Synthesis, Crystal Structures, Lipophilic Properties and Antimicrobial Activity of 5-Pyridylmethylidene-3-rhodanine-carboxyalkyl Acids Derivatives
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Lipophilicity
2.2.1. Lipophilicity Parameters
2.2.2. Lipinski Rule of Five and Veber Rules
- MW (molecular weight) < 500;
- Log p < 5;
- NOHBD (numbers of hydrogen bond donors) < 5;
- NOHBA (numbers of hydrogen bond acceptors) < 10.
- number of rotable bonds (NORB) < 14;
- total polar surface area (TPSA) < 140 Å2.
2.2.3. Bioactivity
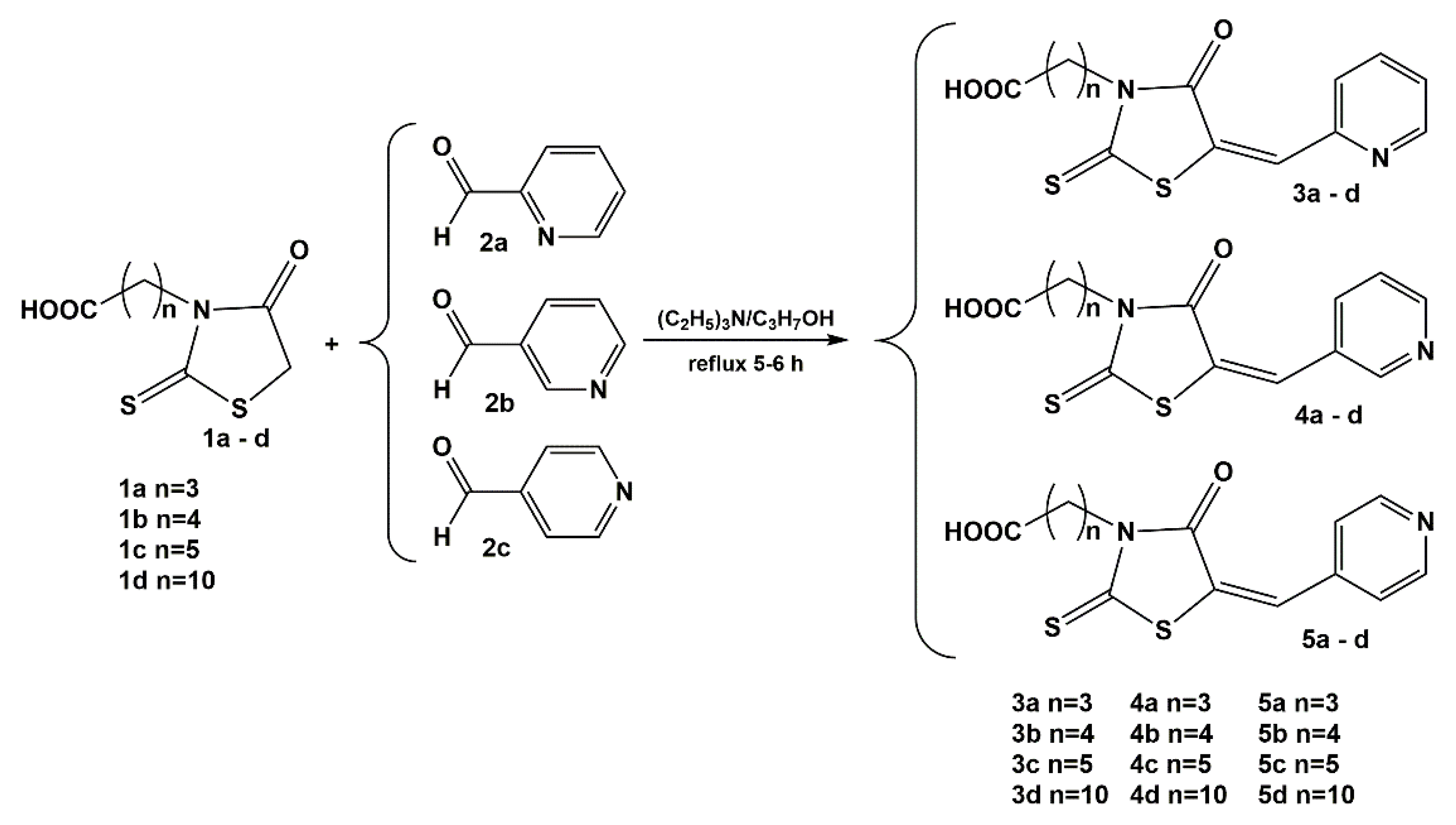
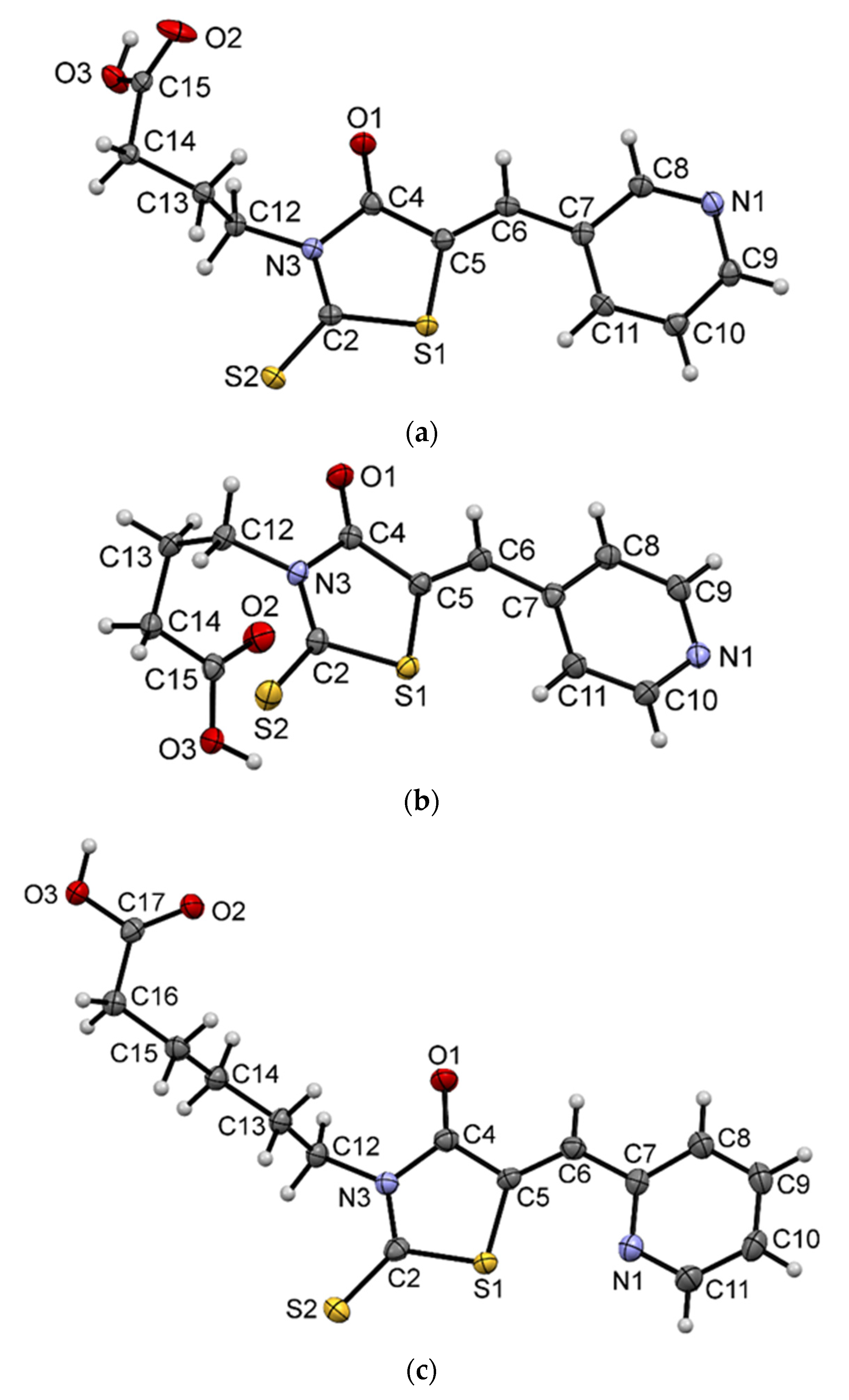
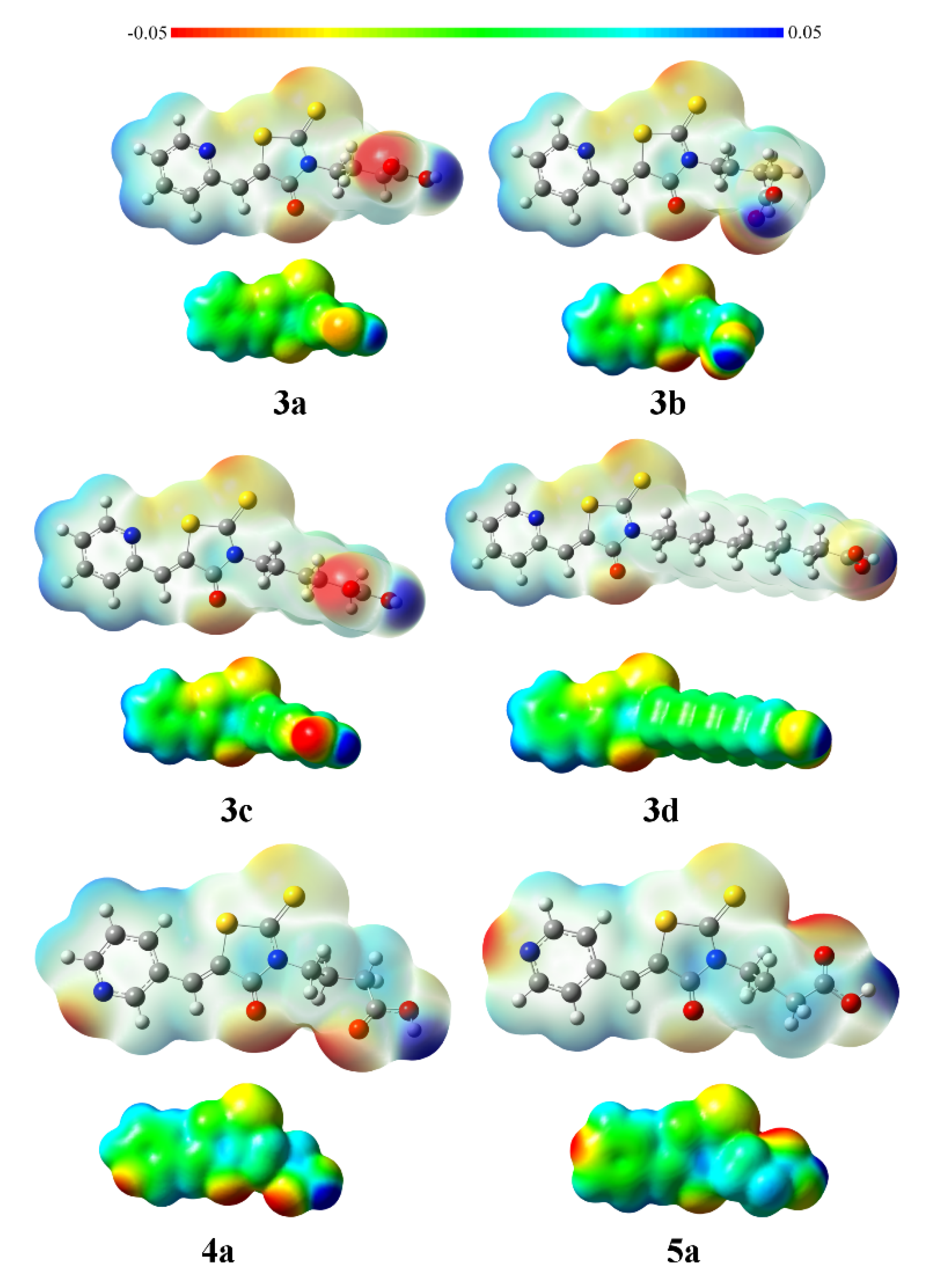
2.3. Crystal and Molecular Structures of / 4a /, / 5a / and / 3c /
2.4. Antimicrobial Activity
3. Experimental Sections
3.1. Materials and Methods
3.2. General Procedure of Knoevenagel Condensation of Rhodanine 3-Alcanoic Acids with Aldehydes
3.3. Antimicrobial Activity In Vitro Assay
3.4. Determination of Lipophilicity Parameters
3.5. X-ray Analysis
3.6. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nencki, M. Ueber die Einwirkung der Monochloressigsäure auf Sulfocyansäure und ihre Salze. J. Prakt. Chem. 1877, 16, 1–17. [Google Scholar] [CrossRef]
- Kaminsky, D.; Kryshchyshyn, A.; Lesyk, R. 5-ene-4-thiazolidinones—An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 40, 542–594. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Babapoor, A.; Amani, A.M. A conceptual review of rhodanine: Current applications of antiviral drugs, anticancer and antimicrobial activities. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Y.; Piao, H.; Zhao, X.; Zhang, W.; Wang, Y.; Liu, M. A Comprehensive Review on the Biological and Pharmacological Activities of Rhodanine Based Compounds for Research and Development of Drugs. Mini Rev. Med. Chem. 2018, 18, 948–961. [Google Scholar] [CrossRef]
