Tripeptide IRW Protects MC3T3-E1 Cells against Ang II Stress in an AT2R Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Antibodies
2.2. Cell Culture
2.3. Western Blot Analysis
2.4. Brdu Incorporation Assay
2.5. Mineralization Assay
2.6. ALP Activity Assay
2.7. Superoxide Detection
2.8. Apoptosis Assay
2.9. RNA Extraction and qPCR
2.10. Statistical Analysis
3. Results
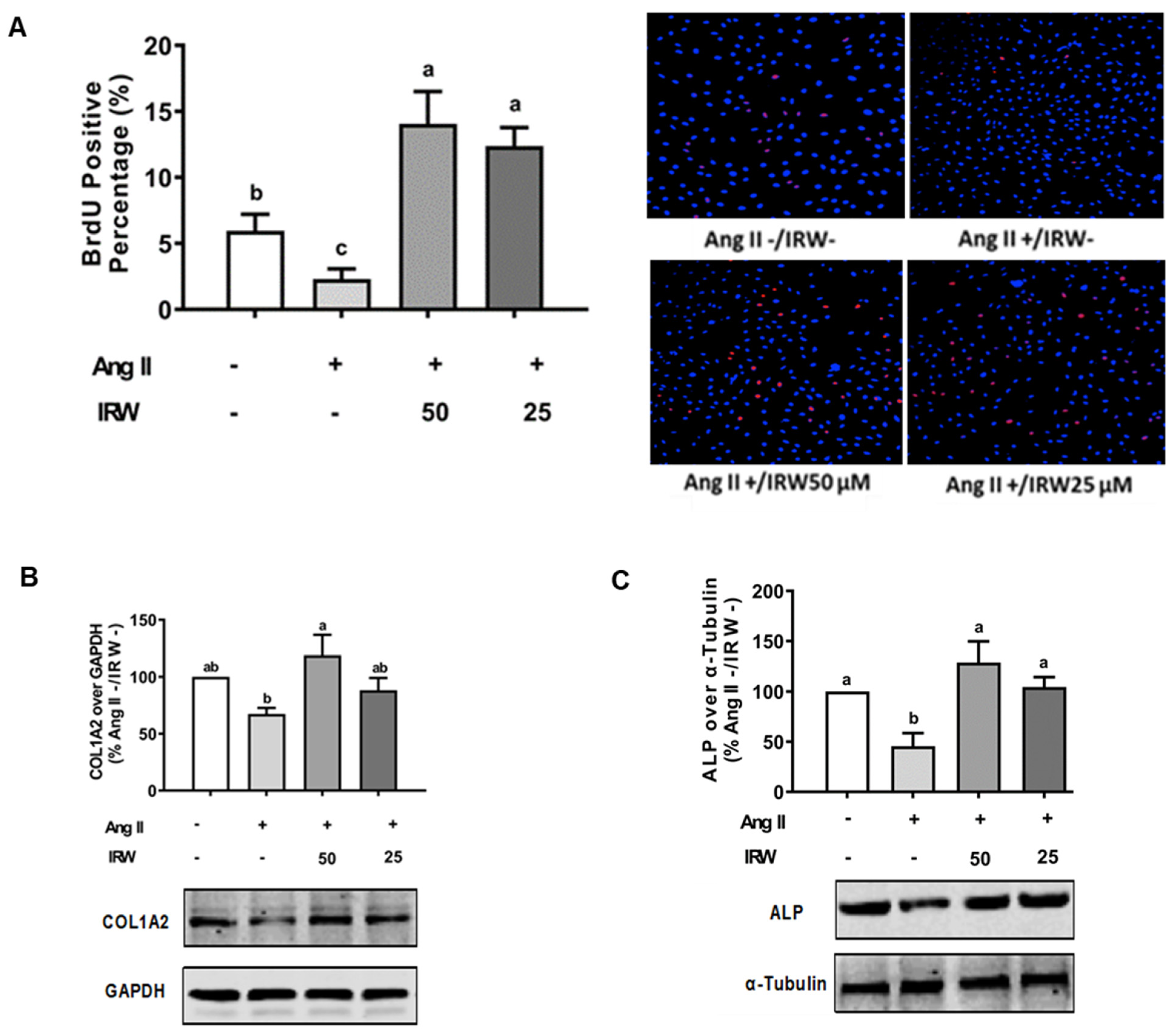
3.1. Impact of IRW Osteoblastic Activity against Ang II Stress in Bone Cells
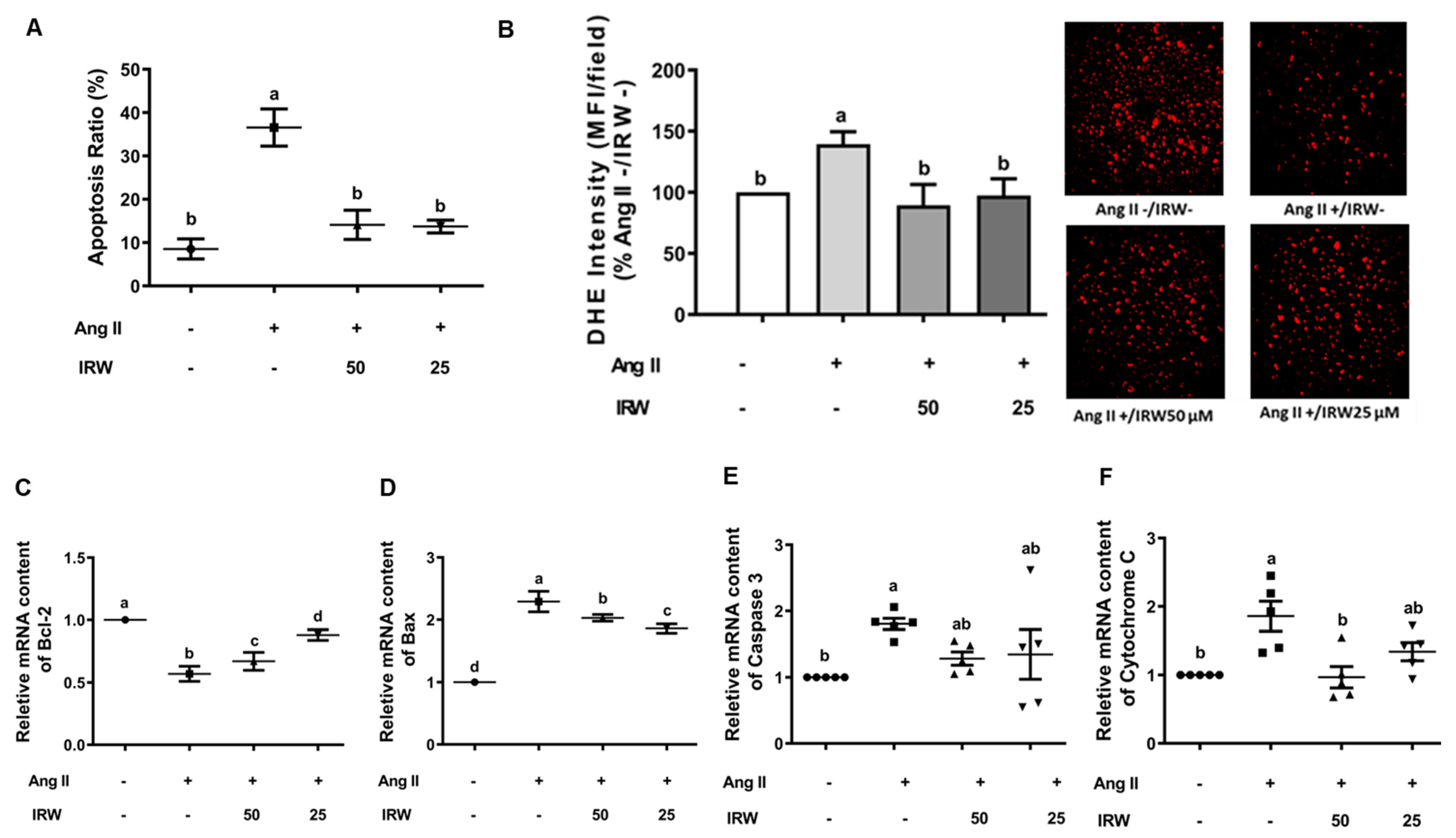
3.2. Cytoprotective Role of IRW against Apoptotic Activity of Ang II in Bone Cells
3.3. IRW Modulates RAAS Factors against Ang II Stress in Bone Cells
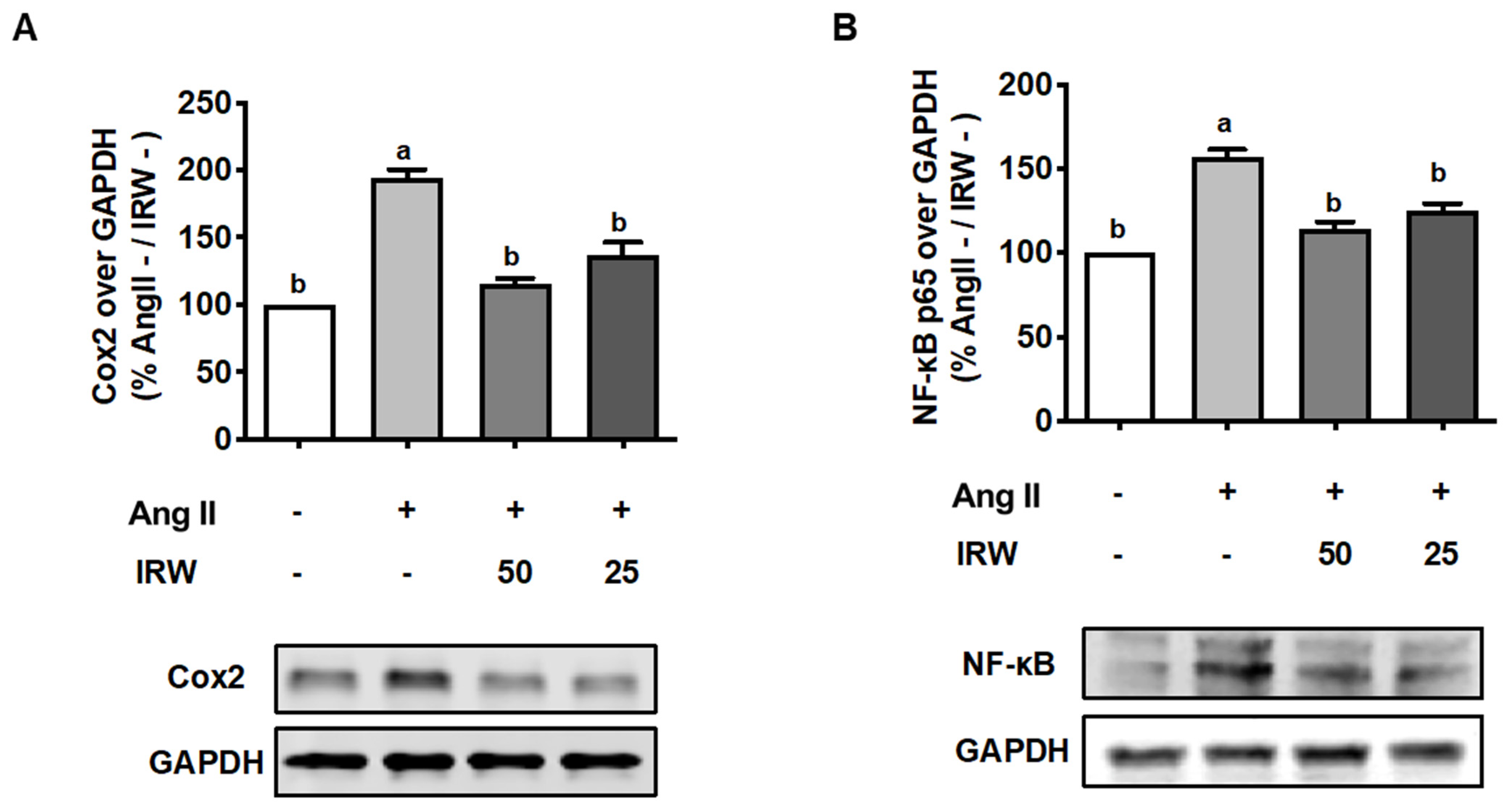
