Supercritical CO2 Extract from Microalga Tetradesmus obliquus: The Effect of High-Pressure Pre-Treatment
Abstract
:1. Introduction
2. Results and Discussion
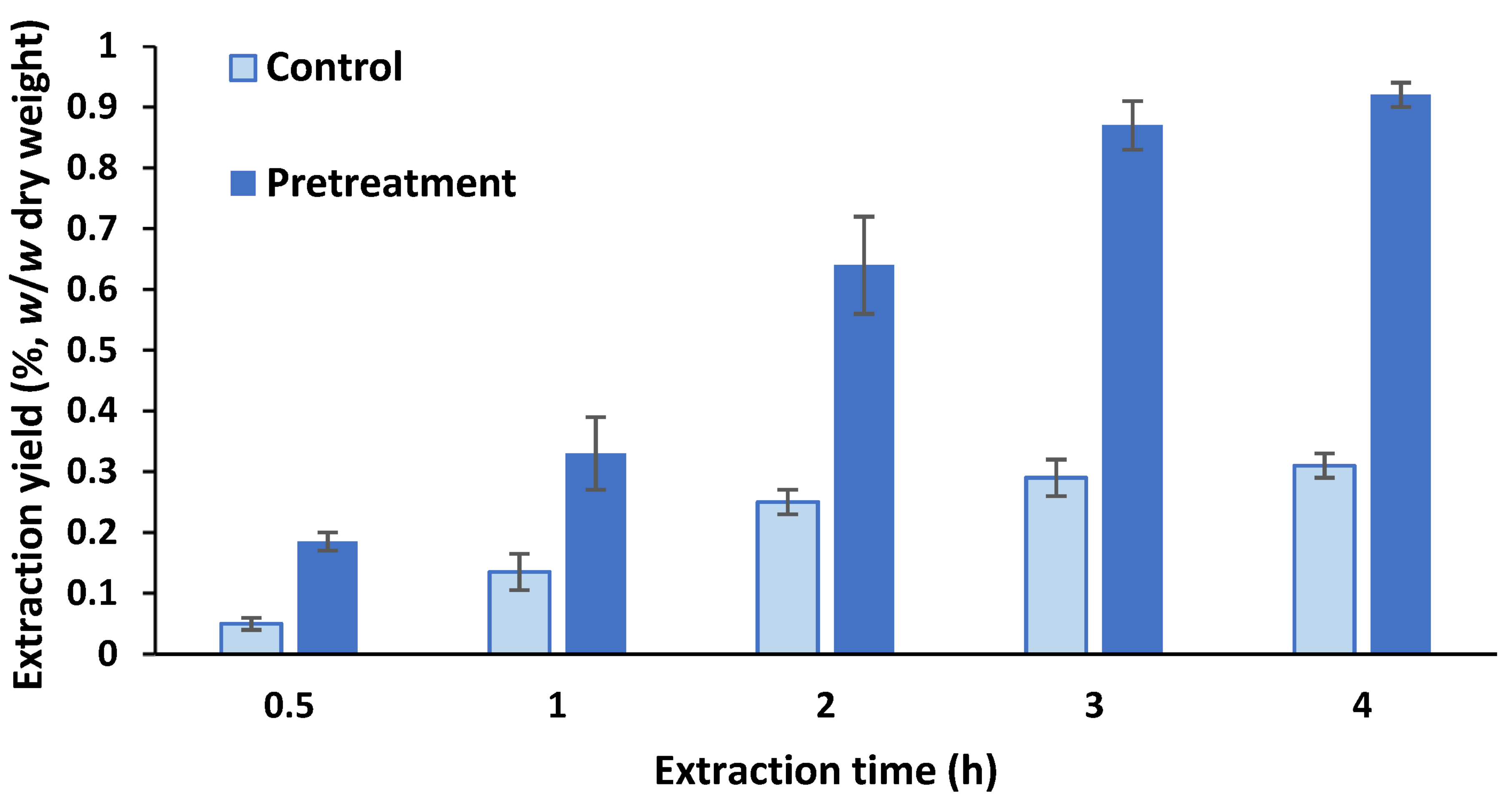
2.1. Supercritical Carbon Dioxide (ScCO2) Extraction
2.2. Microbiological Profile
2.3. Determination of Metal Content
3. Materials and Methods
3.1. Microalgae Cultivation
3.2. Supercritical Carbon Dioxide Extraction
3.3. Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry (UHPLC-ESI-HRMS) Analyses of CO2 Extract
3.4. Determination of Moisture and Lipid Content
3.5. Microbiological Analysis
3.6. Determination of Metal Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, A.; Reis, A.; Vidovic, S.; Vladic, J.; Gkelis, S.; Melkonyan, L.; Avetisova, G.; Congestri, R.; Acién, G.; Muñoz, R.; et al. Combining microalgae-based wastewater treatment with biofuel and bio-based production in the frame of a biorefinery. In Grand Challenges in Algae Biotechnology; Springer: Cham, Switzerland, 2019; pp. 319–369. [Google Scholar]
- Katiyar, R.; Arora, A. Health promoting functional lipids from microalgae pool: A review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2018, 59, 1880–1902. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Prasad, R.; Ong, H.C.; Araujo, E.S.; Shabnam, N.; Gálvez, A.O. A multidisciplinary review of Tetradesmus obliquus: A microalga suitable for large-scale biomass production and emerging environmental applications. Rev. Aquac. 2021, 13, 1594–1618. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.A.; Vladic, J.; Vidović, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V.; et al. Scenedesmus obliquus microalga-based biorefinery—From brewery effluent to bioactive compounds, biofuels and biofertilizers—Aiming at a circular bioeconomy. Biofuels Bioprod. Biorefin. 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Patias, L.D.; Fernandes, A.S.; Petry, F.C.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res. Int. 2017, 100, 260–266. [Google Scholar] [CrossRef]
- Gavarić, A.; Vidović, S.; Aladić, K.; Jokić, S.; Vladić, J. Supercritical CO2 extraction of Marrubium vulgare: Intensification of marrubiin. RSC Adv. 2021, 11, 9067–9075. [Google Scholar] [CrossRef]
- Solana, M.; Rizza, C.S.; Bertucco, A. Exploiting microalgae as a source of essential fatty acids by supercritical fluid extraction of lipids: Comparison between Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina. J. Supercrit. Fluids 2014, 92, 311–318. [Google Scholar] [CrossRef]
