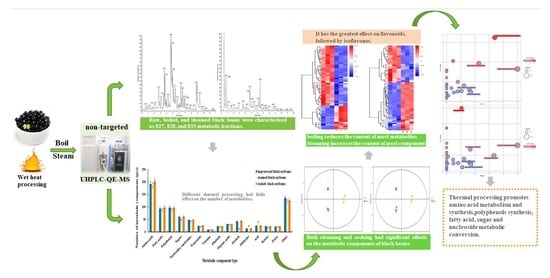
Effect of Thermal Processing on the Metabolic Components of Black Beans on Ultra-High-Performance Liquid Chromatography Coupled with High-Field Quadrupole-Orbitrap High-Resolution Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Qualitative Results
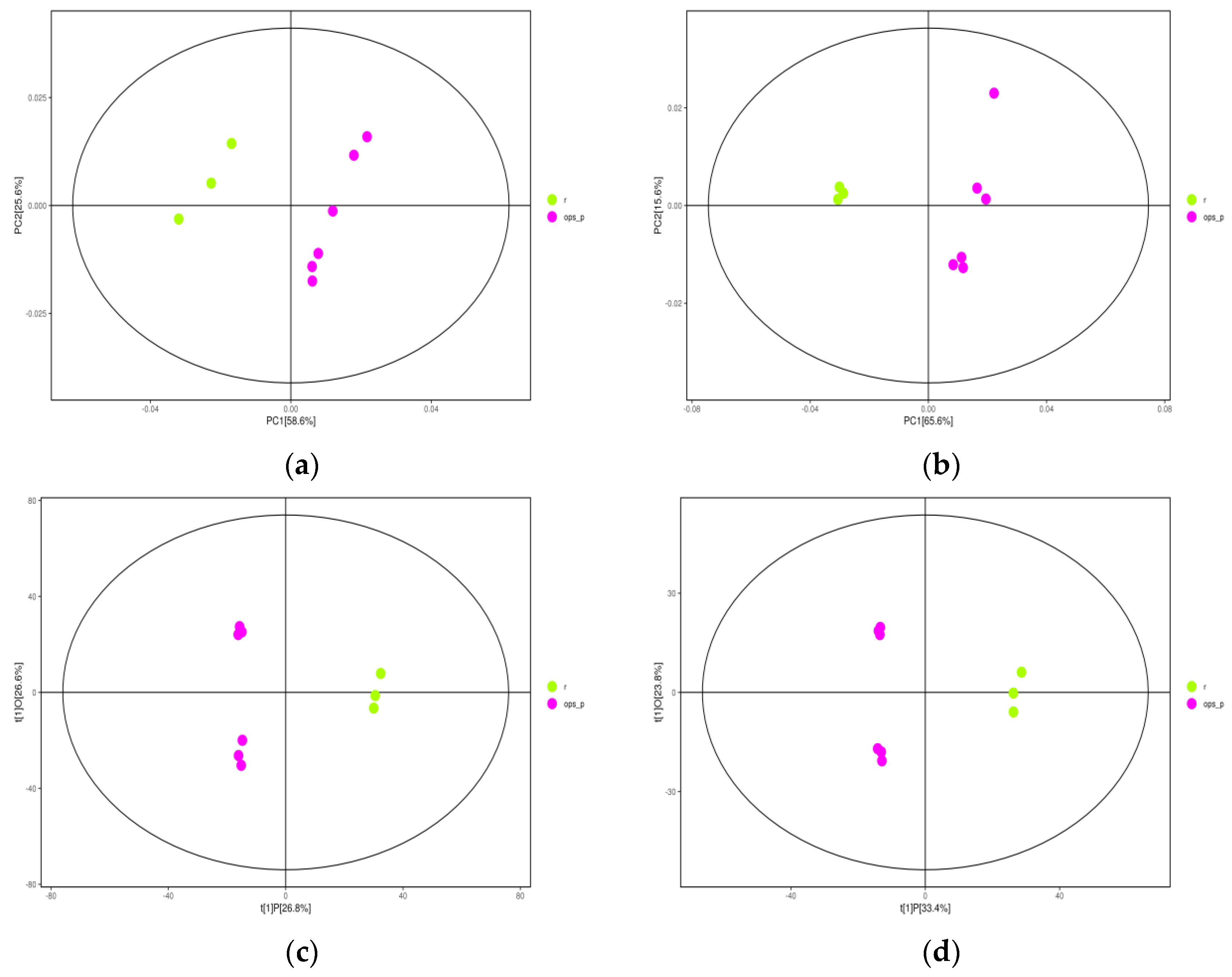
2.2. Orthogonal Projections to Latent Structures-Discriminant Analysis (OPLS-DA)
2.3. Screening of Differential Metabolic Components of Black Beans during Heat Processing
2.4. Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Reagents and Instruments
4.3. Sample Processing
4.3.1. Processing of Raw Black Beans
4.3.2. Cooking Method
4.3.3. Steaming Method
4.4. QEXACTIVE HF Detection
4.4.1. Metabolite Extraction
4.4.2. LC-MS/MS Analysis
4.5. Data Analysis
4.5.1. Data Preprocessing and Annotation
4.5.2. Principal Component Analysis (PCA)
4.5.3. Orthogonal Partial Least Squares-Discriminant Analysis (OPLS-DA)
4.5.4. Screening of Differential Metabolites
4.5.5. Hierarchical Cluster Analysis of Differential Metabolites
4.5.6. KEGG Annotation and Metabolic Pathway Analysis of Differential Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siah, S.D.; Konczak, I.; Agboola, S.; Wood, J.A.; Blanchard, C.L. In vitro investigations of the potential health benefits of Australian-grown faba beans (Vicia faba L.): Chemopreventive capacity and inhibitory effects on the angiotensin-converting enzyme, α-glucosidase, and lipase. Br. J. Nutr. 2012, 108 (suppl. 1), S123–S134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.T.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Luna-Vital, D.A.; Liang, K.; González de Mejía, E.; Loarca-Piña, G. Dietary peptides from the non-digestible fraction of Phaseolus vulgaris L. Decrease angiotensin II-dependent proliferation in HCT116 human colorectal cancer cells through the blockade of the renin-angiotensin system. Food Funct. 2016, 7, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Agric. Food Chem. 2017, 57, 4754–4764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, K.; Xu, B. A critical review of polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Jiménez, A.K.; Reynoso-Camacho, R.; Tejero, M.E.; León-Galván, F.; Loarca-Piña, G. Potential Role of Bioactive Compounds of Phaseolus vulgaris L. on Lipid-Lowering Mechanisms. Food Res. Int. 2015, 76, 92–104. