Novel Silver-Plated Nickel-Coated Graphite Powder with Excellent Heat and Humidity Resistance: Facile Preparation and Performance Investigation
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Material Fabrication Procedures
2.3. Characterization Methods
3. Results and Discussions
3.1. Loading Amount of Ag
3.2. Characterization
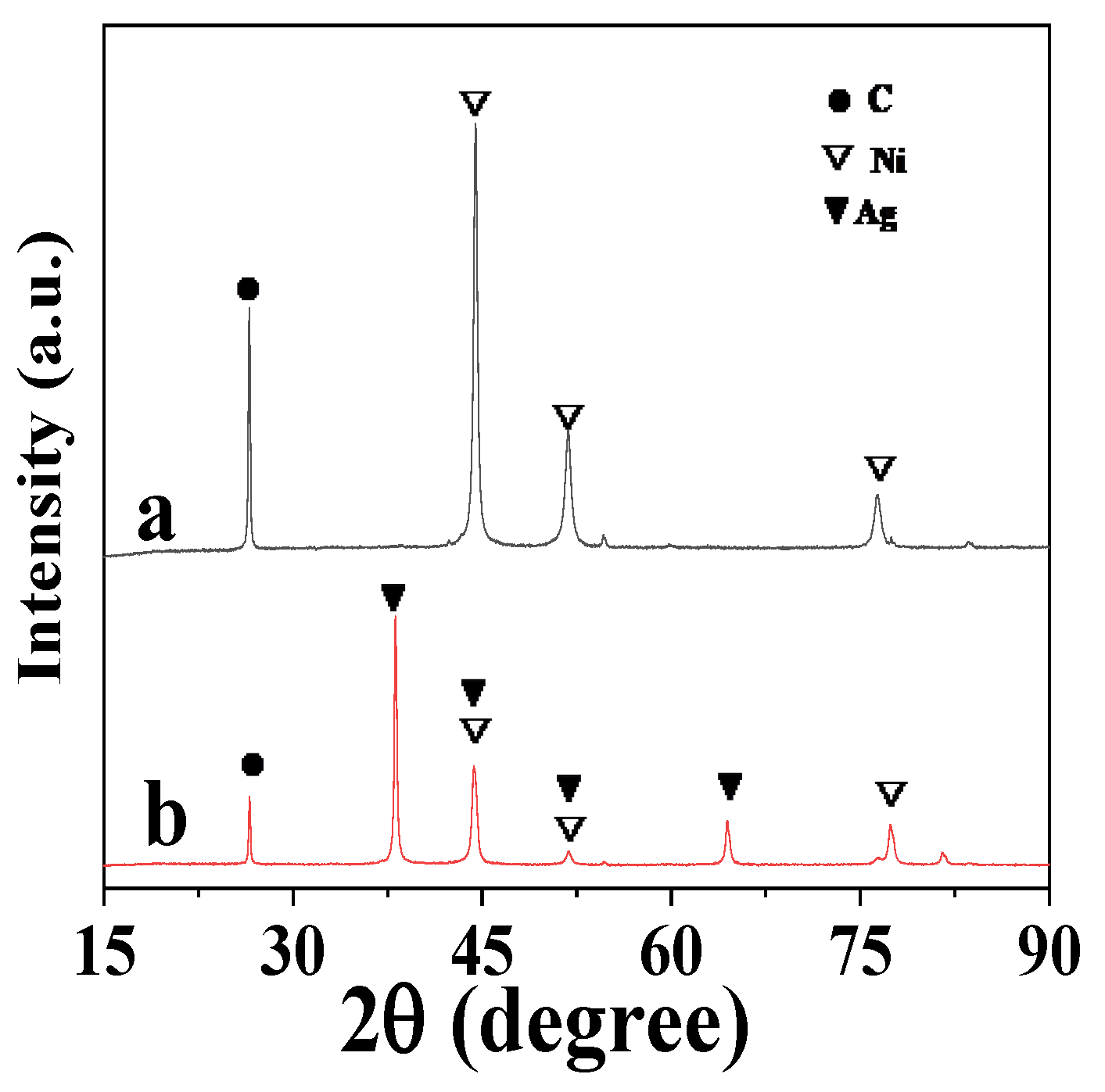
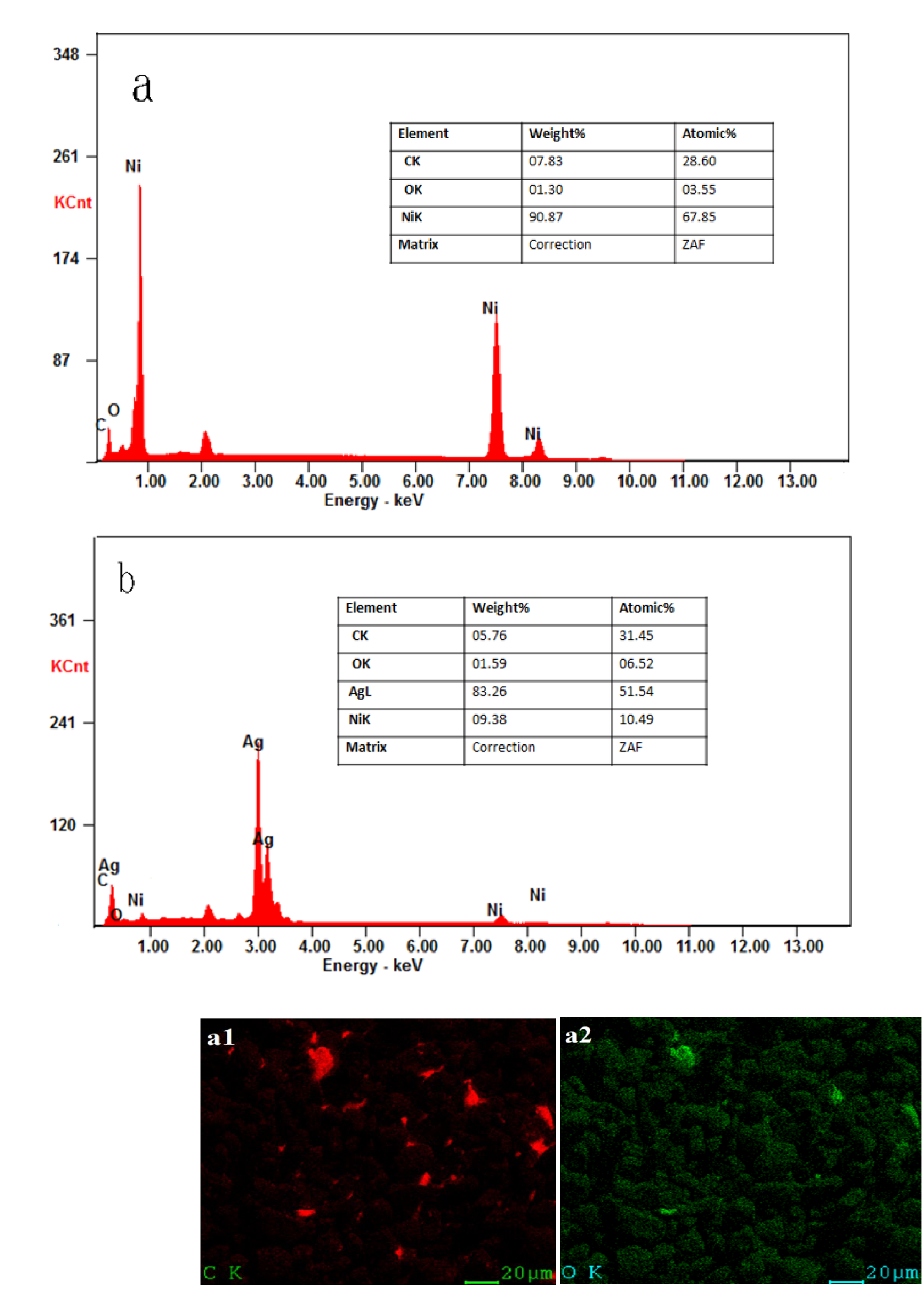
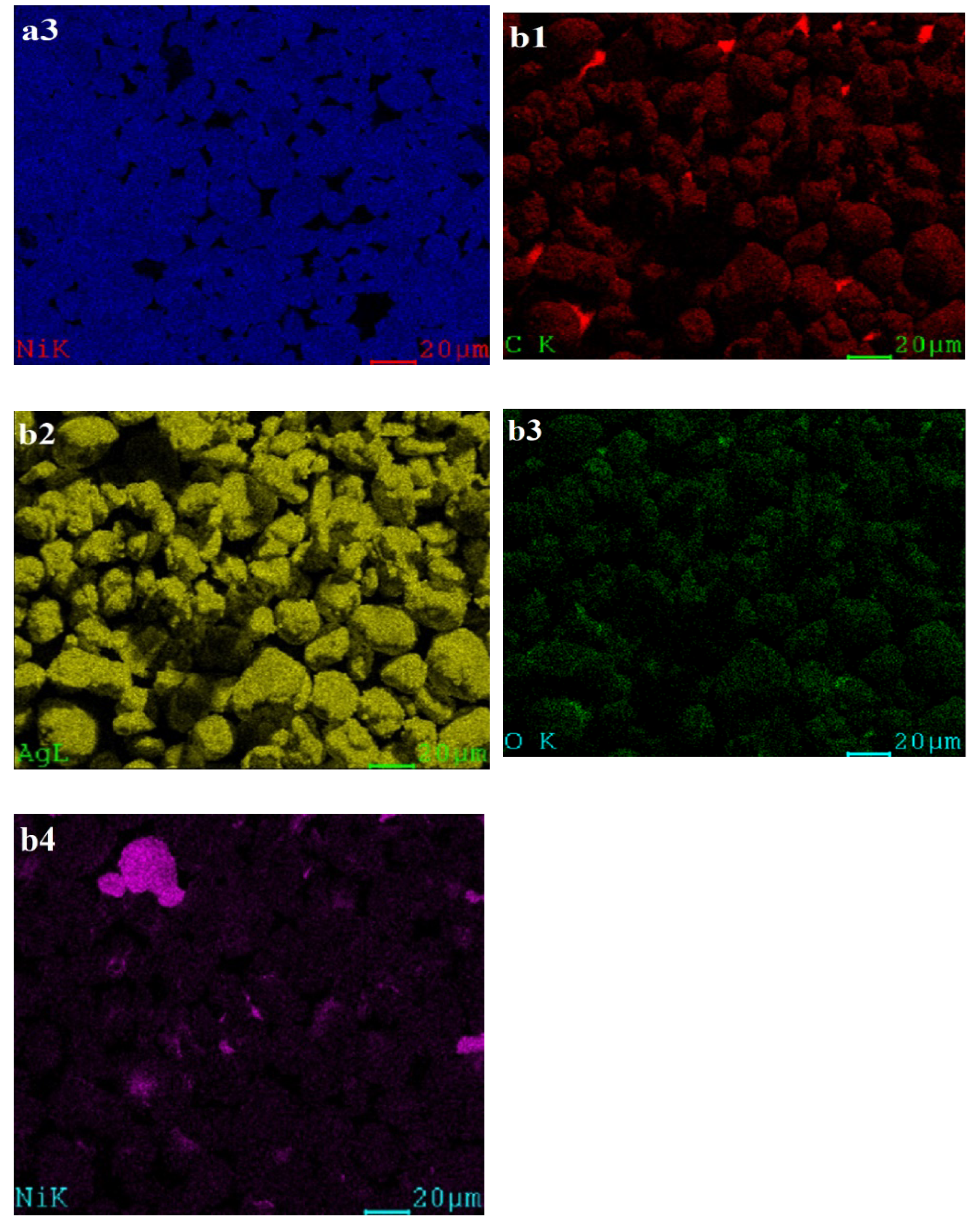
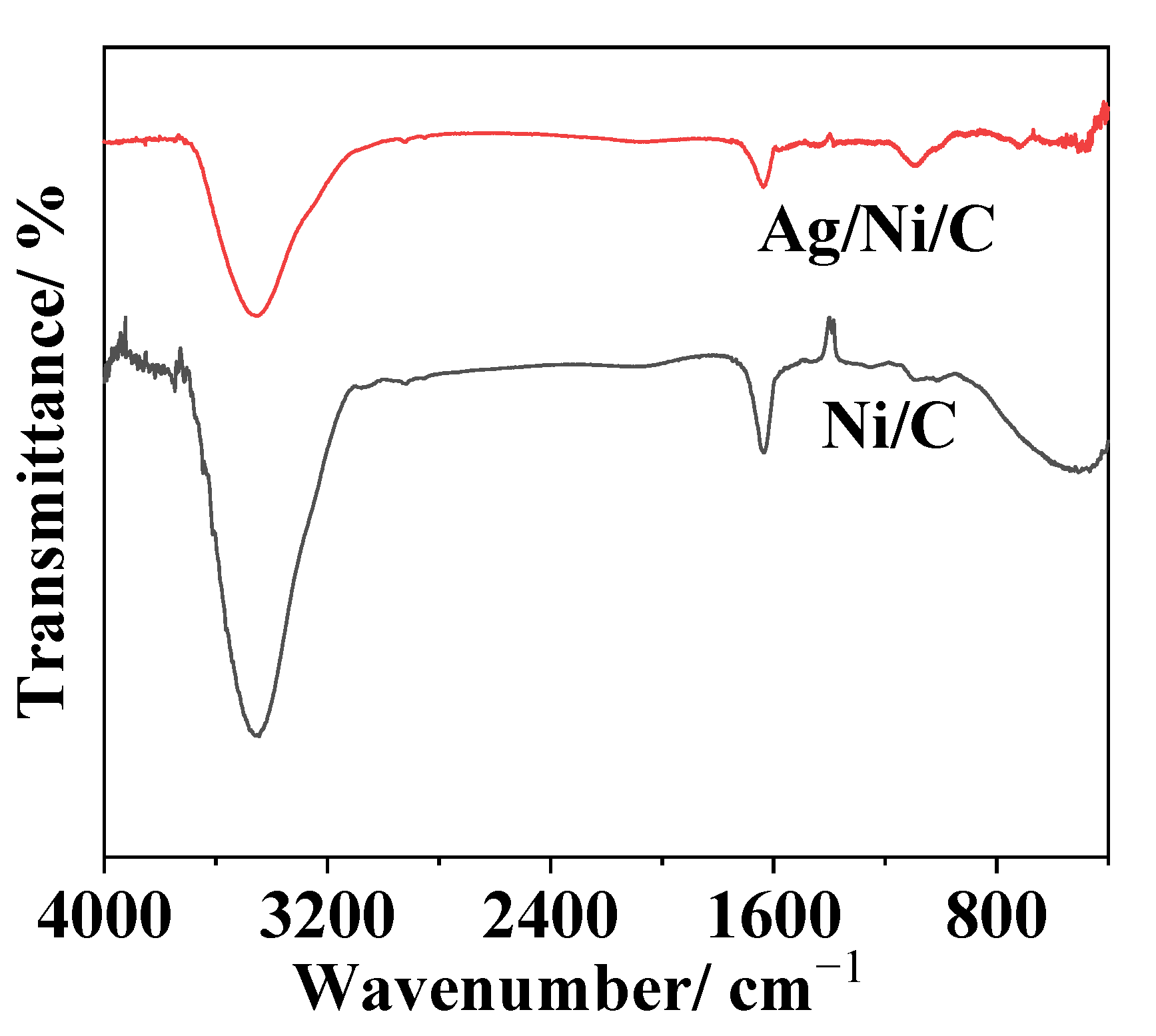
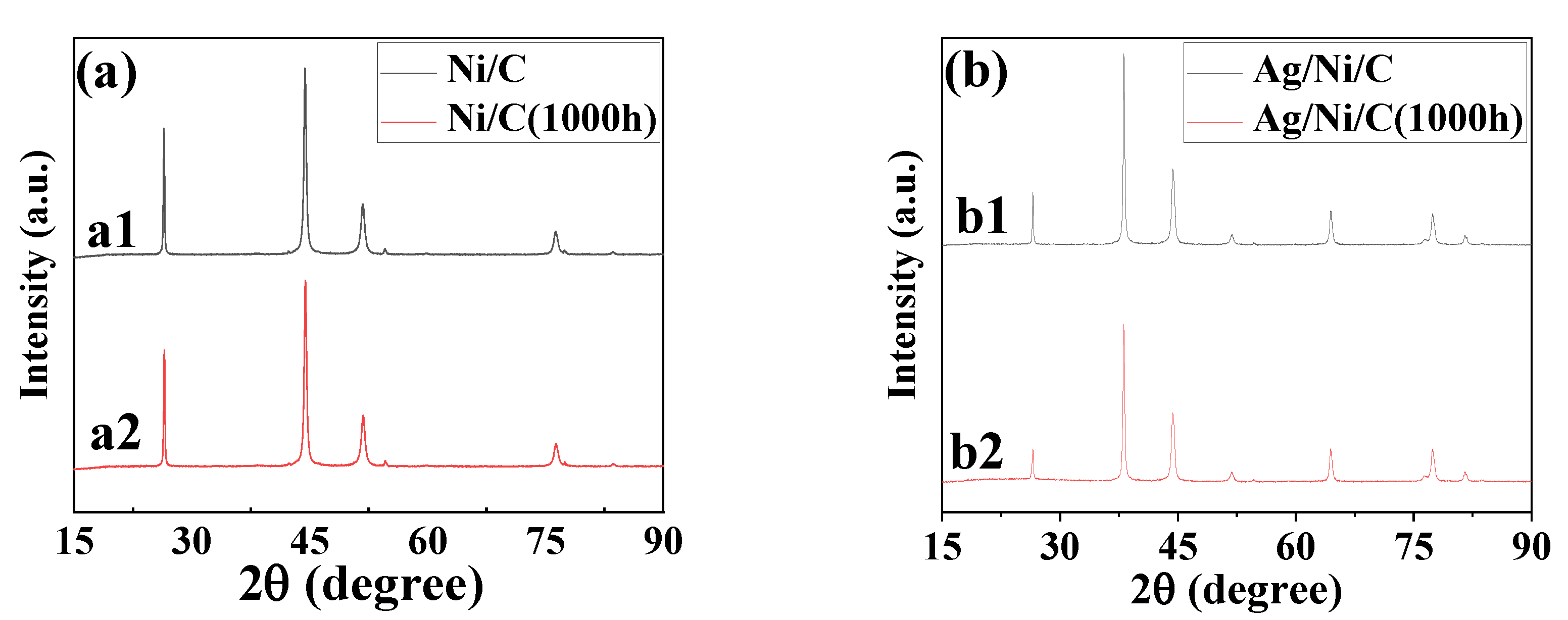
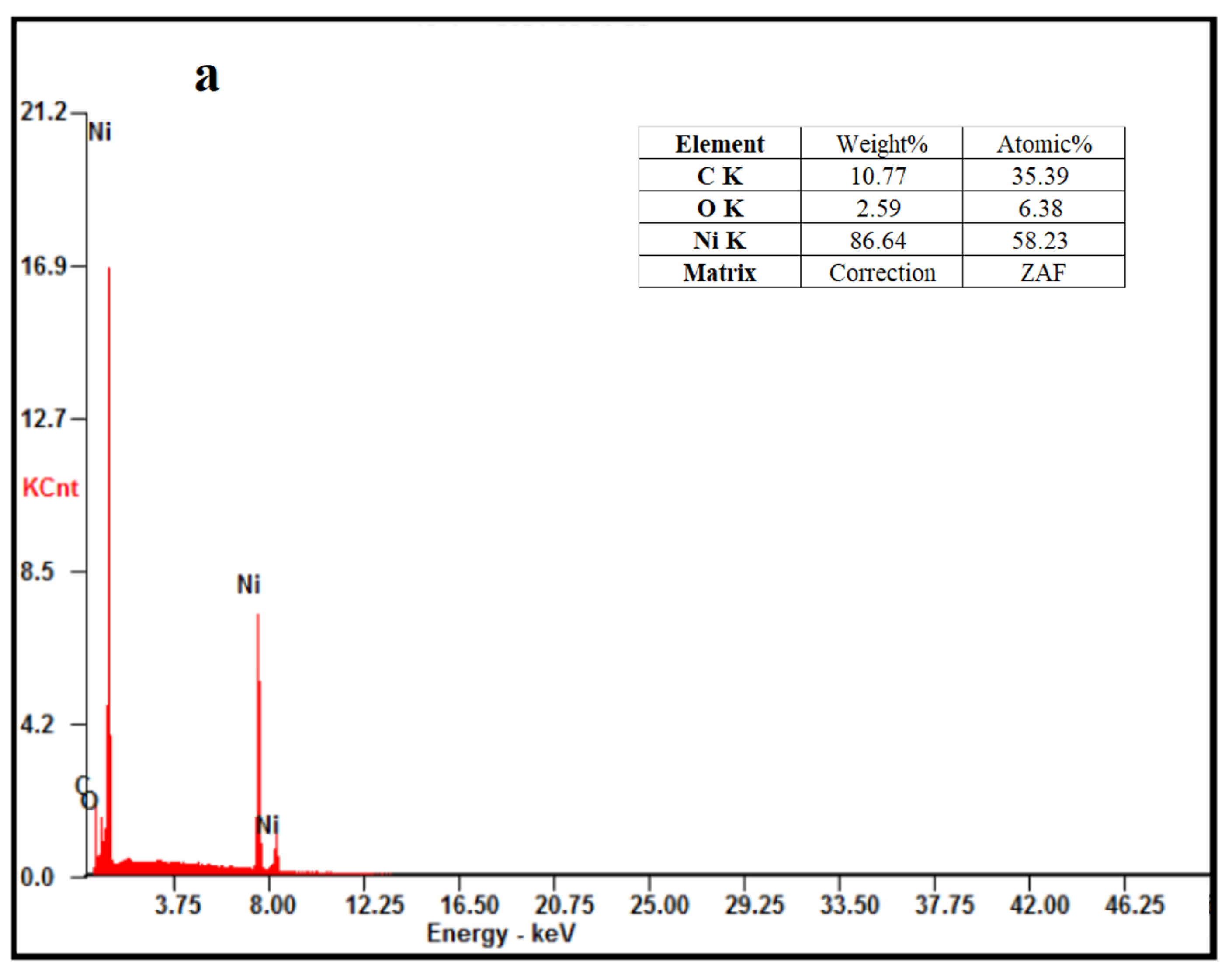
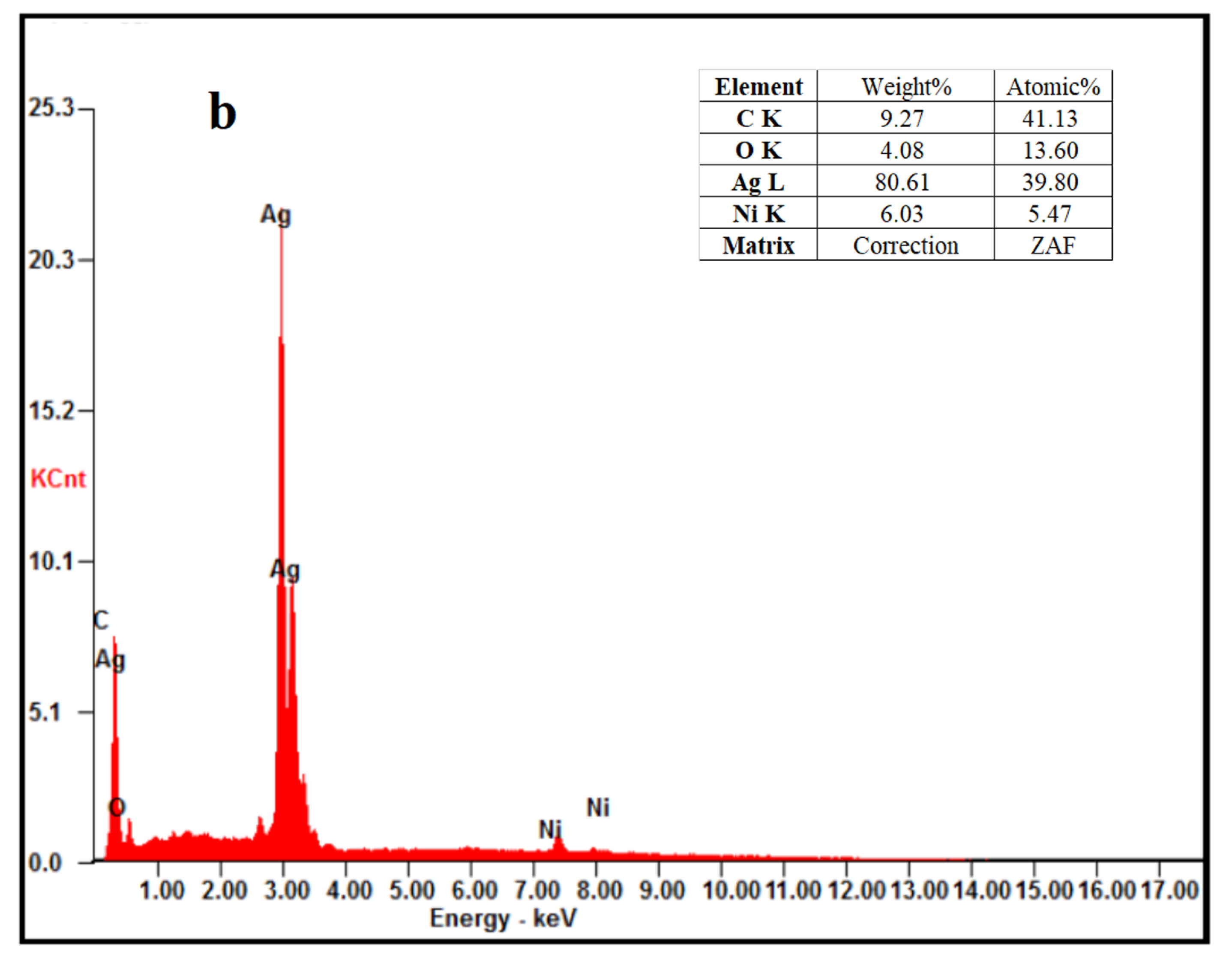
3.2.1. Investigation of Crystal Structure, Morphology, Composition, and Particle Sizes
3.2.2. Particle Size Distribution Analysis
3.3. Effects of Reaction Time on Silver Coating
3.4. Anti-Oxidation Properties of Ag/Ni/C Powder
3.4.1. TG Analyses
3.4.2. Effects of Heating Temperature on Ag/Ni/C Powders
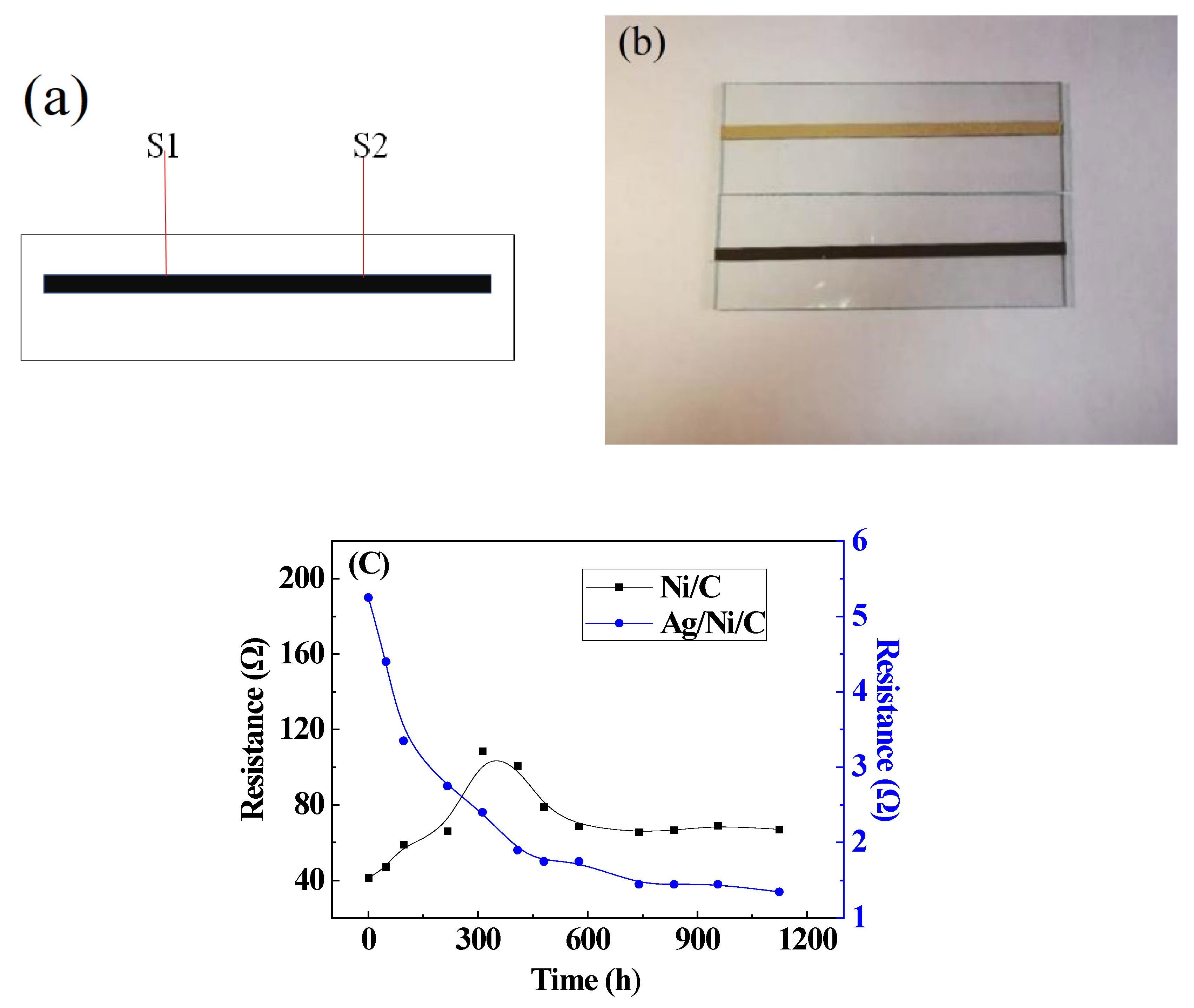
3.5. Hydrothermal Resistance and Electromagnetic Shielding Effectiveness of Ag/Ni/C and Ni/C Powders
3.5.1. Evaluation of the Electrical Conductivity