- Guedes, A.C.; Gião, M.S.; Matias, A.A.; Nunes, A.V.M.; Pintado, M.E.; Duarte, C.M.M.; Malcata, F.X. Supercritical fluid extraction of carotenoids and chlorophylls a, b and c, from a wild strain of Scenedesmus obliquus for use in food processing. J. Food Eng. 2013, 116, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Chronopoulou, L.; Dal Bosco, C.; Di Caprio, F.; Prosini, L.; Gentili, A.; Pagnanelli, F.; Palocci, C. Extraction of carotenoids and fat-soluble vitamins from Tetradesmus Obliquus microalgae: An optimized approach by using supercritical CO2. Molecules 2019, 24, 2581. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, J.; Igl, N.; Tippelt, M.; Stege, A.; Qoura, F.; Sohling, U.; Brück, T. Extraction of microalgae derived lipids with supercritical carbon dioxide in an industrial relevant pilot plant. Bioprocess Biosyst. Eng. 2017, 40, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, L.; Molnar Jazić, J.; Ferreira, A.; Maletić, S.; Cvetković, D.; Vidović, S.; Vladić, J. Green approach for the valorization of microalgae Tetradesmus obliquus. Sustain. Chem. Pharm. 2021, 24, 100556. [Google Scholar] [CrossRef]
- Soares, G.C.; Learmonth, D.A.; Vallejo, M.C.; Davila, S.P.; González, P.; Sousa, R.A.; Oliveira, A.L. Supercritical CO2 technology: The next standard sterilization technique? Mater. Sci. Eng. C 2019, 99, 520–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, X.; Wang, Z.; Sun, Y.; Zhu, S.; Li, L.; Lv, P. Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp. Renew. Energy 2018, 125, 1049–1057. [Google Scholar] [CrossRef]
- Vladić, J.; Duarte, A.R.C.; Radman, S.; Simić, S.; Jerković, I. Enzymatic and microwave pretreatments and supercritical CO2 extraction for improving extraction efficiency and quality of Origanum vulgare L. spp. hirtum extracts. Plants 2022, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Evaluation of various cell drying and disruption techniques for sustainable metabolite extractions from microalgae grown in wastewater: A multivariate approach. J. Clean. Prod. 2018, 182, 634–643. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, I.L.D.; Kentish, S.E.; Scales, P.J.; Martin, G.J.O. Low solvent, low temperature method for extracting biodiesel lipids from concentrated microalgal biomass. Bioresour. Technol. 2013, 148, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.L.; Soares, J.; Vieira, B.B.; de Leite, M.O.; Rocha, D.N.; Aleixo, P.E.; Falconí, J.H.H.; de Xavier Júnior, M.L.; Albino, L.F.T.; Martins, M.A. Pilot-scale biorefining of Scenedesmus obliquus for the production of lipids and proteins. Sep. Purif. Technol. 2021, 270, 118775. [Google Scholar] [CrossRef]
- Zbinden, M.D.A.; Sturm, B.S.M.; Nord, R.D.; Carey, W.J.; Moore, D.; Shinogle, H.; Stagg-Williams, S.M. Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol. Bioeng. 2013, 110, 1605–1615. [Google Scholar] [CrossRef]
- Gaspar, F.; Santos, R.; King, M.B. Disruption of glandular trichomes with compressed CO2: Alternative matrix pre-treatment for CO2 extraction of essential oils. J. Supercrit. Fluids 2001, 21, 11–22. [Google Scholar] [CrossRef]
- Meyer, F.; Jaeger, P.; Eggers, R.; Stamenic, M.; Milovanovic, S.; Zizovic, I. Effect of CO2 pre-treatment on ScCO2 extraction of natural material. Chem. Eng. Process. Process Intensif. 2012, 56, 37–45. [Google Scholar] [CrossRef]
