Quantification of Salicylates and Flavonoids in Poplar Bark and Leaves Based on IR, NIR, and Raman Spectra
Abstract
:1. Introduction
2. Results and Discussion
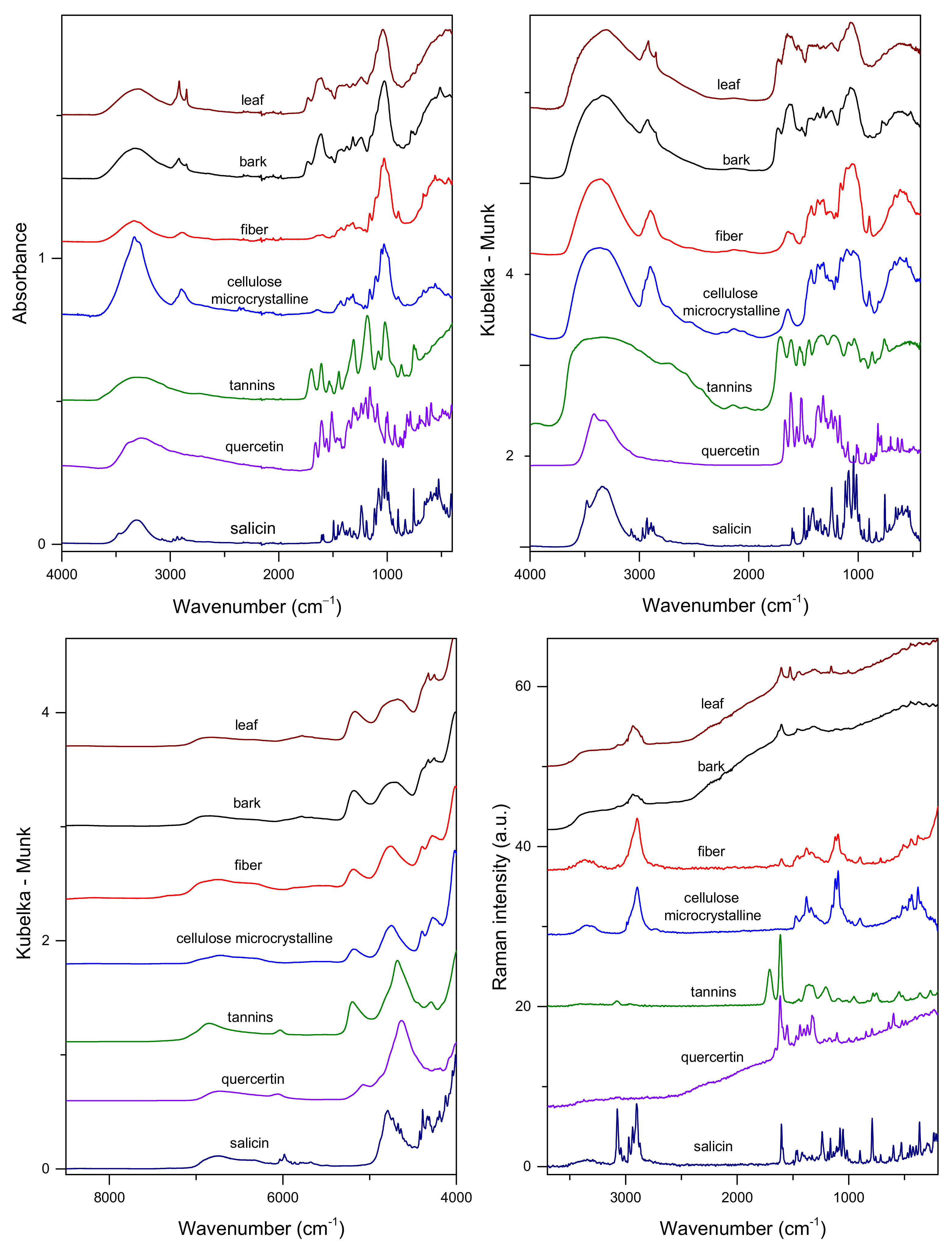
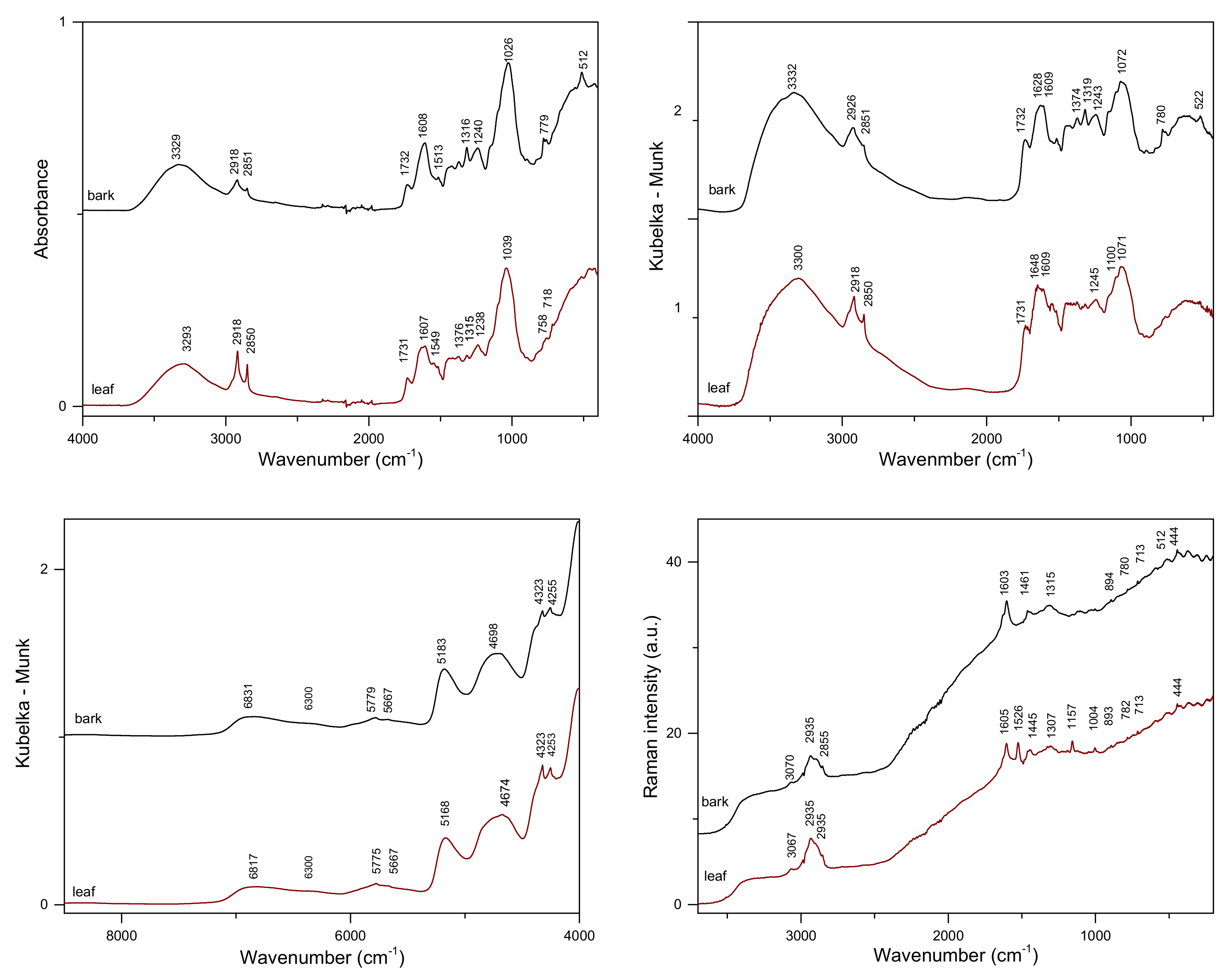
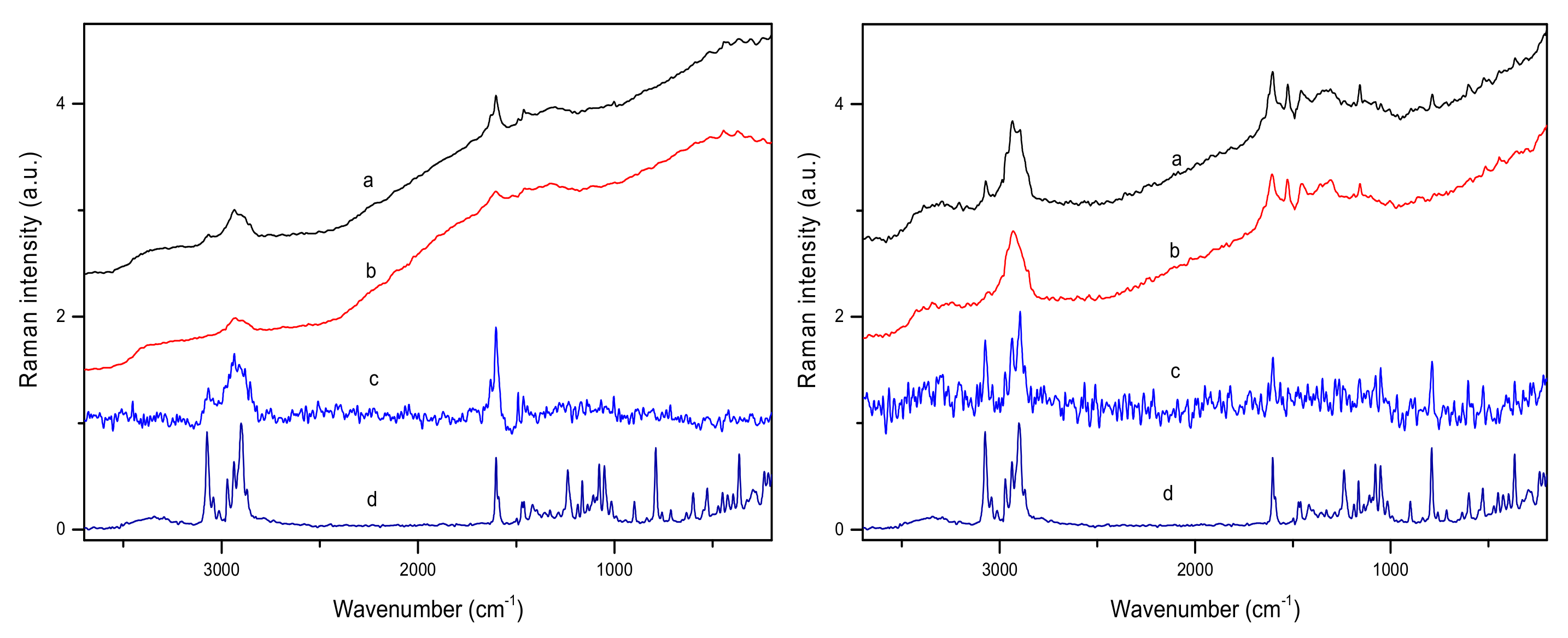
2.1. Vibrational Spectra of Poplar Bark and Leaves
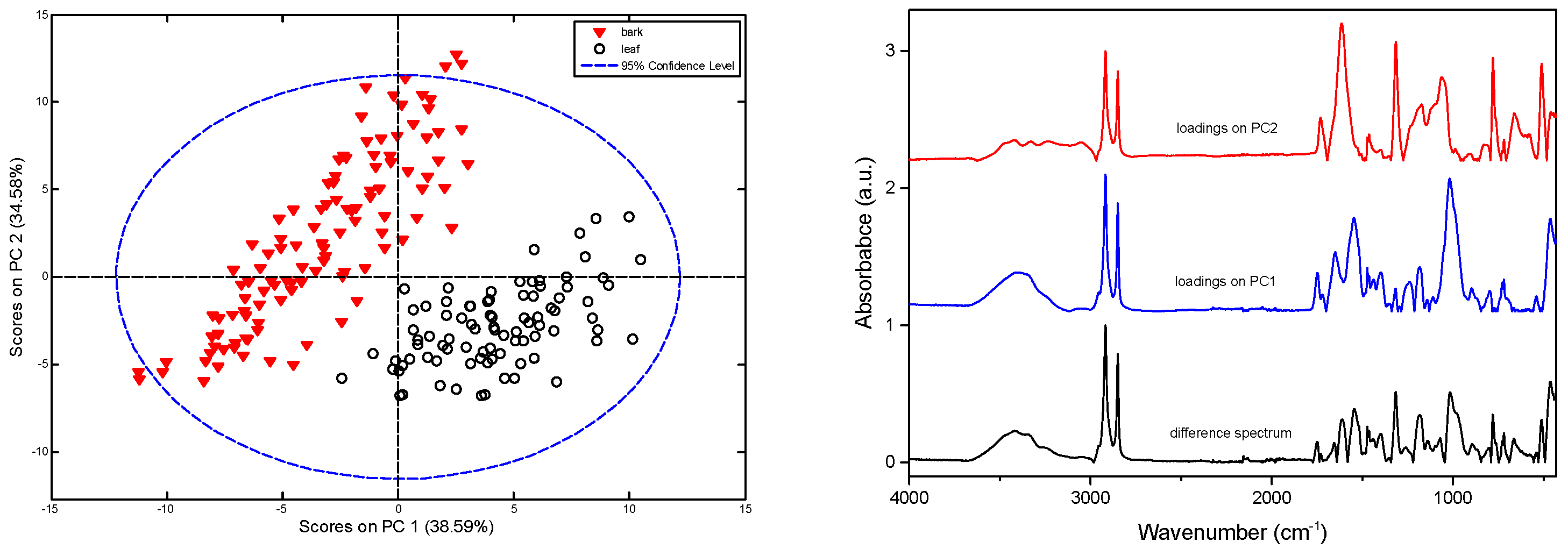
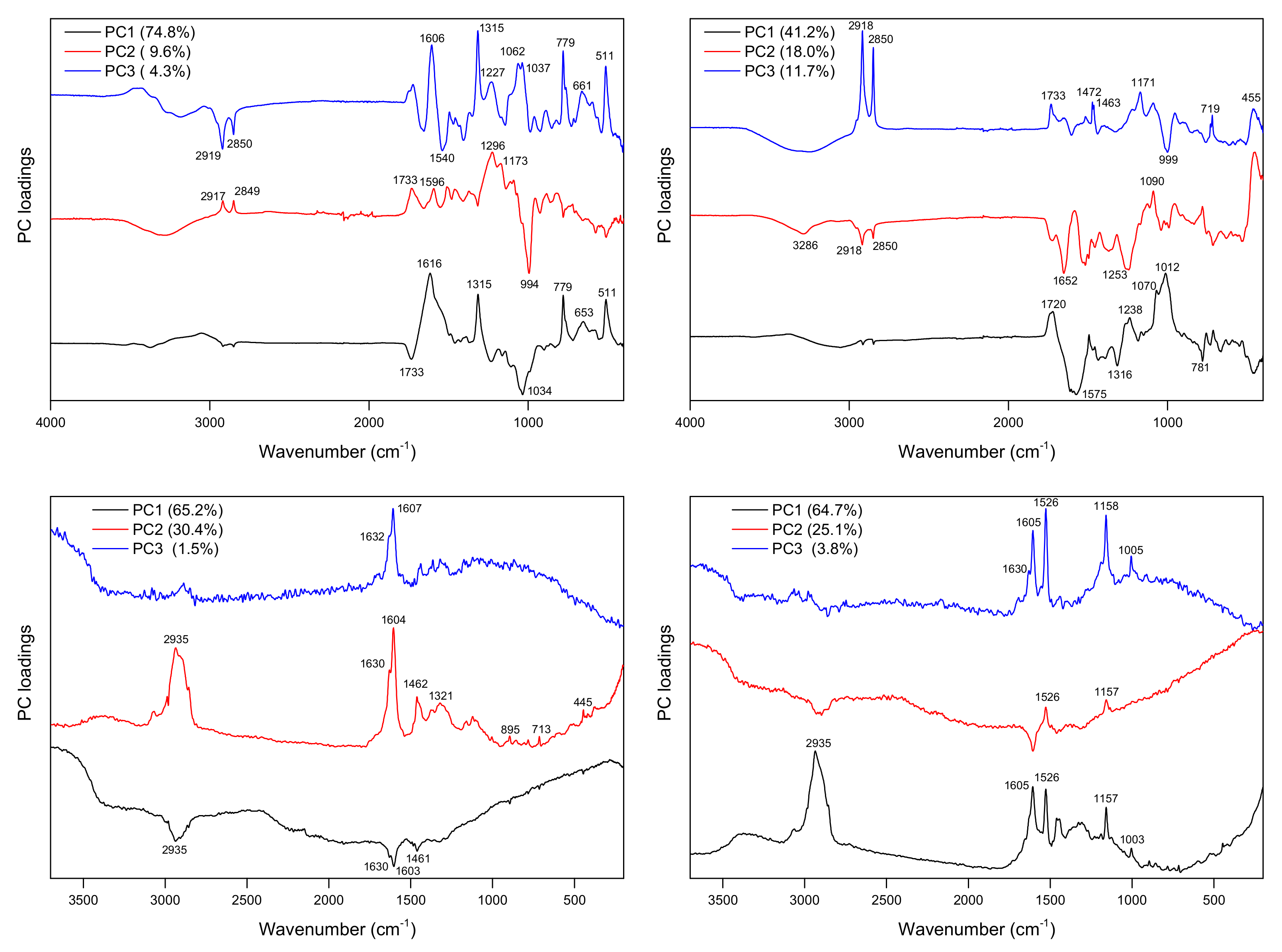
2.2. Principal Component Analysis (PCA)
2.3. PLS Quantification of Active Compounds in Poplar Material
2.3.1. Determination of Total Salicylates (TSA)
