Synthesis, Characterization, DFT and Photocatalytic Studies of a New Pyrazine Cadmium(II) Tetrakis(4-methoxy-phenyl)-porphyrin Compound
Abstract
:1. Introduction
2. Results
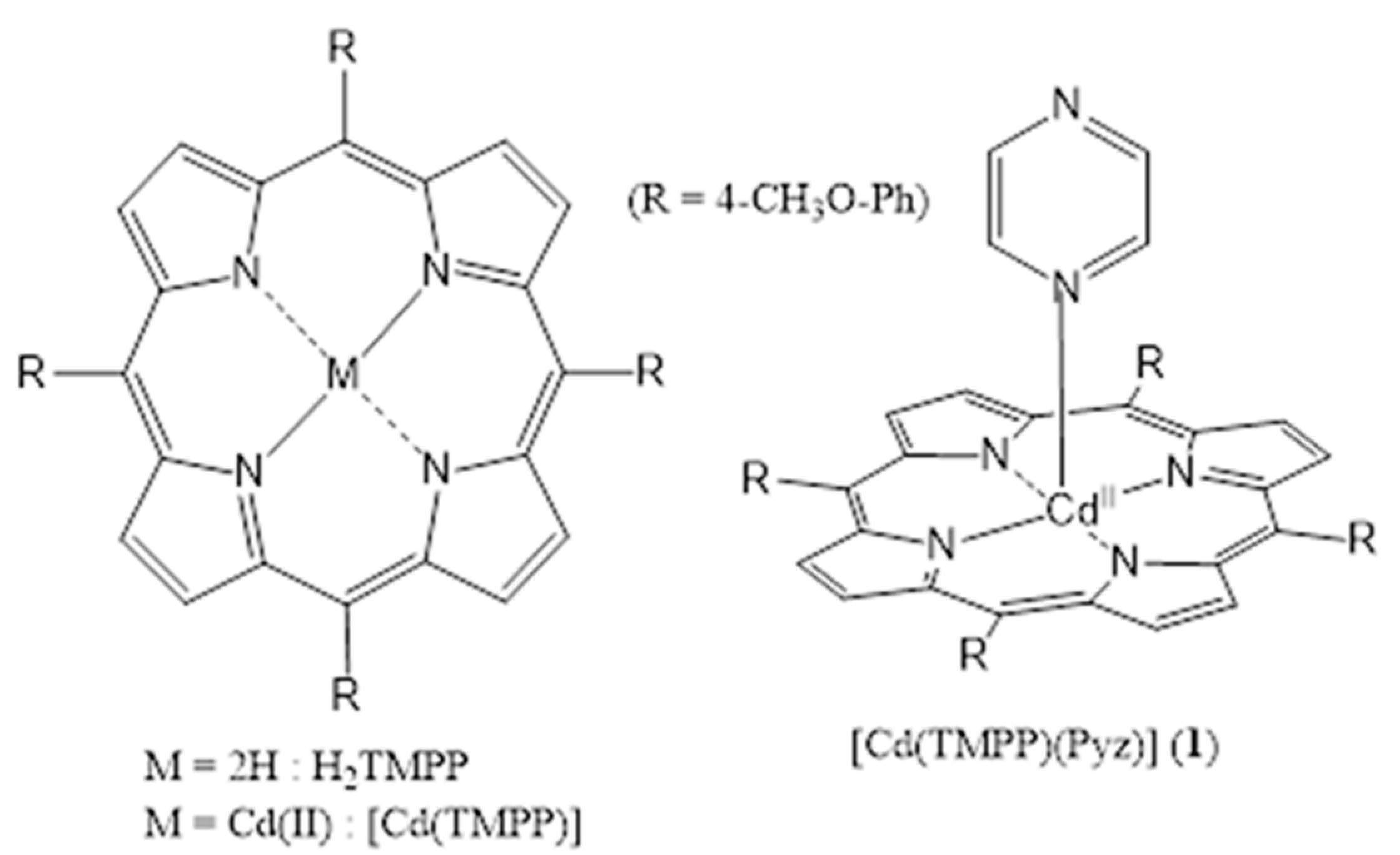
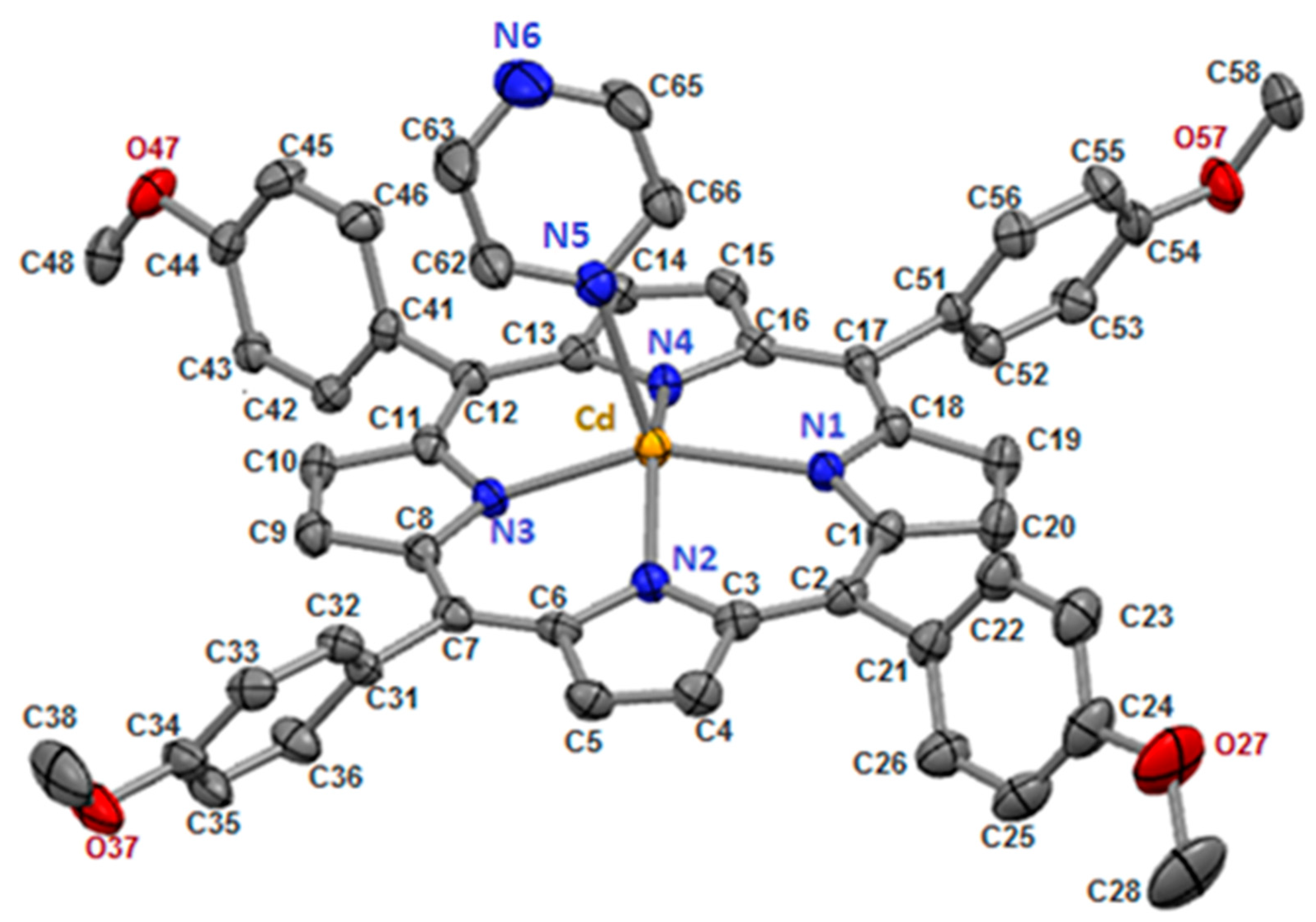
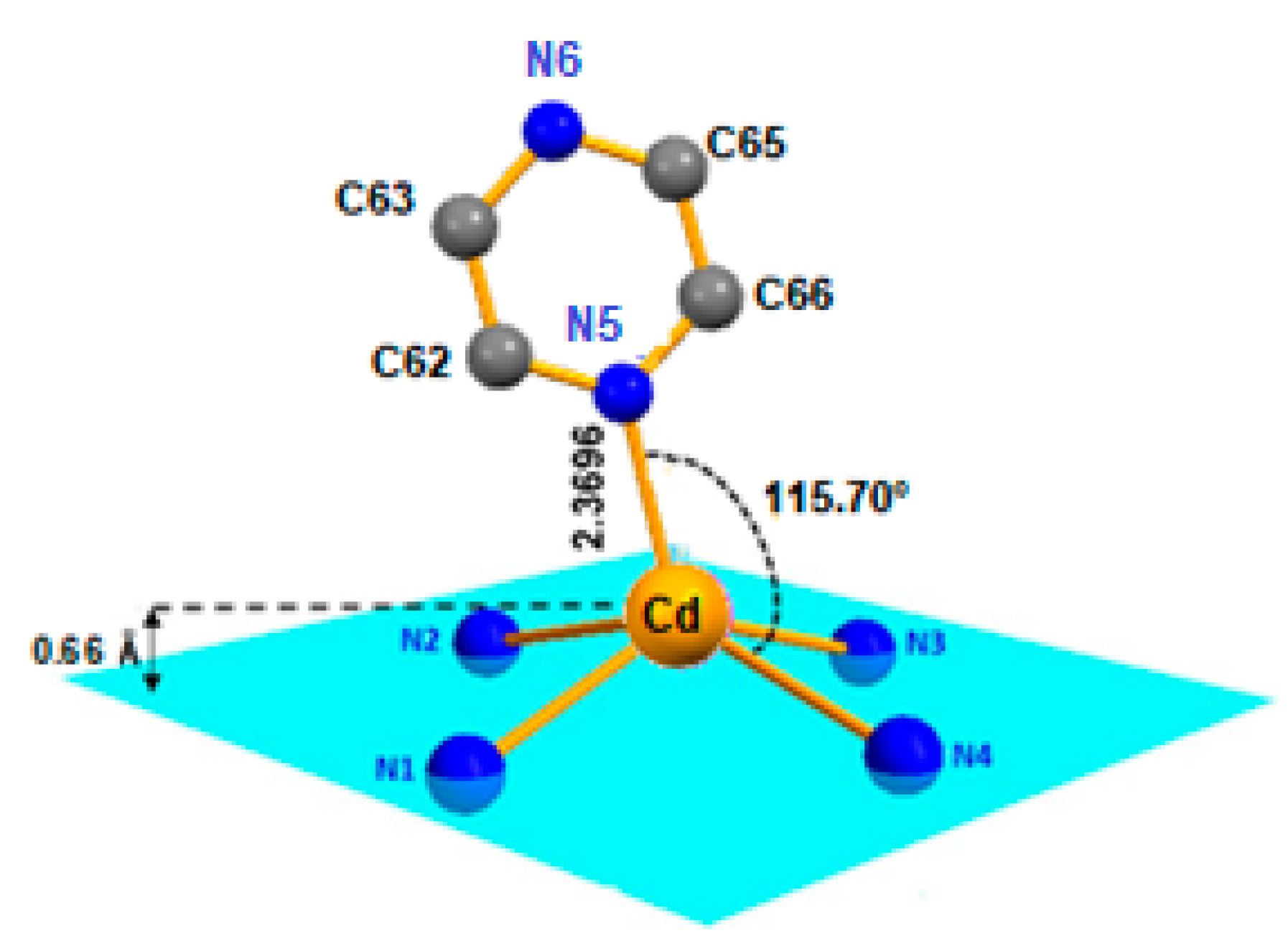
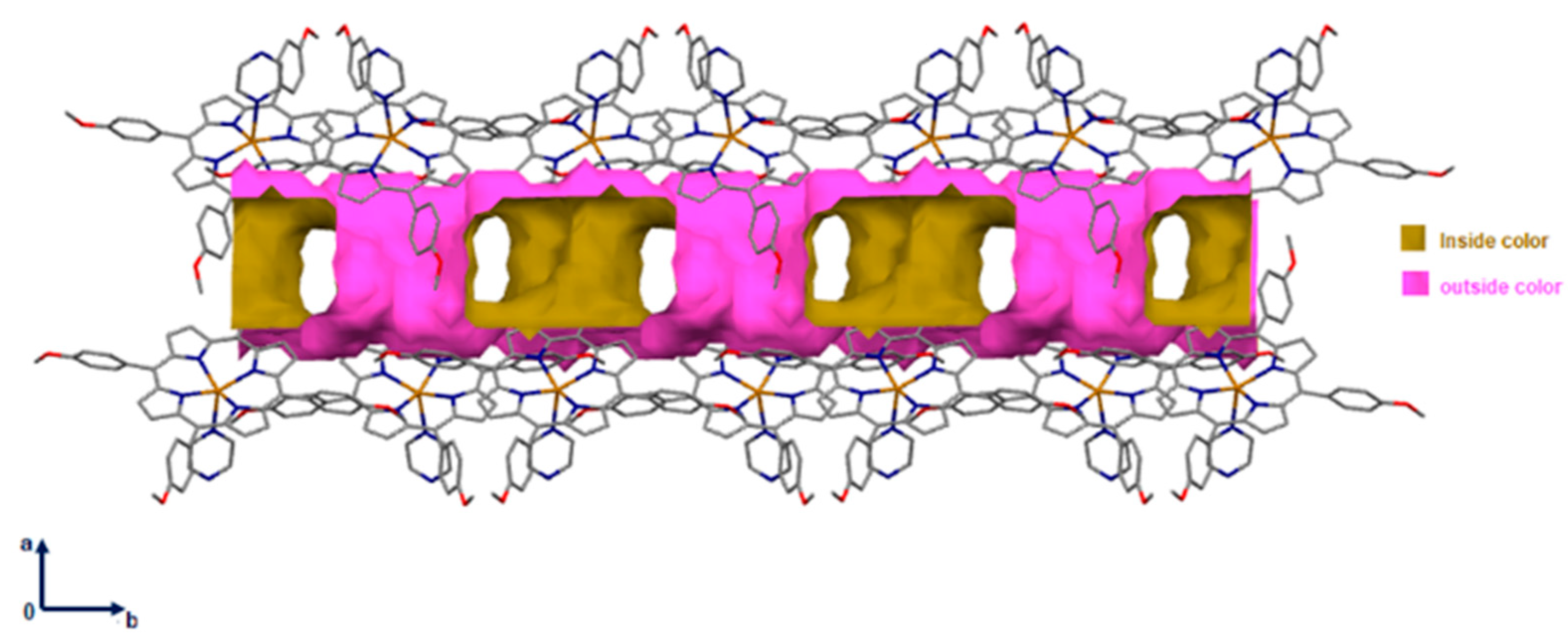
2.1. Crystal Structure of [Cd(TMPP)(Pyz)] (1)
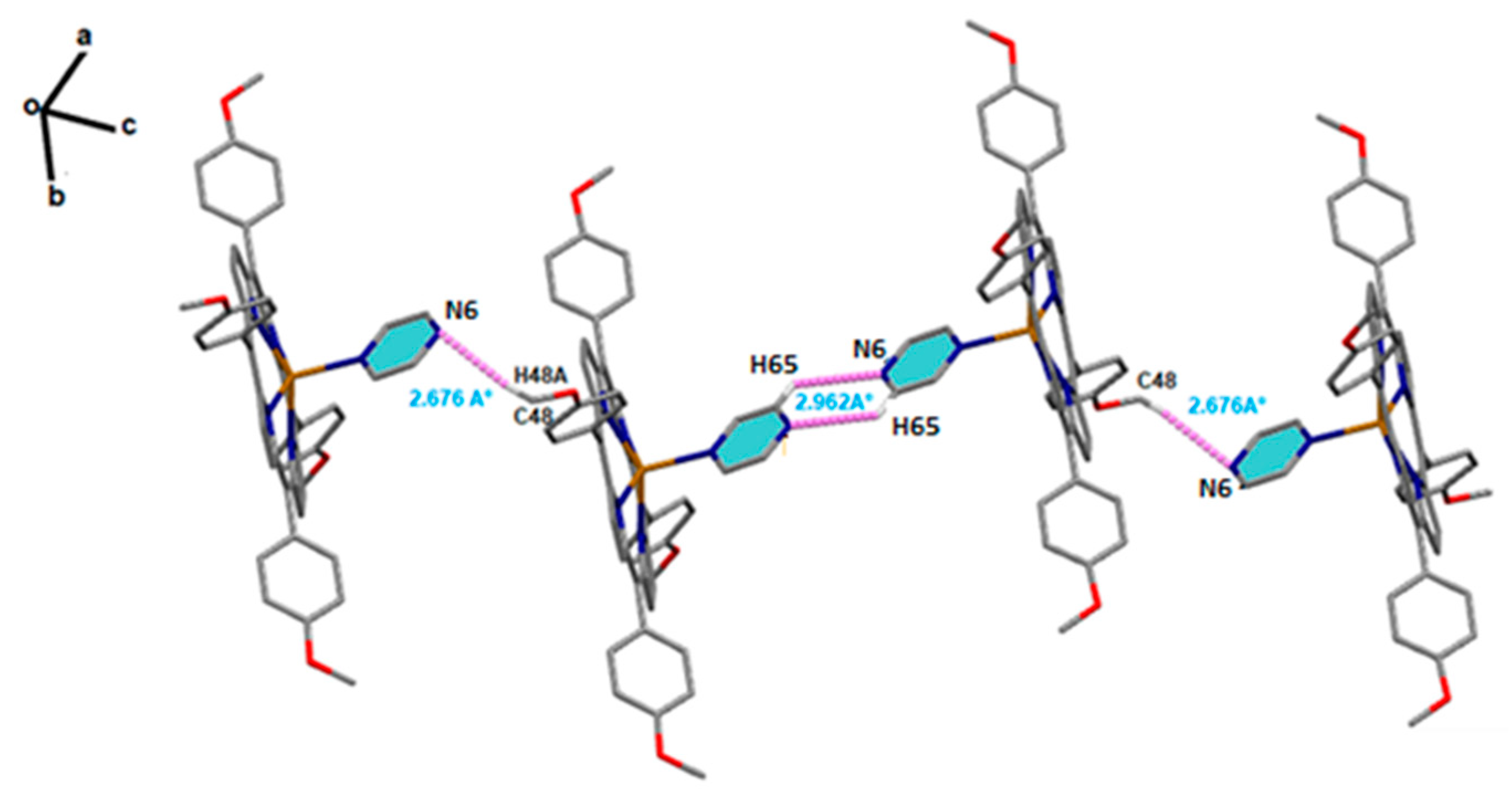
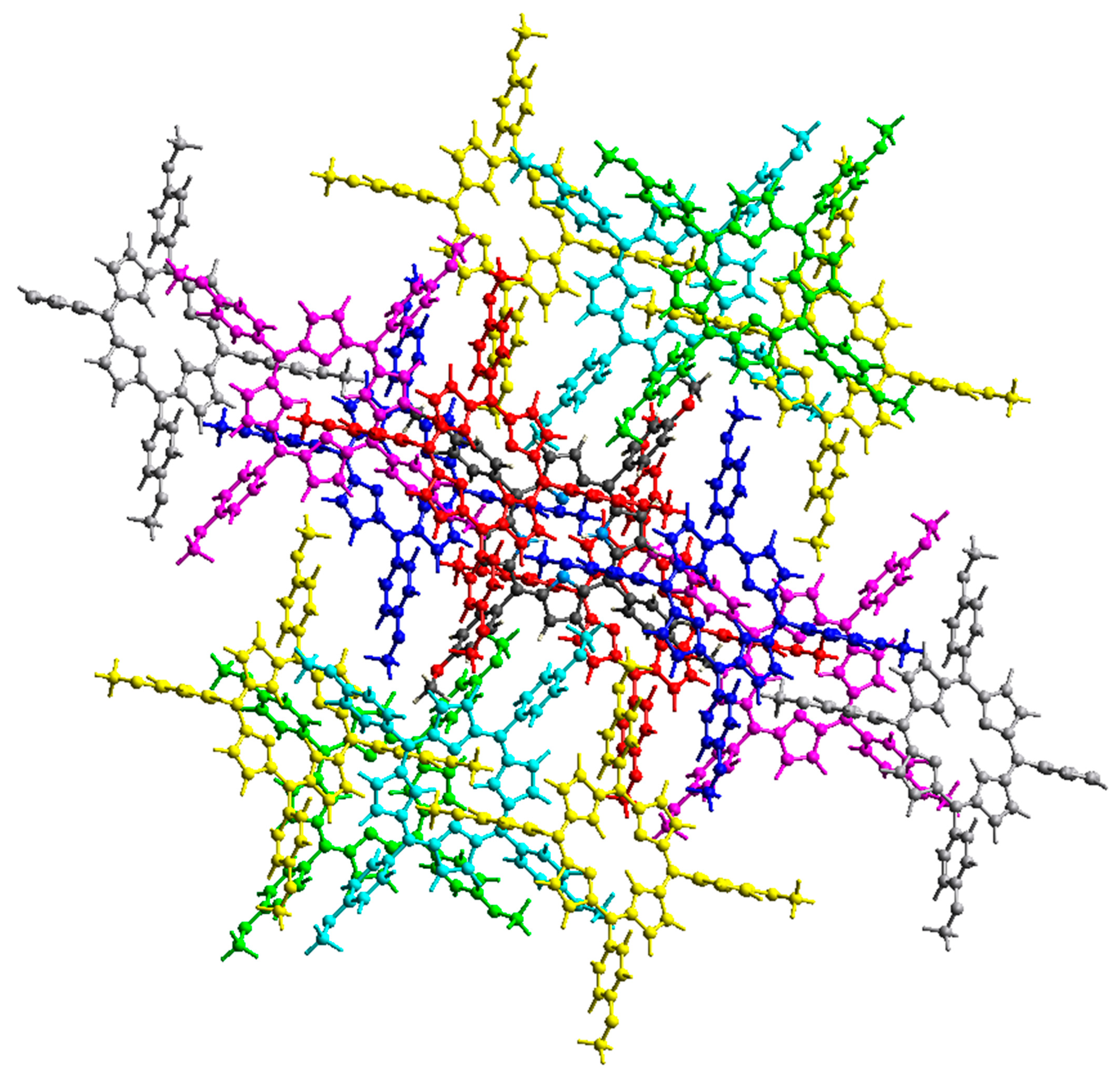
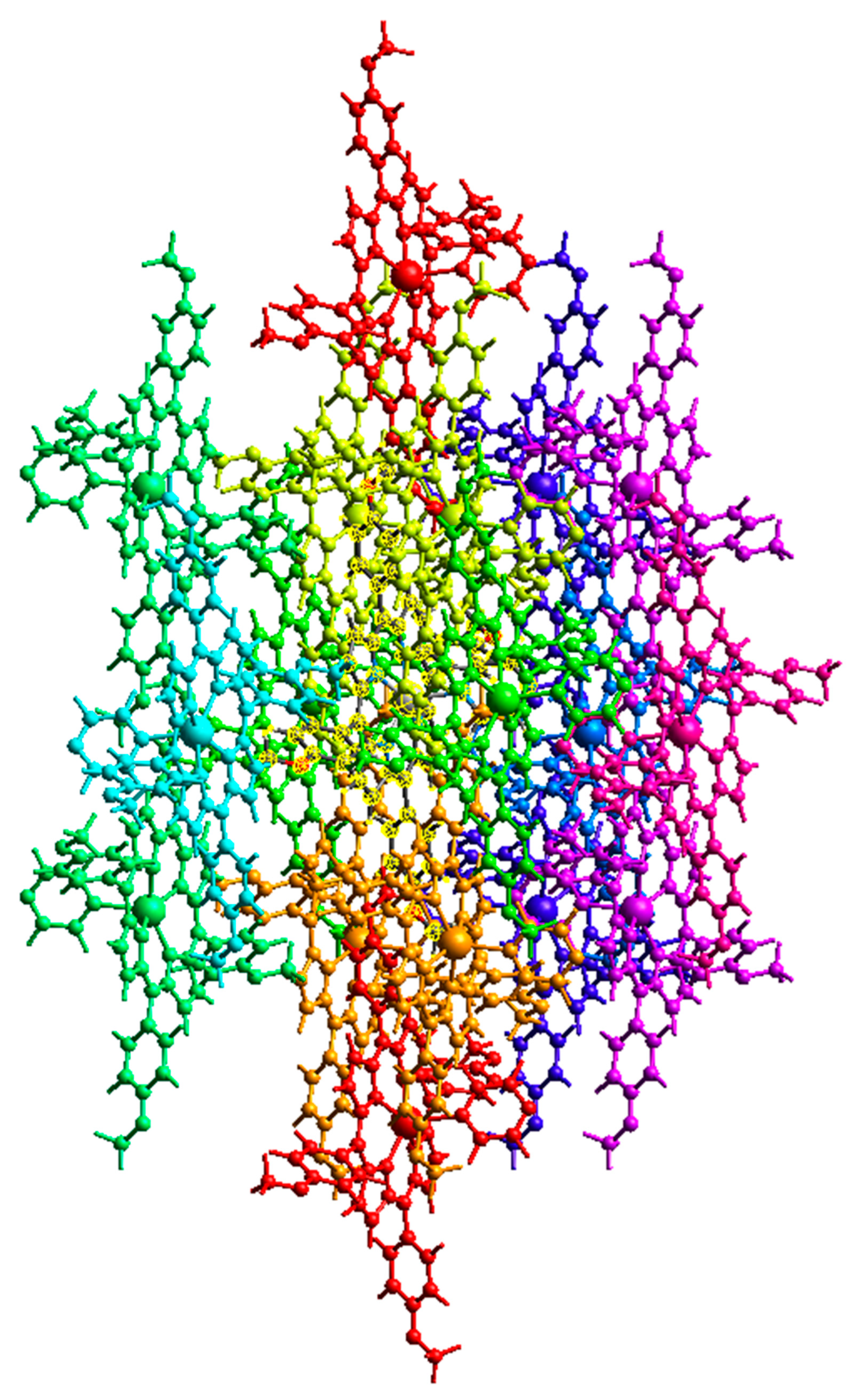
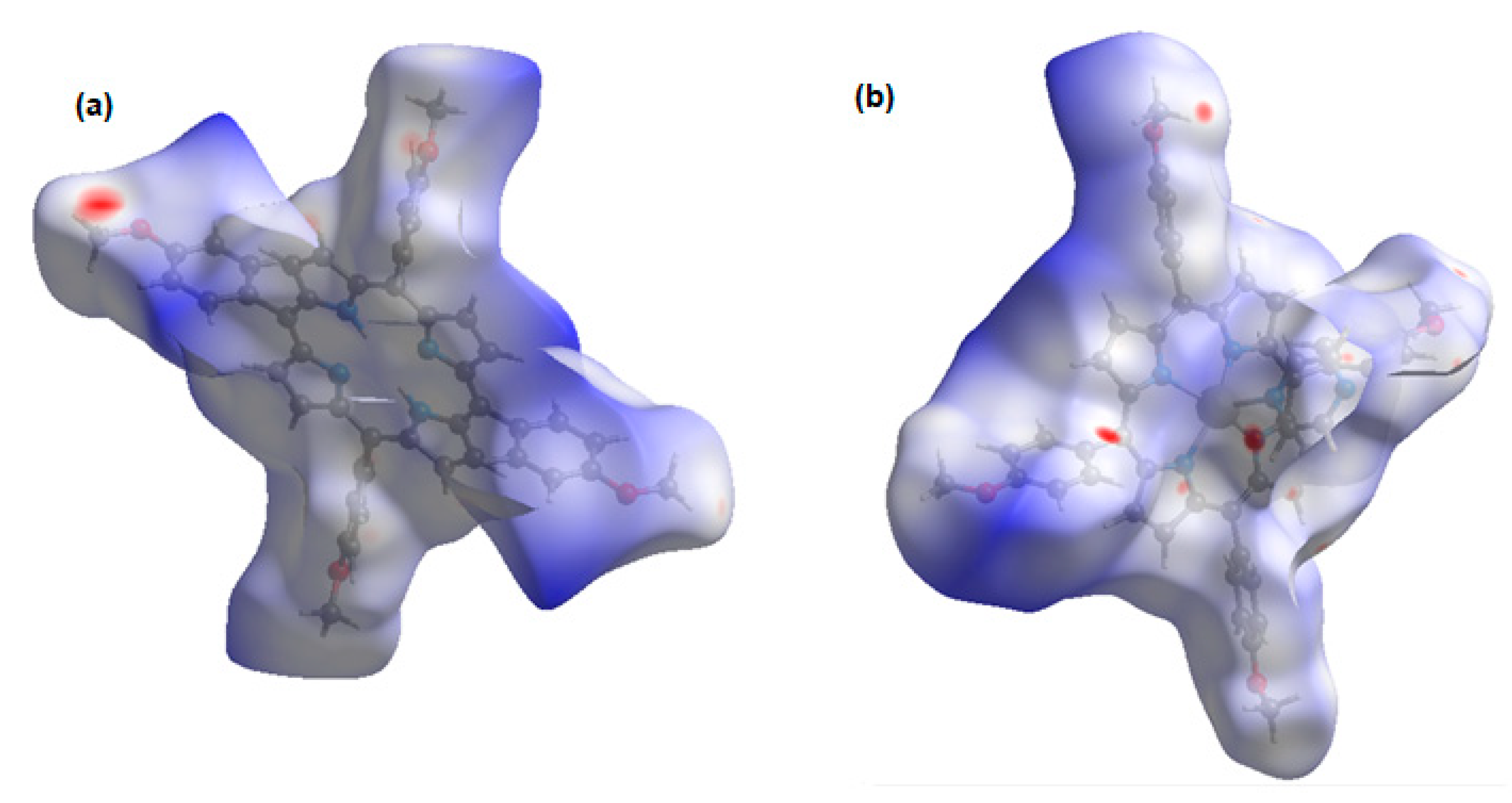
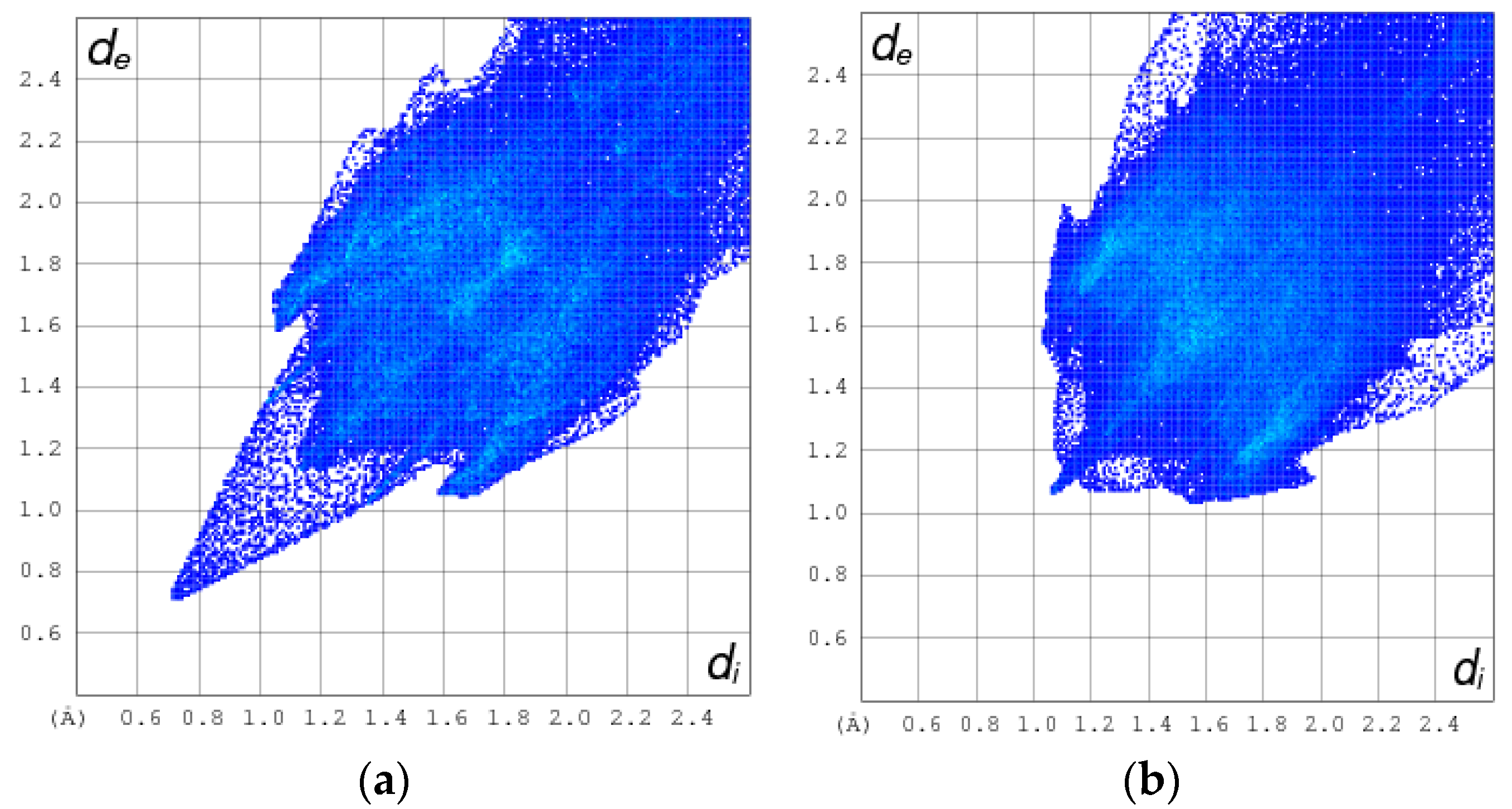
2.2. Non-Covalent-Interactions (NCI) and Hirshfeld Surface for Intermolecular Systems
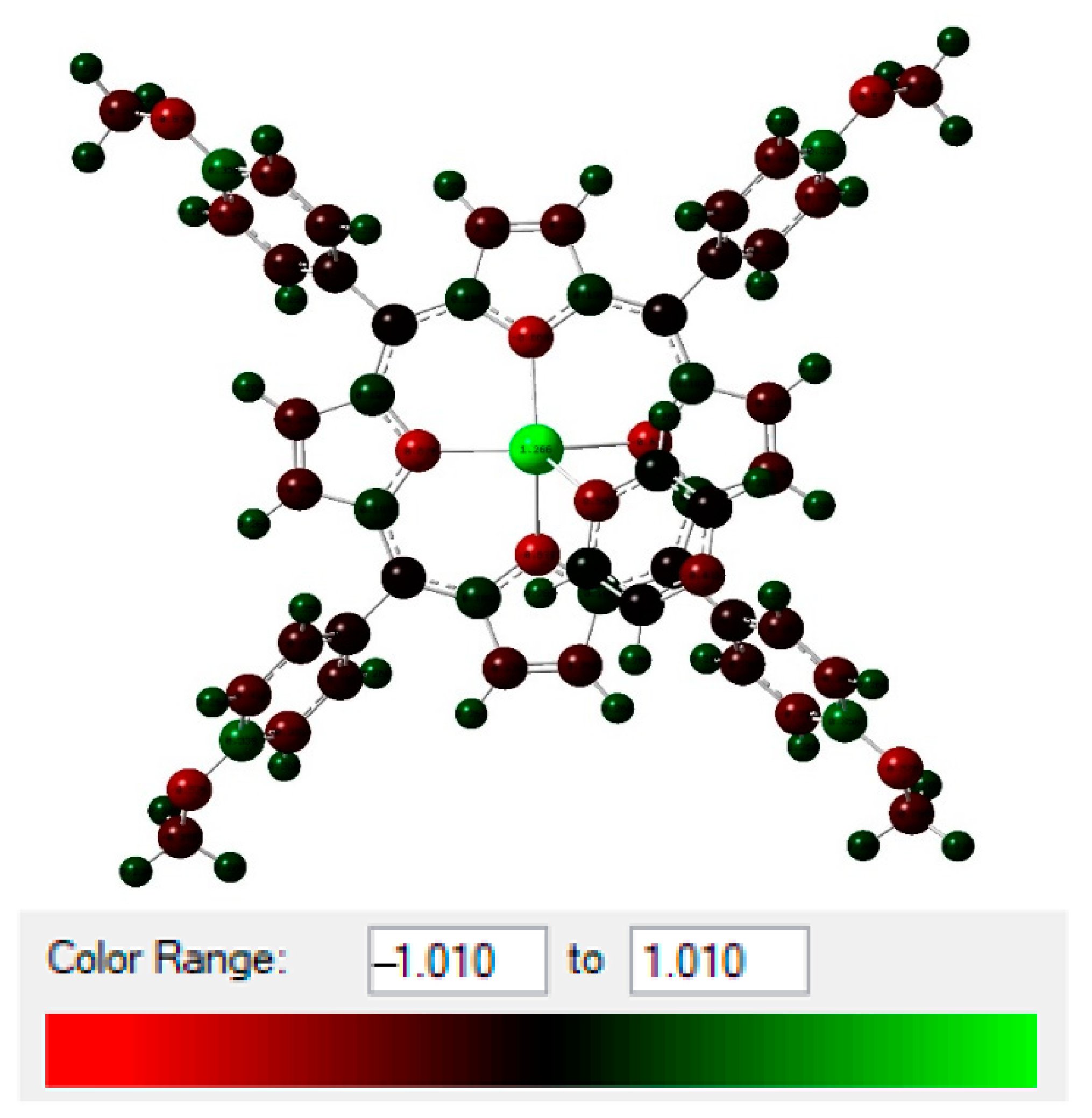
2.3. Natural Bond Orbital Analysis (NBO)
2.4. IR and 1H NMR Spectroscopies
2.5. Photophysical Properties
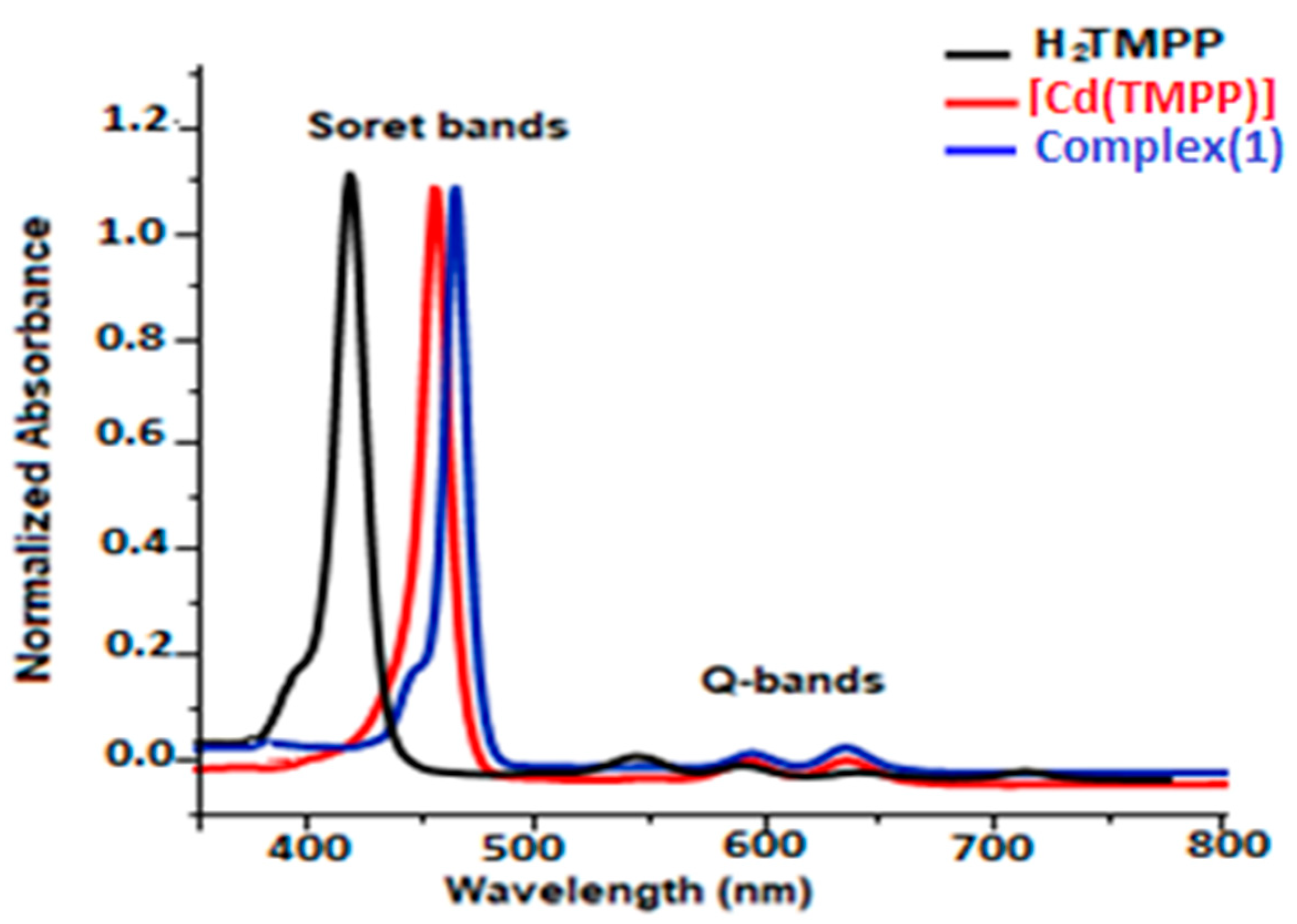
2.5.1. UV-Visible Absorption
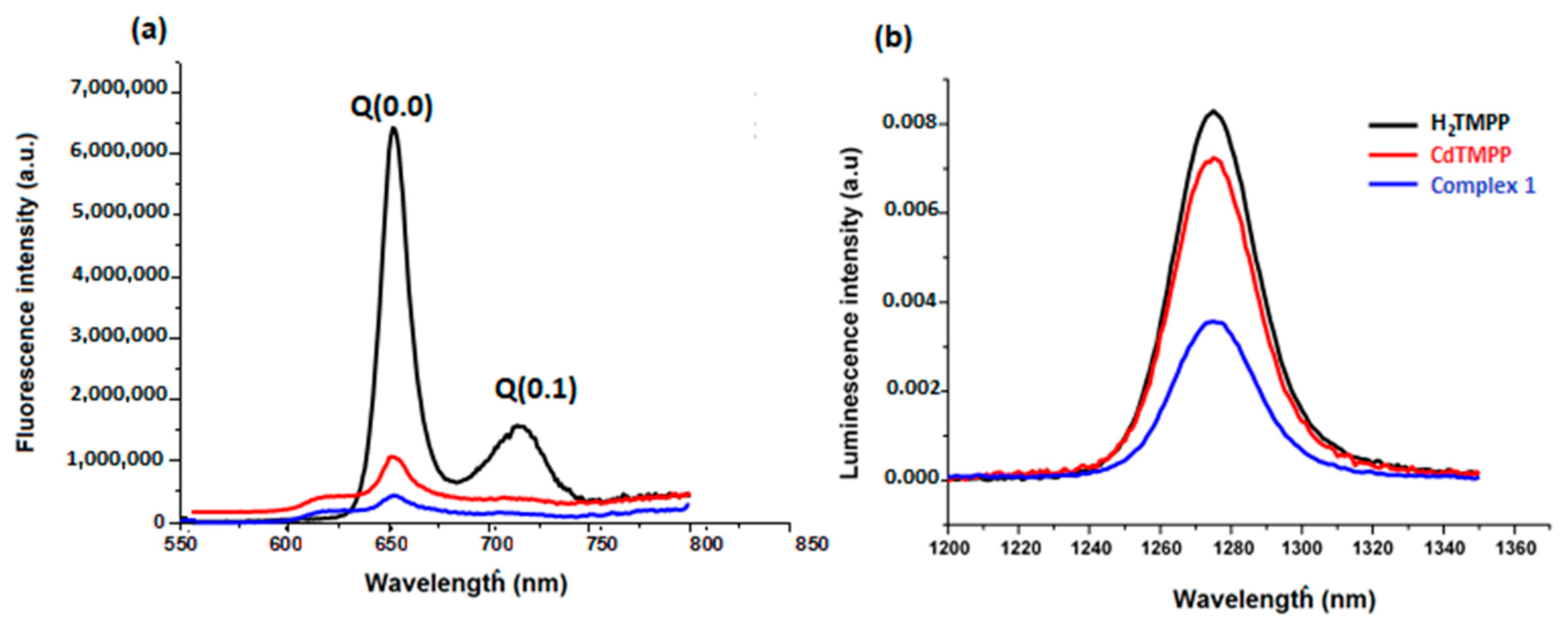
2.5.2. Fluorescence and Singlet Oxygen Studies
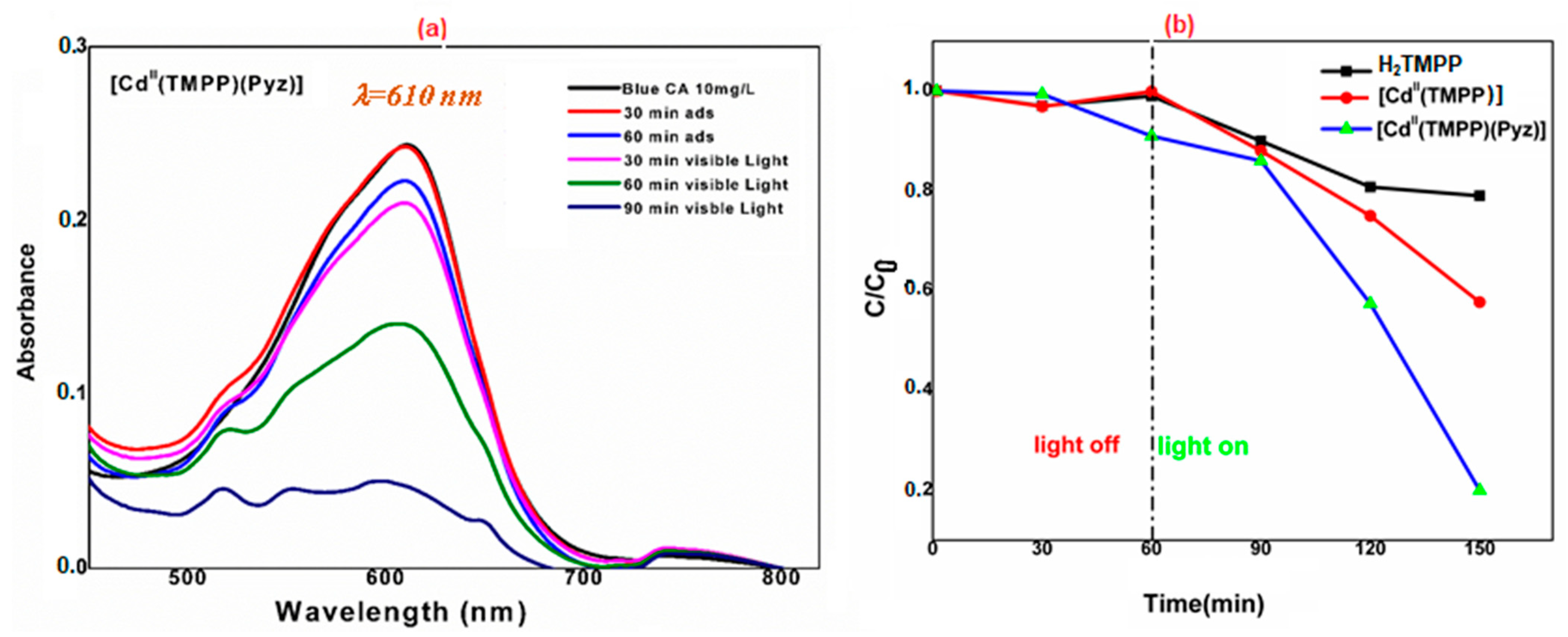
2.6. Photocatalytic Activity
3. Materials and Methods
3.1. Instrumentation
3.2. Crystallography
3.3. Computational Methods
3.4. Photocatalytic Measurements
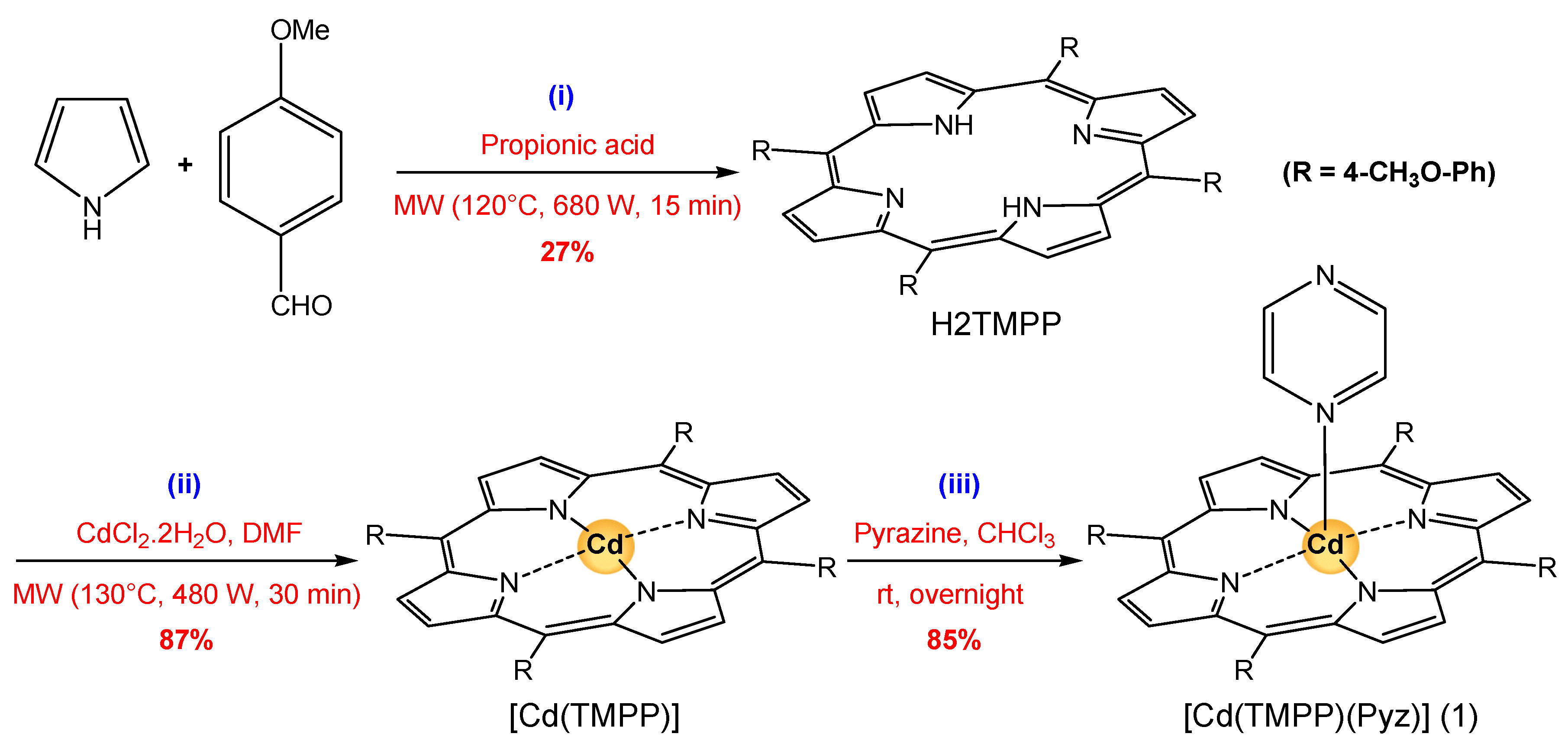
3.5. Synthesis
3.5.1. Synthesis of Meso-Tetrakis(para-methoxyphenyl) Porphyrin, H2-TMPP
3.5.2. Synthesis of Meso-Tetrakis(para-methoxyphenyl)porphyrinato)-cadmium(II), [Cd(TMPP)]
3.5.3. Synthesis of the (Pyrazine)[Meso-tetrakis(para-methoxyphenyl)-porphyrinato)-cadmium(II), [Cd(TMPP)(Pyz)] (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Day, N.U.; Wamser, C.C.; Walter, M.G. Porphyrin polymers and organic frameworks. Polym. Int. 2015, 64, 833–857. [Google Scholar] [CrossRef]
- Liu, T.; Jing, L.; Cui, L.; Liu, Q.; Zhang, X. Facile one-pot synthesis of a porphyrin based hydrophilic porous organic polymer and application as recyclable absorbent for selective separation of methylene blue. Chemosphere 2018, 212, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, Q.; Zhao, D.; Zhang, W.; Wang, N.; Li, J. Synthesis and characterization of porphyrin-based porous coordination polymers obtained by supercritical CO2 extraction. J. Mater. Sci. 2018, 53, 10534–11054. [Google Scholar] [CrossRef]
