Synthesis, Anticancer Potential and Comprehensive Toxicity Studies of Novel Brominated Derivatives of Bacterial Biopigment Prodigiosin from Serratia marcescens ATCC 27117
Abstract
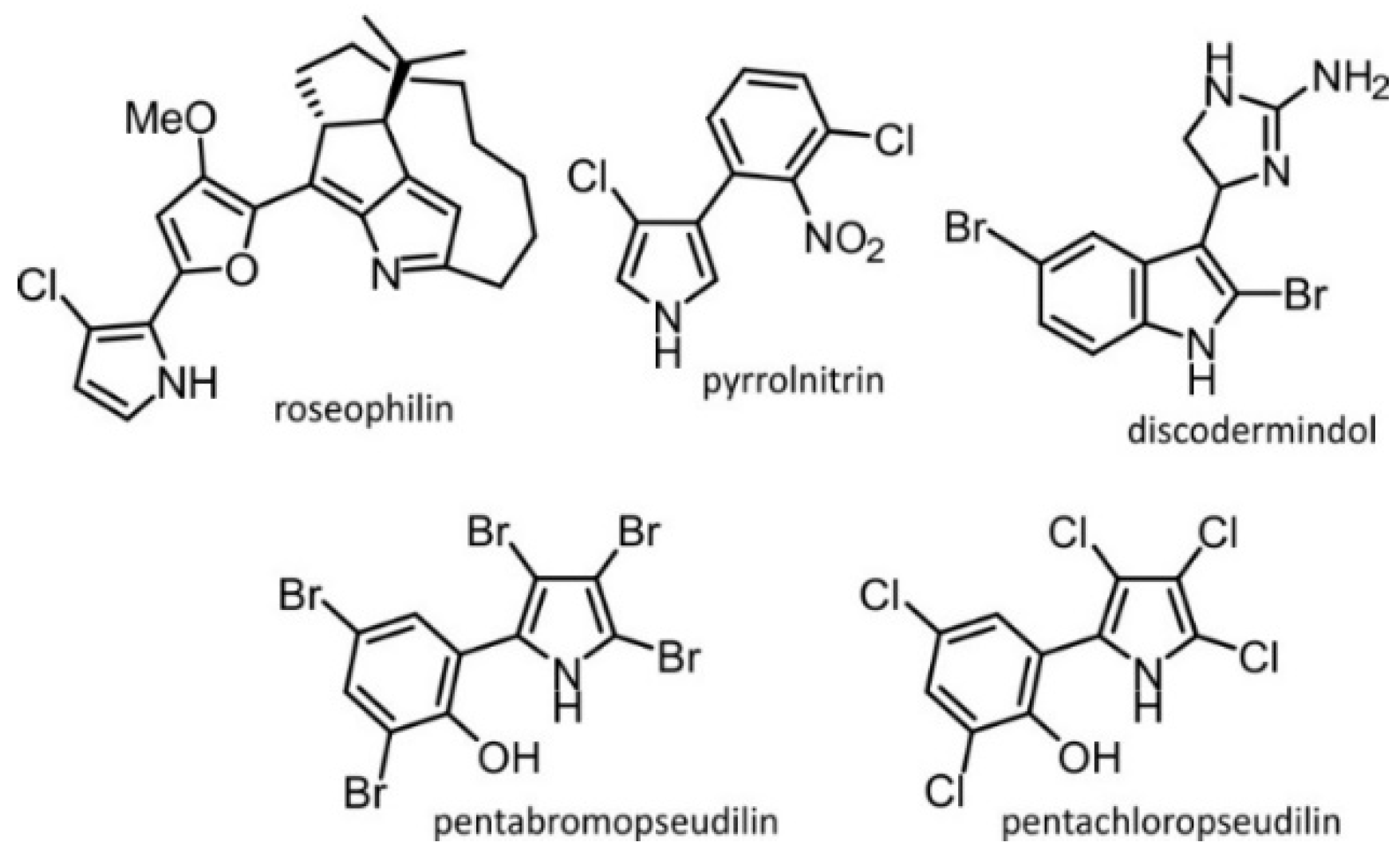
:1. Introduction
2. Results and Discussion
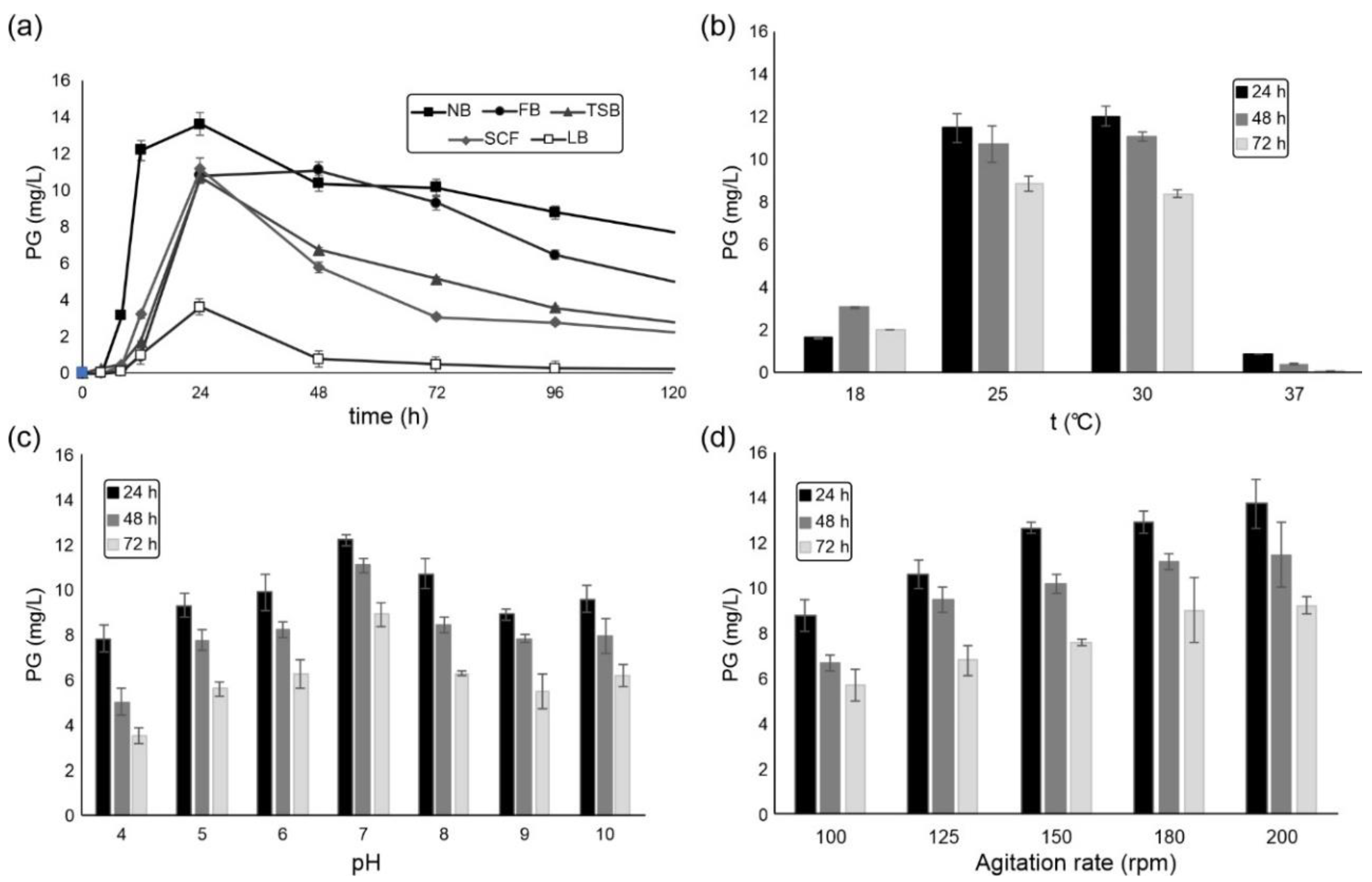
2.1. S. marcescens ATCC 27117 Cultivation for Prodigiosin Production
2.2. Oxidative Prodigiosin Bromination
2.3. Anticancer Potential of Prodigiosin and Its Br Derivatives
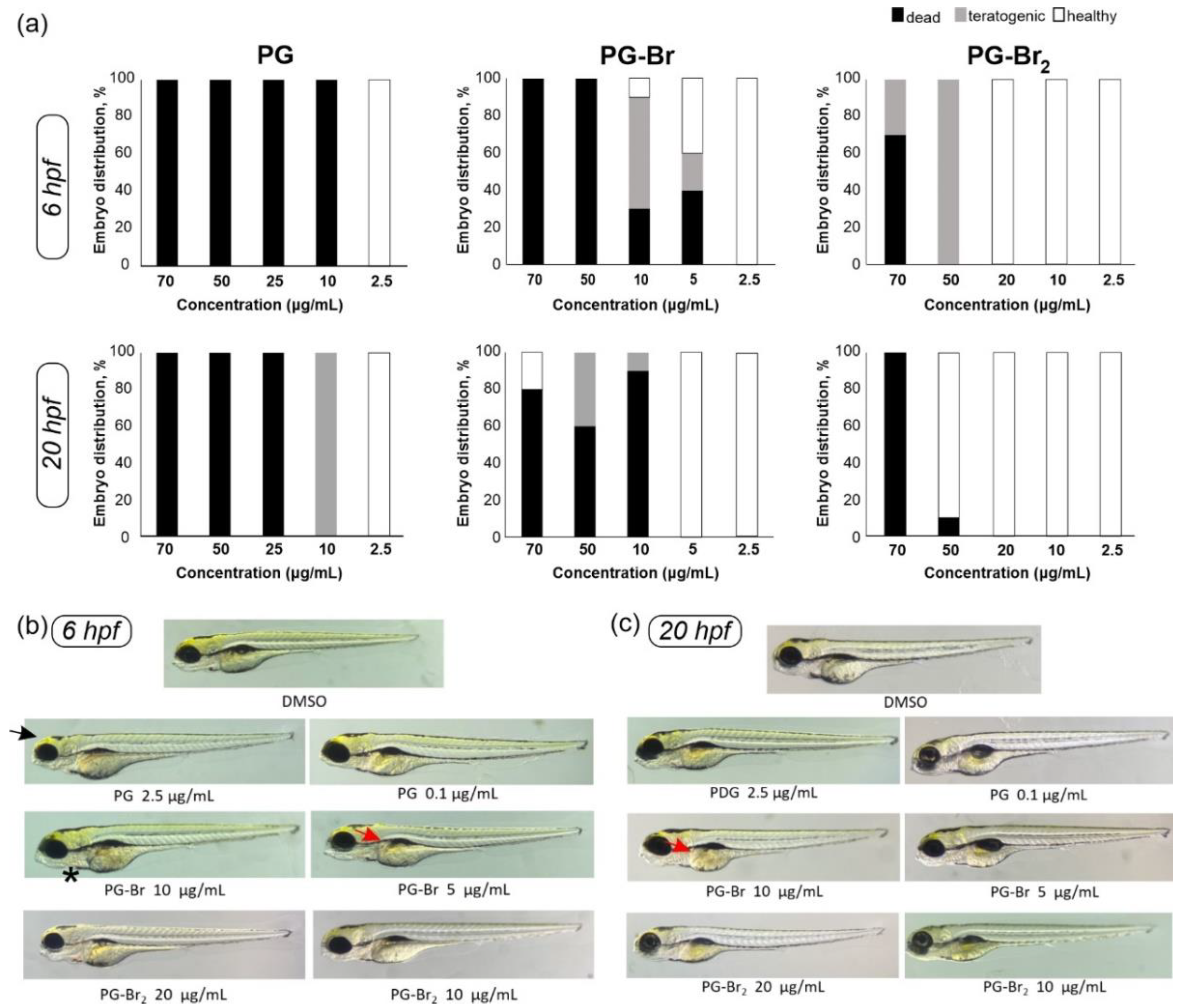
2.4. Toxicity Evaluation of Prodigiosin and Its Br Derivatives in C. elegans and D. rerio
2.5. Drug-Likeness Assessment of Prodigiosin and Its Br Derivatives
3. Materials and Methods
3.1. Reagents
3.2. Prodigiosin Production
3.2.1. Bacterial Strain Cultivation
3.2.2. Bioreactor Design and Experimental Setup
3.2.3. Prodigiosin Extraction and Purification
3.3. Prodigiosin Derivatization
3.3.1. Monobromination of Prodigiosin
3.3.2. Dibromination of Prodigiosin
3.3.3. Structural Characterization of Prodigiosin and its Br derivatives
3.4. Biological Assays
3.4.1. Cytotoxicity and Flow Cytometry Analysis
3.4.2. Roundworm (C. elegans) Survival Assay
3.4.3. Zebrafish (D. rerio) Embryotoxicity
3.5. Drug-Likeness Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williamson, N.R.; Fineran, P.C.; Leeper, F.J.; Salmond, G.P.C. The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. Microbiol. 2006, 4, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Bentley, R. Seeing red: The story of prodigiosin. Adv. Appl. Microbiol. 2000, 47, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Chander, R.; Sainis, K.B. Prodigiosins as anti cancer agents: Living upto their name. Curr. Pharm. Des. 2009, 15, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, N.; Senerovic, L.; Ilic-Tomic, T.; Vasiljevic, B.; Nikodinovic-Runic, J. Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Appl. Microbiol. Biotechnol. 2014, 98, 3841–3858. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, A.N.; Hegazy, W.A.H.; Shaldam, M.A.; Mosbah, R.; Almalki, A.J.; Ibrahim, T.S.; Khayat, M.T.; Khafagy, E.-S.; Soliman, W.E.; Abbas, H.A. Xylitol inhibits growth and blocks virulence in Serratia marcescens. Microorganisms 2021, 9, 1083–1096. [Google Scholar] [CrossRef]
- Soenens, A.; Imperial, J. Biocontrol capabilities of the genus Serratia. Phytochem. Rev. 2020, 19, 577–587. [Google Scholar] [CrossRef]
- Harned, R.L. The production of prodigiosin by submerged growth of Serratia marcescens. Appl. Microbiol. 1954, 2, 365–368. [Google Scholar] [CrossRef]