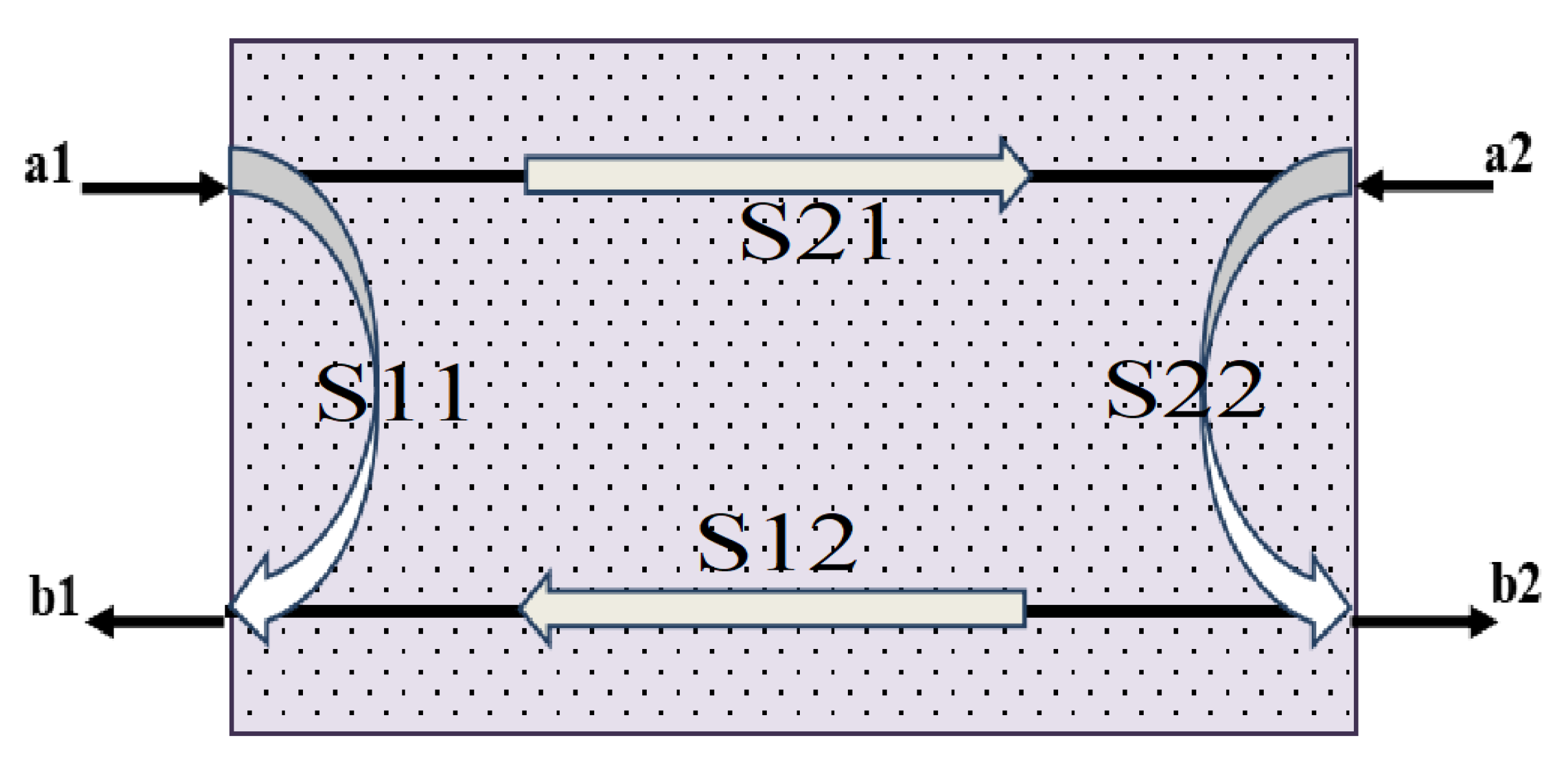
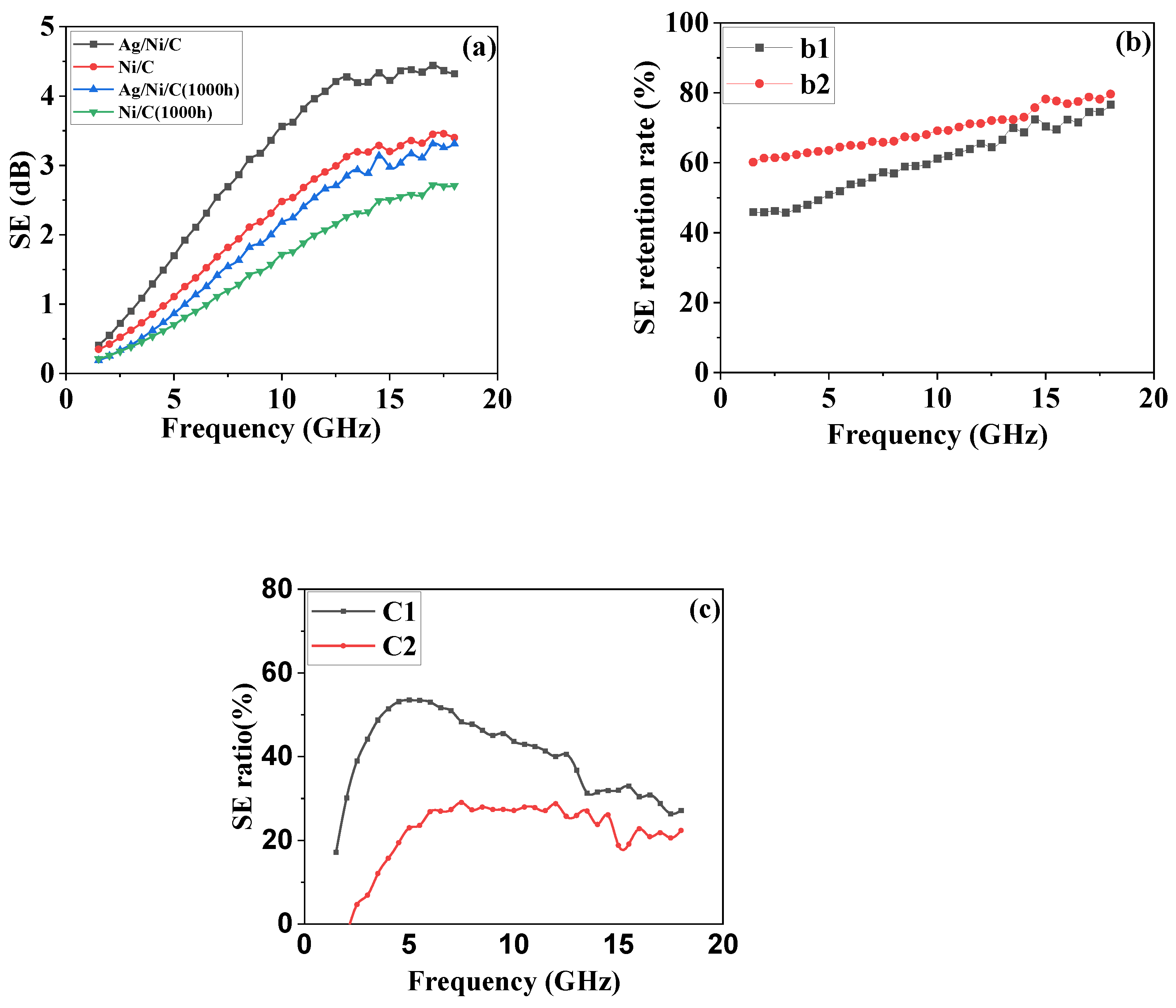
3.5.2. The Electromagnetic Shielding Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kumar, K.S.; Rengaraj, R.; Venkatakrishnan, G.R.; Chandramohan, A. Polymeric materials for electromagnetic shielding—A review. Mater. Today-Proc. 2021, 47, 4925–4928. [Google Scholar] [CrossRef]
- Park, Y.S.; An, C.Y.; Kannan, P.K.; Seo, N.; Zhuo, K.; Yoo, T.K.; Chung, C.-H. Fabrication of dendritic silver-coated copper powders by galvanic displacement reaction and their thermal stability against oxidation. Appl. Surf. Sci. 2016, 389, 865–873. [Google Scholar] [CrossRef]
- Wu, L.; Yang, H.; Cheng, J.; Hu, C.; Wu, Z.; Feng, Y. Review in preparation and application of nickel-coated graphite composite powder. J. Alloys Compd. 2021, 862, 158014. [Google Scholar] [CrossRef]
- Yim, Y.J.; Rhee, K.Y.; Park, S.J. Electromagnetic interference shielding effectiveness of nickel-plated MWCNTs/high-density polyethylene composites. Compos. Part B-Eng. 2016, 98, 120–125. [Google Scholar] [CrossRef]