- Feng, X.; Liu, L.; Honsho, Y.; Saeki, A.; Seki, S.; Irle, S.; Dong, Y.; Nagai, A.; Jiang, D. High-rate charge-carrier transport in porphyrin covalent organic frameworks. Angew. Chem. Int. Ed. Engl. 2012, 51, 2618–2622. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Q.; Luo, J.; Wang, B.; Feng, T.; Zhou, X.; Liu, X.; Xie, Y. Solar cells sensitized with porphyrin dyes containing oligo (Ethylene Glycol) units: A high efficiency beyond 12%. ChemSusChem 2019, 12, 2802–2809. [Google Scholar] [CrossRef]
- Zeng, K.W.; Chen, Y.Y.; Zhu, W.H.; Tian, H.; Xie, Y.S. Efficient solar cells based on concerted companion dyes containing two complementary components: An alternative approach for cosensitization. J. Am. Chem. Soc. 2020, 142, 4–61. [Google Scholar] [CrossRef]
- Hsieh, C.P.; Lu, H.P.; Chiu, C.L.; Lee, C.W.; Chuang, S.H.; Mai, C.L.; Yen, W.N.; Hsu, S.H.; Diau, E.W.G.; Yeh, C.W. Synthesis and characterization of porphyrin sensitizers with various electron donating substituents for highly efficient dye sensitized solar cells. J. Mater. Chem. 2010, 20, 1127–1134. [Google Scholar] [CrossRef]
- Yingyan, S.; Fuming, Z.; Linhardt, R.J. Porphyrin-based compounds and their applications in materials and medicine. Dye Pigment. 2021, 188, 109136. [Google Scholar]
- Min, K.S.; Kumar, R.S.; Lee, J.H.; Kim, K.S.; Lee, S.G.; Son, Y. Synthesis of new TiO2/porphyrin-based composites and photocatalytic studies on methylene blue degradation. Dye Pigment. 2019, 160, 37–47. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.; Jin, J.H.; Kim, H.J.; Choi, W. Visible Light Photocatalysts Based on Homogeneous and Heterogenized Tin Porphyrins. J. Phys. Chem. C 2008, 112, 491–499. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.; Mackeyev, Y.; Lee, G.; Tachikawa, T.; Hong, S.; Lee, S.; Kim, J.; Wilson, L.J. Selective oxidative degradation of organic pollutants by singlet oxygen mediated photosensitization: Tin porphyrin versus C60 Aminofullerene systems. Environ. Sci. Technol. 2012, 46, 9606–9613. [Google Scholar] [CrossRef] [PubMed]
- Belpaire, C.; Reyns, T.; Geeraerts, C.; Van Loco, J. Toxic textile dyes accumulate in wild European eel Anguilla Anguilla. Chemosphere 2015, 138, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Azha, S.F.; Sellaoui, L.; Yunus, E.H.E.; Yee, C.J.; Bonilla-Petriciolet, A.; Ben Lamine, A.; Ismail, S. Iron-modified composite adsorbent coating for azo dye removal and its regeneration by photo-Fenton process: Synthesis, characterization and adsorption mechanism interpretation. Chem. Eng. J. 2019, 361, 31–40. [Google Scholar] [CrossRef]