- El-Bialy, H.A.; El-Nour, S.A.A. Physical and chemical stress on Serratia marcescens and studies on prodigiosin pigment production. Ann. Microbiol. 2015, 65, 59–68. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Swarnalatha, S.; Mary, R.R.; Sekaran, G. Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate. J. Food Sci. Technol. 2018, 55, 2661–2670. [Google Scholar] [CrossRef]
- Chen, W.-C.; Yu, W.-J.; Chang, C.-C.; Chang, J.-S.; Huang, S.-H.; Chang, C.-H.; Chen, S.-Y.; Chien, C.-C.; Yao, C.-L.; Chen, W.-M.; et al. Enhancing production of prodigiosin from Serratia marcescens C3 by statistical experimental design and porous carrier addition strategy. Biochem. Eng. J. 2013, 78, 93–100. [Google Scholar] [CrossRef]
- Andreyeva, I.N.; Ogorodnikova, T.I. Pigmentation of Serratia marcescens and spectral properties of prodigiosin. Microbiology 2015, 84, 28–33. [Google Scholar] [CrossRef]
- Ryazantseva, I.N.; Saakov, V.S.; Andreyeva, I.N.; Ogorodnikova, T.I.; Zuev, Y.F. Response of pigmented Serratia marcescens to the illumination. J. Photochem. Photobiol. B 2012, 106, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Lim, S.; Yoon, K.-h.; Lee, J.I.; Mitchell, R.J. Biotechnological activities and applications of bacterial pigments violacein and prodigiosin. J. Biol. Eng. 2021, 15, 10–25. [Google Scholar] [CrossRef]
- Hong, B.; Prabhu, V.V.; Zhang, S.; van den Heuvel, A.P.J.; Dicker, D.T.; Kopelovich, L.; El-Deiry, W.S. Prodigiosin rescues deficient p53 signaling and antitumor effects via upregulating p73 and disrupting its interaction with mutant p53. Cancer Res. 2014, 74, 1153–1165. [Google Scholar] [CrossRef]
- Chiu, W.-J.; Lin, S.-R.; Chen, Y.-H.; Tsai, M.-J.; Leong, M.K.; Weng, C.-F. Prodigiosin-emerged PI3K/Beclin-1-independent pathway elicits autophagic cell death in doxorubicin-sensitive and -resistant lung cancer. J. Clin. Med. 2018, 7, 321–335. [Google Scholar] [CrossRef]
- Herráez, R.; Quesada, R.; Dahdah, N.; Viñas, M.; Vinuesa, T. Tambjamines and prodiginines: Biocidal activity against Trypanosoma cruzi. Pharmaceutics 2021, 13, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Papireddy, K.; Smilkstein, M.; Xu, J.; Shweta, K.; Salem, S.M.; Alhamadsheh, M.; Haynes, S.W.; Challis, G.L.; Reynolds, K.A. Antimalarial activity of natural and synthetic prodiginines. J. Med. Chem. 2011, 54, 5296–5306. [Google Scholar] [CrossRef]
- Han, S.B.; Park, S.H.; Jeon, Y.J.; Kim, Y.K.; Kim, H.M.; Yang, K.H. Prodigiosin blocks T cell activation by inhibiting interleukin-2Rα expression and delays progression of autoimmune diabetes and collagen-induced arthritis. J. Pharmacol. Exp. Ther. 2001, 299, 415–425. [Google Scholar]
