Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends
Abstract
:1. Introduction
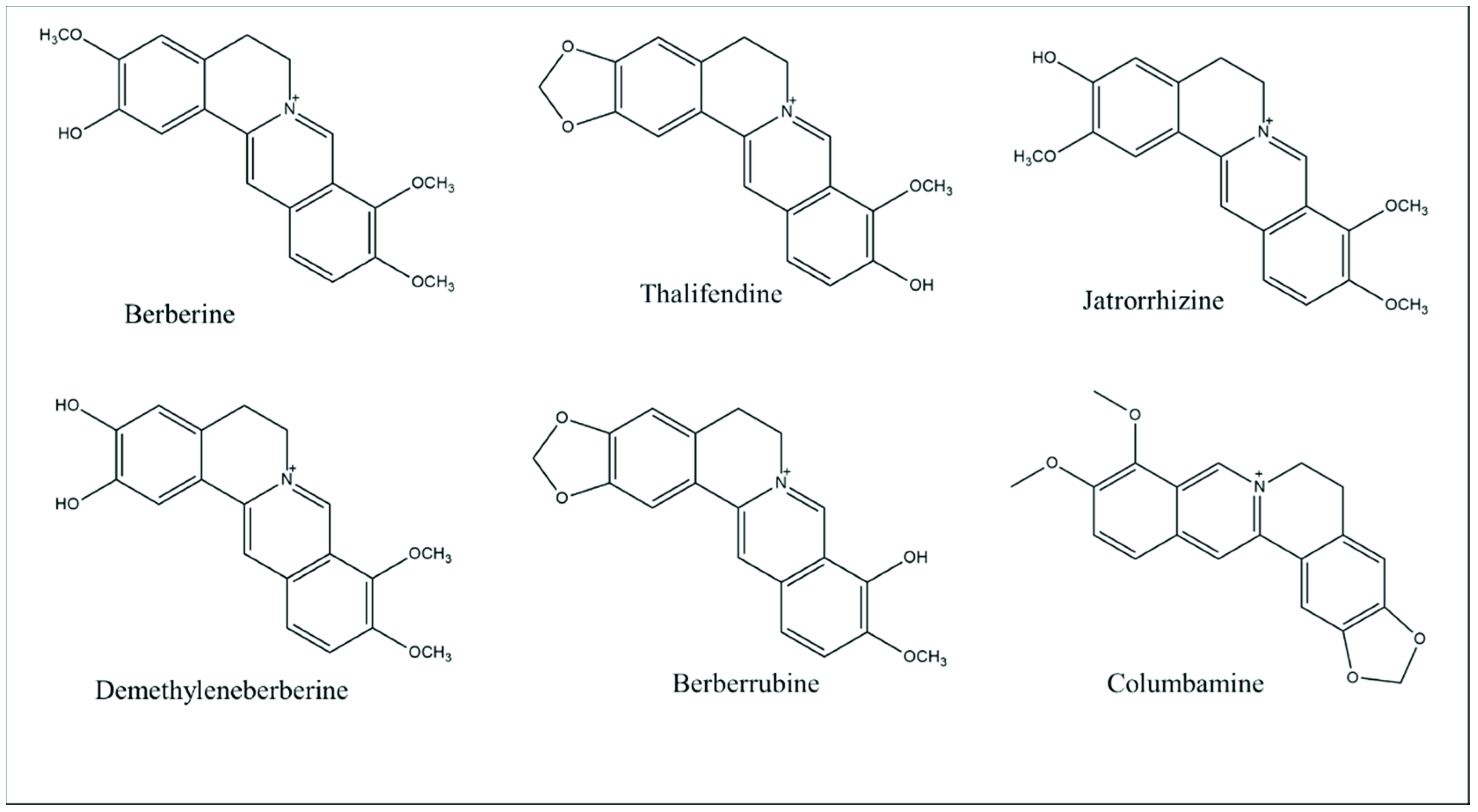
2. Berberine: Chemical Properties and Pharmacokinetic Profile
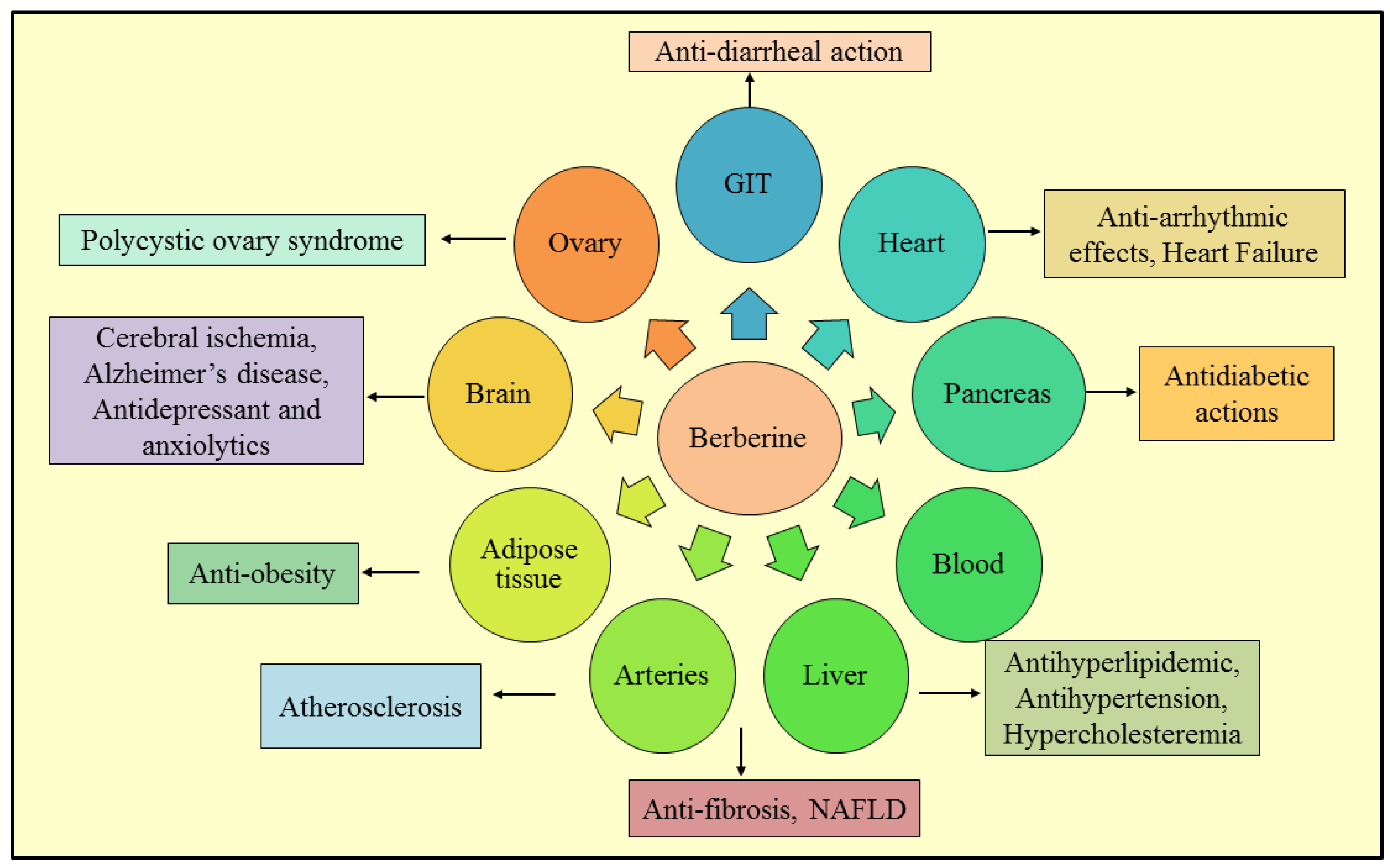
3. Therapeutic Roles of Berberine
3.1. Non-Alcoholic Fatty Liver Disease
3.2. Anti-Diarrheal Action
3.3. Anti-Fibrotic Effect
3.4. Alzheimer’s Disease
3.5. Antidepressant and Anxiolytic Effects
3.6. Cerebral Ischemia
3.7. Anti-Obesity
3.8. Polycystic Ovary Syndrome
3.9. Antidiabetic Action
3.10. Atherosclerosis
3.11. Antihyperlipidemic Effects
3.12. Antihypertensive Activity
3.13. Antiarrhythmic Effects
3.14. Congestive Heart Failure
3.15. Hypercholesterolemia
3.16. Anti-Inflammatory Activity
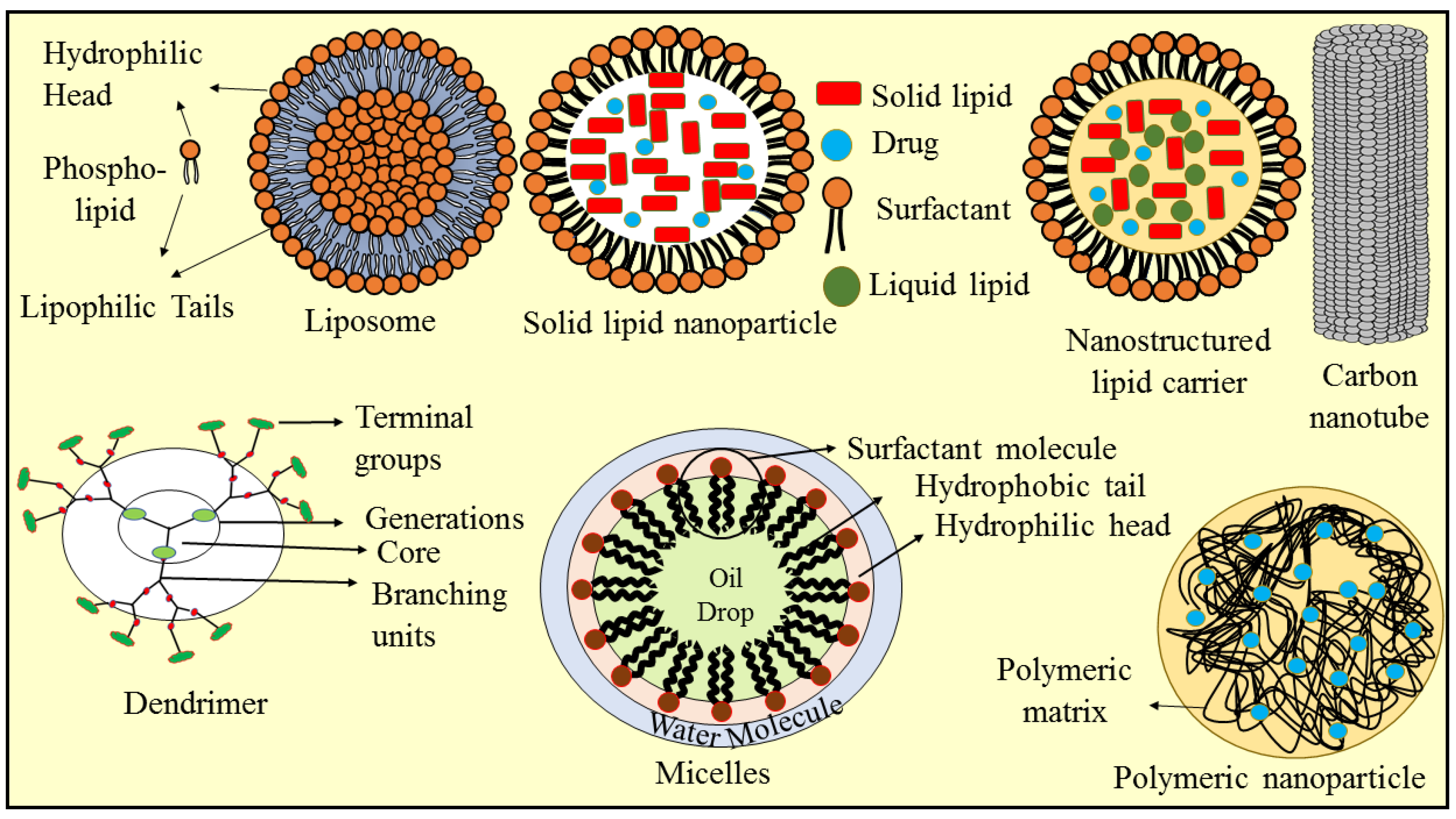
4. Nanotechnology-Based Approaches for Berberine Delivery
4.1. Solid Lipid Nanoparticles
4.2. Nanostructured Lipid Carriers
4.3. Liposomes
4.4. Micelles
4.5. Polymeric Nanoparticles
4.6. Dendrimer
4.7. Carbon Nanotube
| Technique | Dosage Form | Excipients | Outcomes | References |
| Polymeric Nanoparticles | ||||
| Co-precipitation method | Nano-hydroxyapatite/chitosan (n-HA/CS) bone cement | Hydroxyapatite, chitosan, citric acid, calcium hydroxide, orthophosphoric acid, acetic acid, potassium dihydrogen phosphate, zinc oxide, calcium chloride | The n-HA/CS particles with BBR were effective in treating bone deformities, and n-HA/CS particles with 1 wt. % BBR were found to be an efficacious antibiotic drug delivery method. | [175] |
| Freeze-drying process | Fucose-chitosan/heparin nanoparticle | Sodium cyanoborohydride, fucose, chitosan, heparin, trehalose | These nanoparticles produced a prolonged BBR release at the site of infection as a result of their pH sensitivity, leading to an increased level of BBR in the mucus/epithelium layer and inhibiting H. pylori growth. | [176] |
| Ionic cross-linking method | Drug-loaded chitosan nanoparticles | Chitosan, sodium tripolyphosphate | BBR-loaded chitosan nanoparticles had a prolonged retention duration in synovial fluid and exhibited a stronger anti-apoptotic effect than free BBR in the treatment of osteoarthritis. | [177] |
| Solvent evaporation and freeze-drying | O-hexadecyl-dextran-entrapped BBR chloride nanoparticles (BC-HDD NPs) | Fetal bovine serum, rhodamine 123, dextran, sodium hydroxide, | BC-HDD NPs are as efficacious as BBR at reducing oxidative stress, apoptotic cell death, and mitochondrial depolarization when used at ~20-fold lower dose. | [178] |
| Emulsification method | PLGA nanoparticles | Polylactide glycolic acid (PLGA), didodecyl dimethyl ammonium bromide, polyvinyl alcohol (PVA) | The highest encapsulation efficiency (58%) of the nanoparticles was found at pH 8, using a water-immiscible solvent, dichloromethane | [179] |
| Solvent evaporation technique | Polymer–lipid hybrid nanoparticles | Soybean phosphatidylcholine, 4′,6-diamidino-2-phenylindole, polyethylene glycol | PEG–lipid–PLGA NPs/BBR–SPC’s oral bioavailability was dramatically increased by 343% after oral administration to rats in comparison to the suspension of BBR. | [180] |
| Emulsion solvent evaporation method | Polymeric nanoparticles | Sodium alginate, Tween 80 | BBR-loaded polymeric nanoparticles had better antibacterial effectiveness than unloaded polymeric nanoparticles and BBR aqueous solution and were more effective against Bacillus cereus 240. | [181] |
| Ionic gelation method | Chitosan nanoparticles | Sodium tripolyphosphate, chitosan, glacial acetic acid | The combination use of chitosan nanoparticles and BBR provides synergistic action which allows for the efficient use of lower doses and increases their inhibitory effects against strains of Bacillus subtilis and Staphylococcus aureus | [182] |
| Dual emulsion method | PLGA polymeric nanoparticles | PLGA polymer, dichloromethane, polyvinyl alcohol | BBR-containing PLGA polymer nanoparticles had An encapsulation efficiency of 85.2% and will improve their efficiency on MCF-7 cancer cells. | [183] |
| Acid hydrolysis | Rod-shaped keratin nanoparticles (KNPs) | Hair, monopotassium phosphate, sodium chloride, disodium phosphate, potassium chloride | A large amount of BBR is released from KNPs at pH 1.2, showing that the photo-thermal action favors controlled release, and the NPs/BBR system exhibited anticancer activity against colon cancer cells | [184] |
| Nanosuspension | ||||
| High-pressure homogenization | Nanosuspension | Sodium lauryl sulfate, ceric ammonium nitrate, polyvinylpyrrolidone, azobisisobutyronitrile, calcium alginate, N,N-dimethylformamide | The administration of BBR nanosuspension-encapsulated HGFs greatly enhanced the healing process of S. aureus-infected wounds by its antibacterial action, stimulation of capillary development, and granulation, wound healing, hemostasis, and moisture regulation. | [185] |
| Nanogel | ||||
| Swelling/deswelling technique | Nanogel | Poly (diallyldimethylammonium chloride), Fluorescein diacetate, poly (allylamine hydrochloride), Carbopol Aqua SF1 | Significant enhancement in antimicrobial activity at smaller incubation durations in comparison to non-coated nanogel particles loaded with BBR | [186] |
| Solid lipid nanoparticle | ||||
| High-pressure homogenization | Solid lipid nanoparticle | Propidium iodide, glyceryl monostearate, ethylene diamine tetraacetic acid, coumarin 6, paraformaldehyde, penicillin-streptomycin, | BBR-HCl-loaded SLNs had a significant influence on MCF-7 breast cancer cells compared to free BH in terms of lowering the growth rate and inducing arrest of cell cycle and apoptosis. | [152] |
| Nanostructured lipid carriers | ||||
| Hot melting followed by high-pressure homogenization | Nanostructured lipid carriers (NLCs) | Compritol 888, cremophor EL, d-α-tocopheryl polyethylene glycol 1000 succinate, oleic acid | BBR-NLCs effectively suppressed H22 cell growth, and in vivo testing revealed superior antitumor activity, with inhibition rates of 68.3%. | [156] |
| Hot homogenization and ultrasonication strategy | BBR-loaded NLCs overlaid with chitosan (BER-CTS-NLCs) | Poloxamer 407, glycerol monostearate, oleic acid | In comparison to BBR solution, BER-CTS-NLCs showed higher drug levels in the brain, showing that CTS-NLCs might be employed to target the brain via the intranasal route. | [187] |
| High-pressure homogenization | Drug-loaded NLCs | Compritol 888 ATO, olive oil, TPGS | BBR-NLCs, upon oral administration, greatly reduced colitis symptoms by inhibiting NF-κB nuclear translocation and lowering pro-inflammatory cytokine expression | [188] |
| Hot-melt dispersion/homogenization procedure | Selenium-coated NLCs | Sodium selenite, Precirol® ATO 5, oleic acid | In comparison to regular NLCs and BBR solution, the BBR-loaded selenium-coated NLCs had a much better hypoglycemic action and also have a 6.63 times higher oral bioavailability than BBR solution. | [189] |
| Reverse micelle | ||||