- Xiong, G.; Wang, B.B.; You, L.X.; Ren, B.Y.; He, Y.K.; Ding, F.; Dragutan, I.; Dragutan, V.; Sun, Y.G. Hypervalent silicon-based, anionic porous organic polymers with solid microsphere or hollow nanotube morphologies and exceptional capacity for selective adsorption of cationic dyes. J. Mater. Chem. 2019, 7, 393–404. [Google Scholar] [CrossRef]
- Tan, H.L.; Abdi, F.F.; Hau, Y. Heterogeneous photocatalysts: An overview of classic and modern approaches for optical, electronic, and charge dynamics evaluation. Chem. Soc. Rev. 2019, 48, 1255–1271. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Quan, X.; Wang, H.; Chen, S.; Su, Y.; Li, Z. Constructing a visible-light-driven photocatalytic membrane by g-C3N4 quantum dots and TiO2 nanotube array for enhanced water treatment. Sci. Rep. 2017, 7, 3128. [Google Scholar] [CrossRef] [Green Version]
- Soury, R.; Jabli, M.; Saleh, T.A.; Abdul-Hassan, W.; Saint-Aman, S.E.; Loiseau, F.; Philouze, A.; Bujacz, C.; Nasri, H. Synthesis of the (4,4′-bipyridine)(5,10,15,20-tetratolylphenylporphyrinato)-zinc(II) bis(4,4-bipyridine) disolvate dehydrate and Evaluation of its interaction with organic dyes. J. Mol. Liq. 2018, 264, 134–142. [Google Scholar] [CrossRef]
- Wen, T.; Zhang, D.X.; Zhang, J. Unprecedented (3,10)-Connected 2-D Metal−Organic Framework Constructed from Octanuclear Cobalt(II) Clusters and a New Bifunctional Ligand. J. Inorg. Chem. 2013, 52, 12–14. [Google Scholar] [CrossRef]
- Rahman, M.F.; Ara, N.J.; Uddin, M.M.; Sultan, M.Z. Toxic Effects of Levafix Blue CA and Levafix Amber CA Reactive Dyes on Liver and Kidney in Mice. Int. J. Food Sci. Nutr. 2017, 6, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Bahadır, K.; Korbahti, A.; Tanyolac, A. Electrochemical treatment of simulated textile wastewater with industrial components and Levafix Blue CA reactive dye: Optimization through response surface methodology. J. Hazard. Mater. 2008, 151, 422–431. [Google Scholar]
- Mchiri, C.; Ouakouak, A.; Nasri, S.; Jedidi, A.; Turowska-Tyrk, I.; Acherar, S.; Frochot, C.; Roisnel, T.; Nasri, H. DABCO Cadmium(II) Tetrakis(4-methoxyphenyl)porphyrin Complex–Structure, Photophysical properties, and Adsorpion removal of methylene blue dye. Inorg. Chim. Acta 2020, 515, 120046. [Google Scholar] [CrossRef]
