Evaluation of the Intestinal Permeability of Rosmarinic Acid from Thunbergia laurifolia Leaf Water Extract in a Caco-2 Cell Model
Abstract
1. Introduction
2. Results
2.1. Determination of RA in TL Water Extract
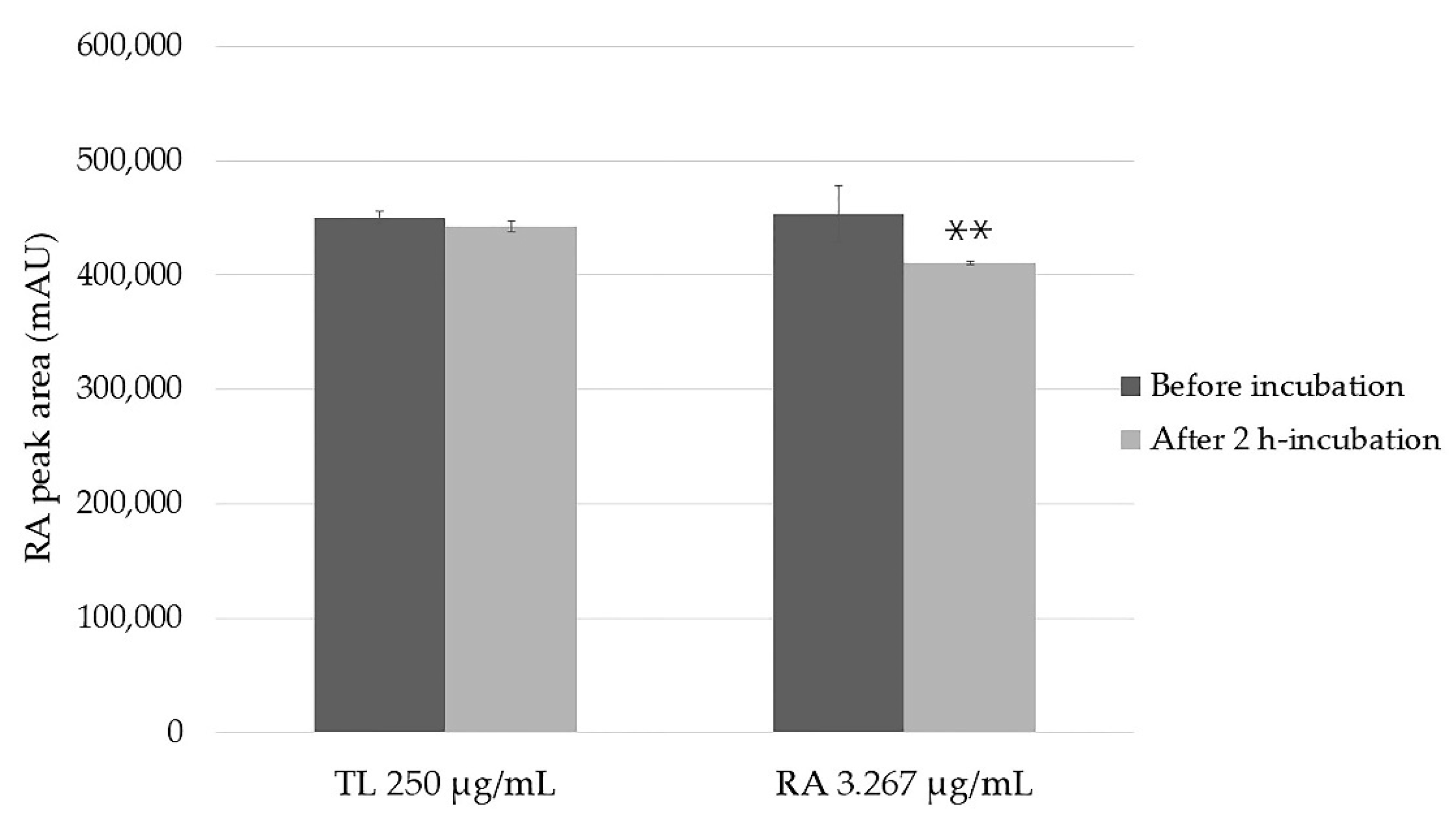
2.2. Stability of the Samples
2.3. Cytotoxicity Test
2.4. The Impact of Post Permeability Deconjugation Treatment with β-Glucuronidase/Sulfatase on Apparent Permeability Coefficient (Papp) of RA from TL Extract and of Pure Compound
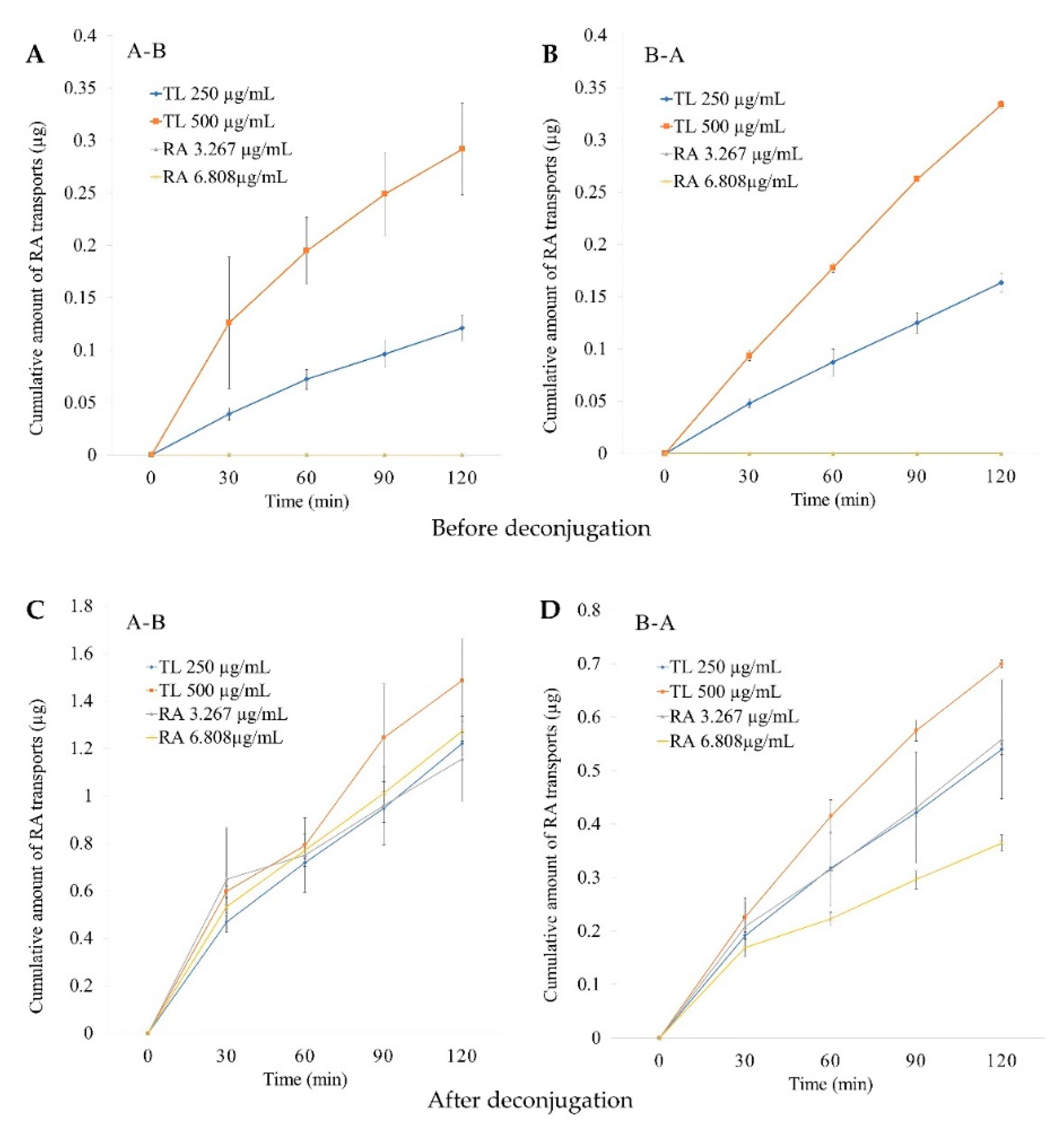
2.4.1. In Vitro Permeability Study
2.4.2. Cumulative Amounts
2.4.3. Basolateral Recovery
2.4.4. ER Values
3. Discussion
4. Materials and Methods
4.1. Plant Extraction
4.2. Chemicals and Reagents
4.3. Experimental Procedures
4.3.1. HPLC Analysis
4.3.2. Stability Study
4.3.3. Transepithelial Transport Experiment
4.3.4. Transepithelial Transfer of RA in TL Extract and Pure RA after Treating with β-Glucuronidase/Sulfatase
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chan, E.W.; Eng, S.Y.; Tan, Y.P.; Wong, Z.C. Phytochemistry and pharmacological properties of Thunbergia laurifolia: A Review. Phcog. J. 2011, 3, 1–6. [Google Scholar] [CrossRef]
- Thai-FDA. Thai National List of Essential Medicine (NLEM) B.E. 2563 (List of Herbal Medicinal Products); Bureau of Drug Control, Ministry of Public Health: Nonthaburi, Thailand, 2020. [Google Scholar]
- Rana, M.N.; Karim, N.; Changlek, S.; Rahman, M.A.; Tangpong, J.; Hajjar, D.; Alelwani, W.; Makki, A.A. Thunbergia laurifolia leaf extract partially recovers lead-induced renotoxicity through modulating the cell signaling pathways. Saudi J. Biol. Sci. 2020, 27, 3700–3710. [Google Scholar] [CrossRef] [PubMed]
- Palipoch, S.; Punsawad, C.; Koomhin, P.; Poonsawat, W. Thunbergia laurifolia aqueous leaf extract ameliorates paraquat-induced kidney injury by regulating NADPH oxidase in rats. Heliyon 2022, 8, e09234. [Google Scholar] [CrossRef] [PubMed]
- Junsi, M.; Siripongvutikorn, S. Anti-insecticides activity in cell-lines model of Thunbergia laurifolia leaf extract aiming for functional drink. Food Sci. Technol. 2022, 42, 1–11. [Google Scholar] [CrossRef]