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Tavaria, F.; Vasconcelos, M.; Gomes, A.M. In vitro fermentation of lupin seeds (Lupinus albus) and road beans (Vicia faba): Dynamic modulation of the intestinal microbiota and metabolomic output. Food Funct. 2015, 6, 3316–3322. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Thavarajah, D.; Combs, G.F.; Thavarajah, P. Lentil (Lens culinaris L.): A prebiotic-Rich whole food legume. Food Res. Int. 2013, 51, 107–113. [Google Scholar] [CrossRef]
- Ha, V.; Sievenpiper, J.L.; de Souza, R.J.; Jayalath, V.H.; Mirrahimi, A.; Agarwal, A.; Chiavaroli, L.; Mejia, S.B.; Sacks, F.M.; Di Buono, M.; et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: A systematic review and meta-analysis of randomized controlled trials. CMAJ 2014, 186, E252–E262. [Google Scholar] [CrossRef] [Green Version]
- Jayalath, V.H.; de Souza, R.J.; Sievenpiper, J.L.; Ha, V.; Chiavaroli, L.; Mirrahimi, A.; Di Buono, M.; Bernstein, A.M.; Leiter, L.A.; Kris-Etherton, P.M.; et al. Effect of dietary pulses on blood pressure: A systematic review and meta-analysis of controlled feeding trials. Am. J. Hypertens. 2014, 27, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liener, I.E.; Thompson, R.M. In vitro and in vivo studies on the digestibility of the major storage protein of navy bean (Phaseolus vulgaris). Plant Food. Hum. Nutr. 1980, 30, 13–25. [Google Scholar] [CrossRef]
- Lovato, F.; Kowaleski, J.; Silva, S.Z.; Heldt, L.F.S. Composição centesimal e conteúdo mineral de diferentes cultivares de feijão biorfortificado (Phaseolus vulgaris L.). Braz. J. Food Technol. 2018, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chau, C.-F.; Cheung, P.C.-K. Effect of various processing methods on Antinutrients and in vitro digestibility of protein and starch in two indigenous ChineseIndigenous legume seeds. J. Agric. Food Chem. 1997, 45, 4773–4776. [Google Scholar] [CrossRef]
- Hongli, W.; Chunying, L.; Tianwei, Y. Effects of different heat-treatment processes on soybean quality. J. Heilongjiang Bayi Agric. Univ. 2003, 15, 82–85. [Google Scholar]
- Institute Medicine (US) Panel on Micronutrients. Dietary Reference Intake for Vitamins A, K, As, Boron, Cr, Cu, I, Fe, Mn, Mo, Ni, Si, Vanadium, and Zn. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222312/ (accessed on 8 March 2018).