- Tarahomi, M.; Baharfar, R.; Mohseni, M. Synthesis and antibacterial activity evaluation of novel rhodamine based amide derivatives. Clin. Microbiol. Infect. Dis. 2019, 4, 1–5. [Google Scholar] [CrossRef]
- Song, M.X.; Zheng, C.J.; Deng, X.Q.; Wei, Z.Y.; Piao, H.R. The synthesis and antibacterial activities of N-carboxymethyl rhodanines. Med. Chem. 2014, 4, 441–448. [Google Scholar] [CrossRef]
- Kapoor, A.; Khare, N. Antibacterial and antifungal evaluation of Mannich bases of 2,4-thiazolidinedione and rhodanine. Der. Pharm. Lett. 2016, 8, 143–148. [Google Scholar]
- Shelke, R.N.; Pansare, D.N.; Dake, S.A.; Pawar, R.P.; Bembalker, S.R. Synthesis of new substituted thiazolidine-4-one analogues with anticancer and antimicrobial activity. Acta Chim. Pharm. Indica 2017, 7, 119–131. [Google Scholar]
- Kaminskyy, D.V. Screening of the antiviral activity in the range of C5 and N3 substituted 4-thiazolidinone derivatives. J. Org. Pharm. Chem. 2015, 13, 64–69. [Google Scholar] [CrossRef][Green Version]
- Tintori, C.; Iovenitti, G.; Ceresola, E.R.; Ferrarese, R.; Zamperini, C.; Brai, A.; Poli, G.; Dreassi, E.; Cagno, V.; Lembo, D.; et al. Rhodanine derivatives as potent anti-HIV and antiHSV microbicides. PLoS ONE 2018, 13, e0198478. [Google Scholar] [CrossRef]
- Dofe, V.S.; Sarkate, A.P.; Azad, R.; Gill, C.H. Green synthesis and inhibitory effect of novel quinoline based thiazolidinones on the growth of MCF-7 human breast cancer cell line by G2/M cell cycle arrest. Res. Chem. Intermed. 2018, 44, 1149–1160. [Google Scholar] [CrossRef]
- Pansare, D.N.; Shelke, R.N.; Khade, M.C.; Jadhav, V.N.; Pawar, C.D.; Jadhav, R.A.; Bembalkar, S.R. New thiazolone derivatives: Design, synthesis, anticancer and antimicrobial activity. Eur. Chem. Bull. 2019, 8, 7–14. [Google Scholar] [CrossRef]
- Liang, H.X.; Yu, Y.H.; Li, X.H.; Tang, N.F.; Hu, G.Q.; Liu, B. Apoptosis of human hepatocellular carcinoma cells SMMC-7721 induced by C-3 methylidene thiazolidinedione acetic acid. Int. J. Clin. Exp. Med. 2019, 12, 371–377. [Google Scholar]