- Jung, D.S.; Lee, H.M.; Kang, Y.C.; Park, S.B. Air-stable silver-coated copper particles of sub-micrometer size. J. Colloid Interf. Sci. 2011, 364, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, W.; Gao, C.; Tian, W.; Sun, B.; Yu, D. A novel preparation of silver-plated polyacrylonitrile fibers functionalized with antibacterial and electromagnetic shielding properties. Appl. Surf. Sci. 2015, 342, 120–126. [Google Scholar] [CrossRef]
- Liu, A.; Ren, X.; Zhang, J.; Yuan, G.; Yang, P.; Zhang, J.; An, M. A composite additive used for an excellent new cyanide-free silver plating bath. New J. Chem. 2015, 39, 2409–2412. [Google Scholar] [CrossRef]
- Peng, Y.H.; Yang, C.H.; Chen, K.T.; Popuri, S.R.; Lee, C.H.; Tang, B.S. Study on synthesis of ultrafine Cu–Ag core–shell powders with high electrical conductivity. Appl. Surf. Sci. 2012, 263, 38–44. [Google Scholar] [CrossRef]
- Varol, T.; Güler, O.; Akçay, S.B.; Aksa, H.C. The effect of silver coated copper particle content on the properties of novel Cu-Ag alloys prepared by hot pressing method. Powder Technol. 2021, 384, 236–246. [Google Scholar] [CrossRef]