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Alvim, R.; Santos, P.C.J.L.; Bortolotto, L.A.; Mill, J.G.; da Costa Pereira, A. Arterial stiffness: Pathophysiological and genetic aspects. Int. J. Cardiovasc. Sci. 2017, 30, 433–441. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Comparative study on antiproliferative properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef]
- Cuevas-Tena, M.; Gómez del Pulgar, E.M.; Benítez-Páez, A.; Sanz, Y.; Alegría, A.; Lagarda, M.J. Plant Sterols and Human Gut Microbiota Relationship: An In Vitro Colonic Fermentation Study. J. Funct. Foods. 2018, 44, 322–329. [Google Scholar] [CrossRef]
- Chávez-Santoscoy, R.A.; Tovar, A.R.; Serna-Saldivar, S.O.; Torres, N.; Gutiérrez-Uribe, J.A. Conjugated and free sterols from black bean (Phaseolus vulgaris L.) seed coat as cholesterol micelle disruptors and their effect on lipid metabolism and cholesterol transport in rat primary hepatocytes. Genes Nutr. 2014, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Granados, O.; Torre-Villalvazo, I.; Serna-Saldivar, S.O.; Torres, N.; Palacios-González, B.; Tovar, A.R. Flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats modulate lipid metabolism and biliary cholesterol secretion in C57BL/6 mice. Br. J. Nutr. 2014, 112, 886–899. [Google Scholar] [CrossRef] [Green Version]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojica, L.; Meyer, A.; Berhow, M.A.; de Mejía, E.G. Bean Cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase, and diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Johnson, M.H. Anthocyanins from Berries: Chemistry and Roles in Inflammation and Diabetes. In Nutraceuticals and Fundamental Foods. Paris, France: Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO; Jayaprakasha, G.K., Patil, B.S., Eds.; Eolss Publishers: Oxford, UK, 2013; Available online: http://www.eolss.net (accessed on 5 December 2021).
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamin, R.; Morgan, K.G. Deciphering Actin Cytoskeletal Function in the Contractile Vascular Smooth Muscle Cell. J. Physiol. 2012, 590, 4145–4154. [Google Scholar] [CrossRef] [PubMed]
- Vaz Patto, M.C.; Amarowicz, R.; Aryee, A.N.A.; Boye, J.I.; Chung, H.J.; Martín-Cabrejas, M.A.; Domoney, C. Achievements and Challenges in Improving the Nutritional Quality of Food Legumes. Crit. Rev. Plant Sci. 2015, 34, 105–143. [Google Scholar] [CrossRef]
- Van Der Poel, A.F.B. Effect of Processing on Antinutritional Factors and Protein Nutritional Value of Dry Beans (Phaseolus vulgaris L.). A Review. Anim. Feed Sci. Technol. 1990, 29, 179–208. [Google Scholar] [CrossRef]
- Rehman, Z.; Shah, W.H. Thermal Heat Processing Effects on Antinutrients, Protein and Starch Digestibility of Food Legumes. Food Chem. 2005, 91, 327–331. [Google Scholar] [CrossRef]
- Dong, L.; Wu, Y.; Wang, W.; Wu, Y.; Zhang, Y.; Wang, S. Structural Modification and Digestibility Change of β-Lactoglobulin Modified by Methylglyoxal with the Simulated Reheating of Dairy Products. Food Chem. 2019, 288, 276–282. [Google Scholar] [CrossRef]