- Rocejanasaroj, A.; Tencomnao, T.; Sangkitikomol, W. Thunbergia laurifolia extract minimizes the adverse effects of toxicants by regulating P-glycoprotein activity, CYP450, and lipid metabolism gene expression in HepG2 cells. GMR Genet. Mol. Res. 2014, 13, 205–219. [Google Scholar] [CrossRef]
- Wonkchalee, O.; Boonmars, T.; Aromdee, C.; Laummaunwai, P.; Khunkitti, W.; Vaeteewoottacharn, K.; Sriraj, P.; Aukkanimart, R.; Loilome, W.; Chamgramol, Y. Anti-inflammatory, antioxidant and hepatoprotective effects of Thunbergia laurifolia Linn. on experimental opisthorchiasis. Parasitol. Res. 2012, 111, 353–359. [Google Scholar] [CrossRef]
- Jetawattana, S.; Boonsirichai, K.; Charoen, S.; Martin, S.M. Radical intermediate generation and cell cycle arrest by an aqueous extract of Thunbergia laurifolia Linn. In human breast cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 4357–4361. [Google Scholar] [CrossRef]
- Aritajat, S.; Wutteerapol, S.; Saenphet, K. Anti-diabetic effect of Thunbergia laurifolia Linn. aqueous extract. Southeast Asian J. Trop. Med. Public Health 2004, 35, 53–58. [Google Scholar]
- Junsi, M.; Takahashi Yupanqui, C.; Usawakesmanee, W.; Slusarenko, A.; Siripongvutikorn, S. Thunbergia laurifolia leaf extract increased levels of antioxidant enzymes and protected human cell-lines in vitro against cadmium. Antioxidants 2020, 9, 47. [Google Scholar] [CrossRef]
- Vongthip, W.; Sillapachaiyaporn, C.; Kim, K.-W.; Sukprasansap, M.; Tencomnao, T. Thunbergia laurifolia Leaf Extract Inhibits Glutamate-Induced Neurotoxicity and Cell Death through Mitophagy Signaling. Antioxidants 2021, 10, 1678. [Google Scholar] [CrossRef]
- Oonsivilai, R.; Cheng, C.; Bomser, J.; Ferruzzi, M.G.; Ningsanond, S. Phytochemical profiling and phase II enzyme-inducing properties of Thunbergia laurifolia Lindl. (RC) extracts. J. Ethnopharmacol. 2007, 114, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Oonsivilai, R. Functional and Nutraceutical Properties of Rang Chuet (Thunbergia laurifolia lindl.) Extracts. Ph.D. Thesis, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 2006. [Google Scholar]
