On the Traceability of the Hazelnut Production Chain by Means of Trace Elements
Abstract
:1. Introduction
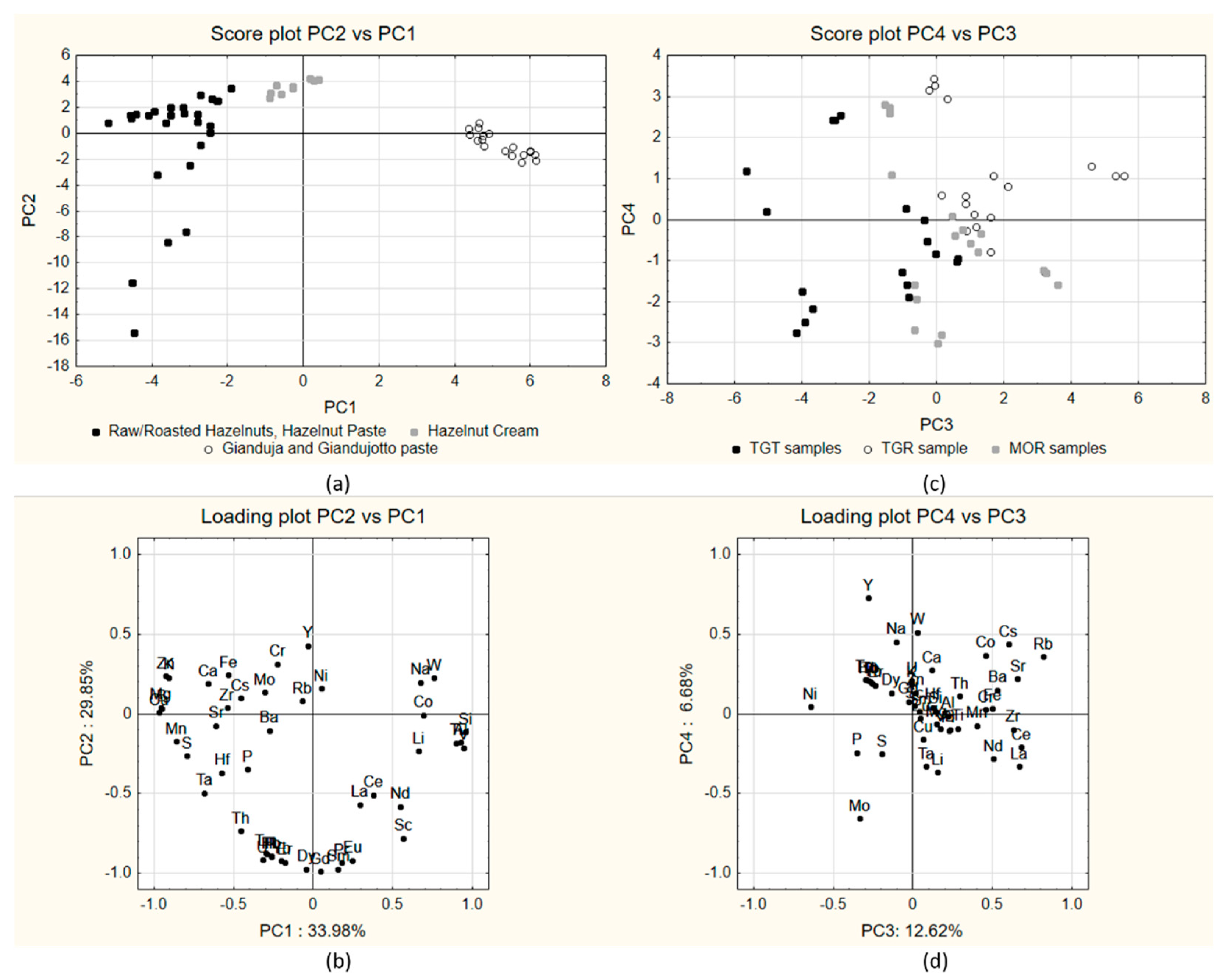
2. Results and Discussion
- Cluster 1, including products containing only hazelnuts (raw, roasted and paste, hereafter termed hazelnuts-only products), clustering at negative values of PC1 and negative and positive values on PC2 (black dots in figure);
- Cluster 2, including hazelnut cream samples, characterized by the presence of small amounts of cocoa powder, clustered at intermediate values on PC1 and positive scores on PC2;
- Cluster 3, including Gianduja and Gianduiotto paste samples (white dots in figure), characterized by higher amounts of cocoa powder and clustering mostly at positive values of PC1 and negative or slightly negative values of PC2.