- Nguyen, C.T.; Nguyen, Q.T.; Dao, P.H.; Nguyen, T.L.; Nguyen, P.T.; Nguyen, H.H. Synthesis and cytotoxic activity against K562 and MCF7 cell lines of some N-(5-arylidene-4-oxo-2-thioxothiazolidin-3-yl)-2-((4-oxo-3-phenyl-3,4-dihydroquinazoline-2-yl)thio) acetamide compounds. Hindawi J. Chem. 2019, 2019, 8. [Google Scholar] [CrossRef]
- Sarkate, A.P.; Lokwani, D.K.; Karnik, K.S.; Shinde, D.B. Novel 2-(nitrooxy)ethyl 2-(4-(substituted phenyl)-2-((substituted phenyl)amino)thiazol-5-yl)acetate as Anti-inflammatory, Analgesic and Nitric Oxide Releasing Agents: Synthesis and Molecular Docking Studies. Antiinflamm. Antiallergy Agents Med. Chem. 2017, 16, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Tejchman, W.; Kołodziej, P.; Kalinowska-Tłuścik, J.; Nitek, W.; Żuchowski, G.; Bogucka-Kocka, A.; Żesławska, E. Discovery of Cinnamylidene Derivative of Rhodanine with High Anthelmintic Activity against Rhabditis sp. Molecules 2022, 27, 2155. [Google Scholar] [CrossRef]
- Sivanadanam, J.; Mukkamala, R.; Mandal, S.; Vedarajan, R.; Matsumi, N.; Aidhen, I.S.; Ramanujam, K. Exploring the role of the spacers and acceptors on the triphenylamine-based dyes for dye-sensitized solar cells. Int. J. Hydrog. Energy 2018, 43, 4691–4705. [Google Scholar] [CrossRef]
- El-sayed, S.; Metwally, K.; El-Shanawani, A.A.; Abdel-Aziz, L.M.; El-Rashedy, A.A.; Soliman, M.E.S.; Quattrini, L.; Coviello, V.; Motta, C. Quinazolinone-based rhodanine-3-acetic acids as potent aldose reductase inhibitors: Synthesis, functional evaluation and molecular modeling study. Bioorg. Med. Chem. Lett. 2017, 27, 4760–4764. [Google Scholar] [CrossRef]
- Bacha, M.M.; Nadeem, H.; Zaib, S.; Sarwar, S.; Imran, A.; Ur Rahman, S.; Saqib Ali, H.; Arif, M.; Iqbal, J. Rhodanine-3-acetamide derivatives as aldose and aldehyde reductase inhibitors to treat diabetic complications: Synthesis, biological evaluation, molecular docking and simulation studies. BMC Chem. 2021, 15, 28. [Google Scholar] [CrossRef]