- Akinmutimi, A.H.; Njaka, G.O.; Archibong, I.M.; Ewa, E.U. The Effect of Boiling Periods on Nutrients Composition and Antinutrient of African Nutmeg (Monodora myristica). Int. J. Curr. Res. 2011, 3, 133–139. [Google Scholar]
- Khalil, M.M. Effect of Soaking, Germination, Autoclaving and Cooking on Chemical and Biological Value of Guar Compared with Faba Bean. Nahrung/Food 2001, 45, 246–250. [Google Scholar] [CrossRef]
- Hefnawy, T.H. Effect of Processing Methods on Nutritional Composition and Anti-Nutritional Factors in Lentils (Lens Culinaris). Ann. Agric. Sci. 2011, 56, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Chopra, N.; Hira, C.K. Effect of Roasting on Protein Quality of Cereals. J. Food Sci. Technol. 1986, 23, 233–235. [Google Scholar]
- Yagoub, A.E.G.A.; Mohammed, M.A.; Baker, A.A.A. Effect of Soaking, Sprouting and Cooking on Chemical Composition, Bioavailability of Minerals and In Vitro Protein Digestibility of Roselle (Hibiscus sabdariffa L.) Seed. Pak. J. Nutr. 2008, 7, 50–56. [Google Scholar]
- Hwang, H.S. NMR spectroscopy for assessing lipid oxidation. Lipid Technol. 2015, 27, 187–189. [Google Scholar] [CrossRef]
- Zamora, R.; León, M.M.; Hidalgo, F.J. Oxidative versus Non-Oxidative Decarboxylation of Amino Acids: Conditions for the Preferential Formation of Either Strecker Aldehydes or Amines in Amino Acid/Lipid-Derived Reactive Carbonyl Model Systems. J. Agric. Food Chem. 2015, 63, 8037–8043. [Google Scholar] [CrossRef]
- Delgado, R.M.; Hidalgo, F.J.; Zamora, R. Antagonism between Lipid-Derived Reactive Carbonyls and Phenolic Compounds in the Strecker Degradation of Amino Acids. Food Chem. 2016, 194, 1143–1148. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K. Aldehyde Adducts Generated during Lipid Peroxidation Modification of Proteins. Free Radic. Res. 2015, 49, 896–904. [Google Scholar] [CrossRef]
- Capuano, E.; Oliviero, T.; Açar, Ö.Ç.; Gökmen, V.; Fogliano, V. Lipid Oxidation Promotes Acrylamide Formation in Fat-Rich Model Systems. Food Res. Int. 2010, 43, 1021–1026. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.; Owczarek, A.; De Meulenaer, B.; De Kimpe, N. Impact of Various Food Ingredients on the Retention of Furan in Foods. Mol. Nutr. Food Res. 2009, 53, 1505–1511. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Contribution of Lipid Oxidation Products to Acrylamide Formation in Model Systems. J. Agric. Food Chem. 2008, 56, 6075–6080. [Google Scholar] [CrossRef]
- Silvagni, A.; Franco, L.; Bagno, A.; Rastrelli, F. Thermoinduced Lipid Oxidation of a Culinary Oil: A Kinetic Study of the Oxidation Products by Magnetic Resonance Spectroscopies. J. Phys. Chem. A 2010, 114, 10059–10065. [Google Scholar] [CrossRef]
- Kumari, S.; Krishnan, V.; Sachdev, A. Impact of Soaking and Germination Durations on Antioxidants and Anti-Nutrients of Black and Yellow Soybean (Glycine max L.). Varieties 2015, 24, 355–358. [Google Scholar] [CrossRef]