- Xu, X.; Luo, X.; Zhuang, H.; Li, W.; Zhang, B. Electroless silver coating on fine copper powder and its effects on oxidation resistance. Mater. Lett. 2003, 57, 3987–3991. [Google Scholar] [CrossRef]
- Liu, A.; Ren, X.; An, M. A composite additive used for a new cyanide-free silver plating bath (II): An insight by electrochemical measurements and quantum chemical calculation. New J. Chem. 2017, 41, 11104–11112. [Google Scholar] [CrossRef]
- Cao, X.G.; Zhang, H.Y. Fabrication and performance of silver coated copper powder. Electron. Mater. Lett. 2012, 8, 467–470. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, B.; Wang, K.; Song, S.; Chen, G. Studies on Preparation and properties of silver coated nickel powder. Mate. Sci. 2017, 7, 507–514. [Google Scholar]
- Joseph, N.; Singh, S.K.; Sirugudu, R.K.; Murthy, V.R.K.; Ananthakumar, S.; Sebastian, M.T. Effect of silver incorporation into PVDF-barium titanate composites for EMI shielding applications. Mater. Res. Bull. 2013, 48, 1681–1687. [Google Scholar] [CrossRef]
- Bi, S.; Zhang, L.; Mu, C.; Liu, M.; Hu, X. Electromagnetic interference shielding properties and mechanisms of chemically reduced graphene aerogels. Appl. Surf. Sci. 2017, 412, 529–536. [Google Scholar] [CrossRef]