3.4. IRW Mitigates Inflammation Induced by Ang II Stress in Bone Cells
3.5. IRW Mitigates Cellular Stress Induced by Ang II in AT2R Dependent Manner in Bone Cells
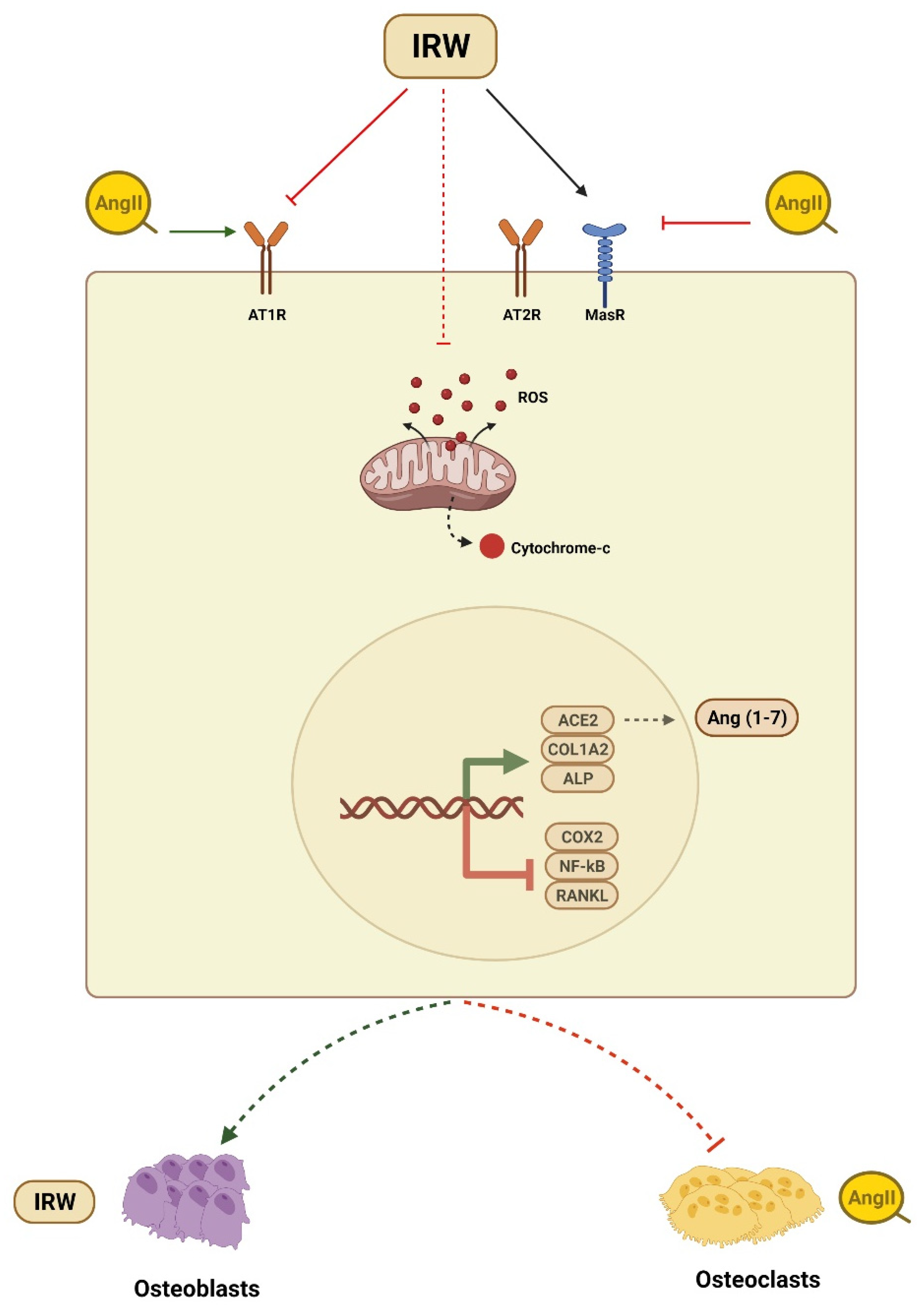
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Morgan, E.F.; Gerstenfeld, L.C. The bone organ system: Form and function. In Marcus and Feldman’s Osteoporosis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 15–35. [Google Scholar]
- Bislev, L.S.; Sikjær, T.; Rolighed, L.; Rejnmark, L. Relationship Between Aldosterone and Parathyroid Hormone, and the Effect of Angiotensin and Aldosterone Inhibition on Bone Health. Clin. Rev. Bone Miner. Metab. 2015, 13, 194–205. [Google Scholar] [CrossRef]