2.3.2. Determination of Total Flavonoids (TFL)
2.4. Comparison of Vibrational Techniques
- The Raman, ATR, and NIR spectra of plant materials can be recorded without any additional sample treatment, but collecting DRIFTS spectra in the MIR range requires diluting the sample in KBr.
- A sample mass of a few milligrams is required to register the spectra. The same sample can be used applying various methods. On the other hand, repeated measurements for different portions of the same sample are required for heterogeneous materials.
- Salicylates can be effectively quantified in poplar bark and leaves by using any of the applied spectroscopic techniques; the most robust model for bark was constructed based on Raman spectra, while, in the case of leaf materials, the lowest RSEPval were found for NIR data. The flavonoids content in the leaves can be accurately determined through all four techniques, but for the bark samples, Raman spectroscopy gives the lowest errors of prediction.
- Raman calibration models for TSA and TFL determination were developed using mean-centered spectra. In the case of IR data, better models were obtained based on the first or second spectra derivatives.
- Raman spectra of the studied plant materials are characterized by a relatively low signal-to-noise ratio value. Therefore, to obtain reliable data, a longer data acquisition time is required compared to the IR techniques.
- In the case of the Raman experiment, fluorescence can significantly affect the registered spectra.
- Vibrational spectroscopy can be applied to quantify various groups of chemical compounds simultaneously, significantly accelerating and simplifying the analysis of the plant materials.