- Siah, S.; Wood, J.A.; Agboola, S.; Konczak, I.; Blanchard, C.L. Effects of Soaking, Boiling and Autoclaving on the Phenolic Contents and Antioxidant Activities of Faba Beans (Vicia faba L.) Differing in Seed Coat Colours. Food Chem. 2014, 142, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Total Phenolics, Phenolic Acids, Isoflavones, and Anthocyanins and Antioxidant Properties of Yellow and Black Soybeans as Affected by Thermal Processing. J. Agric. Food Chem. 2008, 56, 7165–7175. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Phytochemical Profiles and Health-Promoting Effects of Cool-Season Food Legumes as Influenced by Thermal Processing. J. Agric. Food Chem. 2009, 57, 10718–10731. [Google Scholar] [CrossRef]
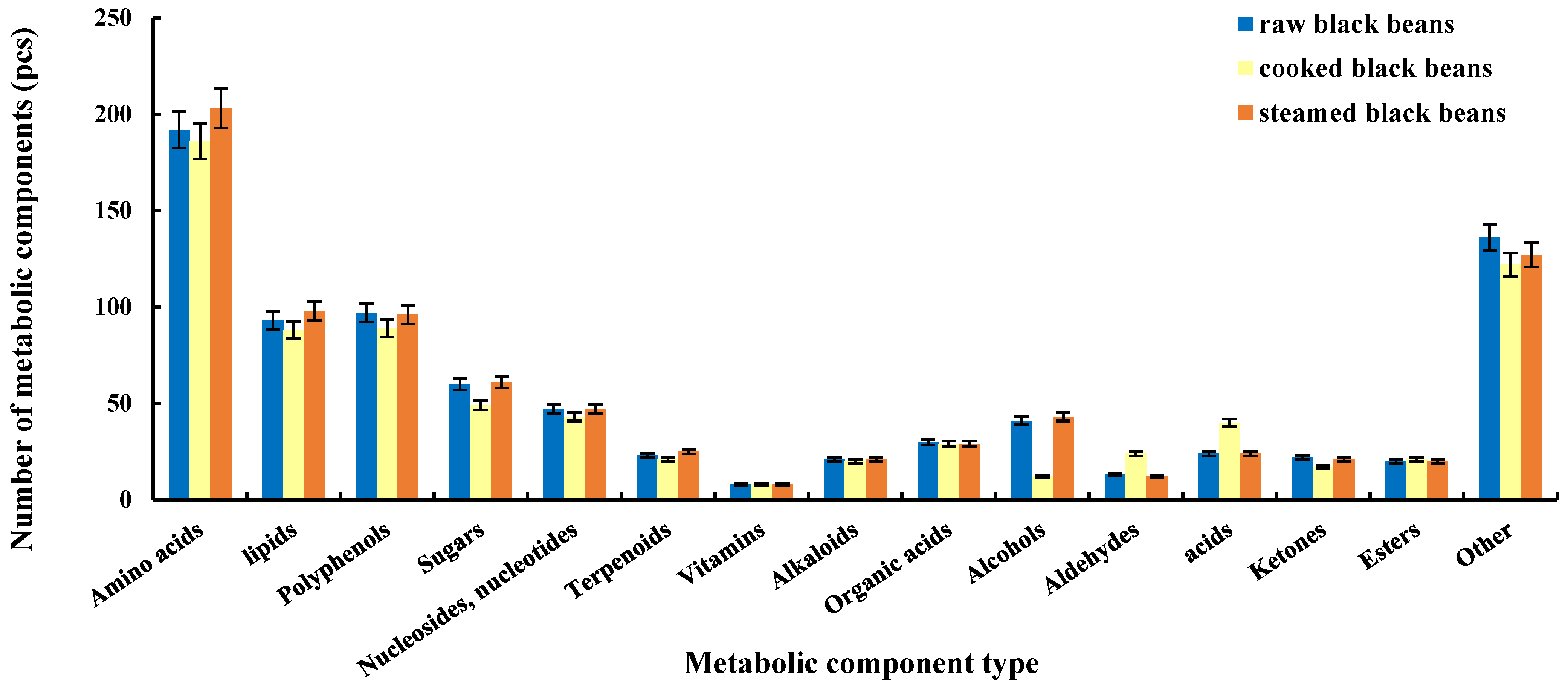



| Raw−Cooked | Raw−Steamed | Steamed−Cooked | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reduced Content | Increased Content | Total | Reduced Content | Increased Content | Total | High Content | Low Content | Total | |
| Amino acids | 21 | 13 | 34 | 15 | 17 | 32 | 34 | 9 | 43 |
| Sugars | 10 | 3 | 13 | 7 | 9 | 16 | 11 | 1 | 12 |
| Lipids | 12 | 11 | 23 | 19 | 6 | 25 | 9 | 20 | 29 |
| Polyphenols | 24 | 19 | 43 | 20 | 30 | 50 | 29 | 2 | 31 |
| Nucleosides | 8 | 3 | 11 | 5 | 16 | 21 | 18 | 2 | 20 |
| Other | 20 | 9 | 29 | 9 | 11 | 20 | 15 | 7 | 22 |
| Aldehydes | 3 | 3 | 6 | 2 | 2 | 4 | 2 | 0 | 2 |
| Alkaloids | 5 | 0 | 5 | 3 | 3 | 6 | 4 | 0 | 4 |
| Acids | 10 | 1 | 11 | 7 | 6 | 13 | 8 | 0 | 8 |
| Terpenes | 2 | 5 | 7 | 3 | 4 | 7 | 1 | 5 | 6 |
| Ketones | 2 | 2 | 4 | 2 | 4 | 6 | 1 | 1 | 2 |
| Vitamins | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 2 |
| Organic acids | 0 | 3 | 3 | 0 | 2 | 2 | 4 | 1 | 5 |
| Esters | 6 | 1 | 7 | 3 | 1 | 4 | 1 | 1 | 2 |
| Alcohols | 0 | 0 | 0 | 0 | 3 | 3 | 4 | 1 | 5 |
| Total | 124 | 73 | 197 | 96 | 114 | 210 | 143 | 50 | 193 |
| Pathway | Total | Hits | Raw p | −ln(p) | Holm Adjust | FDR | Impact | Hits Cpd | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Glycerophospholipid metabolism | 25 | 2 | 0.153 | 1.879 | 1 | 1 | 0.186 | O-Phosphoethanolamine cpd:C00346; Glycerol 3-phosphate cpd:C00093; |
| 2 | Glycerolipid metabolism | 13 | 1 | 0.309 | 1.173 | 1 | 1 | 0.079 | Glycerol 3-phosphate cpd:C00093 |
| 3 | Sphingolipid metabolism | 13 | 1 | 0.309 | 1.173 | 1 | 1 | 0.333 | O-Phosphoethanolamine cpd:C00346 |
| 4 | alpha-Linolenic acid metabolism | 23 | 1 | 0.562 | 0.577 | 1 | 1 | 0.160 | Alpha-Linolenic acid cpd:C06427 |
| 5 | Biosynthesis of unsaturated fatty acids | 42 | 1 | 0.781 | 0.247 | 1 | 1 | 0.000 | Alpha-Linolenic acid cpd:C06427 |
| 6 | Citrate cycle (TCA cycle) | 20 | 1 | 0.512 | 0.670 | 1 | 1 | 0.034 | Fumaric acid cpd:C00122 |
| 7 | Butanoate metabolism | 18 | 1 | 0.475 | 0.744 | 1 | 1 | 0.000 | L-Glutamic acid cpd:C00025 |
| 8 | beta-Alanine metabolism | 12 | 1 | 0.289 | 1.240 | 1 | 1 | 0.000 | Dihydrouracil cpd:C00429 |