- Radwan, A.; Elhenawy, A.A.; Kadh, M.S.; Hashem, F.A.; Abd-Elghany, R.D. Rhodanine-3-acetic acid derivatives as a new class of potent α-amylase inhibitors. J. Chem. Biol. Phys. Sci. 2020, 10, 245–249. [Google Scholar] [CrossRef]
- Abusetta, A.; Alumairi, J.; Alkaabi, M.Y.; Al Ajeil, R.; Abu Shkaidim, A.; Akram, D.; Pajak, J.; Ghattas, M.A.; Atatreh, N.; Al Neyadi, S.S. Design, synthesis, in vitro antibacterial activity, and docking studies of new rhodanine derivatives. Open J. Med. Chem. 2020, 10, 15–34. [Google Scholar] [CrossRef]
- Chauhan, D.; Ginson, G.; Sridhar, S.N.C.; Bhatia, R.; Paul, A.T.; Monga, V. Design, synthesis, biological evaluation, and molecular modeling studies of rhodanine derivatives as pancreatic lipase inhibitors. Arch. Pharm. 2019, 352, 1900029. [Google Scholar] [CrossRef] [PubMed]
- Körner, H. Über einige Derivate der Dithiocarbamino-essigsäure. Ber. Dtsch. Chem. Ges. 1908, 41, 1901–1905. [Google Scholar] [CrossRef]
- Reddy, T.N.; Ravinder, M.; Bagul, P.; Ravikanti, K.; Bagul, C.; Nanubolu, J.B.; Srinivas, K.; Banerjee, S.K.; Rao, V.J. Synthesis and biological evaluation of new epalrestat analogues as aldose reductase inhibitors (ARIs). Eur. J. Med. Chem. 2014, 71, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.P.; Agrawal, M.Y.; Gupta, A.K. Design, synthesis and evaluation of rhodanine derivatives as aldose reductase inhibitors. Chem. Biol. Drug Des. 2015, 85, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Gilhotra, R.; Gupta, A.K. Design, synthesis and evaluation of substituted 5-(2-methoxybenzylidene)-rhodanine ester analogs as aldose reductase inhibitors. Biol. Forum 2019, 11, 217–221. [Google Scholar]
- Tejchman, W.; Korona-Glowniak, I.; Malm, A.; Zylewski, M.; Suder, P. Antibacterial properties of 5-substituted derivatives of rhodanine-3-carboxyalkyl acids. Med. Chem. Res. 2017, 26, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Tejchman, W.; Korona-Glowniak, I.; Kwietniewski, L.; Żesławska, E.; Nitek, W.; Suder, P.; Żylewski, M.; Malm, A. Antibacterial properties of 5-substituted derivatives of rhodanine-3-carboxyalkyl acids. Part II. Saudi Pharm. J. 2020, 28, 414–426. [Google Scholar] [CrossRef]