| Lyophilization of water-in-oil emulsions | Anhydrous reverse micelle | Soybean phosphatidylcholine, medium-chain triglyceride | When compared to the BBR solution, the BER-loaded ARMs lowered diabetic mice’s blood glucose levels (BGLs) by 57% and increased oral bioavailability by a factor of 2.4. | [164] |
| Liposomes | ||||
| Ionophore A23187-mediated ZnSO4 gradient method | Liposomes | Hydrogenated soybean phospholipids, egg yolk lecithin, cholesterol, ammonium sulfate, soybean phospholipids, | The optimized liposomes of BBR hydrochloride have an encapsulation efficiency of 94.3 ± 2.1%. | [190] |
| Thin film hydration followed by sonication | Nano-liposome | Lecithin, chitosan, dihexadecyl phosphate | In the simulated gastrointestinal condition, chitosan-coated nano-liposomes showed superior stability and slower drug release than uncoated ones | [191] |
| Thin film hydration method | Liposome | Soyphsophatidylcholine, cholesterol | The vesicle diameter and entrapment efficiency results were reported to be extremely close to predicted values, and the observed particles are spherical with a zeta potential and an average diameter of −1.93 mV and 0.823 nm, respectively. | [192] |
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | α-smooth muscle actin |
| AChE | Acetylcholinesterase enzyme |
| AD | Alzheimer’s disease |
| AMPK | Adenosine 5′ monophosphate-activated protein kinase |
| ARMs | Anhydrous reverse micelle |
| BBR | Berberine |
| BGLs | Blood glucose levels |
| CHF | Congestive heart failure |
| CNTs | Carbon nanotubes |
| COX-2 | Cyclooxygenase-2 |
| FCCD | Factorial central composite design |
| HSC | Hepatic stellate cells |
| InsR | Insulin receptor |
| LDLR | Low-density lipoprotein-cholesterol receptor |
| MAO | Monoamine oxidase |
| MAPK/ERK | Mitogen-activated protein kinase/extracellular signal-regulated kinase |
| MFB | Myofibroblast-like cells |
| MMP-2 | Matrix metalloproteinase |
| MWCNTs | Multiwalled carbon nanotubes |
| NAFLD | Non-alcoholic fatty liver disease |
| NLCs | Nanostructured lipid carriers |
| oxLDL | Oxidized low-density lipoprotein |
| PCOS | Polycystic ovary syndrome |
| PDGF-A | Platelet-related growth factor A |
| PGs | Prostaglandins |
| SLNs | Solid lipid nanoparticles |
| T2DM | Type 2 diabetes mellitus |
| TGF-β1 | Transforming growth factor |
| TNF-α | Tumor necrosis factor-alpha |
| VSMCs | Vascular smooth muscle cells |
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lou, H. Strategies to Diversify Natural Products for Drug Discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [PubMed]
- Rishton, G.M. Natural Products as a Robust Source of New Drugs and Drug Leads: Past Successes and Present Day Issues. Am. J. Cardiol. 2008, 101, S43–S49. [Google Scholar] [CrossRef]
- Rungsung, W.; Ratha, K.K.; Dutta, S.; Dixit, A.K.; Hazra, J. Secondary Metabolites of Plants in Drugs Discovery. World J. Pharm. Res. 2015, 4, 604–613. [Google Scholar]
- Christodoulou, M.-I.; Tchoumtchoua, J.; Skaltsounis, A.-L.; Scorilas, A.; Halabalaki, M. Natural Alkaloids Intervening the Insulin Pathway: New Hopes for Anti-Diabetic Agents? Curr. Med. Chem. 2019, 26, 5982–6015. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.I.U.; Hui, S.U.N.; Zhang, A.-H.; Hong-Ying, X.U.; Guang-Li, Y.A.N.; Ying, H.A.N.; Xi-Jun, W. Natural Alkaloids: Basic Aspects, Biological Roles, and Future Perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar]
- Qing, Z.-X.; Yang, P.; Tang, Q.; Cheng, P.; Liu, X.-B.; Zheng, Y.; Liu, Y.-S.; Zeng, J.-G. Isoquinoline Alkaloids and Their Antiviral, Antibacterial, and Antifungal Activities and Structure-Activity Relationship. Curr. Org. Chem. 2017, 21, 1920–1934. [Google Scholar] [CrossRef]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and Therapeutic Effects of Berberis Vulgaris and Its Active Constituent, Berberine. Phyther. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of Berberine and Its Main Metabolites in Conventional and Pseudo Germ-Free Rats Determined by Liquid Chromatography/Ion Trap Mass Spectrometry. Drug Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar]
- Komal, S.; Ranjan, B.; Neelam, C.; Birendra, S.; Kumar, S.N. Berberis Aristata: A Review. Int. J. Res. Ayurveda Pharm. 2011, 2, 383–388. [Google Scholar]
- Caliceti, C.; Franco, P.; Spinozzi, S.; Roda, A.; FG Cicero, A. Berberine: New Insights from Pharmacological Aspects to Clinical Evidences in the Management of Metabolic Disorders. Curr. Med. Chem. 2016, 23, 1460–1476. [Google Scholar] [CrossRef] [PubMed]
- Tillhon, M.; Ortiz, L.M.G.; Lombardi, P.; Scovassi, A.I. Berberine: New Perspectives for Old Remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Suresh Kumar, G. Recent Advances in Nucleic Acid Binding Aspects of Berberine Analogs and Implications for Drug Design. Mini Rev. Med. Chem. 2016, 16, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Bonaventura, A.; Bianchi, L.; Romano, D.; Maffioli, P. Effects of Berberine on Lipid Profile in Subjects with Low Cardiovascular Risk. Expert Opin. Biol. Ther. 2013, 13, 475–482. [Google Scholar] [CrossRef]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical Characterization of Berberine Chloride: A Perspective in the Development of a Solution Dosage Form for Oral Delivery. Aaps Pharmscitech 2010, 11, 1466–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Huang, Z.; Ho, F.; Chan, W. Berberine Impairs Embryonic Development in Vitro and in Vivo through Oxidative Stress-mediated Apoptotic Processes. Environ. Toxicol. 2018, 33, 280–294. [Google Scholar] [CrossRef]
- Gaba, S.; Saini, A.; Singh, G.; Monga, V. An Insight into the Medicinal Attributes of Berberine Derivatives: A Review. Bioorg. Med. Chem. 2021, 38, 116143. [Google Scholar] [CrossRef]
- Feng, X.; Wang, K.; Cao, S.; Ding, L.; Qiu, F. Pharmacokinetics and Excretion of Berberine and Its Nine Metabolites in Rats. Front. Pharmacol. 2021, 11, 2238. [Google Scholar] [CrossRef]
- Pan, G.; Wang, G.; Liu, X.; Fawcett, J.P.; Xie, Y. The Involvement of P-glycoprotein in Berberine Absorption. Pharmacol. Toxicol. 2002, 91, 193–197. [Google Scholar] [CrossRef]
- Ma, J.; Feng, R.; Tan, X.; Ma, C.; Shou, J.; Fu, J.; Huang, M.; He, C.; Chen, S.; Zhao, Z. Excretion of Berberine and Its Metabolites in Oral Administration in Rats. J. Pharm. Sci. 2013, 102, 4181–4192. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Xing, D.; Su, H.; Ma, C.; Ding, Y.; Du, L. Kinetic Difference of Berberine between Hippocampus and Plasma in Rat after Intravenous Administration of Coptidis Rhizoma Extract. Life Sci. 2005, 77, 3058–3067. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, D.; Wang, W.; Su, H.; Tao, J.; Du, L. Pharmacokinetics of Berberine in Rat Thalamus after Intravenous Administration of Coptidis Rhizoma Extract. Am. J. Chin. Med. 2005, 33, 935–943. [Google Scholar] [CrossRef]