2.1. Grouping by Process Stage
2.2. Grouping by Cultivar
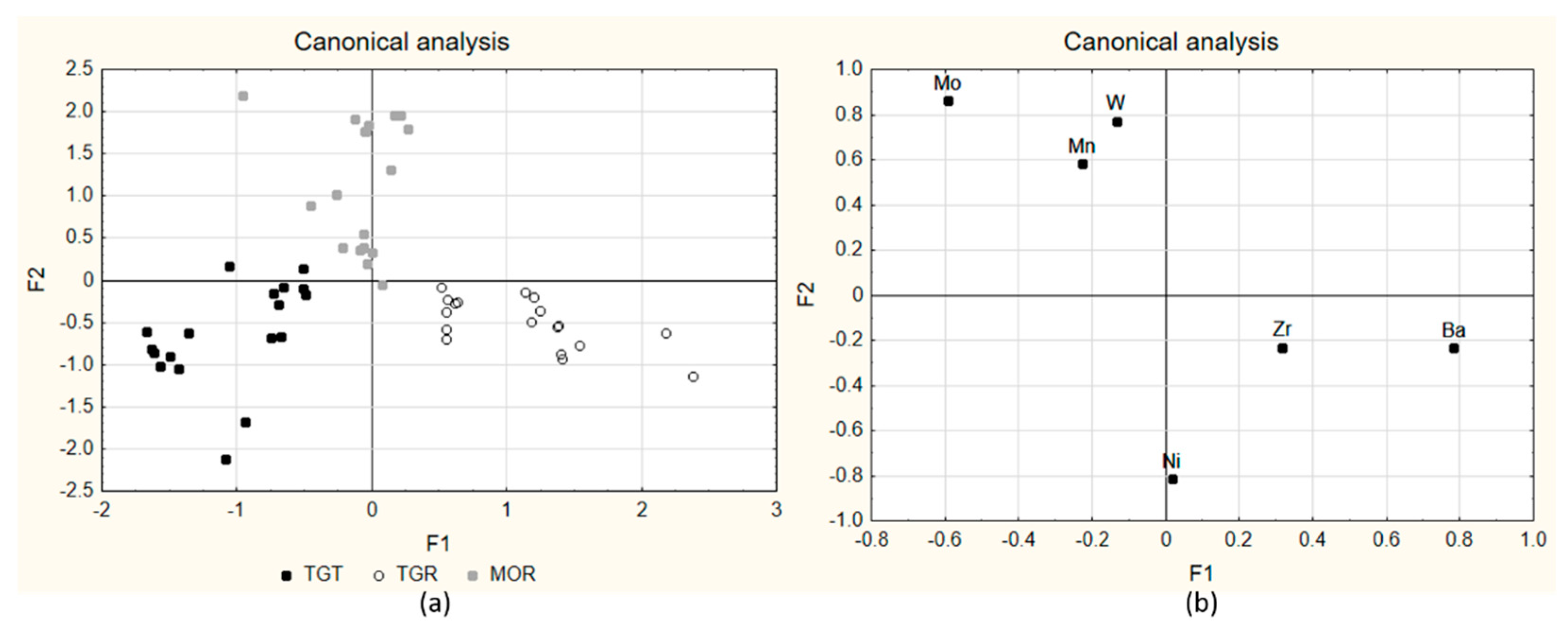
2.3. Verivication with Samples Prepared in Laboratory
3. Materials and Methods
3.1. Materials
3.2. Sample Collection
- Raw hazelnuts: 1 Kg of hazelnuts was collected and placed under vacuum;
- Roasted hazelnuts: after roasting, 1 Kg of hazelnuts was collected and placed under vacuum;
- Hazelnut paste: Roasted hazelnuts were mashed in order to obtain a paste that subsequently divided into a supernatant fraction rich in lipids and with lower solid fraction; both were kept in a polyethylene bottle;
- Hazelnut cream: After processing the hazelnut paste, 0.5 Kg of cream was taken and kept in a polyethylene bottle;
- Gianduja paste: After processing the hazelnut paste, 0.5 Kg of product was taken and kept in a polyethylene bottle;
- Gianduiotto paste: After processing the hazelnut paste, 0.5 Kg of product was taken and kept in a polyethylene bottle.