- Rojsanga, P.; Sithisarn, P.; Sanguansataya, T. Simultaneous determination of caffeic acid and vitexin contents in Thunbergia laurifolia leaf extracts collected from different provinces in Thailand by HPLC. Pharm. Sci. Asia 2018, 45, 205–212. [Google Scholar] [CrossRef]
- Ruangpayungsak, N.; Sithisarn, P.; Rojsanga, P. High performance liquid chromatography fingerprinting and chemometric analysis of antioxidant quality of Thunbergia laurifolia leaves. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 713–721. [Google Scholar] [CrossRef]
- Suwanchaikasem, P.; Chaichantipyuth, C.; Sukrong, S. Antioxidant-guided isolation of rosmarinic acid, a major constituent from Thunbergia laurifolia, and its use as a bioactive marker for standardization. Chiang Mai J. Sci. 2014, 41, 117–127. [Google Scholar]
- Woottisin, N.; Kongkiatpaiboon, S.; Sukprasert, S.; Sathirakul, K. Development and Validation of Stability Indicating HPLC Method for Determination of Caffeic Acid, Vitexin and Rosmarinic Acid in Thunbergia laurifolia Leaf Extract. Pharmacogn. J. 2020, 12, 611–618. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A Comprehensive Review of Rosmarinic Acid: From Phytochemistry to Pharmacology and Its New Insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Alagawany, M.; El-Hack, M.E.A.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic acid–human pharmacokinetics and health benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Sik, B.; Kapcsándi, V.; Székelyhidi, R.; Hanczné, E.L.; Ajtony, Z. Recent advances in the analysis of rosmarinic acid from herbs in the Lamiaceae family. Nat. Prod. Commun. 2019, 14, 1934578X19864216. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Rui, T.; Kang, A.; Li, G.; Fu, T.; Li, J.; Di, L.; Cai, B. Pharmacokinetics of rosmarinic acid in rats by LC-MS/MS: Absolute bioavailability and dose proportionality. RSC Adv. 2017, 7, 9057–9063. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Yasuda, A.; Muto, Y.; Hiyoshi, T.; Takano, H.; Yoshikawa, T.; Terao, J. Absorption, metabolism, degradation and urinary excretion of rosmarinic acid after intake of Perilla frutescens extract in humans. Eur. J. Nutr. 2005, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Therapeutic and nutraceutical potential of rosmarinic acid—Cytoprotective properties and pharmacokinetic profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 1799–1806. [Google Scholar] [CrossRef]
- Sim, D.S.M. Drug absorption and bioavailability. In Pharmacological Basis of Acute Care; Springer: Cham, Switzerland, 2015; pp. 17–26. [Google Scholar]
- Pérez-Sánchez, A.; Borras-Linares, I.; Barrajón-Catalán, E.; Arráez-Román, D.; Gonzalez-Alvarez, I.; Ibanez, E.; Segura-Carretero, A.; Bermejo, M.; Micol, V. Evaluation of the intestinal permeability of rosemary (Rosmarinus officinalis L.) extract polyphenols and terpenoids in Caco-2 cell monolayers. PLoS ONE 2017, 12, e0172063. [Google Scholar] [CrossRef]
- Huang, J.; Chen, P.X.; Rogers, M.A.; Wettig, S.D. Investigating the phospholipid effect on the bioaccessibility of rosmarinic acid-phospholipid complex through a dynamic gastrointestinal in vitro model. Pharmaceutics 2019, 11, 156. [Google Scholar] [CrossRef]
- Blažević, T.; Reznicek, G.; Ding, L.; Yang, G.; Haiss, P.; Heiss, E.H.; Dirsch, V.M.; Liu, R. Short Chain (≤C4) Esterification Increases Bioavailability of Rosmarinic Acid and Its Potency to Inhibit Vascular Smooth Muscle Cell Proliferation. Front. Pharmacol. 2021, 2355, 609756. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Chitchumroonchokchai, C.; Riedl, K.M.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J. Nutr. 2012, 142, 675–680. [Google Scholar] [CrossRef]
- Dechwongya, P.; Limpisood, S.; Boonnak, N.; Mangmool, S.; Takeda-Morishita, M.; Kulsirirat, T.; Rukthong, P.; Sathirakul, K. The Intestinal Efflux Transporter Inhibition Activity of Xanthones from Mangosteen Pericarp: An In Silico, In Vitro and Ex Vivo Approach. Molecules 2020, 25, 5877. [Google Scholar] [CrossRef]