- Mchiri, C.; Acherar, S.; Frochot, C.; Nasri, H. Distorted five-coordinate square pyramidal geometry of a cadmium(II) complex containing a 2-methylimidazole ligand: Crystal structure and axial ligand effect on spectroscopic properties. Polyhedron 2019, 173, 114107. [Google Scholar] [CrossRef]
- Wun, W.-S.; Chen, J.-H.; Wang, S.-S.; Tung, J.-Y.; Liao, F.-L.; Wang, S.-L.; Hwang, L.-P.; Elango, S. Cadmium complexes of meso-tetra-(p-chlorophenyl)-porphyrin: [meso-tetra-(p-chlorophenyl)-porphyrinato](pyridine)cadmium(II) pyridine solvate and [meso-tetra-(p-chlorophenyl)porphyrinato]-(dimethylformamide)cadmium(II) toluene solvate. Inorg. Chem. Commun. 2004, 7, 1233–1237. [Google Scholar] [CrossRef]
- Byrn, M.P.; Curtis, C.J.; Goldberg, I.; Hsiou, Y.; Khan, S.I.; Sawin, P.A.; Tendick, S.K.; Strouse, C.E. Porphyrin Sponges. Structural Systematics of the Host Lattice. J. Am. Chem. Soc. 1991, 113, 6549–6557. [Google Scholar] [CrossRef]
- Zhao, P.S.; Jian, F.F.; Zhang, L. Synthesis, characterization and crystal structure of (α- aminopyridine-N)-(5,10,15,20-tetraphenylporphyrinato) cadmium(II) acetone solvate. Bull. Korean Chem. Soc. 2006, 27, 1053–1055. [Google Scholar]
- Toumi, H.; Amiri, N.; Belkhiria, M.S.; Daran, J.-C.; Nasri, H. Di-μ-azido-bis-(μ-1,4,7,10,13,16-hexa-oxacyclo-octa-deca-ne)bis-(5,10,15,20-tetra-phenyl-porphyrinato)dicadmium-disodium. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, 1557–1558. [Google Scholar] [CrossRef]
- Scheidt, W.R.; Lee, Y. Recent advances in the stereochemistry of metallotetrapyrroles. Struct. Bond. 1987, 64, 1–70. [Google Scholar]
- Shelnutt, J.A.; Ortiz, V. Substituent effects on the electronic structure of metalloporphyrins: A quantitative analysis in terms of four-orbital-model parameters. J. Phys. Chem. 1985, 89, 4733–4739. [Google Scholar] [CrossRef]
- Tiwari, R.; Nath, M. Synthesis of 2-nitro-3-(pyrrol-1-yl)-5,10,15,20-tetraarylporphyrins via a Clauson-Kaas reaction and the study of their electronic properties. New J. Chem. 2015, 39, 5500. [Google Scholar] [CrossRef]
- Majumdar, D.; Frontera, A.; Gomila, R.M.; Das, S.; Bankura, K. Synthesis, spectroscopic findings and crystal engineering of Pb(II)-Salen coordination polymers, and supramolecular architectures engineered by σ-hole/spodium/tetral bonds: A combined experimental and theoretical investigation. RSC. Adv. 2022, 12, 6352–6363. [Google Scholar] [CrossRef]