| 9 | Histidine metabolism | 16 | 2 | 0.366 | 1.004 | 1 | 1 | 0.000 | Imidazole-4-acetaldehyde cpd:C05130; Imidazoleacetic acid cpd:C02835 |
| 10 | Alanine, aspartate, and glutamate metabolism | 22 | 3 | 0.039 | 3.255 | 1 | 1 | 0.339 | L-Glutamic acid cpd:C00025; L-Asparagine cpd:C00152; Fumaric acid cpd:C00122 |
| 11 | Tyrosine metabolism | 18 | 2 | 0.128 | 2.054 | 1 | 1 | 0.045 | Dopamine cpd:C03758; Fumaric acid cpd:C00122 |
| 12 | Cysteine and methionine metabolism | 34 | 1 | 0.623 | 0.473 | 1 | 1 | 0.048 | 5′-Methylthioadenosine cpd:C00170 |
| 13 | Arginine and proline metabolism | 38 | 3 | 0.144 | 1.938 | 1 | 1 | 0.227 | L-Glutamic acid cpd:C00025; L-Proline cpd:C00148; Fumaric acid cpd:C00122 |
| 14 | Glycine, serine, and threonine metabolism | 30 | 1 | 0.660 | 0.415 | 1 | 1 | 0.000 | L-Tryptophan cpd:C00078 |
| 15 | Phenylalanine, tyrosine, and tryptophan biosynthesis | 21 | 1 | 0.529 | 0.637 | 1 | 1 | 0.000 | L-Tryptophan cpd:C00078 |
| 16 | Tryptophan metabolism | 27 | 1 | 0.621 | 0.476 | 1 | 1 | 0.171 | L-Tryptophan cpd:C00078 |
| 17 | Glutathione metabolism | 26 | 1 | 0.607 | 0.499 | 1 | 1 | 0.078 | L-Glutamic acid cpd:C00025 |
| 18 | Nitrogen metabolism | 15 | 1 | 0.415 | 0.879 | 1 | 1 | 0.000 | L-Glutamic acid cpd:C00025 |
| 19 | Aminoacyl-tRNA biosynthesis | 67 | 4 | 0.203 | 1.597 | 1 | 1 | 0.000 | L-Asparagine cpd:C00152; L-Tryptophan cpd:C00078; L-Proline cpd:C00148; L-Glutamic acid cpd:C00025 |
| 20 | Porphyrin and chlorophyll metabolism | 29 | 1 | 0.648 | 0.435 | 1 | 1 | 0.000 | L-Glutamic acid cpd:C00025 |
| 21 | Flavonoid biosynthesis | 43 | 2 | 0.449 | 0.802 | 1 | 1 | 0.122 | Naringenin cpd:C00509; (-)-Epiafzelechin cpd:C12128; Luteolin cpd:C01514 |
| 22 | Stilbenoid, diarylheptanoid, and gingerol biosynthesis | 10 | 1 | 0.300 | 1.203 | 1 | 1 | 0.000 | 3,3′,4′5-Tetrahydroxystilbene cpd:C05901 |
| 23 | Flavone and flavonol biosynthesis | 9 | 1 | 0.226 | 1.488 | 1 | 1 | 0.000 | Luteolin cpd:C01514 |
| 24 | Vitamin B6 metabolism | 11 | 1 | 0.269 | 1.314 | 1 | 1 | 0.000 | Pyridoxine cpd:C00314 |
| 25 | Thiamine metabolism | 11 | 2 | 0.036 | 3.334 | 1 | 1 | 0.471 | 5-(2-Hydroxyethyl)-4-methylthiazole cpd:C04294; Thiamine monophosphate cpd:C01081 |
| 26 | Pantothenate and CoA biosynthesis | 14 | 1 | 0.329 | 1.112 | 1 | 1 | 0.000 | Dihydrouracil cpd:C00429 |
| 27 | Pyrimidine metabolism | 38 | 2 | 0.665 | 0.408 | 1 | 1 | 0.000 | Dihydrouracil cpd:C00429; Cytidine cpd:C00475 |
| 28 | Purine metabolism | 61 | 4 | 0.241 | 1.424 | 1 | 1 | 0.050 | Hypoxanthine cpd:C00262; Phosphoribosyl formamidocarboxamide cpd:C04734; Inosine cpd:C00294; Xanthine cpd:C00385 |
| 29 | Isoquinoline alkaloid biosynthesis | 6 | 1 | 0.192 | 1.648 | 1 | 1 | 0.500 | Dopamine cpd:C03758 |
| 30 | Indole alkaloid biosynthesis | 7 | 1 | 0.221 | 1.510 | 1 | 1 | 0.000 | L-Tryptophan cpd:C00078 |
| 31 | Pentose phosphate pathway | 18 | 1 | 0.475 | 0.744 | 1 | 1 | 0.000 | Gluconic acid cpd:C00257 |
| 32 | Galactose metabolism | 26 | 1 | 0.525 | 0.644 | 1 | 1 | 0.049 | Stachyose cpd:C01613 |