- Tejchman, W.; Skórska—Stania, A.; Żesławska, E. The crystal structures of three rhodanine-3-carboxylic acids. J. Chem. Crystallogr. 2016, 46, 181–187. [Google Scholar] [CrossRef]
- Żesławska, E.; Nitek, W.; Tejchman, W. The synthesis and crystal structures of the homologues of epalrestat. J. Chem. Crystallogr. 2015, 45, 151–157. [Google Scholar] [CrossRef]
- Bate-Smith, E.C.; Westall, R.G. Chromatographic behaviour and chemical structure I. Some naturally occurring phenolic substances. Biochim. Biophys. Acta 1950, 4, 427–440. [Google Scholar] [CrossRef]
- Soczewiński, E.; Wachtmeister, C.A. The relation between the composition of certain ternary two-phase solvent systems and RM values. J. Chromatogr. A 1962, 7, 311–325. [Google Scholar] [CrossRef]
- Gocan, S. Eluotropic series of solvent for TLC. In Encyclopedia of Chromatography, 3rd ed.; Taylor & Francis: New York, NY, USA, 2010; p. 730. [Google Scholar]
- Snyder, L.R. Classification off the solvent properties of common liquids. J. Chromatogr. Sci. 1978, 16, 223–234. [Google Scholar] [CrossRef]
- Karger, B.L.; Snyder, L.R.; Econ, C. An expanded solubility parameter treatment for classification and use of chromatographic solvents and adsorbents: Parameters for dispersion, dipole and hydrogen bonding interactions. J. Chromatogr. A 1976, 125, 71–88. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. Available online: http://www.molinspiration.com (accessed on 7 December 2020).
- Singh, S.; Gupta, A.K.; Verma, A. Molecular Properties and Bioactivity Score of the Aloe Vera Antioxidant Compounds—In Order to Lead Finding. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 876–881. Available online: http://rjpbcs.com/pdf/2013_4(2)/[95].pdf (accessed on 20 November 2020).