- Tan, X.-S.; Ma, J.-Y.; Feng, R.; Ma, C.; Chen, W.-J.; Sun, Y.-P.; Fu, J.; Huang, M.; He, C.-Y.; Shou, J.-W. Tissue Distribution of Berberine and Its Metabolites after Oral Administration in Rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Qiu, F.; Zhu, Z.; Kang, N.; Piao, S.; Qin, G.; Yao, X. Isolation and Identification of Urinary Metabolites of Berberine in Rats and Humans. Drug Metab. Dispos. 2008, 36, 2159–2165. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-S.; Zheng, Y.-R.; Zhang, Y.-F.; Long, X.-Y. Research Progress on Berberine with a Special Focus on Its Oral Bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef]
- Och, A.; Podgórski, R.; Nowak, R. Biological Activity of Berberine—A Summary Update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Xiao, D.; Liu, Z.; Zhang, S.; Zhou, M.; He, F.; Zou, M.; Peng, J.; Xie, X.; Liu, Y.; Peng, D. Berberine Derivatives with Different Pharmacological Activities via Structural Modifications. Mini Rev. Med. Chem. 2018, 18, 1424–1441. [Google Scholar] [CrossRef]
- Kumar, A.; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current Knowledge and Pharmacological Profile of Berberine: An Update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef]
- Kim, M.; Shin, M.S.; Lee, J.M.; Cho, H.S.; Kim, C.J.; Kim, Y.J.; Choi, H.R.; Jeon, J.W. Inhibitory Effects of Isoquinoline Alkaloid Berberine on Ischemia-Induced Apoptosis via Activation of Phosphoinositide 3-Kinase/Protein Kinase B Signaling Pathway. Int. Neurourol. J. 2014, 18, 115. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.-Q.; Zeng, X.-N.; Kong, H.; Sun, X.-L. Neuroprotective Effects of Berberine on Stroke Models in Vitro and in Vivo. Neurosci. Lett. 2008, 447, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Benaissa, F.; Mohseni-Rad, H.; Rahimi-Moghaddam, P.; Mahmoudian, M. Berberine Reduces the Hypoxic-Ischemic Insult in Rat Pup Brain. Acta Physiol. Hung. 2009, 96, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Song, H.; Ji, G. Update on Berberine in Nonalcoholic Fatty Liver Disease. Evid-Based Complement. Altern. Med. 2013, 2013, 308134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Xu, D.; Guo, Y.; Ping, J.; Chen, L.; Wang, H. Protection by and Anti-oxidant Mechanism of Berberine against Rat Liver Fibrosis Induced by Multiple Hepatotoxic Factors. Clin. Exp. Pharmacol. Physiol. 2008, 35, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, Z.; Li, Y.; Fichna, J.; Storr, M. Effects of Berberine in the Gastrointestinal Tract—A Review of Actions and Therapeutic Implications. Am. J. Chin. Med. 2014, 42, 1053–1070. [Google Scholar] [CrossRef] [Green Version]
- Joshi, P.V.; Shirkhedkar, A.A.; Prakash, K.; Maheshwari, V.L. Antidiarrheal Activity, Chemical and Toxicity Profile of Berberis Aristata. Pharm. Biol. 2011, 49, 94–100. [Google Scholar] [CrossRef]
- Zhou, M.; Deng, Y.; Liu, M.; Liao, L.; Dai, X.; Guo, C.; Zhao, X.; He, L.; Peng, C.; Li, Y. The Pharmacological Activity of Berberine, a Review for Liver Protection. Eur. J. Pharmacol. 2021, 890, 173655. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Q.; Tan, H.Y.; Hong, M.; Li, S.; Yuen, M.-F.; Feng, Y. Berberine Inhibition of Fibrogenesis in a Rat Model of Liver Fibrosis and in Hepatic Stellate Cells. Evid-Based Complement. Altern. Med. 2016, 2016, 8762345. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, X.; Hu, H.; Lu, Y.; Chen, J.; Yasuda, K.; Wang, H. Berberine Inhibits Hepatic Stellate Cell Proliferation and Prevents Experimental Liver Fibrosis. Biol. Pharm. Bull. 2009, 32, 1533–1537. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Pan, Y.; Kan, M.; Xiao, X.; Wang, Y.; Guan, F.; Zhang, X.; Chen, L. Hepatoprotective Effects of Berberine on Liver Fibrosis via Activation of AMP-Activated Protein Kinase. Life Sci. 2014, 98, 24–30. [Google Scholar] [CrossRef]
- Eissa, L.A.; Kenawy, H.I.; El-Karef, A.; Elsherbiny, N.M.; El-Mihi, K.A. Antioxidant and Anti-Inflammatory Activities of Berberine Attenuate Hepatic Fibrosis Induced by Thioacetamide Injection in Rats. Chem. Biol. Interact. 2018, 294, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Jakovac, H.; Marchesi, V.V.; Blažeković, B. Resolution of Liver Fibrosis by Isoquinoline Alkaloid Berberine in CCl4-Intoxicated Mice Is Mediated by Suppression of Oxidative Stress and Upregulation of MMP-2 Expression. J. Med. Food 2013, 16, 518–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansod, S.; Saifi, M.A.; Godugu, C. Molecular Updates on Berberine in Liver Diseases: Bench to Bedside. Phyther. Res. 2021, 35, 5459–5476. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, C.; Yang, W. Role of Berberine in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.-F.; Shen, L. Berberine: A Potential Multipotent Natural Product to Combat Alzheimer’s Disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.K.; Nandi, M.K.; Mishra, G.; Maurya, A.; Rai, A.; Rai, G.K.; Awasthi, R.; Sharma, B.; Kulkarni, G.T. Berberine: A Plant-Derived Alkaloid with Therapeutic Potential to Combat Alzheimer’s Disease. Cent. Nerv. Syst. Agents Med. Chem. (Formerly Curr. Med. Chem. Nerv. Syst. Agents) 2019, 19, 154–170. [Google Scholar] [CrossRef]
- Akbar, M.; Shabbir, A.; Rehman, K.; Akash, M.S.H.; Shah, M.A. Neuroprotective Potential of Berberine in Modulating Alzheimer’s Disease via Multiple Signaling Pathways. J. Food Biochem. 2021, 45, e13936. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Dhir, A. Berberine: A Plant Alkaloid with Therapeutic Potential for Central Nervous System Disorders. Phyther. Res. An Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 317–324. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Dhir, A. On the Mechanism of Antidepressant-like Action of Berberine Chloride. Eur. J. Pharmacol. 2008, 589, 163–172. [Google Scholar] [CrossRef]
- Kong, L.D.; Cheng, C.H.K.; Tan, R.X. Monoamine Oxidase Inhibitors from Rhizoma of Coptis Chinensis. Planta Med. 2001, 67, 74–76. [Google Scholar] [CrossRef]
- Peng, W.-H.; Wu, C.-R.; Chen, C.-S.; Chen, C.-F.; Leu, Z.-C.; Hsieh, M.-T. Anxiolytic Effect of Berberine on Exploratory Activity of the Mouse in Two Experimental Anxiety Models: Interaction with Drugs Acting at 5-HT Receptors. Life Sci. 2004, 75, 2451–2462. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Fu, S.; Pi, R.; He, F. Neuropharmacological and Pharmacokinetic Properties of Berberine: A Review of Recent Research. J. Pharm. Pharmacol. 2009, 61, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.-Y.; Hwang, I.K.; Lim, B.O.; Kang, T.-C.; Kim, D.-W.; Kim, S.M.; Lee, H.Y.; Dai Kim, J.; Won, M.H. Berberry Extract Reduces Neuronal Damage and N-Methyl-D-Aspartate Receptor 1 Immunoreactivity in the Gerbil Hippocampus after Transient Forebrain Ischemia. Biol. Pharm. Bull. 2006, 29, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, S.N.; Aboutaleb, N.; Souri, F. Berberine Confers Neuroprotection in Coping with Focal Cerebral Ischemia by Targeting Inflammatory Cytokines. J. Chem. Neuroanat. 2018, 87, 54–59. [Google Scholar] [CrossRef]