- Valicsek, Z.; Horváth, O. Application of the electronic spectra of porphyrins for analytical purposes: The effects of metal ions and structural distortions. Microchem. J. 2013, 107, 47. [Google Scholar] [CrossRef] [Green Version]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Tripathy, U.; Kowalska, D.; Liu, X.; Velate, S.; Steer, R.P. Photophysics of Soret-excited Tetrapyrroles in solution. I. Metalloporphyrins: MgTPP, ZnTPP, and CdTPP. J. Phys. Chem. A 2008, 112, 5824–5833. [Google Scholar] [CrossRef] [PubMed]
- Nifiatis, F.; Jasmin Athas, C.; Gunaratne, K.D.; Gurung, Y.; Monette, K.M.; Shivokevich, P.J. Substituent Effects of Porphyrin on Singlet Oxygen Generation Quantum Yields. Open. Spectros. J. 2011, 5, 1–12. [Google Scholar] [CrossRef]
- Reddy, D.R.; Maiya, B.G. Bis(aryloxo) derivatives of tin (IV) porphyrins: Synthesis, spectroscopy and redox activity. J. Porphyr. Phthalocyanines 2002, 6, 3–11. [Google Scholar] [CrossRef]
- Mchiri, C.; Dhifaoui, S.; Ezzayani, K.; Guergueb, M.; Roisnel, T.; Loiseauc, F.; Nasri, H. Insights into the New Cadmium(II) Metalloporphyrin: Synthesis, X-ray Crystal Structure, Hirshfeld surface analysis, Photophysical and Cyclic voltammetry Characterization of the (Morpholine){(meso-tetra(parachloro-phenyl)porphyrinato}cadmium(II). Polyhedron 2019, 171, 10–19. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Huang, Z.H.; Wang, L.N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Shelnutt, J.A.; Medforth, C.J.; Berber, M.D.; Barkigiaand, K.M.; Smith, K.M. Relationships between Structural Parameters and Raman Frequencies for Some Planar and Nonplanar Nickel(II) Porphyrins. J. Am. Chem. Soc. 1991, 113, 4077–4087. [Google Scholar] [CrossRef]
- Yua, Y.; Lia, Y.; Lia, Y.; Wanga, H.; Zuoa, Q.; Duana, Q. A 1D porphyrin-based rigid conjugated polymer as efficient and recyclable visible-light driven photocatalyst. React. Funct. Polym. 2019, 143, 104340. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Lu, Z.Y. Porphyrin-based porous organic polymer, Py-POP, as a multifunctional platform for efficient selective adsorption and photocatalytic degradation of cationic dyes. Microporous Mesoporous Mater. 2020, 292, 109774. [Google Scholar] [CrossRef]