- Vidović, S.; Zeković, Z.; Marošanović, B.; Todorović, M.P.; Vladić, J. Influence of pre-treatments on yield, chemical composition and antioxidant activity of Satureja montana extracts obtained by supercritical carbon dioxide. J. Supercrit. Fluids 2014, 95, 468–473. [Google Scholar] [CrossRef]
- Pantami, H.A.; Bustamam, M.S.A.; Lee, S.Y.; Ismail, I.S.; Faudzi, S.M.M.; Nakakuni, M.; Shaari, K. Comprehensive GCMS and LC-MS/MS metabolite profiling of Chlorella vulgaris. Mar. Drugs 2020, 18, 367. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E. Noncatalytic biodiesel synthesis under supercritical conditions. Processes 2021, 9, 138. [Google Scholar] [CrossRef]
- Tobar, M.; Núñez, G.A. Supercritical transesterification of microalgae triglycerides for biodiesel production: Effect of alcohol type and co-solvent. J. Supercrit. Fluids 2018, 137, 50–56. [Google Scholar] [CrossRef]
- Heo, H.J.; Park, Y.J.; Suh, Y.M.; Choi, S.J.; Kim, M.J.; Cho, H.Y.; Chang, Y.J.; Hong, B.; Kim, H.K.; Kim, E.; et al. Effects of oleamide on choline acetyltransferase and cognitive activities. Biosci. Biotechnol. Biochem. 2003, 67, 1284–1291. [Google Scholar] [CrossRef] [Green Version]
- Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive effects of a fermented dairy product against Alzheimer’s disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS ONE 2015, 10, e0118512. [Google Scholar] [CrossRef] [Green Version]
- Gilbert-López, B.; Mendiola, J.A.; van den Broek, L.A.M.; Houweling-Tan, B.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Green compressed fluid technologies for downstream processing of Scenedesmus obliquus in a biorefinery approach. Algal Res. 2017, 24, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Shailaja, V.L.; Christina, V.S.; Mohanapriya, C.D.; Sneha, P.; Lakshmi Sundaram, R.; Magesh, R.; George Priya Doss, C.; Gnanambal, K.M.E. A natural anticancer pigment, Pheophytin a, from a seagrass acts as a high affinity human mitochondrial translocator protein (TSPO) ligand, in silico, to reduce mitochondrial membrane Potential (∆ψmit) in adenocarcinomic A549 cells. Phytomedicine 2019, 61, 152858. [Google Scholar] [CrossRef]
- Wang, S.Y.; Tseng, C.P.; Tsai, K.C.; Lin, C.F.; Wen, C.Y.; Tsay, H.S.; Sakamoto, N.; Tseng, C.H.; Cheng, J.C. Bioactivity-guided screening identifies pheophytin a as a potent anti-hepatitis C virus compound from Lonicera hypoglauca Miq. Biochem. Biophys. Res. Commun. 2009, 385, 230–235. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K. Potent anti-inflammatory activity of pheophytin a derived from edible green alga, Enteromorpha prolifera (Sujiao-nori). Int. J. Immunopharmacol. 1997, 19, 355–358. [Google Scholar] [CrossRef]
- Hiqashi-Okaj, K.; Otani, S.; Okai, Y. Potent suppressive effect of a Japanese edible seaweed, Enteromorpha prolifera (Sujiao-nori) on initiation and promotion phases of chemically induced mouse skin tumorigenesis. Cancer Lett. 1999, 140, 21–25. [Google Scholar] [CrossRef]