- Yip, C.-H.; Mahalingam, S.; Wan, K.-L.; Nathan, S. Prodigiosin inhibits bacterial growth and virulence factors as a potential physiological response to interspecies competition. PLoS ONE 2021, 16, e0253445. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Koli, S.H.; Hallsworth, J.E.; Patil, S.V. Antimicrobial activity of prodigiosin is attributable to plasma-membrane damage. Nat. Prod. Res. 2016, 31, 572–577. [Google Scholar] [CrossRef]
- Borić, M.; Danevčič, T.; Stopar, D. Prodigiosin from Vibrio sp. DSM 14379; a new UV-protective pigment. Microb. Ecol. 2011, 62, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A. Chemistry and biology of roseophilin and the prodigiosin alkaloids: A survey of the last 2500 years. Angew. Chem. Int. Ed. 2003, 42, 3582–3603. [Google Scholar] [CrossRef] [PubMed]
- van Pée, K.-H. Biosynthesis of Halogenated Alkaloids; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 71. [Google Scholar]
- Laus, G. Biological Activities of Natural Halogen Compounds; Elsevier B.V.: Amsterdam, The Netherlands, 2001; Volume 25. [Google Scholar]
- Hong, B.; Luo, T.; Lei, X. Late-stage diversification of natural products. ACS Cent. Sci. 2020, 6, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Fejzagić, A.V.; Gebauer, J.; Huwa, N.; Classen, T. Halogenating enzymes for active agent synthesis: First steps are done and many have to follow. Molecules 2019, 24, 4008–4041. [Google Scholar] [CrossRef]
- Jia, Y.; Xu, L.; Yin, B.; Zhou, M.; Song, J. Synthesis and characterization of meso-to-meso directly linked porphyrin-diazaporphyrin triads. J. Porphyr. Phthalocyanines 2018, 22, 814–820. [Google Scholar] [CrossRef]
- Pati, P.B.; Zade, S.S. Selective bromination of 2,5-bis(2-thienyl)pyrroles and solid-state polymerization through the β-carbon of pyrrole. RSC Adv. 2014, 4, 17022–17027. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E. Halogenated Heterocycles as Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2012; Volume 27. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Kajorinne, J.K.; Steers, J.C.M.; Merchant, M.E.; MacKinnon, C.D. Green halogenation reactions for (hetero)aromatic ring systems in alcohol, water, or no solvent. Can. J. Chem. 2018, 96, 1087–1091. [Google Scholar] [CrossRef]
- Podgoršek, A.; Zupan, M.; Iskra, J. Oxidative halogenation with “green” oxidants: Oxygen and hydrogen peroxide. Angew. Chem. Int. Ed. 2009, 48, 8424–8450. [Google Scholar] [CrossRef]
- Song, S.; Sun, X.; Li, X.; Yuan, Y.; Jiao, N. Efficient and practical oxidative bromination and iodination of arenes and heteroarenes with DMSO and hydrogen halide: A mild protocol for late-stage functionalization. Org. Lett. 2015, 17, 2886–2889. [Google Scholar] [CrossRef]
