4 | 41.2 ± 0.5 | 59 ± 5 |
| Sample | k1 × 103 (min−1) | qe (mg g−1) | R2 |
|---|---|---|---|
| SWCNT BP | 5.44 ± 0.07 | 15.3 ± 0.5 | 0.9784 |
| 25% GO-SWCNT BP | 6.07 ± 0.06 | 15.4 ± 0.4 | 0.9864 |
| 50% GO-SWCNT BP | 6.88 ± 0.07 | 15.4 ± 0.4 | 0.9864 |
| 75% GO-SWCNT BP | 8.81 ± 0.05 | 15.9 ± 0.2 | 0.9958 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratta, M.; Tursi, A.; Curcio, M.; Cirillo, G.; Nicoletta, F.P.; De Filpo, G. GO-SWCNT Buckypapers as an Enhanced Technology for Water Decontamination from Lead. Molecules 2022, 27, 4044. https://doi.org/10.3390/molecules27134044
Baratta M, Tursi A, Curcio M, Cirillo G, Nicoletta FP, De Filpo G. GO-SWCNT Buckypapers as an Enhanced Technology for Water Decontamination from Lead. Molecules. 2022; 27(13):4044. https://doi.org/10.3390/molecules27134044
Chicago/Turabian StyleBaratta, Mariafrancesca, Antonio Tursi, Manuela Curcio, Giuseppe Cirillo, Fiore Pasquale Nicoletta, and Giovanni De Filpo. 2022. "GO-SWCNT Buckypapers as an Enhanced Technology for Water Decontamination from Lead" Molecules 27, no. 13: 4044. https://doi.org/10.3390/molecules27134044
APA StyleBaratta, M., Tursi, A., Curcio, M., Cirillo, G., Nicoletta, F. P., & De Filpo, G. (2022). GO-SWCNT Buckypapers as an Enhanced Technology for Water Decontamination from Lead. Molecules, 27(13), 4044. https://doi.org/10.3390/molecules27134044