3.3. Sample Treatment
3.4. ICP-OES Analysis
3.5. ICP-MS Analysis
3.6. Analysis of Certified Samples
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aceto, M.; Calà, E.; Musso, D.; Regalli, N.; Oddone, M. A Preliminary Study on the Authentication and Traceability of Extra Virgin Olive Oil Made from Taggiasca Olives by Means of Trace and Ultra-Trace Elements Distribution. Food Chem. 2019, 298, 125047. [Google Scholar] [CrossRef] [PubMed]
- Aceto, M.; Gulino, F.; Calà, E.; Robotti, E.; Petrozziello, M.; Tsolakis, C.; Cassino, C. Authentication and Traceability Study on Barbera d’asti and Nizza Docg Wines: The Role of Trace-and Ultra-Trace Elements. Beverages 2020, 6, 63. [Google Scholar] [CrossRef]
- Aceto, M.; Robotti, E.; Oddone, M.; Baldizzone, M.; Bonifacino, G.; Bezzo, G.; Di Stefano, R.; Gosetti, F.; Mazzucco, E.; Manfredi, M.; et al. A Traceability Study on the Moscato Wine Chain. Food Chem. 2013, 138, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Aceto, M.; Musso, D.; Calà, E.; Arieri, F.; Oddone, M. Role of Lanthanides in the Traceability of the Milk Production Chain. J. Agric. Food Chem. 2017, 65, 4200–4208. [Google Scholar] [CrossRef] [PubMed]
- Böhme, K.; Calo-Mata, P.; Barros-Velázquez, J.; Ortea, I. Review of Recent DNA-Based Methods for Main Food-Authentication Topics. J. Agric. Food Chem. 2019, 67, 3854–3864. [Google Scholar] [CrossRef] [PubMed]
- Inaudi, P.; Giacomino, A.; Malandrino, M.; La Gioia, C.; Conca, E.; Karak, T.; Abollino, O. The Inorganic Component as a Possible Marker for Quality and for Authentication of the Hazelnut’s Origin. Int. J. Environ. Res. Public Health 2020, 17, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Król, K.; Gantner, M. Morphological Traits and Chemical Composition of Hazelnut from Different Geographical Origins: A Review. Agriculture 2020, 10, 375. [Google Scholar] [CrossRef]
- Oddone, M.; Aceto, M.; Baldizzone, M.; Musso, D.; Osella, D. Authentication and Traceability Study of Hazelnuts from Piedmont, Italy. J. Agric. Food Chem. 2009, 57, 3404–3408. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Coïsson, J.D.; Travaglia, F.; Bordiga, M.; Arlorio, M. Impact of Roasting on Identification of Hazelnut (Corylus avellana L.) Origin: A Chemometric Approach. J. Agric. Food Chem. 2015, 63, 7294–7303. [Google Scholar] [CrossRef] [PubMed]
- Torello Marinoni, D.; Acquadro, A.; Vallauri, G.; Talucci, G.; Valentini, N.; Botta, R. From Field to Snack: Development of a Molecular Marker-Based Method for the Identification of the Hazelnut (Corylus avellana L.) Cultivar along the Production Chain. Acta Hortic. 2020, 1292, 263–268. [Google Scholar] [CrossRef]
- Loureiro, G.A.H.A.; Araujo, Q.R.; Sodré, G.A.; Valle, R.R.; Souza, J.O.; Ramos, E.M.L.S.; Comerford, N.B.; Grierson, P.F. Cacao Quality: Highlighting Selected Attributes. Food Rev. Int. 2017, 33, 382–405. [Google Scholar] [CrossRef]
- Stahl, T.; Taschan, H.; Brunn, H. Aluminium Content of Selected Foods and Food Products. Environ. Sci. Eur. 2011, 23, 37. [Google Scholar] [CrossRef] [Green Version]