- Ina, A.; Hayashi, K.I.; Nozaki, H.; Kamei, Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007, 25, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Uquiche, E.; Antilaf, I.; Millao, S. Enhancement of pigment extraction from B. braunii pretreated using CO2 rapid depressurization. Braz. J. Microbiol. 2016, 47, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Mäki-Arvela, P.; Hachemi, I.; Murzin, D.Y. Comparative study of the extraction methods for recovery of carotenoids from algae: Extraction kinetics and effect of different extraction parameters. J. Chem. Technol. Biotechnol. 2014, 89, 1607–1626. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, C.; Saettler, A.; Schroeder, K.R.; Roegner, M. Use of Myxoxanthophyll and/or Echinenon for the Prophylactic and/or Therapeutic Treatment of Undesirable Physical Conditions Caused or Promoted by Oxidative Processes—Google Patents. DE10046838A1, 4 April 2002. [Google Scholar]
- Ghosh, T.; Bhayani, K.; Paliwal, C.; Maurya, R.; Chokshi, K.; Pancha, I.; Mishra, S. Cyanobacterial pigments as natural anti-hyperglycemic agents: An in vitro study. Front. Mar. Sci. 2016, 3, 146. [Google Scholar] [CrossRef] [Green Version]
- Schwartzel, E.H.; Cooney, J.J. Isolation and Identification of Echinenone from Micrococcus roseus. J. Bacteriol. 1970, 104, 272–274. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, H.; Watanabe, M.M.; Kaya, K. Echinenone production of a dark red-coloured strain of Botryococcus braunii. J. Appl. Phycol. 2012, 24, 973–977. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, A new therapeutic option for neurological diseases? In vitro neuroprotective and anti-inflammatory activities of a monoterpenoid lactone isolated from Codium tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Kim, H.S.; Fernando, I.P.S.; Ahn, G. (−)-Loliolide isolated from Sargassum horneri protects against fine dust-induced oxidative stress in human keratinocytes. Antioxidants 2020, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J.; Maki, N. New Loliolide Derivatives from the Brown Alga Undaria pinnatifida. J. Nat. Prod. 2001, 65, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Cikoš, A.M.; Babić, S.; Čižmek, L.; Bojanić, K.; Aladić, K.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T.; Čož-Rakovac, R.; et al. Bioprospecting of less-polar constituents from endemic brown macroalga Fucus virsoides J. Agardh from the Adriatic sea and targeted antioxidant effects in vitro and in vivo (zebrafish model). Mar. Drugs 2021, 19, 235. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Cikoš, A.M.; Flanjak, I.; Babić, S.; Čižmek, L.; Šubarić, D.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Less polar compounds and targeted antioxidant potential (in vitro and in vivo) of Codium adhaerens C. Agardh 1822. Pharmaceuticals 2021, 14, 944. [Google Scholar] [CrossRef]