- Pulipati, V.P.; Pannain, S. Pharmacotherapy of Obesity in Complex Diseases. Clin. Obes. 2022, 12, e12497. [Google Scholar] [CrossRef]
- Badi, N.; Cruz-Monserrate, Z. Murine Model of Obesity-Induced Cancer. In Cancer Immunoprevention; Springer: Berlin/Heidelberg, Germany, 2022; pp. 195–201. [Google Scholar]
- Jiang, D.; Wang, D.; Zhuang, X.; Wang, Z.; Ni, Y.; Chen, S.; Sun, F. Berberine Increases Adipose Triglyceride Lipase in 3T3-L1 Adipocytes through the AMPK Pathway. Lipids Health Dis. 2016, 15, 214. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.M.; Barish, G.D.; Wang, Y.-X. PPARs and the Complex Journey to Obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Gong, Z.; Sheng, X.; Li, Z.; Zhang, W.; Qin, Y. Berberine Inhibits 3T3-L1 Adipocyte Differentiation through the PPARγ Pathway. Biochem. Biophys. Res. Commun. 2006, 348, 571–578. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, F.; Wei, H.; Liu, L.; Shen, T.; Xu, S.; Wei, J.; Ren, J.; Ni, H. Combination of Berberine and Evodiamine Inhibits Intestinal Cholesterol Absorption in High Fat Diet Induced Hyperlipidemic Rats. Lipids Health Dis. 2017, 16, 239. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yi, X.; Ghanam, K.; Zhang, S.; Zhao, T.; Zhu, X. Berberine Decreases Cholesterol Levels in Rats through Multiple Mechanisms, Including Inhibition of Cholesterol Absorption. Metabolism 2014, 63, 1167–1177. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of Gut Microbiota by Berberine and Metformin during the Treatment of High-Fat Diet-Induced Obesity in Rats. Sci. Rep. 2015, 5, 14405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.Y.; Piao, X.S.; Zhang, Q.; Li, P.; Yi, J.Q.; Liu, J.D.; Li, Q.Y.; Wang, G.Q. The Effects of Forsythia Suspensa Extract and Berberine on Growth Performance, Immunity, Antioxidant Activities, and Intestinal Microbiota in Broilers under High Stocking Density. Poult. Sci. 2013, 92, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Berberine Pharmacology and the Gut Microbiota: A Hidden Therapeutic Link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pathak, N.; Fatima, E.; Negi, A.S. Plant Isoquinoline Alkaloids: Advances in the Chemistry and Biology of Berberine. Eur. J. Med. Chem. 2021, 226, 113839. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Gober, H.-J.; Leung, W.T.; Wang, L. Effect and Mechanism of Berberine against Polycystic Ovary Syndrome. Biomed. Pharmacother. 2021, 138, 111468. [Google Scholar] [CrossRef]
- Wang, Z.; Nie, K.; Su, H.; Tang, Y.; Wang, H.; Xu, X.; Dong, H. Berberine Improves Ovulation and Endometrial Receptivity in Polycystic Ovary Syndrome. Phytomedicine 2021, 91, 153654. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, H.; Wang, A.; Sui, M.; Liang, K.; Deng, H.; Ma, Y.; Zhang, Y.; Zhang, H.; Guan, Y. A Clinical Study on the Short-Term Effect of Berberine in Comparison to Metformin on the Metabolic Characteristics of Women with Polycystic Ovary Syndrome. Eur. J. Endocrinol. 2012, 166, 99. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Infantino, V.; Riva, A.; Petrangolini, G.; Faliva, M.A.; Peroni, G.; Naso, M.; Nichetti, M.; Spadaccini, D.; Gasparri, C. Polycystic Ovary Syndrome Management: A Review of the Possible Amazing Role of Berberine. Arch. Gynecol. Obstet. 2020, 301, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Zhang, D.; Ma, H.; He, H.; Xia, Q.; Shen, W.; Chang, H.; Deng, Y.; Wu, Q.; Cong, J. The Effect of Berberine on Reproduction and Metabolism in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Control Trials. Evid-Based Complement. Altern. Med. 2019, 2019, 7918631. [Google Scholar] [CrossRef] [Green Version]
- Saleem, F.; Rizvi, S.W. New Therapeutic Approaches in Obesity and Metabolic Syndrome Associated with Polycystic Ovary Syndrome. Cureus 2017, 9, e1844. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fu, X.; Xu, J.; Wang, Q.; Kuang, H. Systems Pharmacology to Investigate the Interaction of Berberine and Other Drugs in Treating Polycystic Ovary Syndrome. Sci. Rep. 2016, 6, 28089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Chen, H.; Yan, L.; Wang, W.; Wang, D. Berberine Alleviates Type 2 Diabetic Symptoms by Altering Gut Microbiota and Reducing Aromatic Amino Acids. Biomed. Pharmacother. 2020, 131, 110669. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, N.; Zhao, L.; Lu, F. Berberine in the Treatment of Type 2 Diabetes Mellitus: A Systemic Review and Meta-Analysis. Evid-Based Complement. Altern. Med. 2012, 2012, 591654. [Google Scholar] [CrossRef] [Green Version]
- Abascal, K.; Yarnell, E. Recent Clinical Advances with Berberine. Altern. Complement. Ther. 2010, 16, 281–287. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Zhang, J.; Sun, C.; Lopez, A. Berberine Improves Glucose Homeostasis in Streptozotocin-Induced Diabetic Rats in Association with Multiple Factors of Insulin Resistance. Int. Sch. Res. Not. 2011, 2011, 519371. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Yang, D.; Liang, H.; Ye, J.; Weng, J. Berberine Improves Glucose Metabolism in Diabetic Rats by Inhibition of Hepatic Gluconeogenesis. PLoS ONE 2011, 6, e16556. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Xing, H.; Ye, J. Efficacy of Berberine in Patients with Type 2 Diabetes Mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yin, J.; Gao, H.; Xu, L.; Wang, Y.; Xu, L.; Li, M. Berberine Improves Insulin Sensitivity by Inhibiting Fat Store and Adjusting Adipokines Profile in Human Preadipocytes and Metabolic Syndrome Patients. Evid-Based Complement. Altern. Med. 2012, 2012, 363845. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Chen, L. Berberine in Type 2 Diabetes Therapy: A New Perspective for an Old Antidiarrheal Drug? Acta Pharm. Sin. B 2012, 2, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.-M.; Lee, C.H.; Oh, W.K.; Kim, C.T. Berberine, a Natural Plant Product, Activates AMP-Activated Protein Kinase with Beneficial Metabolic Effects in Diabetic and Insulin-Resistant States. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Kong, W.-J.; Shan, Y.-Q.; Song, D.-Q.; Li, Y.; Wang, Y.-M.; You, X.-F.; Jiang, J.-D. Protein Kinase D Activation Stimulates the Transcription of the Insulin Receptor Gene. Mol. Cell. Endocrinol. 2010, 330, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-J.; Dong, H.; Li, J.-B.; Xu, L.-J.; Zou, X.; Wang, K.-F.; Lu, F.-E.; Yi, P. Berberine Inhibits Hepatic Gluconeogenesis via the LKB1-AMPK-TORC2 Signaling Pathway in Streptozotocin-Induced Diabetic Rats. World J. Gastroenterol. WJG 2015, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y. Structural Changes of Gut Microbiota during Berberine-Mediated Prevention of Obesity and Insulin Resistance in High-Fat Diet-Fed Rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Lin, H.; Huang, W. Modulating Gut Microbiota as an Anti-Diabetic Mechanism of Berberine. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2011, 17, RA164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Kong, W.; Jiang, J. Learning from Berberine: Treating Chronic Diseases through Multiple Targets. Sci. China Life Sci. 2015, 58, 854–859. [Google Scholar] [CrossRef] [Green Version]