- Li, Y.D.; Han, Y.; Jiang, H.L. Photocatalytic Molecular Oxygen Activation by Regulating Excitonic Effects in Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 20763–20771. [Google Scholar]
- Seybold, P.G.; Counterman, M. Fluorescence Spectra and Quantum Yields. J. Mol. Spectrosc. 1969, 31, 1–13. [Google Scholar] [CrossRef]
- Redmond, R.W.; Gamlin, J.N. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 1999, 70, 391–475. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.J.; Coppens, P. Extinction within the Limit of Validity of the Darwin Transfer Equations. I. General Formalisms for Primary and Secondary Extinction and Their Application to Spherical Crystals. Acta Crystallogr. 1974, A30, 129–147. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Gagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIRPOW.92—A program for automatic solution of crystal structures by direct methods optimized for powder data. J. Appl. Crystallogr. 1994, 27, 435–436. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van Der Streek, P.A. Wood, Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Frisch, G.M.; Trucks, J.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Salman, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.R.; Keith, T.; Millam, J. GaussView; Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Simmonett, M.D.; Andrew, C.; Franklin, L.E.; Wesley, A.; Schleyer, D.; Schaefer, P.V.R.; Henry, F. Popular theoretical methods predict benzene and arenes to be nonplanar. J. Am. Chem. Soc. 2006, 128, 9342–9343. [Google Scholar]
- Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the rare earth elements. J. Chem. Phys. 1989, 90, 1730–1734. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Weinhold, F. What Is the Nature of Supramolecular Bonding Comprehensive NBO/NRT Picture of Halogen and Pnicogen Bonding in RPH2·IF/FI Complexes (R = CH3, OH, CF3, CN, NO2). Molecules 2019, 24, 2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Weinhold, F. NBO/NRT Two-State Theory of Bond-Shift Spectral Excitation. Molecules 2020, 25, 4052. [Google Scholar] [CrossRef] [PubMed]
- Glendenning, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO, Version 3.1; Gaussian Inc.: Pittsburg, PA, USA, 2001. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Rahimia, R.; Fayyaz, F.; Rabbani, M.M. Microwave-assisted synthesis of 5,10,15,20-tetrakis(4-nitrophenyl) porphyrin and zinc derivative and study of their bacterial photoinactivation. Iran. Chem. Commun. 2016, 4, 175–185. [Google Scholar]