- Anwar, M.M.; Shalaby, M.; Embaby, A.M.; Saeed, H.; Agwa, M.M.; Hussein, A. Prodigiosin/PU-H71 as a novel potential combined therapy for triple negative breast cancer (TNBC): Preclinical insights. Sci. Rep. 2020, 10, 14706. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.; Pérez-Tomás, R.; Gimènez-Bonafé, P.; Soto-Cerrato, V.; Giménez-Xavier, P.; Ambrosio, S. Mechanisms of prodigiosin cytotoxicity in human neuroblastoma cell lines. Eur. J. Pharmacol. 2007, 572, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Ma, X.; Lin, C.; Lu, L.; Liu, D.; Ma, D.; Gao, X.; Qian, X.Y. Prodigiosin inhibits proliferation, migration, and invasion of nasopharyngeal cancer cells. Cell. Physiol. Biochem. 2018, 48, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Kobet, R.A.; Pan, X.; Zhang, B.; Pak, S.C.; Asch, A.S.; Lee, M.-H. Caenorhabditis elegans: A model system for anti-cancer drug discovery and therapeutic target identification. Biomol. Ther. 2014, 22, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.B.; Hamza, A.; Singh, T.; Flibotte, S.; Hieter, P.; O’Neil, N.J. A multimodal genotoxic anticancer drug characterized by pharmacogenetic analysis in Caenorhabditis elegans. Genetics 2020, 215, 609–621. [Google Scholar] [CrossRef]
- Nathan, J.; Kannan, R.R. Antiangiogenic molecules from marine actinomycetes and the importance of using zebrafish model in cancer research. Heliyon 2020, 6, e05662. [Google Scholar] [CrossRef]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M.; et al. The use of zebrafish (Danio rerio) as biomedical models. Anim. Front. 2019, 9, 68–77. [Google Scholar] [CrossRef]
- Rapoport, H.; Willson, C.D. The preparation and properties of some methoxypyrroles. J. Am. Chem. Soc. 1962, 84, 630–635. [Google Scholar] [CrossRef]
- Hu, D.X.; Withall, D.M.; Challis, G.L.; Thomson, R.J. Structure, chemical synthesis, and biosynthesis of prodiginine natural products. Chem. Rev. 2016, 116, 7818–7853. [Google Scholar] [CrossRef]
- Domröse, A.; Klein, A.S.; Hage-Hülsmann, J.; Thies, S.; Svensson, V.; Classen, T.; Pietruszka, J.; Jaeger, K.-E.; Drepper, T.; Loeschcke, A. Efficient recombinant production of prodigiosin in Pseudomonas putida. Front. Microbiol. 2015, 6, 972. [Google Scholar] [CrossRef]
- Klein, A.S.; Brass, H.U.C.; Klebl, D.P.; Classen, T.; Loeschcke, A.; Drepper, T.; Sievers, S.; Jaeger, K.-E.; Pietruszka, J. Preparation of cyclic prodiginines by mutasynthesis in Pseudomonas putida KT2440. ChemBioChem 2018, 19, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.S.; Domröse, A.; Bongen, P.; Brass, H.U.C.; Classen, T.; Loeschcke, A.; Drepper, T.; Laraia, L.; Sievers, S.; Jaeger, K.-E.; et al. New prodigiosin derivatives obtained by mutasynthesis in Pseudomonas putida. ACS Synth. Biol. 2017, 6, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Xiang, R.; Li, J.; Wang, F.; Wang, C. High-level production of microbial prodigiosin: A review. J. Basic Microbiol. 2021, 61, 506–523. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-l.; Wang, X.-d.; Shen, Y.-l.; Wei, D.-z. Strategy for the improvement of prodigiosin production by a Serratia marcescens mutant through fed-batch fermentation. World J. Microbiol. Biotechnol. 2005, 21, 969–972. [Google Scholar] [CrossRef]