3. Materials and Methods
3.1. Plant Materials
3.2. Reference Analysis
3.3. Chemicals and Reagents
3.4. Spectroscopic Conditions
3.5. Software and Numerical Data Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry 2011, 72, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Ruuhola, T.; Julkunen-Tiitto, R.; Vainiotalo, P. In vitro degradation of willow salicylates. J. Chem. Ecol. 2003, 29, 1083–1097. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Schmidt, M.; Jaggi, R.; Metz, J.; Khayyal, M.T. Willow bark extract: The contribution of polyphenols to the overall effect. Wien Med. Wochenschr. 2007, 157, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Yu, W. Dynamic changes of phenolic compound contents in leaf and bark of poplar during autumn temperature drop. J. For. Res. 2011, 22, 481–485. [Google Scholar] [CrossRef]
- Rubert-Nason, K.F.; Holeski, L.M.; Couture, J.J.; Gusse, A.; Undersander, D.J.; Lindroth, R.L. Rapid phytochemical analysis of birch (Betula) and poplar (Populus) foliage by near-infrared reflectance spectroscopy. Anal. Bioanal. Chem. 2013, 405, 1333–1344. [Google Scholar] [CrossRef]
- Heiska, S.; Tikkanen, O.P.; Rousi, M.; Julkunen-Tiitto, R. Bark salicylates and condensed tannins reduce vole browsing amongst cultivated dark-leaved willows (Salix myrsinifolia). Chemoecology 2007, 17, 245–253. [Google Scholar] [CrossRef]
- Juntheikki, M.R.; Julkunen-Tiito, R. Inhibition of beta-glucosidase and esterase by tannins from Betula, Salix, and Pinus species. J. Chem. Ecol. 2000, 26, 1151–1165. [Google Scholar] [CrossRef]
- Julkunentiitto, R. Phenolic constituents in the leaves of northern willows-methods for the analysis of certain phenolics. J. Agric. Food. Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Głód, D.; Jesionek, A.; Łuczkiewicz, M.; Krauze-Baranowska, M. Studies on the polyphenolic composition and the antioxidant properties of the leaves of poplar (Populus spp.) various species and hybrids. Chem. Biodivers. 2021, 18, e210027. [Google Scholar] [CrossRef]
- Tyskiewicz, K.; Konkol, M.; Kowalski, R.; Roj, E.; Warminski, K.; Krzyzaniak, M.; Gil, L.; Stolarski, M.J. Characterization of bioactive compounds in the biomass of black locust, poplar and willow. Trees-Struct. Funct. 2019, 33, 1235–1263. [Google Scholar] [CrossRef] [Green Version]
- Nissinen, K.; Virjamo, V.; Randriamanana, T.; Sobuj, N.; Sivadasan, U.; Mehtatalo, L.; Beuker, E.; Julkunen-Tiitto, R.; Nybakken, L. Responses of growth and leaf phenolics in European aspen (Populus tremula) to climate change during juvenile phase change. Can. J. For. Res. 2017, 47, 1350–1363. [Google Scholar] [CrossRef]
- Griffiths, P.R. Introduction to vibrational spectroscopy. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Griffiths, P.R., Eds.; John Wiley & Sons: Chichester, UK, 2002. [Google Scholar]
- Schulz, H.; Barańska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Gillon, D.; Houssard, C.; Joffre, R. Using near-infrared reflectance spectroscopy to predict carbon, nitrogen and phosphorus content in heterogeneous plant material. Oecologia 1999, 118, 173–182. [Google Scholar] [CrossRef]
- Petisco, C.; Garcia-Criado, B.; Mediavilla, S.; de Aldana, B.R.V.; Zabalgogeazcoa, I.; Garcia-Ciudad, A. Near-infrared reflectance spectroscopy as a fast and non-destructive tool to predict foliar organic constituents of several woody species. Anal. Bioanal. Chem. 2006, 386, 1823–1833. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Spectral and chemical analysis of tropical forests: Scaling from leaf to canopy levels. Remote Sens. Environ. 2008, 112, 3958–3970. [Google Scholar] [CrossRef]