- El Hadri, K.; Smith, R.; Duplus, E.; El Amri, C. Inflammation, Oxidative Stress, Senescence in Atherosclerosis: Thioredoxine-1 as an Emerging Therapeutic Target. Int. J. Mol. Sci. 2021, 23, 77. [Google Scholar] [CrossRef]
- Noble, C.; Carlson, K.D.; Neumann, E.; Lewis, B.; Dragomir-Daescu, D.; Lerman, A.; Erdemir, A.; Young, M.D. Finite Element Analysis in Clinical Patients with Atherosclerosis. J. Mech. Behav. Biomed. Mater. 2022, 125, 104927. [Google Scholar] [CrossRef]
- Mehu, M.; Narasimhulu, C.A.; Singla, D.K. Inflammatory Cells in Atherosclerosis. Antioxidants 2022, 11, 233. [Google Scholar] [CrossRef]
- Xie, X.; Ma, X.; Zeng, S.; Tang, W.; Xiao, L.; Zhu, C.; Yu, R. Mechanisms of Berberine for the Treatment of Atherosclerosis Based on Network Pharmacology. Evid-Based Complement. Altern. Med. 2020, 2020, 3568756. [Google Scholar] [CrossRef] [Green Version]
- Frismantiene, A.; Philippova, M.; Erne, P.; Resink, T.J. Smooth Muscle Cell-Driven Vascular Diseases and Molecular Mechanisms of VSMC Plasticity. Cell. Signal. 2018, 52, 48–64. [Google Scholar] [CrossRef]
- Ping, S.; Liu, S.; Zhou, Y.; Li, Z.; Li, Y.; Liu, K.; Bardeesi, A.S.A.; Wang, L.; Chen, J.; Deng, L. Protein Disulfide Isomerase-Mediated Apoptosis and Proliferation of Vascular Smooth Muscle Cells Induced by Mechanical Stress and Advanced Glycosylation End Products Result in Diabetic Mouse Vein Graft Atherosclerosis. Cell Death Dis. 2017, 8, e2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, R.; Yang, H.; Liu, Y.; Zhou, Y.; Xu, X.; Li, C.; Liu, S. Effects of Berberine on Atherosclerosis. Front. Pharmacol. 2021, 12, 764175. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lim, H.-J.; Park, J.-H.; Lee, K.-S.; Jang, Y.; Park, H.-Y. Berberine-Induced LDLR up-Regulation Involves JNK Pathway. Biochem. Biophys. Res. Commun. 2007, 362, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Abidi, P.; Zhou, Y.; Jiang, J.-D.; Liu, J. Extracellular Signal-Regulated Kinase–Dependent Stabilization of Hepatic Low-Density Lipoprotein Receptor MRNA by Herbal Medicine Berberine. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2170–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brusq, J.-M.; Ancellin, N.; Grondin, P.; Guillard, R.; Martin, S.; Saintillan, Y.; Issandou, M. Inhibition of Lipid Synthesis through Activation of AMP Kinase: An Additional Mechanism for the Hypolipidemic Effects of Berberine. J. Lipid Res. 2006, 47, 1281–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H. Berberine Is a Novel Cholesterol-Lowering Drug Working through a Unique Mechanism Distinct from Statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P. Treatment of Type 2 Diabetes and Dyslipidemia with the Natural Plant Alkaloid Berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Rovati, L.C.; Setnikar, I. Eulipidemic Effects of Berberine Administered Alone or in Combination with Other Natural Cholesterol-Lowering Agents. Arzneimittelforschung 2007, 57, 26–30. [Google Scholar] [CrossRef]
- Kong, W.-J.; Wei, J.; Zuo, Z.-Y.; Wang, Y.-M.; Song, D.-Q.; You, X.-F.; Zhao, L.-X.; Pan, H.-N.; Jiang, J.-D. Combination of Simvastatin with Berberine Improves the Lipid-Lowering Efficacy. Metabolism 2008, 57, 1029–1037. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Chakraborty, S.; Singh, S. Berberine: A Potential Phytochemical with Multispectrum Therapeutic Activities. Expert Opin. Investig. Drugs 2010, 19, 1297–1307. [Google Scholar] [CrossRef]
- Fatehi-Hassanabad, Z.; Jafarzadeh, M.; Tarhini, A.; Fatehi, M. The Antihypertensive and Vasodilator Effects of Aqueous Extract from Berberis Vulgaris Fruit on Hypertensive Rats. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 222–225. [Google Scholar]
- Derosa, G.; Maffioli, P.; Cicero, A.F.G. Berberine on Metabolic and Cardiovascular Risk Factors: An Analysis from Preclinical Evidences to Clinical Trials. Expert Opin. Biol. Ther. 2012, 12, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Šmejkal, K.; Malaník, M. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 2019, 9, 1923. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-H.; Yao, X.-Q.; Lau, C.-W.; Law, W.-I.; Chen, Z.-Y.; Kwok, W.; Ho, K.; Huang, Y. Vasorelaxant and Antiproliferative Effects of Berberine. Eur. J. Pharmacol. 2000, 399, 187–196. [Google Scholar] [CrossRef]
- Cicero, A.; Ertek, S. Metabolic and Cardiovascular Effects of Berberine: From Preclinical Evidences to Clinical Trial Results. Clin. Lipidol. 2009, 4, 553–563. [Google Scholar] [CrossRef]
- Kang, D.G.; Sohn, E.J.; Kwon, E.K.; Han, J.H.; Oh, H.; Lee, H.S. Effects of Berberine on Angiotensin-Converting Enzyme and NO/CGMP System in Vessels. Vascul. Pharmacol. 2002, 39, 281–286. [Google Scholar] [CrossRef]
- Caliceti, C.; Rizzo, P.; Ferrari, R.; Fortini, F.; Aquila, G.; Leoncini, E.; Zambonin, L.; Rizzo, B.; Calabria, D.; Simoni, P. Novel Role of the Nutraceutical Bioactive Compound Berberine in Lectin-like OxLDL Receptor 1-Mediated Endothelial Dysfunction in Comparison to Lovastatin. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 552–563. [Google Scholar] [CrossRef]
- Knox, M.; Vinet, R.; Fuentes, L.; Morales, B.; Martínez, J.L. A Review of Endothelium-Dependent and-Independent Vasodilation Induced by Phytochemicals in Isolated Rat Aorta. Animals 2019, 9, 623. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.-W.; Yin, S.-C.; Ting, C.-T.; Lin, S.-J.; Hsueh, C.-M.; Chen, C.-Y.; Hsu, S.-L. Berberine Inhibits Platelet-Derived Growth Factor-Induced Growth and Migration Partly through an AMPK-Dependent Pathway in Vascular Smooth Muscle Cells. Eur. J. Pharmacol. 2008, 590, 343–354. [Google Scholar] [CrossRef]
- Firouzi, S.; Malekahmadi, M.; Ghayour-Mobarhan, M.; Ferns, G.; Rahimi, H.R. Barberry in the Treatment of Obesity and Metabolic Syndrome: Possible Mechanisms of Action. Diabetes, Metab. Syndr. Obes. Targets Ther. 2018, 11, 699. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Tang, F.; Deng, Y.; Li, X.; Lan, T.; Zhang, X.; Huang, H.; Liu, P. Berberine Reduces Fibronectin and Collagen Accumulation in Rat Glomerular Mesangial Cells Cultured under High Glucose Condition. Mol. Cell. Biochem. 2009, 325, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.-J.; Im, E.K.; Kwon, J.H.; Lee, K.-H.; Shin, H.-J.; Oh, J.; Kang, S.-M.; Chung, J.H.; Jang, Y. Berberine Inhibits the Production of Lysophosphatidylcholine-Induced Reactive Oxygen Species and the ERK1/2 Pathway in Vascular Smooth Muscle Cells. Mol. Cells 2005, 20, 429–434. [Google Scholar] [PubMed]