- Haddix, P.L.; Shanks, R.M.Q. Production of prodigiosin pigment by Serratia marcescens is negatively associated with cellular ATP levels during high-rate, low-cell-density growth. Can. J. Microbiol. 2020, 66, 243–255. [Google Scholar] [CrossRef]
- Casullo de Araújo, H.W.; Fukushima, K.; Takaki, G.M.C. Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low cost substrate. Molecules 2010, 15, 6931–6940. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Wang, S.-L.; Nguyen, D.-N.; Nguyen, A.-D.; Nguyen, T.-H.; Doan, M.-D.; Ngo, V.-A.; Doan, C.-T.; Kuo, Y.-H.; Nguyen, V.-B. Bioprocessing of marine chitinous wastes for the production of bioactive prodigiosin. Molecules 2021, 26, 3138–3151. [Google Scholar] [CrossRef]
- Paul, T.; Bandyopadhyay, T.K.; Mondal, A.; Tiwari, O.N.; Muthuraj, M.; Bhunia, B. A comprehensive review on recent trends in production, purification, and applications of prodigiosin. Biomass Conv. Bioref. 2020, 12, 1409–1431. [Google Scholar] [CrossRef]
- Chen, G.; Shi, K.; Song, D.; Quan, L.; Wu, Z. The pigment characteristics and productivity shifting in high cell density culture of Monascus anka mycelia. BMC Biotechnol. 2015, 15, 72. [Google Scholar] [CrossRef]
- Tran, L.T.; Techato, K.; Nguyen, V.B.; Wang, S.-L.; Nguyen, A.D.; Phan, T.Q.; Doan, M.D.; Phoungthong, K. Utilization of cassava wastewater for low-cost production of prodigiosin via Serratia marcescens TNU01 fermentation and its novel potent α-glucosidase inhibitory effect. Molecules 2021, 26, 6270. [Google Scholar] [CrossRef]
- Song, M.-J.; Bae, J.; Lee, D.-S.; Kim, C.-H.; Kim, J.-S.; Kim, S.-W.; Hong, S.-I. Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. J. Biosci. Bioeng. 2006, 101, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.-H.; Yarkoni, O.; Ajioka, J.; Wan, K.-L.; Nathan, S. Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl. Microbiol. Biotechnol. 2019, 103, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Seel, C.J.; Groll, M.; Gulder, T. Characterization of a cyanobacterial haloperoxidase and evaluation of its biocatalytic halogenation potential. ChemBioChem 2016, 17, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-R.; Chen, Y.-H.; Tseng, F.-J.; Weng, C.-F. The production and bioactivity of prodigiosin: Quo vadis? Drug Discov. Today 2020, 25, 828–836. [Google Scholar] [CrossRef]