- Ahmed, I.; Leach, D.N.; Wohlmuth, H.; De Voss, J.J.; Blanchfield, J.T. Caco-2 cell permeability of flavonoids and saponins from gynostemma pentaphyllum: The immortal herb. ACS Omega 2020, 5, 21561–21569. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Herranz-López, M.; Barrajón-Catalán, E.; Arráez-Román, D.; Gonzálezlvarez, I.; Bermejo, M.; Gutiérrez, A.F.; Micol, V.; Segura-Carretero, A. Permeability study of polyphenols derived from a phenolic-enriched Hibiscus sabdariffa extract by UHPLC-ESI-UHR-Qq-TOF-MS. Int. J. Mol. Sci. 2015, 16, 18396–18411. [Google Scholar] [CrossRef]
- Bernardi, S.; Del Bo’, C.; Marino, M.; Gargari, G.; Cherubini, A.; Andrés-Lacueva, C.; Hidalgo-Liberona, N.; Peron, G.; González-Dominguez, R.; Kroon, P. Polyphenols and intestinal permeability: Rationale and future perspectives. J. Agric. Food Chem. 2020, 68, 1816–1829. [Google Scholar] [CrossRef]
- Hithamani, G.; Kizhakayil, D.; Srinivasan, K. Uptake of phenolic compounds from plant foods in human intestinal Caco-2 cells. J. Biosci. 2017, 42, 603–611. [Google Scholar] [CrossRef]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of rosmarinic acid in intestinal Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2005, 69, 583–591. [Google Scholar] [CrossRef]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.-H.; Zhao, J.; Zhang, W.-D.; Ma, B.-L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: Focus on natural occurring nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Qiang, Z.; Ye, Z.; Hauck, C.; Murphy, P.A.; McCoy, J.-A.; Widrlechner, M.P.; Reddy, M.B.; Hendrich, S. Permeability of rosmarinic acid in Prunella vulgaris and ursolic acid in Salvia officinalis extracts across Caco-2 cell monolayers. J. Ethnopharmacol. 2011, 137, 1107–1112. [Google Scholar] [CrossRef]
- Achour, M.; Saguem, S.; Sarriá, B.; Bravo, L.; Mateos, R. Bioavailability and metabolism of rosemary infusion polyphenols using Caco-2 and HepG2 cell model systems. J. Sci. Food Agric. 2018, 98, 3741–3751. [Google Scholar] [CrossRef]