- Mazza, A.; Lenti, S.; Schiavon, L.; Zuin, M.; D’Avino, M.; Ramazzina, E.; Casiglia, E. Nutraceuticals for Serum Lipid and Blood Pressure Control in Hypertensive and Hypercholesterolemic Subjects at Low Cardiovascular Risk. Adv. Ther. 2015, 32, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, T.; Peng, Q.; Yue, S.; Wang, S. Berberine via Suppression of Transient Receptor Potential Vanilloid 4 Channel Improves Vascular Stiffness in Mice. J. Cell. Mol. Med. 2015, 19, 2607–2616. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Gu, Y.; Liu, P.; Jiang, X.; Yu, W.; Ye, P.; Chao, Y.; Yang, H.; Zhu, L.; Zhou, L. Berberine Attenuates Pulmonary Arterial Hypertension via Protein Phosphatase 2A Signaling Pathway Both in Vivo and in Vitro. J. Cell. Physiol. 2018, 233, 9750–9762. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Akhter, M.H.; Alam, M.; Ali, M.D.; Hussain, A. An Updated Review on Therapeutic Potential and Recent Advances in Drug Delivery of Berberine: Current Status and Future Prospect. Curr. Pharm. Biotechnol. 2022, 23, 60–71. [Google Scholar] [CrossRef]
- Lau, C.; Yao, X.; Chen, Z.; Ko, W.; Huang, Y. Cardiovascular Actions of Berberine. Cardiovasc. Drug Rev. 2001, 19, 234–244. [Google Scholar] [CrossRef]
- Caruso, L.; Nadur, N.F.; da Fonseca, M.B.; Peixoto Ferreira, L.d.A.; Lacerda, R.B.; Graebin, C.S.; Kümmerle, A.E. The Design of Multi-Target Drugs to Treat Cardiovascular Diseases: Two (or More) Birds on One Stone. Curr. Top. Med. Chem. 2022, 22, 366–394. [Google Scholar] [CrossRef]
- Zhou, Z.-W.; Zheng, H.-C.; Zhao, L.-F.; Li, W.; Hou, J.-W.; Yu, Y.; Miao, P.-Z.; Zhu, J.-M. Effect of Berberine on Acetylcholine-Induced Atrial Fibrillation in Rabbit. Am. J. Transl. Res. 2015, 7, 1450. [Google Scholar]
- Jun-xian, C.; Lu, F.; Yu-hui, D.; Yan-li, D. The Effect of Berberine on Arrhythmia Caused by Stretch of Isolated Myocardial Infarcted Hearts in Rats. Heart 2012, 98, E84–E85. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; FU, L.; Sun, D. The Effect of Berberine on Arrhythmia Caused by Stretch of Myocardium, in Vitro, of Rats after Myocardial Ischemia. Chin. J. Emerg. Med. 2012, 12, 683–686. [Google Scholar]
- Yu, F.; Lin, M.; Zhang, W. Inhibition of Berberine on IKr, IKs and IK1 in Thyroxine Induced Cardiomyopathic Guinea Pig Ventricular Myocytes. J. China Pharm. Univ. 2009, 40, 244–249. [Google Scholar]
- Wang, L.; Yu, C.; Fu, Y.; Li, Q.; Sun, Y. Berberine Elicits Anti-arrhythmic Effects via IK1/Kir2. 1 in the Rat Type 2 Diabetic Myocardial Infarction Model. Phyther. Res. 2011, 25, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Y.; Tang, Y.; Yang, J.; Wang, D.; Yu, T.; Huang, C. Berberine Attenuates Spontaneous Action Potentials in Sinoatrial Node Cells and the Currents of Human HCN4 Channels Expressed in Xenopus Laevis Oocytes. Mol. Med. Rep. 2014, 10, 1576–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosterd, A.; Hoes, A.W. Clinical Epidemiology of Heart Failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Hao, J.; Fan, D. Biological Properties and Clinical Applications of Berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Xiong, C.; Wu, Y.; Zhang, Y.; Wu, Z.; Chen, X.; Jiang, P.; Guo, H.; Xie, K.; Wang, K.; Su, S. Protective Effect of Berberine on Acute Cardiomyopathy Associated with Doxorubicin Treatment. Oncol. Lett. 2018, 15, 5721–5729. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.-M.; Luo, M.-H. Study Progress of Berberine for Treating Cardiovascular Disease. Chronic Dis. Transl. Med. 2015, 1, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, X.; Liu, H.; Luo, F.; Li, G. Effects of Ginseng Total Saponins with Berberine on Plasma Brain Natriuretic Peptide and Ca2+ Concentration in Experimental Rats with Chronic Congestive Heart Failure. China J. Chin. Mater. Medica 2009, 34, 324–327. [Google Scholar]
- Dong, S.-F.; Hong, Y.; Liu, M.; Hao, Y.-Z.; Yu, H.-S.; Liu, Y.; Sun, J.-N. Berberine Attenuates Cardiac Dysfunction in Hyperglycemic and Hypercholesterolemic Rats. Eur. J. Pharmacol. 2011, 660, 368–374. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, J.-D.; Kong, W.-J. Berberine Upregulates Hepatic Low-Density Lipoprotein Receptor through Ras-Independent but AMP-Activated Protein Kinase-Dependent Raf-1 Activation. Biol. Pharm. Bull. 2014, 37, b14-00412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, J.; Ranheim, T.; Kulseth, M.A.; Leren, T.P.; Berge, K.E. Berberine Decreases PCSK9 Expression in HepG2 Cells. Atherosclerosis 2008, 201, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, X.; Yang, Y.; Wu, L.; Li, F.; Zhang, R.; Yuan, G.; Wang, N.; Chen, M.; Ning, G. Berberine Attenuates CAMP-Induced Lipolysis via Reducing the Inhibition of Phosphodiesterase in 3T3-L1 Adipocytes. Biochim. Biophys. Acta (BBA)-Molecular Basis Dis. 2011, 1812, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Dong, B.; Park, S.W.; Lee, H.-S.; Chen, W.; Liu, J. Hepatocyte Nuclear Factor 1α Plays a Critical Role in PCSK9 Gene Transcription and Regulation by the Natural Hypocholesterolemic Compound Berberine. J. Biol. Chem. 2009, 284, 28885–28895. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.-J.; Xu, R.-X.; Sun, J.; Tang, Y.; Li, J.-J. Enhanced Circulating PCSK9 Concentration by Berberine through SREBP-2 Pathway in High Fat Diet-Fed Rats. J. Transl. Med. 2014, 12, 103. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zidichouski, J.A. Update on the Benefits and Mechanisms of Action of the Bioactive Vegetal Alkaloid Berberine on Lipid Metabolism and Homeostasis. Cholesterol 2018, 2018, 7173920. [Google Scholar] [CrossRef]
- Gu, L.; Li, N.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine Ameliorates Intestinal Epithelial Tight-Junction Damage and down-Regulates Myosin Light Chain Kinase Pathways in a Mouse Model of Endotoxinemia. J. Infect. Dis. 2011, 203, 1602–1612. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.-H.; Ahn, I.-S.; Kim, Y.-H.; Park, J.-W.; Lee, S.-Y.; Hyun, C.-K.; Do, M.-S. Berberine Reduces the Expression of Adipogenic Enzymes and Inflammatory Molecules of 3T3-L1 Adipocyte. Exp. Mol. Med. 2006, 38, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Wang, J.; Liu, L. Advance of Studies on Anti-Atherosclerosis Mechanism of Berberine. Chin. J. Integr. Med. 2010, 16, 188–192. [Google Scholar] [CrossRef]
- Lou, T.; Zhang, Z.; Xi, Z.; Liu, K.; Li, L.; Liu, B.; Huang, F. Berberine Inhibits Inflammatory Response and Ameliorates Insulin Resistance in Hepatocytes. Inflammation 2011, 34, 659–667. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, P.; Wu, X.; Liu, W.; Shen, X.; Lan, T.; Xu, S.; Peng, J.; Xie, X.; Huang, H. Berberine Attenuates Lipopolysaccharide-Induced Extracelluar Matrix Accumulation and Inflammation in Rat Mesangial Cells: Involvement of NF-ΚB Signaling Pathway. Mol. Cell. Endocrinol. 2011, 331, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, M.; Fromm, A.; Krug, S.M.; Amasheh, S.; Andres, S.; Zeitz, M.; Fromm, M.; Schulzke, J.-D. TNFα-Induced and Berberine-Antagonized Tight Junction Barrier Impairment via Tyrosine Kinase, Akt and NFκB Signaling. J. Cell Sci. 2010, 123, 4145–4155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.W.; Hsu, K.C.; Lee, J.-W.; Ham, M.; Huh, J.Y.; Shin, H.J.; Kim, W.S.; Kim, J.B. Berberine Suppresses Proinflammatory Responses through AMPK Activation in Macrophages. Am. J. Physiol. Metab. 2009, 296, E955–E964. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-Y.; Tseng, Y.-T.; Lo, Y.-C. Berberine, a Natural Antidiabetes Drug, Attenuates Glucose Neurotoxicity and Promotes Nrf2-Related Neurite Outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano Strategies for Berberine Delivery, a Natural Alkaloid of Berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef]