- Manderville, R.A. Synthesis, proton-affinity and anti-cancer properties of the prodigiosin-group natural products. Curr. Med. Chem.-Anti-Cancer Agents 2001, 1, 195–218. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Zhou, L.; Yu, S.; Su, Z.; Song, J.; Sun, Q.; Sha, O.; Wang, X.; Jiang, W.; et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13150–13155. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Y.; Liu, J.; Wei, D. Antimetastatic effect of prodigiosin through inhibition of tumor invasion. Biochem. Pharmacol. 2005, 69, 407–414. [Google Scholar] [CrossRef]
- Baldino, C.M.; Parr, J.; Wilson, C.J.; Ng, S.-C.; Yohannesa, D.; Wasserman, H.H. Indoloprodigiosins from the C-10 bipyrrolic precursor: New antiproliferative prodigiosin analogs. Bioorg. Med. Chem. Lett. 2006, 16, 701–704. [Google Scholar] [CrossRef]
- Abrahantes-Pérez, M.C.; Reyes-González, J.; Véliz Ríos, G.; Bequet-Romero, M.; Gómez Riera, R.; Anais Gasmury, C.; Huerta, V.; González, L.J.; Canino, C.; Suarez, J.G.; et al. Cytotoxic proteins combined with prodigiosin obtained from Serratia marcescens have both broad and selective cytotoxic activity on tumor cells. J. Chemother. 2006, 18, 172–181. [Google Scholar] [CrossRef]
- Leong, S.W.; Chia, S.L.; Abas, F.; Yuso, K. In-vitro and in-silico evaluations of heterocyclic-containing diarylpentanoids as Bcl-2 inhibitors against LoVo colorectal cancer cells. Molecules 2020, 25, 3877–3892. [Google Scholar] [CrossRef]
- Lin, P.-B.; Shen, J.; Ou, P.-Y.; Liu, L.-Y.; Chen, Z.-Y.; Chu, F.-J.; Wang, J.; Jin, X.-B. Prodigiosin isolated from Serratia marcescens in the Periplaneta americana gut and its apoptosis-inducing activity in HeLa cells. Oncol. Rep. 2019, 41, 3377–3385. [Google Scholar] [CrossRef] [PubMed]
- Melvin, M.S.; Tomlinson, J.T.; Saluta, G.R.; Kucera, G.L.; Lindquist, N.; Manderville, R.A. Double-strand DNA cleavage by copper∙prodigiosin. J. Am. Chem. Soc. 2000, 122, 6333–6334. [Google Scholar] [CrossRef]
- Park, G.; Tomlinson, J.T.; Melvin, M.S.; Wright, M.W.; Day, C.S.; Manderville, R.A. Zinc and copper complexes of prodigiosin: Implications for copper-mediated double-strand DNA cleavage. Org. Lett. 2003, 5, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Espona-Fiedler, M.; Soto-Cerrato, V.; Quesada, R.; Pérez-Tomás, R.; Guallar, V. Molecular interactions of prodiginines with the BH3 domain of anti-apoptotic Bcl-2 family members. PLoS ONE 2013, 8, e57562. [Google Scholar] [CrossRef] [PubMed]
- Espona-Fiedler, M.; Soto-Cerrato, V.; Hosseini, A.; Lizcano, J.M.; Guallar, V.; Quesada, R.; Gao, T.; Pérez-Tomás, R. Identification of dual mTORC1 and mTORC2 inhibitors in melanoma cells: Prodigiosin vs. obatoclax. Biochem. Pharmacol. 2012, 83, 489–496. [Google Scholar] [CrossRef]