- Kleinebecker, T.; Schmidt, S.R.; Fritz, C.; Smolders, A.J.P.; Holzel, N. Prediction of delta 13C and delta 15N in plant tissues with near-infrared reflectance spectroscopy. New Phytol. 2009, 184, 732–739. [Google Scholar] [CrossRef]
- Martin-Tornero, E.; Pascoa, R.; Espinosa-Mansilla, A.; Martin-Meras, I.D.; Lopes, J.A. Comparative quantification of chlorophyll and polyphenol levels in grapevine leaves sampled from different geographical locations. Sci. Rep. 2020, 10, 6246. [Google Scholar] [CrossRef] [Green Version]
- Schulz, H.; Engelhardt, U.H.; Wegent, A.; Drews, H.H.; Lapczynski, S. Application of near-infrared reflectance spectroscopy to the simultaneous prediction of alkaloids and phenolic substances in green tea leaves. J. Agric. Food. Chem. 1999, 47, 5064–5067. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Shen, Y.; Chen, J.; Xiao, P.; Bao, J.S. Nondestructive prediction of total phenolics, flavonoid contents, and antioxidant capacity of rice grain using near-infrared spectroscopy. J. Agric. Food. Chem. 2008, 56, 8268–8272. [Google Scholar] [CrossRef]
- Smith, K.F.; Kelman, W.M. Predicting condensed tannin concentrations in Lotus uliginosus Schkuhr using near-infrared reflectance spectroscopy. J. Sci. Food Agric. 1997, 75, 263–267. [Google Scholar] [CrossRef]
- Frizon, C.N.T.; Oliveira, G.A.; Perussello, C.A.; Peralta-Zamora, P.G.; Camlofski, A.M.O.; Rossa, U.B.; Hoffmann-Ribani, R. Determination of total phenolic compounds in yerba mate (Ilex paraguariensis) combining near infrared spectroscopy (NIR) and multivariate analysis. LWT-Food Sci. Technol. 2015, 60, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Meacham-Hensold, K.; Montes, C.M.; Wu, J.; Guan, K.Y.; Fu, P.; Ainsworth, E.A.; Pederson, T.; Moore, C.E.; Brown, K.L.; Raines, C.; et al. High-throughput field phenotyping using hyperspectral reflectance and partial least squares regression (PLSR) reveals genetic modifications to photosynthetic capacity. Remote Sens. Environ. 2019, 231, 111176. [Google Scholar] [CrossRef]
- Toscano, G.; Rinnan, A.; Pizzi, A.; Mancini, M. The use of near-infrared (NIR) spectroscopy and principal component analysis (PCA) to discriminate bark and wood of the most common species of the pellet sector. Energy Fuels 2017, 31, 2814–2821. [Google Scholar] [CrossRef]
- Couture, J.J.; Singh, A.; Rubert-Nason, K.F.; Serbin, S.P.; Lindroth, R.L.; Townsend, P.A. Spectroscopic determination of ecologically relevant plant secondary metabolites. Methods Ecol. Evol. 2016, 7, 1402–1412. [Google Scholar] [CrossRef] [Green Version]
- Traore, M.; Kaal, J.; Cortizas, A.M. Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci. Technol. 2018, 52, 487–504. [Google Scholar] [CrossRef] [Green Version]
- Fasoli, M.; Dell’Anna, R.; Dal Santo, S.; Balestrini, R.; Sanson, A.; Pezzotti, M.; Monti, F.; Zenoni, S. Pectins, hemicelluloses and celluloses show specific dynamics in the internal and external surfaces of grape berry skin during ripening. Plant Cell Physiol. 2016, 57, 1332–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falamas, A.; Pinzaru, S.C.; Dehelean, C.A.; Peev, C.I.; Soica, C. Betulin and its natural resource as potential anticancer drug candidate seen by FT-Raman and FT-IR spectroscopy. J. Raman Spectrosc. 2011, 42, 97–107. [Google Scholar] [CrossRef]
- Pinzaru, S.C.; Leopold, N.; Kiefer, W. Vibrational spectroscopy of betulinic acid HIV inhibitor and of its birch bark natural source. Talanta 2002, 57, 625–631. [Google Scholar] [CrossRef]
- Cinta-Pinzaru, S.; Dehelean, C.A.; Soica, C.; Culea, M.; Borcan, F. Evaluation and differentiation of the Betulaceae birch bark species and their bioactive triterpene content using analytical FT-vibrational spectroscopy and GC-MS. Chem. Cent. J. 2012, 6, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostapiuk, A.; Kurach, L.; Strzemski, M.; Kurzepa, J.; Hordyjewska, A. Evaluation of antioxidative mechanisms in vitro and triterpenes composition of extracts from silver birch (Betula pendula roth) and black birch (Betula obscura kotula) barks by FT-IR and HPLC-PDA. Molecules 2021, 26, 4633. [Google Scholar] [CrossRef] [PubMed]