- Falé, P.L.; Ascensão, L.; Serralheiro, M.L. Effect of luteolin and apigenin on rosmarinic acid bioavailability in Caco-2 cell monolayers. Food Funct. 2013, 4, 426–431. [Google Scholar] [CrossRef]
- EMA. Guideline on Bioanalytical Method Validation; Committee for Medicinal Products for Human Use (CHMP): London, UK, 2011. [Google Scholar]
- Järvinen, E.; Deng, F.; Kiander, W.; Sinokki, A.; Kidron, H.; Sjöstedt, N. The Role of Uptake and Efflux Transporters in the Disposition of Glucuronide and Sulfate Conjugates. Front. Pharmacol. 2022, 12, 802539. [Google Scholar] [CrossRef]
- Awortwe, C.; Fasinu, P.; Rosenkranz, B. Application of Caco-2 cell line in herb-drug interaction studies: Current approaches and challenges. J. Pharm Pharm. Sci. 2014, 17, 1. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Iwasa, K.; Nagai, T.; Kobayashi, S.; Nakamura, H.; Yamada, M. Pharmacokinetics, Safety and Tolerability of Melissa officinalis Extract which Contained Rosmarinic Acid in Healthy Individuals: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0126422. [Google Scholar] [CrossRef]
- Rukthong, P.; Sereesongsang, N.; Kulsirirat, T.; Nimprayoon, B.; Sathirakul, K. Effect of α-mangostin on enhanced transdermal bioavailability of Gartanin via efflux transporters. J. Bioequiv. Availab. 2017, 9, 455–462. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Shekhawat, P.B.; Pokharkar, V.B. Understanding peroral absorption: Regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm. Sin. B 2017, 7, 260–280. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Casanova, F.; Estevinho, B.; Santos, L. Preliminary studies of rosmarinic acid microencapsulation with chitosan and modified chitosan for topical delivery. Powder Technol. 2016, 297, 44–49. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef]
- Wang, X.-D.; Meng, M.-X.; Gao, L.-B.; Liu, T.; Xu, Q.; Zeng, S. Permeation of astilbin and taxifolin in Caco-2 cell and their effects on the P-gp. Int. J. Pharm. 2009, 378, 1–8. [Google Scholar] [CrossRef]