- Seah, S.-W.; Nathan, S.; Wan, K.-L. Toxicity Evaluation of Prodigiosin from Serratia marcescens in a Caenorhabditis elegans Model. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; p. 020015. [Google Scholar]
- Habash, S.S.; Brass, H.U.C.; Klein, A.S.; Klebl, D.P.; Weber, T.M.; Classen, T.; Pietruszka, J.; Grundler, F.M.W.; Schleker, A.S.S. Novel prodiginine derivatives demonstrate bioactivities on plants, nematodes, and fungi. Front. Plant Sci. 2020, 11, 579807. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.-L.; Doan, M.D.; Nguyen, T.H.; Tran, T.H.T.; Tran, T.N.; Doan, C.T.; Ngo, V.A.; Ho, N.D.; Do, V.C.; et al. Utilization of by-product of groundnut oil processing for production of prodigiosin by microbial fermentation and its novel potent anti-nematodes effect. Agronomy 2022, 12, 41–66. [Google Scholar] [CrossRef]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Chandrakant, N.; Laxmikant, S.; Satish, P. Nematicidal activity of microbial pigment from Serratia marcescens. Nat. Prod. Res. 2014, 28, 1399–1404. [Google Scholar] [CrossRef]
- Smithen, D.A.; Forrester, A.M.; Corkery, D.P.; Dellaire, G.; Colpitts, J.; McFarland, S.A.; Berman, J.N.; Thompson, A. Investigations regarding the utility of prodigiosenes to treat leukemia. Org. Biomol. Chem. 2013, 11, 62–68. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Faraag, A.H.; El-Batal, A.I.; El-Hendawy, H.H. Characterization of prodigiosin produced by Serratia marcescens strain isolated from irrigation water in Egypt. Nat. Sci. 2017, 15, 55–68. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to poliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. In WormBook: The Online Review of C. elegans Biology; Oxford University Press: Oxford, UK, 2006; pp. 1–11. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]
- Buschmann, J. The OECD Guidelines for the Testing of Chemicals and Pesticides; Springer: Berlin/Heidelberg, Germany, 2013; Volume 947. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The embryo test with the zebrafish Danio rerio—A general model in ecotoxicology and toxicology. ALTEX 2002, 19, 38–48. [Google Scholar]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems; OECD: Paris, France, 2013. [Google Scholar] [CrossRef]



| IC50 (µg/mL) | |||
|---|---|---|---|
| Cell Line | PG | PG-Br | PG-Br2 |
| MRC-5 | 1.20 ± 0.04 | 5.50 ± 0.02 | 10.00 ± 0.09 |
| A549 | 1.30 ± 0.02 | 8.00 ± 0.06 | 16.00 ± 0.08 |
| A375 | 1.25 ± 0.04 | 6.00 ± 0.05 | 12.00 ± 0.04 |
| MDA-MB-231 | 0.62 ± 0.01 | 6.25 ± 0.04 | 17.00 ± 0.05 |
| HCT116 | 0.70 ± 0.02 | 5.00 ± 0.05 | 10.00 ± 0.08 |
| Exposure Time (h) | IC50 (µg/mL) | ||
|---|---|---|---|
| PG | PG-Br | PG-Br2 | |
| Cell line MRC-5 | |||
| 24 | 1.80 ± 0.04 | 7.60 ± 0.08 | 15.00 ± 0.05 |