- Yaseen, M.; Ali, M.; Muhammad, B.; Najeeb Ullah, A.; Munawar, A.; Khokhar, I. Microwave-Assisted Synthesis, Metallation, and Duff Formylation of Porphyrins. J. Heterocyclic Chem. 2009, 46, 251. [Google Scholar] [CrossRef]
- Milanesio M., E.; Alvarez, M.G.; Yslas, E.I.; Borsarelli, C.D.; Silber, J.J.; Rivarola, V.; Durantini, E.N. Photodynamic Studies of Metallo 5,10,15,20-Tetrakis(4-methoxyphenyl) porphyrin: Photochemical Characterization and Biological Consequences in a Human Carcinoma Cell Line. Photochem. Photobiol. 2001, 74, 14–21. [Google Scholar] [CrossRef]
- Wun, W.S.; Chen, J.H.; Wang, S.S.; Tung, J.Y.; Liao, F.L.; Wang, S.L.; Hwang, L.P.; Elango, S. Cadmium complexes of meso-tetra-(p-chlorophenyl) porphyrin:[meso-tetra-(p-chlorophenyl)porphyrinato](pyridine)cadmium(II)pyridine solvate and [meso-tetra-(p-chlorophenyl)porphyrinato](dimethylformamide)cadmium(II) toluene solvate. Inorg. Chem. Commun. 2004, 7, 1233–1237. [Google Scholar] [CrossRef]
- Gentemann, S.; Medforth, C.J.; Ema, T.; Nelson, N.Y.; Smith, K.M.; Fajer, J.; Holten, D. Unusual picosecond 1 (π, π *) deactivation of ruffled nonplanar porphyrins. Chem. Phys. Lett. 1995, 245, 441–447. [Google Scholar] [CrossRef]

| Complex | Cd-Np a | Cd···Ct b | Cd-Lax c | Ref. |
|---|---|---|---|---|
| [Cd(TMPP)(Pyz)] (1) | 2.188 (17) | 0.708 | 2.369 (19) | This work |
| [Cd(TMPP)(DABCO)] | 2.192(5) | 0.746 | 2.320(5) | [21] |
| [Cd(TBPP)(2-MeHIm)] | 2.215(7) | 0.860 | 2.31(4)/2.28(6) | [22] |
| [Cd(TClPP)(py)] | 2.203(4) | 0.729 | 2.316(4) | [23] |
| [Cd(TClPP)(DMF)] | 2.197 | 0.824 | 2.280 | [23] |
| [Cd(TPP)(4-picoline)] | 2.195 | 0.744 | 2.298 | [24] |
| [Cd(TPP)(2-NH2-py)] | 2.204(3) | 0.739 | 2.316(3) | [25] |
| [Cd(TPP)(N3)]- | 2.215(1) | 0.796 | 2.238(2) | [26] |
| H2-TMPP | |
|---|---|
| Types of intermolecular interactions | Percentage contribution (%) |
| C-H weak interaction | 12.8 |
| C-O weak interaction | 0.4 |
| H-bond (O and outside H) | 5.4 |
| H-bond (N and outside H) | 2.7 |
| Cd-TMPP-Pyz | |
| Types of intermolecular interactions | Percentage contribution (%) |
| C-H weak interaction | 13.6 |
| C-O weak interaction | 0.5 |
| N-O weak interaction | 0.1 |
| H-bond (N and outside H) | 4.3 |