- Jia, Y.; Li, K.; Xue, L.; Ren, J.; Zhang, S.; Li, H. Mechanical and electromagnetic shielding performance of carbon fiber reinforced multilayered (PyC-SiC) n matrix composites. Carbon 2017, 111, 299–308. [Google Scholar] [CrossRef]
- Hu, S.; Li, H.; Chen, X.; Zhang, C.; Liu, Z. The electrical conductive effect of nickel-coated graphite/two-component silicone-rubber sealant. J. Wuhan Univ. Technol. 2013, 28, 429–436. [Google Scholar] [CrossRef]
- Aradhana, R.; Mohanty, S.; Nayak, S.K. A review on epoxy-based electrically conductive adhesives. Int. J. Adhes. Adhes. 2020, 99, 102596. [Google Scholar] [CrossRef]
- Luo, X.; Wen, G. NiO@C and Ni@C nanoparticles: Synthesis, characterization and magnetic properties. Nano 2020, 15, 2050072. [Google Scholar] [CrossRef]
- Hai, H.T.; Ahn, J.G.; Kim, D.J.; Lee, J.R.; Chung, H.S.; Kim, C.O. Developing process for coating copper particles with silver by electroless plating method. Surf. Coat. Technol. 2006, 201, 3788–3792. [Google Scholar] [CrossRef]