- Özkutlu, F.; Doğru, Y.Z.; Özenç, N.; Yazici, G.; Turan, M.; Akçay, F. The Importance of Turkish Hazelnut Trace and Heavy Metal Contents for Human Nutrition. J. Soil Sci. Environ. Manag. 2011, 2, 25–33. [Google Scholar] [CrossRef]
- Bertoldi, D.; Barbero, A.; Camin, F.; Caligiani, A.; Larcher, R. Multielemental Fingerprinting and Geographic Traceability of Theobroma Cacao Beans and Cocoa Products. Food Control 2016, 65, 46–53. [Google Scholar] [CrossRef]
- Simsek, A.; Aykut, O. Evaluation of the Microelement Profile of Turkish Hazelnut (Corylus avellana L.) Varieties for Human Nutrition and Health. Int. J. Food Sci. Nutr. 2007, 58, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, U.; Şahin, Y.; Türkez, H. Comparison of Element Contents in Hazelnuts Grown under Organic and Conventional Farming Regimes for Human Nutrition and Health. Fresenius Environ. Bull. 2011, 20, 1660–1665. [Google Scholar]


| Product | Hazelnut % | Cocoa % |
|---|---|---|
| raw hazelnuts | 100 | 0 |
| roasted hazelnuts | 100 | 0 |
| hazelnut paste | 100 | 0 |
| hazelnut cream | 45 | 12 |
| Gianduja paste | 20 | 34 |
| Gianduiotto paste | 26 | 24 |
| Element | Hazelnut (mg/Kg) | Cocoa (mg/Kg) |
|---|---|---|
| Al | 0.9–18 [13] | 41–275 [14] |
| Co | 0.07–0.6 [13] | 0.4–0.6 [14] |
| Li | 0.035–0.042 [15] | 0.01–0.05 [14] |
| Na | 0.06–5 [6] | 10–32 [14] |
| Si | 13 [16] | 400–4700 [11] |
| Ba | 2–23 [6] | 5.9–22.2 [14] |
| Mo | 0.09–0.31 [13] | 0.1–0.4 [14] |
| Ni | 0.58–2.58 [15] | 4.9–12.1 [14] |
| Sr | 4–23 [6] | 6.8–18.1 [14] |
| Samples | Precision | Sensitivity | Specificity |
|---|---|---|---|
| TGT | 94.92% | 93.33% | 97.57% |
| TGR | 95.69% | 95.97% | 98.01% |
| MOR | 93.97% | 95.17% | 96.61% |
| Sample | Piemonte % | Mortarella % | Romana % | Hazelnut % |
|---|---|---|---|---|
| Gianduja mix 1 | 50 | 25 | 25 | 21 |
| Gianduja mix 2 | 33 | 33 | 33 | 27 |
| Gianduiotto mix 1 | 50 | 25 | 25 | 27 |
| Gianduiotto mix 2 | 33 | 33 | 33 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calà, E.; Fracchia, A.; Robotti, E.; Gulino, F.; Gullo, F.; Oddone, M.; Massacane, M.; Cordone, G.; Aceto, M. On the Traceability of the Hazelnut Production Chain by Means of Trace Elements. Molecules 2022, 27, 3854. https://doi.org/10.3390/molecules27123854
Calà E, Fracchia A, Robotti E, Gulino F, Gullo F, Oddone M, Massacane M, Cordone G, Aceto M. On the Traceability of the Hazelnut Production Chain by Means of Trace Elements. Molecules. 2022; 27(12):3854. https://doi.org/10.3390/molecules27123854
Chicago/Turabian StyleCalà, Elisa, Andrea Fracchia, Elisa Robotti, Federica Gulino, Francesca Gullo, Matteo Oddone, Marco Massacane, Gianluigi Cordone, and Maurizio Aceto. 2022. "On the Traceability of the Hazelnut Production Chain by Means of Trace Elements" Molecules 27, no. 12: 3854. https://doi.org/10.3390/molecules27123854
APA StyleCalà, E., Fracchia, A., Robotti, E., Gulino, F., Gullo, F., Oddone, M., Massacane, M., Cordone, G., & Aceto, M. (2022). On the Traceability of the Hazelnut Production Chain by Means of Trace Elements. Molecules, 27(12), 3854. https://doi.org/10.3390/molecules27123854