- Brangule, A.; Sukele, R.; Bandere, D. Herbal medicine characterization perspectives using advanced FTIR sample techniques-diffuse reflectance (DRIFT) and photoacoustic spectroscopy (PAS). Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Saltas, D.; Pappas, C.S.; Daferera, D.; Tarantilis, P.A.; Polissiou, M.G. Direct determination of rosmarinic acid in lamiaceae herbs using diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and chemometrics. J. Agric. Food. Chem. 2013, 61, 3235–3241. [Google Scholar] [CrossRef]
- Barreto, M.S.C.; dos Reis, J.V.; Muraoka, T.; Jemo, M.; Vergutz, L.; Alleoni, L.R.F. Diffuse reflectance infrared Fourier transform spectroscopy for a qualitative evaluation of plant leaf pigment extraction. Analyst 2021, 146, 3440–3448. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Polissiou, M.G. Assessing saffron (Crocus sativus L.) adulteration with plant-derived adulterants by diffuse reflectance infrared Fourier transform spectroscopy coupled with chemometrics. Talanta 2017, 162, 558–566. [Google Scholar] [CrossRef]
- Liu, N.; Yu, P.Q. Using DRIFT molecular spectroscopy with uni- and multivariate spectral techniques to detect protein molecular structure differences among different genotypes of barley. J. Agric. Food. Chem. 2010, 58, 6264–6269. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, D.R.; Larondelle, Y.; Rogez, H.; Abbas, O.; Pierna, J.A.F.; Baeten, V. Characterization and discrimination of phenolic compounds using Fourier transform Raman spectroscopy and chemometric tools. Biotechnol. Agron. Soc. 2018, 22, 13–28. [Google Scholar] [CrossRef]
- Agarwal, U.P. Analysis of Cellulose and Lignocellulose Materials by Raman Spectroscopy: A Review of the Current Status. Molecules 2019, 24, 1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frosch, T.; Schmitt, M.; Popp, J. In situ UV resonance Raman micro-spectroscopic localization of the antimalarial quinine in cinchona bark. J. Phys. Chem. B 2007, 111, 4171–4177. [Google Scholar] [CrossRef] [PubMed]
- Numata, Y.; Tanaka, H. Quantitative analysis of quercetin using Raman spectroscopy. Food Chem. 2011, 126, 751–755. [Google Scholar] [CrossRef]
- Baranska, M.; Schulz, H.; Joubert, E.; Manley, M. In situ flavonoid analysis by FT-Raman spectroscopy: Identification, distribution, and quantification of aspalathin in green rooibos (Aspalathus linearis). Anal. Chem. 2006, 78, 7716–7721. [Google Scholar] [CrossRef]
- Zeng, J.J.; Ping, W.; Sanaeifar, A.; Xu, X.; Luo, W.; Sha, J.J.; Huang, Z.X.; Huang, Y.F.; Liu, X.M.; Zhan, B.S.; et al. Quantitative visualization of photosynthetic pigments in tea leaves based on Raman spectroscopy and calibration model transfer. Plant Methods 2021, 17, 4. [Google Scholar] [CrossRef]
- Gao, W.L.; Shu, T.; Liu, Q.; Ling, S.J.; Guan, Y.; Liu, S.Q.; Zhou, L. Predictive modeling of lignin content for the screening of suitable poplar genotypes based on Fourier transform-Raman spectrometry. ACS Omega 2021, 6, 8578–8587. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Fecka, I.; Weglinska, M.; Szostak, R. Quantification of active ingredients in Potentilla tormentilla by Raman and infrared spectroscopy. Talanta 2018, 189, 308–314. [Google Scholar] [CrossRef]
- Węglińska, M.; Szostak, R.; Kita, A.; Nemś, A.; Mazurek, S. Determination of nutritional parameters of bee pollen by Raman and infrared spectroscopy. Talanta 2020, 212, 8. [Google Scholar] [CrossRef]