- Verma, A. Lead finding from Phyllanthus debelis with hepatoprotective potentials. Asian Pac. J. Trop. Biomed. 2012, 3, 1735–1737. [Google Scholar] [CrossRef]
- Stawoska, I.; Tejchman, W.; Mazuryk, O.; Lyčka, A.; Nowak-Sliwinska, P.; Żesławska, E.; Nitek, W.; Kania, A. Spectral Characteristic and Preliminary Anticancer Activity in vitro of Selected Rhodanine-3-carboxylic Acids Derivatives. J. Heterocycl. Chem. 2017, 54, 2889–2897. [Google Scholar] [CrossRef]
- Beno, B.R.; Yeung, K.S.; Bartberger, M.D.; Pennington, L.D.; Meanwell, N.A. A survey of the role of noncovalent sulfur interactions in drug design. J. Med. Chem. 2015, 58, 4383–4438. [Google Scholar] [CrossRef]
- Popelier, P. Atoms in Molecules. An Introduction; Pearson Education Limited: Harlow, UK, 2000. [Google Scholar]
- Esswein, A.; Schaefer, W.; Tsaklakidis, C.; Honold, K.; Kaluza, K. Rhodanine Carboxylic Acid Derivatives for the Treatment and Prevention of Metabolic Bone Disorders. Patent WO 00/18747, 6 April 2000. [Google Scholar]
- Dolezel, J.; Hirsova, P.; Opletalova, V.; Dohnal, J.; Vejsova, M.; Kunes, J.; Jampilek, J. Rhodeanineacetic acid derivatives as potential drugs: Preparation, hydrophobic properties and antifungal activity of (5-arylalkylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)acetic acids. Molecules 2009, 14, 4197–4212. [Google Scholar] [CrossRef] [PubMed]
- Zvarec, O.; Polyak, S.W.; Tieu, W.; Kuan, K.; Dai, H.; Pedersen, D.S.; Morona, R.; Zhang, L.; Booker, G.W.; Abell, A.D. 5-benzylidenerhodanine and 5-benzylidene-2,4-thiazolidinedione based antibacterials. Bioorg. Med. Chem. Lett. 2012, 22, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Kratky, M.; Vinsova, J.; Stolarikova, J. Antimicrobial activity of rhodanine-3-acetic acid derivatives. Bioorg. Med. Chem. 2017, 25, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Hawrył, H.M.; Świeboda, R.S.; Gawroński, M.S.; Wójciak-Kosior, M.A.; Popiołek, Ł.P.; Kocjan, R.B. Determination of lipophilicity of some new 1,2,3-triazole derivatives by RP-HPLC and RP-TLC and calculated methods. Curr. Chem. Lett. 2015, 4, 101–110. [Google Scholar] [CrossRef]
- Sortino, M.; Delgado, P.; Juarez, S.; Quiroga, J.; Abonia, R.; Insuasty, B.; Nogueras, M.; Rodero, L.; Garibotto, F.M.; Enriz, R.D.; et al. Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorg. Med. Chem. 2007, 15, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, M.M.; Mingos, D.M.P.; White, A.J.P.; Williams, D.J. Syntheses and characterization of 5-substituted hydantoins and thiazolines-implications for crystal engineering of hydrogen bonded assemblies. Crystal structures of 5-(2-pyridylmethylene)-hydantoin, 5-(2-pyridylmethylene)-2-thiohydantoin, 5-(2-pyridyl-methylene)thiazolidine-2,4-dione, 5-(2-pyridylmethylene)rhodanine and 5-(2-pyridylmethylene)pseudothiohydantoin. J. Chem. Soc. Perkin Trans. 2000, 1, 3495–3504. [Google Scholar] [CrossRef]
- Chowdhry, M.M.; Burrows, A.D.; Mingos, D.M.P.; White, A.J.P.; Williams, D.J. Synthesis and crystal structure of 5-(2-pyridylmethylene)hydantoin(Hpyhy) and complexes of pyhy with nickel(II) and copper(II). J. Chem. Soc. Chem. Commun. 1995, 15, 1521–1522. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Ravoof, T.B.S.A.; Tiekink, E.R.T.; Veerakumarasivam, A.; Crouse, K.A.; Tahir, M.I.M.; Ahmad, H. Synthesis, Characterization and Biological Evaluation of Transition Metal Complexes Derived from N, S Bidentate Ligands. Int. J. Mol. Sci. 2015, 26, 11034–11054. [Google Scholar] [CrossRef]
- Islam, M.H.; Sheikh, M.C.; Islam, M.A.A.A.A. Studies on coordination chemistry and antibacterial activity of bidentate NS Schiff base derived from SBDTC and 4-benzyloxybenzaldehyde. J. Sci. Res. 2019, 11, 121–132. [Google Scholar] [CrossRef]
- Virtual Computational Chemistry Laboratory. Available online: http://www.vcclab.org/lab/alogps/ (accessed on 7 December 2020).
- Organic Chemistry Portal. Available online: https://www.organic-chemistry.org/prog/peo/ (accessed on 7 December 2020).
- XLOGP. Available online: https://www.ics.uci.edu/~dock/manuals/xlogp2.1/ (accessed on 7 December 2020).
- XLOGP3. Available online: http://www.sioc-ccbg.ac.cn/skins/ccbgwebsite/software/xlogp3/ (accessed on 7 December 2020).
- Acd/Labs. Available online: https://www.acdlabs.com/ (accessed on 7 December 2020).