- Mohanraj, V.J.; Chen, Y. Nanoparticles—A review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Kayser, O.; Lemke, A.; Hernandez-Trejo, N. The Impact of Nanobiotechnology on the Development of New Drug Delivery Systems. Curr. Pharm. Biotechnol. 2005, 6, 3–5. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. Aaps Pharmscitech 2011, 12, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Zhang, L.; Yang, M.; Zhang, W.; Li, X.; Ou, Z.; Li, Z.; Liu, S.; Li, X.; Yang, S. Berberine-Loaded Solid Lipid Nanoparticles Are Concentrated in the Liver and Ameliorate Hepatosteatosis in Db/Db Mice. Int. J. Nanomed. 2015, 10, 5049. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Yang, M.; Zhang, W.; Li, X.; Gao, D.; Ou, Z.; Li, Z.; Liu, S.; Li, X.; Yang, S. Characterization, Pharmacokinetics, and Hypoglycemic Effect of Berberine Loaded Solid Lipid Nanoparticles. Int. J. Nanomed. 2013, 8, 4677. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the Antitumor Activity of Berberine Hydrochloride by Solid Lipid Nanoparticle Encapsulation. Aaps Pharmscitech 2014, 15, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Zahoor, I.; Sachdeva, M.; Subramaniyan, V.; Fuloria, S.; Fuloria, N.K.; Naved, T.; Bhatia, S.; Al-Harrasi, A.; Aleya, L. Deciphering the Role of Nanoparticles for Management of Bacterial Meningitis: An Update on Recent Studies. Environ. Sci. Pollut. Res. 2021, 28, 60459–60476. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.I.; Devesa, S.S. Cancer Burden in the Year 2000. The Global Picture. Eur. J. Cancer 2001, 37, 4–66. [Google Scholar] [CrossRef]
- Meng, X.-P.; Fan, H.; Wang, Y.; Wang, Z.; Chen, T. Anti-Hepatocarcinoma Effects of Berberine-Nanostructured Lipid Carriers against Human HepG2, Huh7, and EC9706 Cancer Cell Lines. In Proceedings of the Optics in Health Care and Biomedical Optics VII, Beijing, China, 12–14 October 2016; Volume 10024, p. 1002417. [Google Scholar]
- Wang, Z.; Wu, J.; Chen, T.; Zhou, Q.; Wang, Y. In Vitro and in Vivo Antitumor Efficacy of Berberine-Nanostructured Lipid Carriers against H22 Tumor. In Proceedings of the Biophotonics and Immune Responses X, International Society for Optics and Photonics, San Francisco, CA, USA, 7–12 February 2015; Volume 9324, p. 93240Y. [Google Scholar]
- Raju, M.; Kunde, S.S.; Auti, S.T.; Kulkarni, Y.A.; Wairkar, S. Berberine Loaded Nanostructured Lipid Carrier for Alzheimer’s Disease: Design, Statistical Optimization and Enhanced in Vivo Performance. Life Sci. 2021, 285, 119990. [Google Scholar] [CrossRef]
- Gendy, A.M.; Elnagar, M.R.; Allam, M.M.; Mousa, M.R.; Khodir, A.E.; El-Haddad, A.E.; Elnahas, O.S.; Fayez, S.M.; El-Mancy, S.S. Berberine-Loaded Nanostructured Lipid Carriers Mitigate Warm Hepatic Ischemia/Reperfusion-Induced Lesion through Modulation of HMGB1/TLR4/NF-ΚB Signaling and Autophagy. Biomed. Pharmacother. 2022, 145, 112122. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Calvo, A.; Moreno, E.; Larrea, E.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Berberine-Loaded Liposomes for the Treatment of Leishmania Infantum-Infected BALB/c Mice. Pharmaceutics 2020, 12, 858. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Kuo, J.-Y.; Hsu, C.-C.; Tsai, W.-C.; Li, W.-C.; Yu, M.-C.; Wen, H.-W. Optimizing Manufacture of Liposomal Berberine with Evaluation of Its Antihepatoma Effects in a Murine Xenograft Model. Int. J. Pharm. 2013, 441, 381–388. [Google Scholar] [CrossRef]
- Allijn, I.E.; Czarny, B.M.S.; Wang, X.; Chong, S.Y.; Weiler, M.; Da Silva, A.E.; Metselaar, J.M.; Lam, C.S.P.; Pastorin, G.; De Kleijn, D.P. V Liposome Encapsulated Berberine Treatment Attenuates Cardiac Dysfunction after Myocardial Infarction. J. Control. Release 2017, 247, 127–133. [Google Scholar] [CrossRef]
- Barbosa-Barros, L.; Barba, C.; Rodríguez, G.; Cócera, M.; Coderch, L.; López-Iglesias, C.; De La Maza, A.; López, O. Lipid Nanostructures: Self-Assembly and Effect on Skin Properties. Mol. Pharm. 2009, 6, 1237–1245. [Google Scholar] [CrossRef]
- Wang, T.; Wang, N.; Song, H.; Xi, X.; Wang, J.; Hao, A.; Li, T. Preparation of an Anhydrous Reverse Micelle Delivery System to Enhance Oral Bioavailability and Anti-Diabetic Efficacy of Berberine. Eur. J. Pharm. Sci. 2011, 44, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lim, D.Y.; Lee, C.H.; Jeon, J.-H.; Choi, M.-K.; Song, I.-S. Enhanced Intestinal Absorption and Pharmacokinetic Modulation of Berberine and Its Metabolites through the Inhibition of P-Glycoprotein and Intestinal Metabolism in Rats Using a Berberine Mixed Micelle Formulation. Pharmaceutics 2020, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Nagavarma, B.V.N.; Yadav, H.K.S.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different Techniques for Preparation of Polymeric Nanoparticles-a Review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Vuddanda, P.R.; Mishra, A.; Singh, S.K.; Singh, S. Development of Polymeric Nanoparticles with Highly Entrapped Herbal Hydrophilic Drug Using Nanoprecipitation Technique: An Approach of Quality by Design. Pharm. Dev. Technol. 2015, 20, 579–587. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, W.-Y.; Lai, C.-H.; Hsu, Y.-M.; Yao, Y.-H.; Chen, T.-Y.; Wu, J.-Y.; Peng, S.-F.; Lin, Y.-H. Development of Novel Nanoparticles Shelled with Heparin for Berberine Delivery to Treat Helicobacter Pylori. Acta Biomater. 2011, 7, 593–603. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Lin, Y.-C.; Lu, H.-T.; Ho, Y.-C.; Weng, S.-C.; Tsai, M.-L.; Mi, F.-L. A Novel Injectable in Situ Forming Gel Based on Carboxymethyl Hexanoyl Chitosan/Hyaluronic Acid Polymer Blending for Sustained Release of Berberine. Carbohydr. Polym. 2019, 206, 664–673. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A Versatile Nanocarrier for Drug Delivery and Targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Gupta, L.; Sharma, A.K.; Gothwal, A.; Khan, M.S.; Khinchi, M.P.; Qayum, A.; Singh, S.K.; Gupta, U. Dendrimer Encapsulated and Conjugated Delivery of Berberine: A Novel Approach Mitigating Toxicity and Improving in Vivo Pharmacokinetics. Int. J. Pharm. 2017, 528, 88–99. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon Nanotubes: Applications in Pharmacy and Medicine. Biomed Res. Int. 2013, 2013, 578290. [Google Scholar] [CrossRef] [Green Version]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.C.; Wong, E.H. An Overview of Recent Development in Therapeutic Drug Carrier System Using Carbon Nanotubes. J. Drug Deliv. Sci. Technol. 2020, 59, 101855. [Google Scholar] [CrossRef]