- Paloukis, F.; Papazisi, K.M.; Dintzer, T.; Papaefthimiou, V.; Saveleva, V.A.; Balomenou, S.P.; Tsiplakides, D.; Bournel, F.; Gallet, J.J.; Zafeiratos, S. Insights into the surface reactivity of Cermet and Perovskite electrodes in oxidizing, reducing, and humid environments. ACS Appl. Mater. Inter. 2017, 9, 25265–25277. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.Y.; Chang, T.F.; Lu, S.Y.; Chang, Y.C. Preparation of monolithic silica aerogel of low thermal conductivity by ambient pressure drying. J. Am. Ceram. Soc. 2007, 90, 2003–2007. [Google Scholar] [CrossRef]
- Morel, B.; Autissier, L.; Autissier, D.; Lemordant, D.; Yrieix, B.; Quenard, D. Pyrogenic silica ageing under humid atmosphere. Powder Technol. 2009, 190, 225–229. [Google Scholar] [CrossRef]
- Moglie, F.; Micheli, D.; Laurenzi, S.; Marchetti, M.; Primiani, V.M. Electromagnetic shielding performance of carbon foams. Carbon 2012, 50, 1972–1980. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Wen, B. An ingenious strategy to construct helical structure with excellent electromagnetic shielding performance. Adv. Mater. Inter. 2019, 6, 1900375. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, T.; Yang, Z. Flexible polyethylene terephthalate/polyaniline composite paper with bending durability and effective electromagnetic shielding performance. Chem. Eng. J. 2020, 389, 124433. [Google Scholar] [CrossRef]
- Karteri, I.; Altun, M.; Gunes, M. Electromagnetic interference shielding performance and electromagnetic properties of wood-plastic nanocomposite with graphene nanoplatelets. J. Mate. Sci. Mater. El. 2017, 28, 6704–6711. [Google Scholar] [CrossRef]
- Chen, Y.H.; Huang, C.Y.; Lai, F.D.; Roan, M.L.; Chen, K.N.; Yeh, J.T. Electroless deposition of the copper sulfide coating on polyacrylonitrile with a chelating agent of triethanolamine and its EMI Shielding Effectiveness. Thin Solid Films 2009, 517, 4984–4988. [Google Scholar] [CrossRef]
- Colaco, J.; Lohani, R.B. Study of radiation shielding materials on microstrip patch antenna for sustainability. Mater. Today-Proc. 2022, 49, 1625–1630. [Google Scholar] [CrossRef]
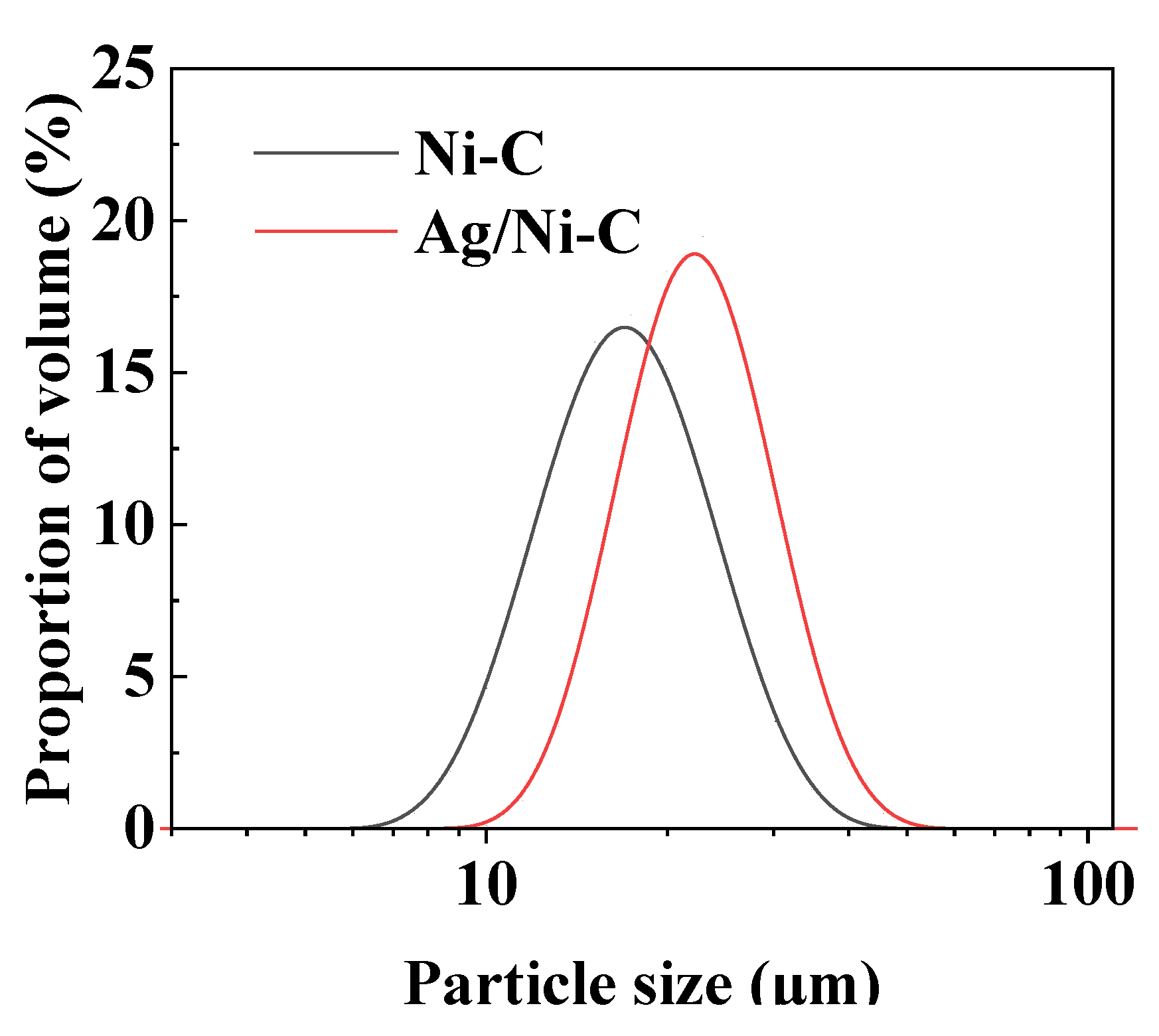
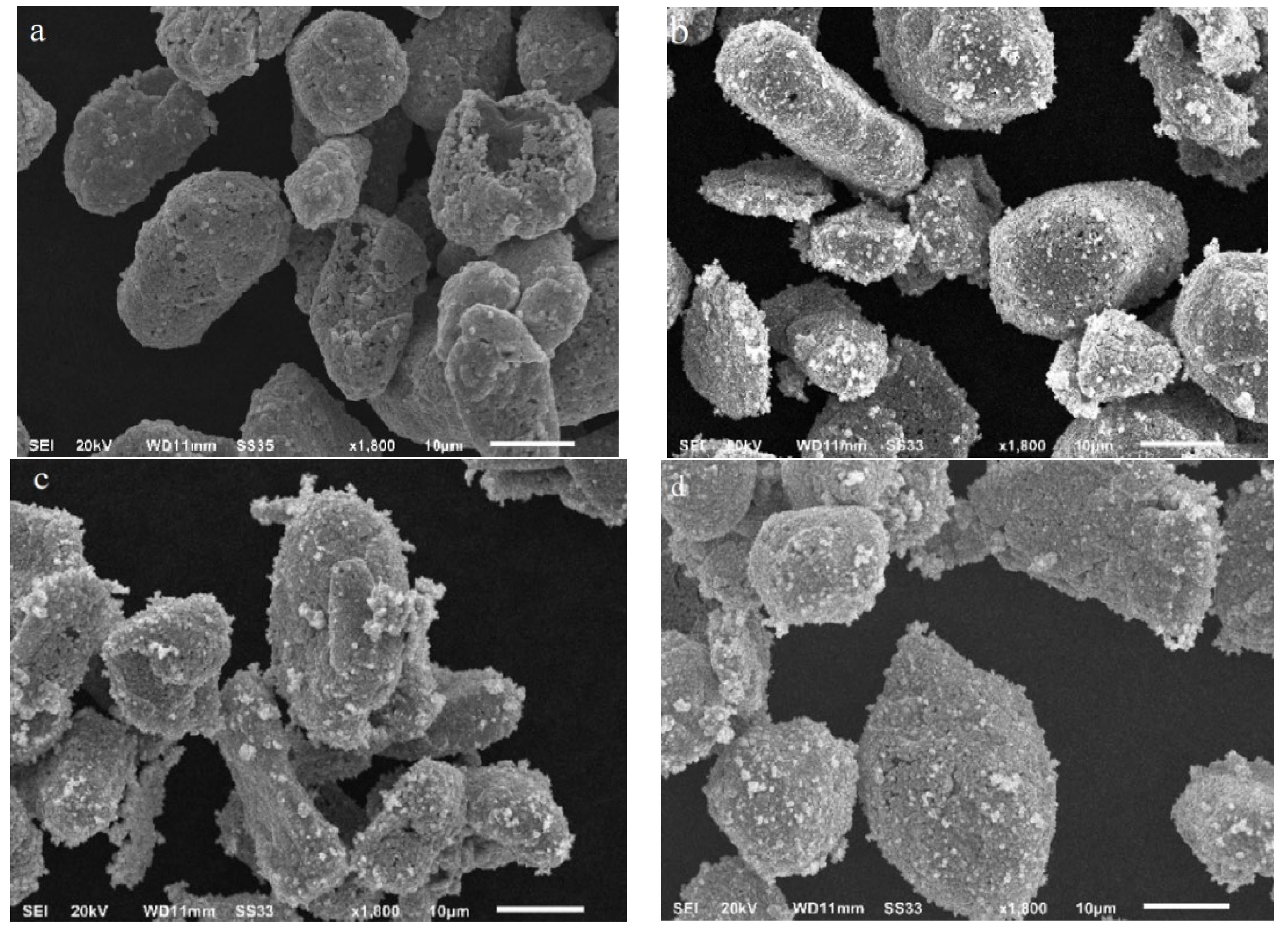
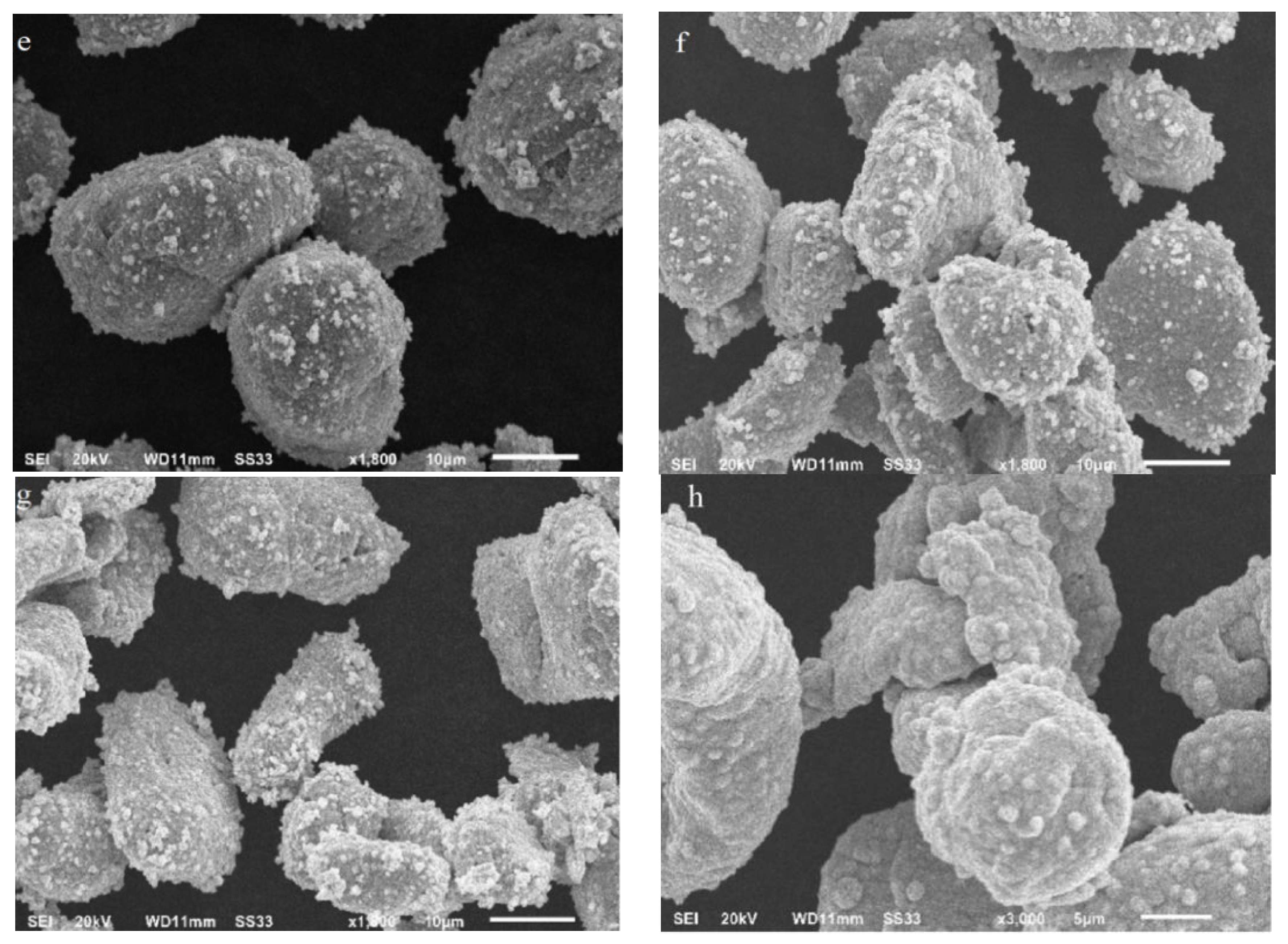
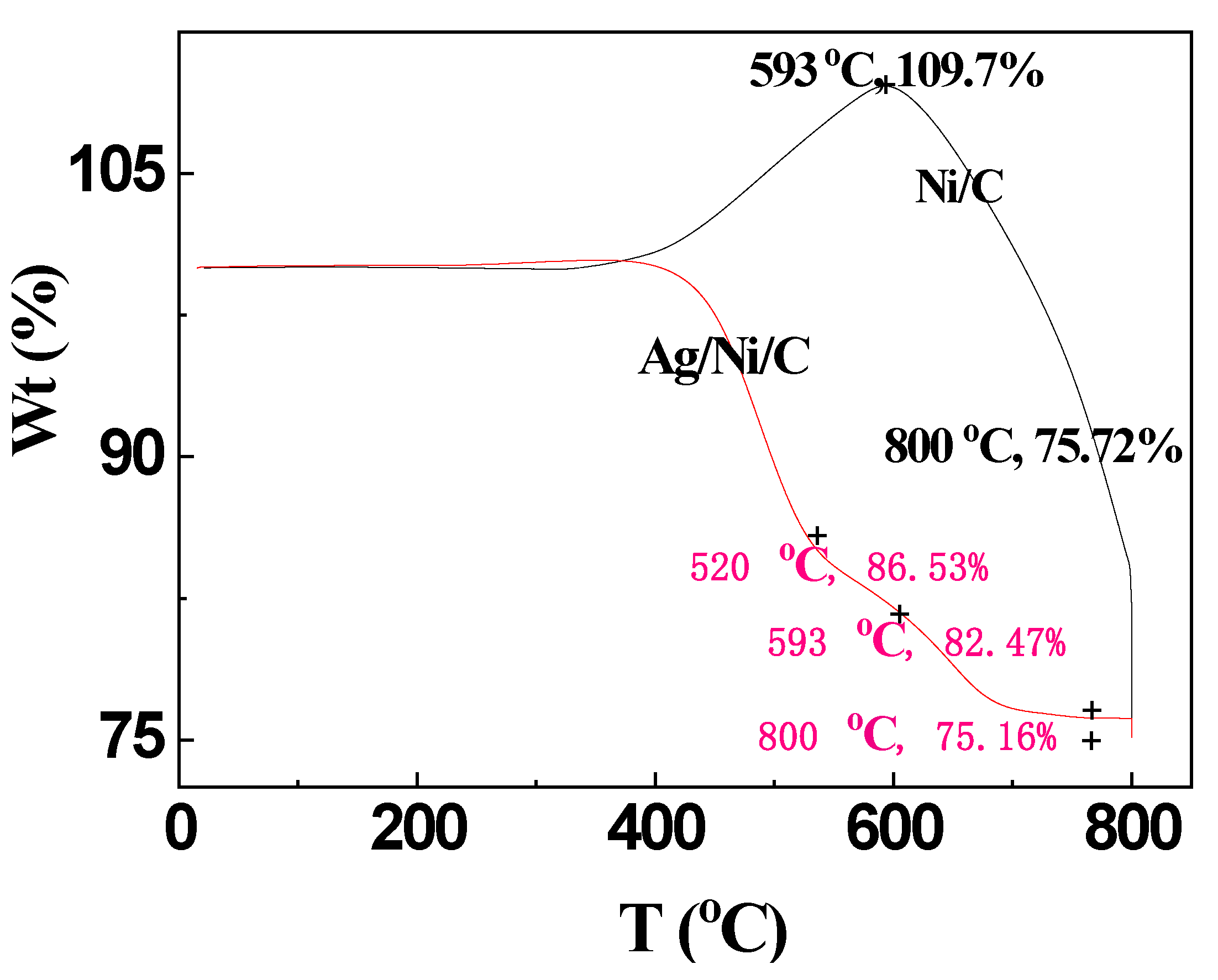

| Sample | d10 (μm) | d50 (μm) | d90 (μm) |