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELX. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2000. [Google Scholar]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre Binkley, J.S.; DeFrees, D.J.; Pople, J.A.; Gordon, M.S. Self-Consistent Molecular Orbital Methods. XXIII. A polarization-type basis set for 2nd-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis-sets for anion calculations. 3. The 3-21+G basis set for 1st-row elements, Li-F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, UK, 2013. [Google Scholar]
- Bader, R.W.F. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Murray, J.S. Molecular Electrostatic Potentials: Concepts and Applications; Elsevier Science, B.V.: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Keith, T.A. AIMAll; Version 11.10.16; TK Gristmill Software: Overland Park, KA, USA, 2011. [Google Scholar]
- Wiberg, K.B. Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Mukaka, M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]


| Compound | D-H···A | H···A [Å] | D···A [Å] | D-H···A [°] | Symmetry Codes |
|---|---|---|---|---|---|
| /4a/ | O3-H3···N1 | 1.84 | 2.622 | 163.4 | −x − 2, −y + 1, −z + 2 |
| C6-H6···O1 | 2.62 | 3.533 | 161.5 | −x − 2, −y + 1, −z + 2 | |
| C11-H11···S2 | 2.90 | 3.572 | 128.7 | −x, −y, −z + 2 | |
| C14-H14A···O2 | 2.33 | 3.269 | 159.0 | x + 1, y, z | |
| C14-H14B···O2 | 2.65 | 3.452 | 137.9 | −x − 1, −y + 1, −z + 1 | |
| /5a/ | O3-H3···N1 | 1.76 | 2.649 | 169.9 | −x + 1, −y + 1, −z + 1 |
| C6-H6···O2 | 2.52 | 3.380 | 153.6 | −x + 1, y − 1/2, −z + 3/2 | |
| C9-H9···S2 | 2.96 | 3.761 | 144.9 | x + 1, y, z | |
| C10-H10···O1 | 2.47 | 3.285 | 147.1 | x, −y + 1/2, z − 1/2 | |
| C12-H12B···O3 | 2.63 | 3.462 | 144.3 | −x, y − 1/2, −z + 3/2 | |
| C14-H14B···S2 | 2.91 | 3.843 | 162.2 | x, −y + 1/2, z + 1/2 | |
| /3c/ | O3-H3···O2 | 1.77 | 2.681 | 172.7 | −x + 2, −y − 1, −z |
| C6-H6···O1 | 2.30 | 3.210 | 160.7 | −x + 2, −y, −z + 1 | |
| C11-H11···S1 | 2.78 | 3.592 | 143.4 | −x + 1, −y + 1, −z + 1 | |
| C15-H15A···S2 | 2.80 | 3.63 | 141.3 | −x, −y, −z |
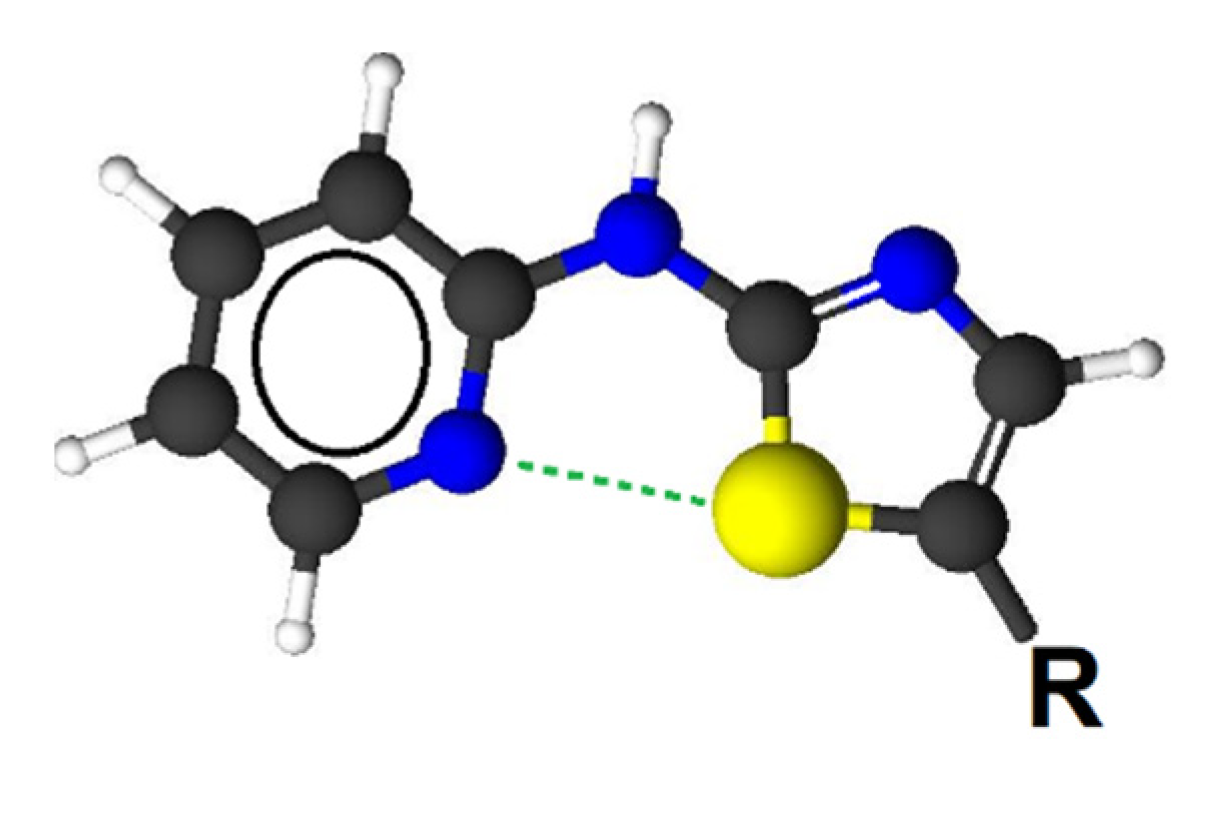
| Compound | N-S Distance [Å] | Electron Density at BCP [a.u.] | Charge N | Charge S | Force Constant |
|---|---|---|---|---|---|
| 3a | 2.8551 | 0.0180 | −1.1503 | 0.3120 | 0.1517 |
| 3b | 2.8574 | 0.0179 | −1.1504 | 0.3084 | 0.1512 |
| 3c | 2.8563 | 0.0179 | −1.1503 | 0.3095 | 0.1515 |
| 3d | 2.8582 | 0.0179 | −1.1502 | 0.3076 | 0.1511 |
| Microorganism | 3a | 3b | 3c | 3d | 4a | 4b | 4c | 4d | 5a | 5b | 5c | 5d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | 62.5 | 62.5 | 62.5 | 62.5 | 250 | 250 | 250 | 500 | 500 | 250 | 125 | 250 |