- Grabska, J.; Beć, K.B.; Ishigaki, M.; Huck, C.W.; Ozaki, Y. NIR Spectra simulations by anharmonic DFT-saturated and nsaturated Long-Chain Fatty Acids. J. Phys. Chem. B 2018, 122, 6931–6944. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Fackler, K. A review of band assignments in near infrared spectra of wood and wood components. J. Near Infrared Spectrosc. 2011, 19, 287–308. [Google Scholar] [CrossRef]
- Schulz, H.; Barańska, M.; Barański, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Willow Bark (Salicis cortex) Monograph. In European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020; pp. 1671–1672.
- Oses, S.M.; Pascual-Mate, A.; Fernandez-Muino, M.A.; Lopez-Diaz, T.M.; Sancho, M.T. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Geladi, P.; Macdougall, D.; Martens, H. Linearization and scatter-correction for near-infrared reflectance spectra of meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing & differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar]
- Wise, B.M.; Kowalski, B.R. Process chemometrics. In Process Analytical Chemistry; McLennan, F., Kowalski, B.R., Eds.; Chapman & Hall: London, UK, 1996; pp. 259–312. [Google Scholar]

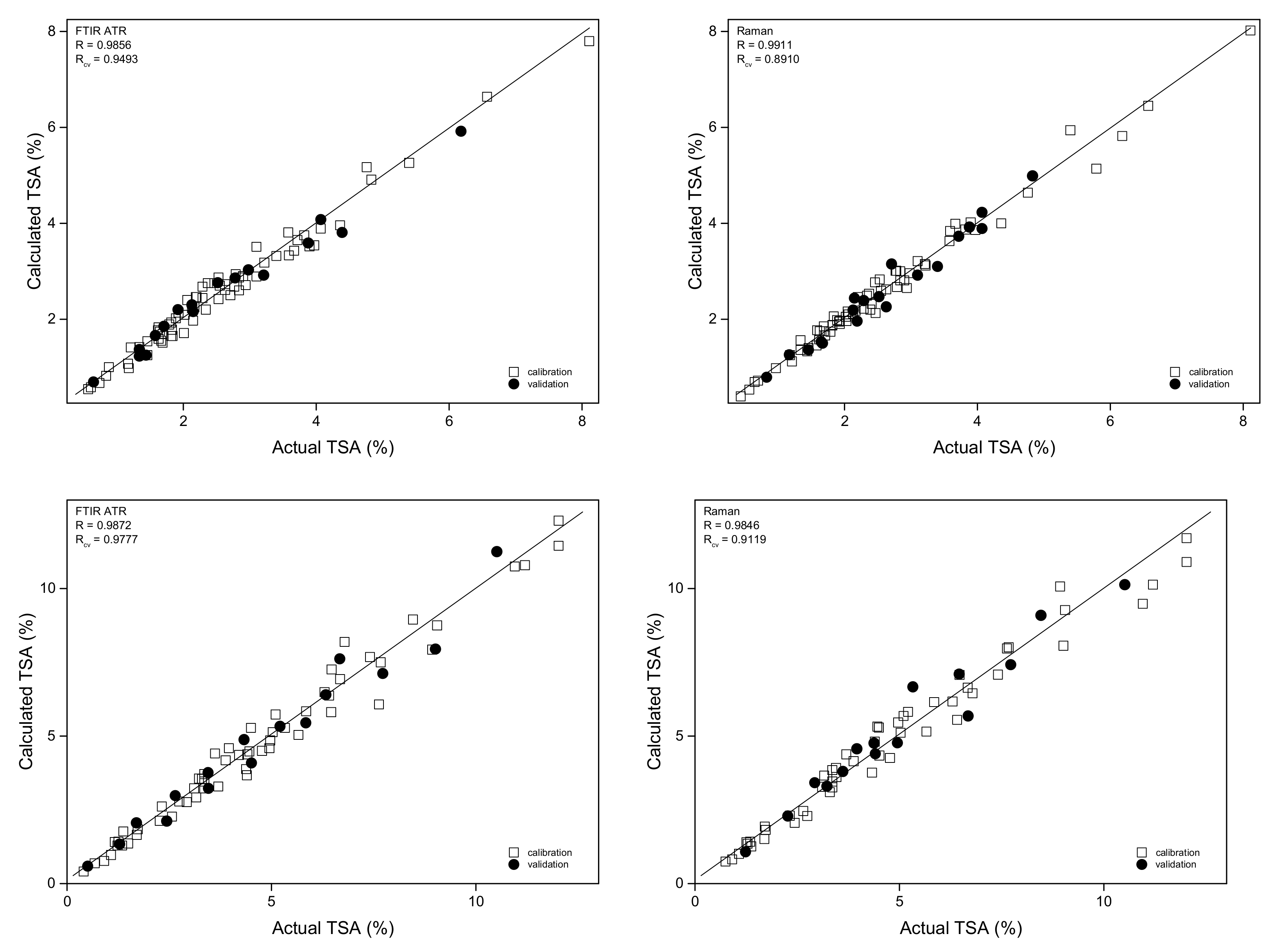
| Plant Material | Active Compound | Technique | Preprocessing | Rcal | Rval | RSEPcal | RSEPval | Rcv | LV |
|---|---|---|---|---|---|---|---|---|---|
| TSA | ATR | 2nd der | 0.989 | 0.986 | 7.17 | 7.25 | 0.949 | 6 | |
| TSA | DRIFTS/MIR | 2nd der | 0.989 | 0.979 | 6.88 | 8.09 | 0.928 | 5 | |
| TSA | NIR | MSC | 0.984 | 0.972 | 8.01 | 8.85 | 0.948 | 8 | |
| TSA | RAMAN | none | 0.991 | 0.983 | 6.03 | 6.67 | 0.891 | 10 | |
| bark | |||||||||
| TFL | ATR | 2nd der | 0.985 | 0.971 | 6.99 | 9.14 | 0.891 | 6 | |
| TFL | DRIFTS/MIR | 1st der | 0.973 | 0.972 | 9.17 | 9.18 | 0.834 | 7 | |
| TFL | NIR | MSC | 0.979 | 0.965 | 8.25 | 8.64 | 0.838 | 7 | |
| TFL | RAMAN | none | 0.982 | 0.983 | 7.35 | 7.45 | 0.873 | 8 | |
| TSA | ATR | 2nd der | 0.987 | 0.983 | 8.48 | 9.20 | 0.977 | 5 | |
| TSA | DRIFTS/MIR | 1st der | 0.987 | 0.980 | 8.36 | 9.56 | 0.963 | 4 | |
| TSA | NIR | 1st der | 0.986 | 0.989 | 8.53 | 8.07 | 0.982 | 5 | |
| TSA | RAMAN | none | 0.985 | 0.975 | 9.26 | 9.95 | 0.912 | 8 | |
| leaves | |||||||||
| TFL | ATR | 2nd der | 0.988 | 0.978 | 4.79 | 5.20 | 0.885 | 7 | |
| TFL | DRIFTS/MIR | 1st der | 0.985 | 0.975 | 5.98 | 5.69 | 0.857 | 6 | |
| TFL | NIR | 1st der | 0.995 | 0.983 | 3.40 | 4.47 | 0.856 | 5 | |
| TFL | RAMAN | none | 0.986 | 0.973 | 5.48 | 6.59 | 0.910 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, S.; Włodarczyk, M.; Pielorz, S.; Okińczyc, P.; Kuś, P.M.; Długosz, G.; Vidal-Yañez, D.; Szostak, R. Quantification of Salicylates and Flavonoids in Poplar Bark and Leaves Based on IR, NIR, and Raman Spectra. Molecules 2022, 27, 3954. https://doi.org/10.3390/molecules27123954
Mazurek S, Włodarczyk M, Pielorz S, Okińczyc P, Kuś PM, Długosz G, Vidal-Yañez D, Szostak R. Quantification of Salicylates and Flavonoids in Poplar Bark and Leaves Based on IR, NIR, and Raman Spectra. Molecules. 2022; 27(12):3954. https://doi.org/10.3390/molecules27123954
Chicago/Turabian StyleMazurek, Sylwester, Maciej Włodarczyk, Sonia Pielorz, Piotr Okińczyc, Piotr M. Kuś, Gabriela Długosz, Diana Vidal-Yañez, and Roman Szostak. 2022. "Quantification of Salicylates and Flavonoids in Poplar Bark and Leaves Based on IR, NIR, and Raman Spectra" Molecules 27, no. 12: 3954. https://doi.org/10.3390/molecules27123954
APA StyleMazurek, S., Włodarczyk, M., Pielorz, S., Okińczyc, P., Kuś, P. M., Długosz, G., Vidal-Yañez, D., & Szostak, R. (2022). Quantification of Salicylates and Flavonoids in Poplar Bark and Leaves Based on IR, NIR, and Raman Spectra. Molecules, 27(12), 3954. https://doi.org/10.3390/molecules27123954