- Ochi, M.; Watanabe, M.; Miura, I.; Taniguchi, M.; Tokoroyama, T. Amijiol, isoamijiol, and 14-deoxyamijiol, three new diterpenoids from the brown seaweed Dictyota linearis. Chem. Lett. 1980, 9, 1229–1232. [Google Scholar] [CrossRef]
- De Freitas Araújo, M.G.; Bauab, T.M. Microbial quality of medicinal plant materials. In Latest Research into Quality Control; IntechOpen: London, UK, 2012; ISBN 978-953-51-0868-9. [Google Scholar]
- Omar, A.M.; Norsalwani, T.T.; Asmah, M.S.; Badrulhisham, Z.Y.; Easa, A.M.; Omar, F.M.; Hossain, M.S.; Zuknik, M.H.; Norulaini, N.N. Implementation of the supercritical carbon dioxide technology in oil palm fresh fruits bunch sterilization: A review. J. CO2 Util. 2018, 25, 205–215. [Google Scholar] [CrossRef]
- Buszewski, B.; Wrona, O.; Mayya, R.P.; Zakharenko, A.M.; Kalenik, T.K.; Golokhvast, K.S.; Piekoszewskih, W.; Rafińska, K. The potential application of supercritical CO2 in microbial inactivation of food raw materials and products. Crit. Rev. Food Sci. Nutr. 2021, 1–14. [Google Scholar] [CrossRef]
- Norulaini, N.N.; Ahmad, A.; Omar, F.M.; Banana, A.A.S.; Zaidul, I.M.; Kadir, M.O.A. Sterilization and extraction of palm oil from screw pressed palm fruit fiber using supercritical carbon dioxide. Sep. Purif. Technol. 2008, 60, 272–277. [Google Scholar] [CrossRef]
- Hosseinizand, H.; Sokhansanj, S.; Lim, C.J. Studying the drying mechanism of microalgae Chlorella vulgaris and the optimum drying temperature to preserve quality characteristics. Dry. Technol. 2018, 36, 1049–1060. [Google Scholar] [CrossRef]
- de Farias Neves, F.; Demarco, M.; Tribuzi, G. Drying and quality of microalgal powders for human alimentation. In Microalgae—From Physiology to Application; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- European Parliament; The Council of the EU. Directive 2002/32/EC of The European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed; European Parliament: Strasbourg, France, 2002; pp. 1–15.
- Council of the European Communities Protection of the Environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Communities 1986, 4, 6–12.
- Miranda, J.R.; Passarinho, P.C.; Gouveia, L. Bioethanol production from Scenedesmus obliquus sugars: The influence of photobioreactors and culture conditions on biomass production. Appl. Microbiol. Biotechnol. 2012, 96, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0.95. ISO: Geneva, Switzerland, 2008.
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. ISO: Geneva, Switzerland, 2008.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. ISO: Geneva, Switzerland, 2017.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucur. ISO: Geneva, Switzerland, 2001.
- USEPA EPA Method 3051A; Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils, Revision 1. USEPA: Washington, DC, USA, 2007; pp. 119–122.
- ISO 11352:2012; Water Quality—Estimation of Measurement Uncertainty Based on Validation and Quality Control Data. ISO: Geneva, Switzerland, 2012.