| S. aureus ATCC 6538 | 125 | 62.5 | 62.5 | 62.5 | 250 | 250 | 250 | 500 | 500 | 250 | 125 | 250 |
| S. aureus ATCC 43300 | 62.5 | 125 | 62.5 | 62.5 | 250 | 250 | 250 | 250 | 500 | 250 | 125 | 250 |
| S. epidermidis ATCC 12228 | 125 | 62.5 | 62.5 | 31.3 | 250 | 125 | 125 | 250 | 125 | 125 | 125 | 125 |
| M. luteus ATCC 10240 | 125 | 62.5 | 125 | 7.8 | 250 | 15.6 | 31.3 | 31.3 | 125 | 7.8 | 31.3 | 7.8 |
| B. subtilis ATCC 6633 | 62.5 | 62.5 | 31.3 | 125 | 250 | 250 | 125 | 500 | 500 | >1000 | 125 | 500 |
| B. cereus ATCC 10876 | 125 | 62.5 | 31.3 | 62.5 | 250 | 125 | 125 | 62.5 | 250 | >1000 | 62.5 | 125 |
| S. pyogenes ATCC 19615 | 500 | >1000 | >1000 | 1000 | >1000 | 500 | >1000 | >1000 | 500 | 500 | 250 | 1000 |
| S. pneumoniae ATCC 49619 | 1000 | >1000 | >1000 | >1000 | >1000 | 500 | >1000 | >1000 | 1000 | >1000 | 1000 | >1000 |
| S. mutans ATCC 25175 | 500 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 1000 | >1000 | >1000 | >1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żesławska, E.; Zakrzewski, R.; Nowicki, A.; Korona-Głowniak, I.; Lyčka, A.; Kania, A.; Zborowski, K.K.; Suder, P.; Skórska-Stania, A.; Tejchman, W. Synthesis, Crystal Structures, Lipophilic Properties and Antimicrobial Activity of 5-Pyridylmethylidene-3-rhodanine-carboxyalkyl Acids Derivatives. Molecules 2022, 27, 3975. https://doi.org/10.3390/molecules27133975
Żesławska E, Zakrzewski R, Nowicki A, Korona-Głowniak I, Lyčka A, Kania A, Zborowski KK, Suder P, Skórska-Stania A, Tejchman W. Synthesis, Crystal Structures, Lipophilic Properties and Antimicrobial Activity of 5-Pyridylmethylidene-3-rhodanine-carboxyalkyl Acids Derivatives. Molecules. 2022; 27(13):3975. https://doi.org/10.3390/molecules27133975
Chicago/Turabian StyleŻesławska, Ewa, Robert Zakrzewski, Arkadiusz Nowicki, Izabela Korona-Głowniak, Antonín Lyčka, Agnieszka Kania, Krzysztof Kazimierz Zborowski, Piotr Suder, Agnieszka Skórska-Stania, and Waldemar Tejchman. 2022. "Synthesis, Crystal Structures, Lipophilic Properties and Antimicrobial Activity of 5-Pyridylmethylidene-3-rhodanine-carboxyalkyl Acids Derivatives" Molecules 27, no. 13: 3975. https://doi.org/10.3390/molecules27133975
APA StyleŻesławska, E., Zakrzewski, R., Nowicki, A., Korona-Głowniak, I., Lyčka, A., Kania, A., Zborowski, K. K., Suder, P., Skórska-Stania, A., & Tejchman, W. (2022). Synthesis, Crystal Structures, Lipophilic Properties and Antimicrobial Activity of 5-Pyridylmethylidene-3-rhodanine-carboxyalkyl Acids Derivatives. Molecules, 27(13), 3975. https://doi.org/10.3390/molecules27133975