| 33 | Glucosinolate biosynthesis | 54 | 1 | 0.859 | 0.152 | 1 | 1 | 0.000 | L-Tryptophan cpd:C00078 |
| Pathway | Total | Hits | Raw p | −ln(p) | Holm Adjust | FDR | Impact | Hits Cpd | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Thiamine metabolism | 11 | 3 | 0.006 | 5.157 | 0.50088 | 0.5009 | 0.471 | 5-Aminoimidazole ribonucleotide cpd:C03373; 5-(2-Hydroxyethyl)-4-methylthiazole cpd:C04294; Thiamine monophosphate cpd:C01081 |
| 2 | Vitamin B6 metabolism | 11 | 2 | 0.056 | 2.884 | 1 | 1 | 0.000 | Pyridoxine cpd:C00314; Pyridoxal cpd:C00250 |
| 3 | Nicotinate and nicotinamide metabolism | 12 | 1 | 0.355 | 1.036 | 1 | 1 | 0.000 | Nicotinic acid cpd:C00253 |
| 4 | Glycerophospholipid metabolism | 25 | 2 | 0.224 | 1.498 | 1 | 1 | 0.081 | O-Phosphoethanolamine cpd:C00346; Citicoline cpd:C00307 |
| 5 | Sphingolipid metabolism | 13 | 1 | 0.378 | 0.973 | 1 | 1 | 0.333 | O-Phosphoethanolamine cpd:C00346 |
| 6 | alpha-Linolenic acid metabolism | 23 | 1 | 0.570 | 0.562 | 1 | 1 | 0.160 | Alpha-Linolenic acid cpd:C06427 |
| 7 | Biosynthesis of unsaturated fatty acids | 42 | 1 | 0.788 | 0.238 | 1 | 1 | 0.000 | Alpha-Linolenic acid cpd:C06427 |
| 8 | Cysteine and methionine metabolism | 34 | 2 | 0.345 | 1.065 | 1 | 1 | 0.138 | 2-Oxo-4-methylthiobutanoic acid cpd:C01180; 5′-Methylthioadenosine cpd:C00170 |
| 9 | beta-Alanine metabolism | 12 | 1 | 0.355 | 1.036 | 1 | 1 | 0.000 | Dihydrouracil cpd:C00429 |
| 10 | Glycine, serine, and threonine metabolism | 30 | 2 | 0.668 | 0.403 | 1 | 1 | 0.000 | Betaine cpd:C00719; L-Tryptophan cpd:C00078 |
| 11 | Arginine and proline metabolism | 38 | 1 | 0.754 | 0.282 | 1 | 1 | 0.008 | N-Acetyl-L-glutamate 5-semialdehyde cpd:C01250 |
| 12 | Phenylalanine metabolism | 8 | 1 | 0.253 | 1.374 | 1 | 1 | 0.167 | Phenylpyruvic acid cpd:C00166 |
| 13 | Histidine metabolism | 16 | 1 | 0.443 | 0.814 | 1 | 1 | 0.000 | Imidazoleacetic acid cpd:C02835 |
| 14 | Phenylalanine, tyrosine, and tryptophan biosynthesis | 21 | 2 | 0.171 | 1.767 | 1 | 1 | 0.000 | Phenylpyruvic acid cpd:C00166; L-Tryptophan cpd:C00078 |
| 15 | Tyrosine metabolism | 18 | 1 | 0.483 | 0.728 | 1 | 1 | 0.045 | Dopamine cpd:C03758 |
| 16 | Pyruvate metabolism | 21 | 1 | 0.537 | 0.622 | 1 | 1 | 0.000 | 2-Isopropylmalic acid cpd:C02504 |
| 17 | Valine, leucine, and isoleucine biosynthesis | 26 | 1 | 0.615 | 0.486 | 1 | 1 | 0.048 | 2-Isopropylmalic acid cpd:C02504 |
| 18 | Tryptophan metabolism | 27 | 1 | 0.629 | 0.463 | 1 | 1 | 0.171 | L-Tryptophan cpd:C00078 |
| 19 | Aminoacyl-tRNA biosynthesis | 67 | 1 | 0.918 | 0.085 | 1 | 1 | 0.000 | L-Tryptophan cpd:C00078 |
| 20 | Purine metabolism | 61 | 7 | 0.019 | 3.981 | 1 | 1 | 0.105 | Xanthine cpd:C00385; Guanosine monophosphate cpd:C00144; Guanine cpd:C00242; Deoxyinosine cpd:C05512; Inosine cpd:C00294; Guanosine cpd:C00387; 5-Aminoimidazole ribonucleotide cpd:C03373; |
| 21 | Pantothenate and CoA biosynthesis | 14 | 1 | 0.401 | 0.915 | 1 | 1 | 0.000 | Dihydrouracil cpd:C00429 |