- Lohan, S.; Raza, K.; Mehta, S.K.; Bhatti, G.K.; Saini, S.; Singh, B. Anti-Alzheimer’s Potential of Berberine Using Surface Decorated Multi-Walled Carbon Nanotubes: A Preclinical Evidence. Int. J. Pharm. 2017, 530, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Li, Y.; Zhang, L.; Zuo, Y.; Li, J.; Li, J. Antibiotic Delivery System Using Nano-hydroxyapatite/Chitosan Bone Cement Consisting of Berberine. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 89, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Lin, J.-H.; Chou, S.-C.; Chang, S.-J.; Chung, C.-C.; Chen, Y.-S.; Chang, C.-H. Berberine-Loaded Targeted Nanoparticles as Specific Helicobacter Pylori Eradication Therapy: In Vitro and in Vivo Study. Nanomedicine 2015, 10, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, S.; Peng, H.; Yu, L.; He, B.; Zhao, Q. In Vivo Anti-Apoptosis Activity of Novel Berberine-Loaded Chitosan Nanoparticles Effectively Ameliorates Osteoarthritis. Int. Immunopharmacol. 2015, 28, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Singh, S.; Tripathi, M.; Bhatnagar, P.; Kakkar, P.; Gupta, K.C. O-Hexadecyl-Dextran Entrapped Berberine Nanoparticles Abrogate High Glucose Stress Induced Apoptosis in Primary Rat Hepatocytes. PLoS ONE 2014, 9, e89124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khemani, M.; Sharon, M.; Sharon, M. Encapsulation of Berberine in Nano-Sized PLGA Synthesized by Emulsification Method. Int. Sch. Res. Not. 2012, 2012, 187354. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Ao, M.; Zheng, X.; Li, N.; Xia, J.; Li, Y.; Li, D.; Hou, Z.; Qi, Z.; Chen, X.D. PEG–Lipid–PLGA Hybrid Nanoparticles Loaded with Berberine–Phospholipid Complex to Facilitate the Oral Delivery Efficiency. Drug Deliv. 2017, 24, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Mehra, M.; Sheorain, J.; Kumari, S. Synthesis of Berberine Loaded Polymeric Nanoparticles by Central Composite Design. In Proceedings of the AIP Conference Proceedings, Penang, Malaysia, 9–11 August 2022; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1724, p. 20060. [Google Scholar]
- Dash, S.; Kumar, M.; Pareek, N. Enhanced Antibacterial Potential of Berberine via Synergism with Chitosan Nanoparticles. Mater. Today Proc. 2020, 31, 640–645. [Google Scholar] [CrossRef]
- Taebpour, M.; Arasteh, F.; Akhlaghi, M.; Haghirosadat, B.F.; Oroojalian, F.; Tofighi, D. Fabrication and Characterization of PLGA Polymeric Nanoparticles Containing Berberine and Its Cytotoxicity on Breast Cancer Cell (MCF-7). Nanomed. Res. J. 2021, 6, 396–408. [Google Scholar]
- Silva, O.A.; Pellá, M.G.; Popat, K.C.; Kipper, M.J.; Rubira, A.F.; Martins, A.F.; Follmann, H.D.M.; Silva, R. Rod-Shaped Keratin Nanoparticles Extracted from Human Hair by Acid Hydrolysis as Photothermally Triggered Berberine Delivery System. Adv. Powder Technol. 2022, 33, 103353. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, X.-D.; Shen, B.-D.; Han, J.; Lv, Q.-Y.; Dai, L.; Lin, M.-G.; Yu, C.; Bai, J.-X.; Yuan, H.-L. Development of Poly (N-Isopropylacrylamide)/Alginate Copolymer Hydrogel-Grafted Fabrics Embedding of Berberine Nanosuspension for the Infected Wound Treatment. J. Biomater. Appl. 2014, 28, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Al-Awady, M.J.; Fauchet, A.; Greenway, G.M.; Paunov, V.N. Enhanced Antimicrobial Effect of Berberine in Nanogel Carriers with Cationic Surface Functionality. J. Mater. Chem. B 2017, 5, 7885–7897. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Enin, H.A.; Elkomy, M.H.; Naguib, I.A.; Ahmed, M.F.; Alsaidan, O.A.; Alsalahat, I.; Ghoneim, M.M.; Eid, H.M. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceuticals 2022, 15, 281. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wu, Z.; Zhao, Z.; Wu, C.; Yuan, M.; Su, Z.; Wang, Y.; Wang, Z. Berberine-Loaded Nanostructured Lipid Carriers Enhance the Treatment of Ulcerative Colitis. Int. J. Nanomed. 2020, 15, 3937. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Yin, Y.; Song, X. Selenium-Coated Nanostructured Lipid Carriers Used for Oral Delivery of Berberine to Accomplish a Synergic Hypoglycemic Effect. Int. J. Nanomed. 2017, 12, 8671. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Li, J.; Guo, L.; Cheng, X.; Zhang, T.; Deng, Y. Preparation of Berberine Hydrochloride Long-Circulating Liposomes by Ionophore A23187-Mediated ZnSO4 Gradient Method. Asian J. Pharm. Sci. 2013, 8, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.X.; Huang, L.; Liu, L.; Abdalla, A.M.E.; Gauthier, M.; Yang, G. Chitosan-Coated Nano-Liposomes for the Oral Delivery of Berberine Hydrochloride. J. Mater. Chem. B 2014, 2, 7149–7159. [Google Scholar] [CrossRef]
- Sailor, G.; Seth, A.K.; Parmar, G.; Chauhan, S.; Javia, A. Formulation and in Vitro Evaluation of Berberine Containing Liposome Optimized by 32 Full Factorial Designs. J. Appl. Pharm. Sci. 2015, 5, 23–28. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Description |
| Source | Family: Berberidaceae and genus: Berberis (~450–500 species) such as Berberis vulgaris, Berberis aristata, Berberis lycium, Berberis buxifolia, Berberis chitria, Berberis darwinii. |
| Form | Powder of chloride or sulfate salt |
| Color | Bright yellow |
| Taste | Bitter |
| Nature | Neutral |
| Solubility | Water-insoluble |
| Melting point | 145 °C |
| Oral bioavailability | Low |
| Organ distribution | Highest concentration in the liver, followed by kidneys, muscle, lungs, brain, heart, and pancreas, and the lowest concentration in adipose tissue, which remains generally stable for 48 h |
| Metabolism | In liver, where it undergoes phase I demethylation before combining with sulfuric acid or glucuronic acid to produce phase II metabolites |
| Excretion | Feces (84%), urine (78%) and bile |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behl, T.; Singh, S.; Sharma, N.; Zahoor, I.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bungau, S. Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends. Molecules 2022, 27, 3705. https://doi.org/10.3390/molecules27123705
Behl T, Singh S, Sharma N, Zahoor I, Albarrati A, Albratty M, Meraya AM, Najmi A, Bungau S. Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends. Molecules. 2022; 27(12):3705. https://doi.org/10.3390/molecules27123705
Chicago/Turabian StyleBehl, Tapan, Sukhbir Singh, Neelam Sharma, Ishrat Zahoor, Ali Albarrati, Mohammed Albratty, Abdulkarim M. Meraya, Asim Najmi, and Simona Bungau. 2022. "Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends" Molecules 27, no. 12: 3705. https://doi.org/10.3390/molecules27123705
APA StyleBehl, T., Singh, S., Sharma, N., Zahoor, I., Albarrati, A., Albratty, M., Meraya, A. M., Najmi, A., & Bungau, S. (2022). Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends. Molecules, 27(12), 3705. https://doi.org/10.3390/molecules27123705