| 48 | 1.20 ± 0.04 | 5.50 ± 0.02 | 10.00 ± 0.09 |
| 72 | 0.70 ± 0.02 | 5.98 ± 0.09 | 10.00 ± 0.08 |
| Cell line HCT116 | |||
| 24 | 5.00 ± 0.06 | 35.00 ± 0.09 | 50.00 ± 0.09 |
| 48 | 0.70 ± 0.02 | 5.00 ± 0.05 | 10.00 ± 0.08 |
| 72 | 0.80 ± 0.02 | 3.20 ± 0.06 | 10.00 ± 0.05 |
| Annexin V Positive Cells | PG | PG-Br | PG-Br2 |
|---|---|---|---|
| (%) | 20.3 ± 0.5 | 21.3 ± 0.8 | 19.6 ± 0.6 |
| Comp. | miLogP a | TPSA b | Natoms c | MW d | NON e | NOHNH f | Nviol. g | Nrotb. h | Vol i |
|---|---|---|---|---|---|---|---|---|---|
| PG | 4.70 | 53.71 | 24 | 323.44 | 4 | 2 | 0 | 7 | 315.55 |
| PG-Br2 | 6.17 | 53.71 | 26 | 481.23 | 4 | 2 | 1 | 7 | 354.32 |
| PG-2-Br | 5.63 | 53.71 | 25 | 402.34 | 4 | 2 | 1 | 7 | 336.44 |
| PG-3-Br | 5.43 | 53.71 | 25 | 402.34 | 4 | 2 | 1 | 7 | 336.44 |
| PG-4-Br | 5.43 | 53.71 | 25 | 402.34 | 4 | 2 | 1 | 7 | 336.44 |
| PG-3′-Br | 5.43 | 53.71 | 25 | 402.34 | 4 | 2 | 1 | 7 | 336.44 |
| PG-6′-Br | 5.49 | 53.71 | 25 | 402.34 | 4 | 2 | 1 | 7 | 336.44 |
| PG-3″-Br | 5.43 | 53.71 | 25 | 402.34 | 4 | 2 | 1 | 7 | 336.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazic, J.; Skaro Bogojevic, S.; Vojnovic, S.; Aleksic, I.; Milivojevic, D.; Kretzschmar, M.; Gulder, T.; Petkovic, M.; Nikodinovic-Runic, J. Synthesis, Anticancer Potential and Comprehensive Toxicity Studies of Novel Brominated Derivatives of Bacterial Biopigment Prodigiosin from Serratia marcescens ATCC 27117. Molecules 2022, 27, 3729. https://doi.org/10.3390/molecules27123729
Lazic J, Skaro Bogojevic S, Vojnovic S, Aleksic I, Milivojevic D, Kretzschmar M, Gulder T, Petkovic M, Nikodinovic-Runic J. Synthesis, Anticancer Potential and Comprehensive Toxicity Studies of Novel Brominated Derivatives of Bacterial Biopigment Prodigiosin from Serratia marcescens ATCC 27117. Molecules. 2022; 27(12):3729. https://doi.org/10.3390/molecules27123729
Chicago/Turabian StyleLazic, Jelena, Sanja Skaro Bogojevic, Sandra Vojnovic, Ivana Aleksic, Dusan Milivojevic, Martin Kretzschmar, Tanja Gulder, Milos Petkovic, and Jasmina Nikodinovic-Runic. 2022. "Synthesis, Anticancer Potential and Comprehensive Toxicity Studies of Novel Brominated Derivatives of Bacterial Biopigment Prodigiosin from Serratia marcescens ATCC 27117" Molecules 27, no. 12: 3729. https://doi.org/10.3390/molecules27123729
APA StyleLazic, J., Skaro Bogojevic, S., Vojnovic, S., Aleksic, I., Milivojevic, D., Kretzschmar, M., Gulder, T., Petkovic, M., & Nikodinovic-Runic, J. (2022). Synthesis, Anticancer Potential and Comprehensive Toxicity Studies of Novel Brominated Derivatives of Bacterial Biopigment Prodigiosin from Serratia marcescens ATCC 27117. Molecules, 27(12), 3729. https://doi.org/10.3390/molecules27123729