| Compound | Structure | tR (min) | Monoisotopic Mass | [M + H]+ | Mass Difference (ppm) | Area (Counts) | |
|---|---|---|---|---|---|---|---|
| Control | Pre-Treatment | ||||||
| Pigments | |||||||
| Pheophytin a | C55H74N4O5 | 20.2 | 870.56592 | 871.5732 | 3.9 | 23,149 | 13,838 |
| Vaucheriaxanthin | C40H58O4 | 14.0 | 602.433533 | 603.44079 | 5.2 | 72,962 | 70,087 |
| Echinenone | C40H54O | 15.2 | 550.417470 | 551.42474 | 4.0 | 10,175 | 31,354 |
| Myxol 2’-fucoside | C46H66O7 | 19.1 | 730.480835 | 731.48813 | 2.5 | 72,962 | 14,927 |
| Phytoene epoxide | C40H64O | 20.2 | 560.495728 | 561.50299 | 2.5 | 72,962 | 138,351 |
| Fatty Acid Derivatives | |||||||
| Palmitamide | C16H33NO | 13.8 | 255.25621 | 256.26349 | 2.4 | 816,302 | 1,062,902 |
| 2,3-Dihydroxypropyl palmitate | C19H38O4 | 14.2 | 330.27701 | 331.28429 | 1.9 | 18,191 | 19,599 |
| Oleamide | C18H35NO | 14.2 | 281.27185 | 282.27914 | 1.7 | 11,626,747 | 13,605,089 |
| 1,3-Dihydroxy-2-propanyl 5,8,11,14-icosatetraenoate | C23H38O4 | 14.5 | 378.27701 | 379.28429 | 0.6 | 35,250 | 10,435 |
| Erucamide | C22H43NO | 16.0 | 337.33447 | 338.34174 | 2.0 | 51,790 | 91,297 |
| 3-Hydroxy-1,2-propanediyl bis(9-octadecenoate) | C39H72O5 | 19.4 | 620.53798 | 621.54525 | 3.6 | 184,023 | 43,933 |
| 3-Phorbinepropanoic acid, 9-acetyl-14-ethylidene-13,14-dihydro-21-(methoxycarbonyl)-4,8,13,18-tetramethyl-20-oxo-, 3,7,11,15-tetramethyl-2-hexadecen-1-yl ester | C55H74N4O6 | 20.0 | 886.56085 | 887.56811 | 3.3 | 11,791 | 1,978,013 |
| Methyl (3R,10Z,14Z,20Z,22S,23S)-12-ethyl-3-hydroxy-13,18,22,27-tetramethyl-5-oxo-23-(3-oxo-3-{[(2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-yl]oxy}propyl)-17-vinyl-4-oxa-8,24,25,26-tetraazahexacycl; o[19.2.1.16,9.111,14.116,19.02,7]heptacosa-1(24),2(7),6(27),8,10,12,14,16,18,20-decaene-3-carboxylate | C55H74N4O7 | 20.0 | 902.55575 | 903.56303 | 3.6 | 16,412 | 193,151 |
| Triacylglycerols | |||||||
| Triacylglycerol 54:7 | C57H96O6 | 21.5 | 876.72069 * | 877.72797 | 3.5 | 2,529,250 | 82,339 |
| Triacylglycerol 54:6 | C57H98O6 | 21.9 | 878.73634 | 879.74362 | 2.1 | 7,576,059 | 99,112 |
| Triacylglycerol 54:4 | C57H102O6 | 22.0 | 882.76764 | 883.77492 | 2.4 | 3,323,899 | 93,081 |
| Triacylglycerol 54:5 | C57H100O6 | 22.4 | 880.75199 | 881.75927 | 1.2 | 8,851,390 | 182,825 |
| Triacylglycerol 54:3 | C57H104O6 | 22.5 | 884.78329 | 885.79057 | 2.1 | 5,891,554 | 8953 |
| Diacylglycerophosphocholines and Diacylglycerophosphoserines | |||||||
| Phosphatidylcholine 33:2 | C41H78NO8P | 17.4 | 743.54651 | 744.55378 | 1.7 | 10,998 | 11,217 |