|---|---|---|---|
| Ni/C | 12.15 | 18.19 | 27.23 |
| Ag/Ni/C | 14.66 | 20.75 | 29.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.-K.; Yu, J.-G. Novel Silver-Plated Nickel-Coated Graphite Powder with Excellent Heat and Humidity Resistance: Facile Preparation and Performance Investigation. Molecules 2022, 27, 4007. https://doi.org/10.3390/molecules27134007
Lv X-K, Yu J-G. Novel Silver-Plated Nickel-Coated Graphite Powder with Excellent Heat and Humidity Resistance: Facile Preparation and Performance Investigation. Molecules. 2022; 27(13):4007. https://doi.org/10.3390/molecules27134007
Chicago/Turabian StyleLv, Xin-Kun, and Jin-Gang Yu. 2022. "Novel Silver-Plated Nickel-Coated Graphite Powder with Excellent Heat and Humidity Resistance: Facile Preparation and Performance Investigation" Molecules 27, no. 13: 4007. https://doi.org/10.3390/molecules27134007
APA StyleLv, X.-K., & Yu, J.-G. (2022). Novel Silver-Plated Nickel-Coated Graphite Powder with Excellent Heat and Humidity Resistance: Facile Preparation and Performance Investigation. Molecules, 27(13), 4007. https://doi.org/10.3390/molecules27134007