| H-bond (O and outside H) | 4.9 |
| No. | Transition | Pyrazine | Complex 1 |
|---|---|---|---|
| 1 | n(1)N1 → n*(6)Cd | 50.42 | 43.00 |
| 2 | n(1)N1 → n*(7)Cd | 46.24 | 26.02 |
| 3 | n(1)N1 → n*(8)Cd | 18.84 | |
| subtotal | 96.66 | 87.86 | |
| 4 | n(1)N2 → n*(6)Cd | 46.16 | 44.25 |
| 5 | n(1)N2 → n*(7)Cd | 6.01 | |
| 6 | n(1)N2 → n*(8)Cd | 44.82 | 28.29 |
| 7 | n(1)N2 → n*(9)Cd | 10.34 | |
| subtotal | 90.98 | 88.89 | |
| 8 | n(1)N3 → n*(6)Cd | 50.41 | 47.63 |
| 9 | n(1)N3 → n*(7)Cd | 8.96 | |
| 10 | n(1)N3 → n*(8)Cd | 46.08 | 11.55 |
| 11 | n(1)N3 → n*(9)Cd | 27.76 | |
| subtotal | 96.49 | 95.90 | |
| 12 | n(1)N4 → n*(6)Cd | 49.99 | 43.29 |
| 13 | n(1)N4 → n*(7)Cd | 46.50 | 22.04 |
| 14 | n(1)N4 → n*(8)Cd | 28.53 | |
| subtotal | 96.49 | 93.86 | |
| 15 | n(1)N5 → n*(6)Cd | 21.55 | |
| 16 | n(1)N5 → n*(7)Cd | 27.68 | |
| 17 | n(1)N5 → n*(8)Cd | 13.73 | |
| subtotal | 62.96 | ||
| Total | 380.62 | 429.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mchiri, C.; Coetzee, L.-C.C.; Chandoul, F.; Jedidi, A.; Adeyinka, A.S.; Magwa, N.; Roisnel, T.; Ben Moussa, S.; Nasri, H. Synthesis, Characterization, DFT and Photocatalytic Studies of a New Pyrazine Cadmium(II) Tetrakis(4-methoxy-phenyl)-porphyrin Compound. Molecules 2022, 27, 3833. https://doi.org/10.3390/molecules27123833
Mchiri C, Coetzee L-CC, Chandoul F, Jedidi A, Adeyinka AS, Magwa N, Roisnel T, Ben Moussa S, Nasri H. Synthesis, Characterization, DFT and Photocatalytic Studies of a New Pyrazine Cadmium(II) Tetrakis(4-methoxy-phenyl)-porphyrin Compound. Molecules. 2022; 27(12):3833. https://doi.org/10.3390/molecules27123833
Chicago/Turabian StyleMchiri, Chadlia, Louis-Charl C. Coetzee, Faycal Chandoul, Abdesslem Jedidi, Adedapo S. Adeyinka, Nomampondo Magwa, Thierry Roisnel, Sana Ben Moussa, and Habib Nasri. 2022. "Synthesis, Characterization, DFT and Photocatalytic Studies of a New Pyrazine Cadmium(II) Tetrakis(4-methoxy-phenyl)-porphyrin Compound" Molecules 27, no. 12: 3833. https://doi.org/10.3390/molecules27123833
APA StyleMchiri, C., Coetzee, L.-C. C., Chandoul, F., Jedidi, A., Adeyinka, A. S., Magwa, N., Roisnel, T., Ben Moussa, S., & Nasri, H. (2022). Synthesis, Characterization, DFT and Photocatalytic Studies of a New Pyrazine Cadmium(II) Tetrakis(4-methoxy-phenyl)-porphyrin Compound. Molecules, 27(12), 3833. https://doi.org/10.3390/molecules27123833