| Phosphatidylserine 40:2 | C46H86NO10P | 18.1 | 843.59893 | 844.60621 | 1.0 | 37,610 | |
| Phosphatidylcholine 38:3 | C46H86NO8P | 19.7 | 811.60911 | 812.61638 | 0.6 | 132,581 | |
| Phosphatidylcholine 37:2 | C45H86NO8P | 19.9 | 799.60911 | 800.61638 | 1.4 | 100,848 | 4865 |
| Phosphatidylcholine 38:2 | C46H88NO8P | 20.0 | 813.62476 | 814.63203 | 1.5 | 570,968 | 25,582 |
| Terpenes and Steroids | |||||||
| Loliolide | C11H16O3 | 6.4 | 196.10994 | 197.11722 | 0.1 | 173,169 | 506,025 |
| Isoamijiol oxidation product | C20H30O2 | 14.9 | 302.22458 | 303.23186 | 0.5 | 122,024 | 452,509 |
| Isoamijiol | C20H32O2 | 15.5 | 304.24023 | 305.24751 | 2.0 | 722,108 | 1,698,141 |
| (3β)-3-Hydroxystigmast-5-en-7-one | C29H48O2 | 17.5 | 428.36543 | 429.37271 | 3.5 | 53,409 | 195,727 |
| Sample | Number of Microorganisms | Molds and Yeasts | Enterobacteriaceae | Escherichia coli | Spores of Anaerobic Bacteria |
|---|---|---|---|---|---|
| Initial biomass | 910 × 104 | <10 | 49 × 103 | <40 | 240 × 102 |
| ScCO2-spent biomass | 310 × 104 | <10 | <10 | <10 | 76 × 102 |
| Metal | Initial Biomass (mg/kg) | ScCO2-Spent Biomass (mg/kg) |
|---|---|---|
| Cr | 2.67 | 2.72 |
| Mn | 1070 | 1050 |
| Fe | 2510 | 3530 |
| Co | 1.66 | 1.64 |
| Ni | 2.63 | 2.82 |
| Cu | 39.4 | 38.0 |
| Zn | 149 | 150 |
| As | <0.1 | <0.1 |
| Cd | 0.083 | 0.080 |
| Pb | 5.50 | 5.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vladić, J.; Jerković, I.; Radman, S.; Molnar Jazić, J.; Ferreira, A.; Maletić, S.; Gouveia, L. Supercritical CO2 Extract from Microalga Tetradesmus obliquus: The Effect of High-Pressure Pre-Treatment. Molecules 2022, 27, 3883. https://doi.org/10.3390/molecules27123883
Vladić J, Jerković I, Radman S, Molnar Jazić J, Ferreira A, Maletić S, Gouveia L. Supercritical CO2 Extract from Microalga Tetradesmus obliquus: The Effect of High-Pressure Pre-Treatment. Molecules. 2022; 27(12):3883. https://doi.org/10.3390/molecules27123883
Chicago/Turabian StyleVladić, Jelena, Igor Jerković, Sanja Radman, Jelena Molnar Jazić, Alice Ferreira, Snežana Maletić, and Luisa Gouveia. 2022. "Supercritical CO2 Extract from Microalga Tetradesmus obliquus: The Effect of High-Pressure Pre-Treatment" Molecules 27, no. 12: 3883. https://doi.org/10.3390/molecules27123883
APA StyleVladić, J., Jerković, I., Radman, S., Molnar Jazić, J., Ferreira, A., Maletić, S., & Gouveia, L. (2022). Supercritical CO2 Extract from Microalga Tetradesmus obliquus: The Effect of High-Pressure Pre-Treatment. Molecules, 27(12), 3883. https://doi.org/10.3390/molecules27123883