| 22 | Pyrimidine metabolism | 38 | 3 | 0.397 | 0.923 | 1 | 1 | 0.012 | Cytidine cpd:C00475; Thymidine cpd:C00214; Dihydrouracil cpd:C00429 |
| 23 | Stilbenoid, diarylheptanoid, and gingerol biosynthesis | 10 | 1 | 0.306 | 1.185 | 1 | 1 | 0.000 | 3,3′,4′5-Tetrahydroxystilbene cpd:C05901 |
| 24 | Flavone and flavonol biosynthesis | 9 | 1 | 0.280 | 1.273 | 1 | 1 | 0.000 | Luteolin cpd:C01514 |
| 25 | Flavonoid biosynthesis | 43 | 3 | 0.460 | 0.776 | 1 | 1 | 0.122 | Naringenin cpd:C00509; (-)-Epiafzelechin cpd:C12128; Luteolin cpd:C01514 |
| 26 | Galactose metabolism | 26 | 2 | 0.615 | 0.486 | 1 | 1 | 0.070 | Melibiose cpd:C05402; Glucose 1-phosphate cpd:C00103 |
| 27 | Glucosinolate biosynthesis | 54 | 2 | 0.866 | 0.144 | 1 | 1 | 0.010 | 2-Oxo-4-methylthiobutanoic acid cpd:C01180; L-Tryptophan cpd:C00078 |
| 28 | Pentose and glucuronate interconversions | 12 | 2 | 0.066 | 2.724 | 1 | 1 | 0.000 | D-Xylose cpd:C00181; Glucose 1-phosphate cpd:C00103 |
| 29 | Glycolysis or gluconeogenesis | 25 | 1 | 0.601 | 0.510 | 1 | 1 | 0.000 | Glucose 1-phosphate cpd:C00103 |
| 30 | Starch and sucrose metabolism | 30 | 1 | 0.668 | 0.403 | 1 | 1 | 0.172 | Glucose 1-phosphate cpd:C00103 |
| 31 | Amino sugar and nucleotide sugar metabolism | 41 | 1 | 0.780 | 0.248 | 1 | 1 | 0.110 | Glucose 1-phosphate cpd:C00103 |
| 32 | Isoquinoline alkaloid biosynthesis | 6 | 1 | 0.196 | 1.628 | 1 | 1 | 0.500 | Dopamine cpd:C03758 |
| 33 | Indole alkaloid biosynthesis | 7 | 1 | 0.225 | 1.491 | 1 | 1 | 0.000 | L-Tryptophan cpd:C00078 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Fan, X.; Zhang, S.; Yu, M.; Wu, T.; Liang, Y.; Wang, C.; Yang, H. Effect of Thermal Processing on the Metabolic Components of Black Beans on Ultra-High-Performance Liquid Chromatography Coupled with High-Field Quadrupole-Orbitrap High-Resolution Mass Spectrometry. Molecules 2022, 27, 3919. https://doi.org/10.3390/molecules27123919
Feng Y, Fan X, Zhang S, Yu M, Wu T, Liang Y, Wang C, Yang H. Effect of Thermal Processing on the Metabolic Components of Black Beans on Ultra-High-Performance Liquid Chromatography Coupled with High-Field Quadrupole-Orbitrap High-Resolution Mass Spectrometry. Molecules. 2022; 27(12):3919. https://doi.org/10.3390/molecules27123919
Chicago/Turabian StyleFeng, Yuchao, Xia Fan, Shu Zhang, Miao Yu, Tong Wu, Ying Liang, Changyuan Wang, and Hongzhi Yang. 2022. "Effect of Thermal Processing on the Metabolic Components of Black Beans on Ultra-High-Performance Liquid Chromatography Coupled with High-Field Quadrupole-Orbitrap High-Resolution Mass Spectrometry" Molecules 27, no. 12: 3919. https://doi.org/10.3390/molecules27123919
APA StyleFeng, Y., Fan, X., Zhang, S., Yu, M., Wu, T., Liang, Y., Wang, C., & Yang, H. (2022). Effect of Thermal Processing on the Metabolic Components of Black Beans on Ultra-High-Performance Liquid Chromatography Coupled with High-Field Quadrupole-Orbitrap High-Resolution Mass Spectrometry. Molecules, 27(12), 3919. https://doi.org/10.3390/molecules27123919