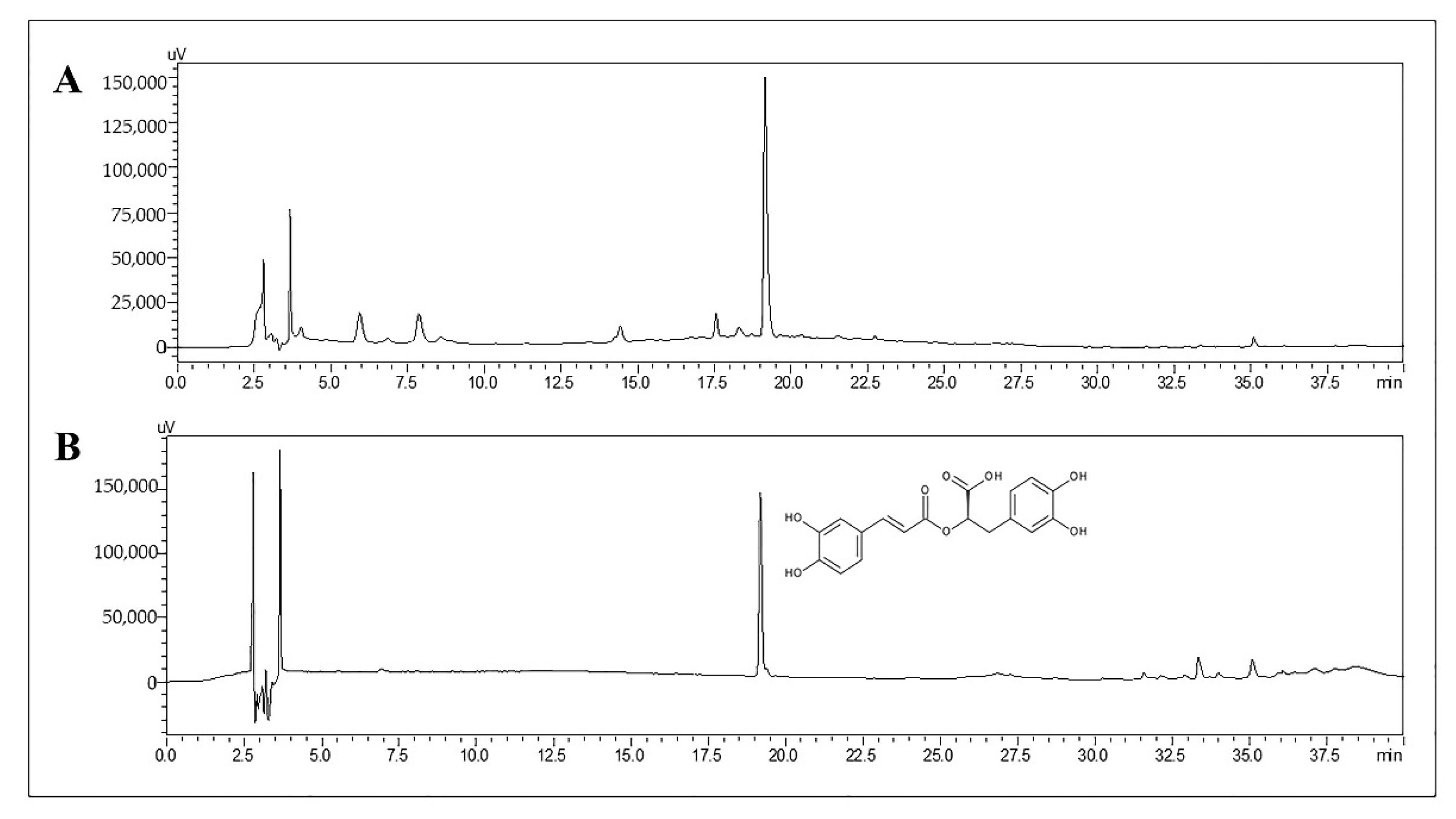



| RA Concentration (µg/mL) | RT, 24 h | −80 °C, 30 Days |
|---|---|---|
| 0.05 | 99% | 97% |
| 0.8 | 99% | 98% |
| Tests | Papp × 10−6 (cm/s) (before Deconjugation) | ER | Papp × 10−6 (cm/s) (after Deconjugation) | ER | ||
|---|---|---|---|---|---|---|
| A-B Direction | B-A Direction | A-B Direction | B-A Direction | |||
| RA in TL extract | ||||||
| 250 µg/mL | 4.7 ± 0.2 | 6.1 ± 0.4 ** | 1.3 | 46.1 ± 1.2 | 19.9 ± 0.3 *** | 0.4 |
| 500 µg/mL | 5.3 ± 0.3 # | 6.1 ± 0.1 ** | 1.1 | 27.3 ± 3.3 ## | 12.7 ± 0.2 *,### | 0.5 |
| RA in pure RA | ||||||
| 3.267 µg/mL | N.A. | N.A. | - | 41.2 ± 2.3 | 20.4 ± 5.2 ** | 0.5 |
| 6.808 µg/mL | N.A. | N.A. | - | 22.9 ± 1.0 ### | 6.2 ± 0.2 ***,# | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woottisin, N.; Sukprasert, S.; Kulsirirat, T.; Tharavanij, T.; Sathirakul, K. Evaluation of the Intestinal Permeability of Rosmarinic Acid from Thunbergia laurifolia Leaf Water Extract in a Caco-2 Cell Model. Molecules 2022, 27, 3884. https://doi.org/10.3390/molecules27123884
Woottisin N, Sukprasert S, Kulsirirat T, Tharavanij T, Sathirakul K. Evaluation of the Intestinal Permeability of Rosmarinic Acid from Thunbergia laurifolia Leaf Water Extract in a Caco-2 Cell Model. Molecules. 2022; 27(12):3884. https://doi.org/10.3390/molecules27123884
Chicago/Turabian StyleWoottisin, Nanthakarn, Sophida Sukprasert, Thitianan Kulsirirat, Thipaporn Tharavanij, and Korbtham Sathirakul. 2022. "Evaluation of the Intestinal Permeability of Rosmarinic Acid from Thunbergia laurifolia Leaf Water Extract in a Caco-2 Cell Model" Molecules 27, no. 12: 3884. https://doi.org/10.3390/molecules27123884
APA StyleWoottisin, N., Sukprasert, S., Kulsirirat, T., Tharavanij, T., & Sathirakul, K. (2022). Evaluation of the Intestinal Permeability of Rosmarinic Acid from Thunbergia laurifolia Leaf Water Extract in a Caco-2 Cell Model. Molecules, 27(12), 3884. https://doi.org/10.3390/molecules27123884