- Högberg, U.; Andersson, J.; Högberg, G.; Thiblin, I. Metabolic bone disease risk factors strongly contributing to long bone and rib fractures during early infancy: A population register study. PLoS ONE 2018, 13, e0208033. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Ke, J.; Zhao, D.; Zhang, B. Role of the renin–angiotensin–aldosterone system in bone metabolism. J. Bone Miner. Metab. 2020, 38, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Fountain, J.H.; Lappin, S.L. Physiology, Renin Angiotensin System; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- McKinley, M.; Albiston, A.; Allen, A.M.; Mathai, M.; May, C.; McAllen, R.; Oldfield, B.; Mendelsohn, F.; Chai, S.Y. The brain renin–angiotensin system: Location and physiological roles. Int. J. Biochem. Cell Biol. 2003, 35, 901–918. [Google Scholar] [CrossRef]
- White, A.J.; Cheruvu, S.C.; Sarris, M.; Liyanage, S.S.; Lumbers, E.; Chui, J.; Wakefield, D.; McCluskey, P. Expression of classical components of the renin-angiotensin system in the human eye. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz-Junior, C.M.; Santos, A.C.P.M.; Galvão, I.; Souto, G.R.; Mesquita, R.A.; Sá, M.A.; Ferreira, A.J. The angiotensin converting enzyme 2/angiotensin-(1-7)/Mas Receptor axis as a key player in alveolar bone remodeling. Bone 2019, 128, 115041. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Nakagami, H.; Osako, M.K.; Hanayama, R.; Kunugiza, Y.; Kizawa, T.; Tomita, T.; Yoshikawa, H.; Ogihara, T.; Morishita, R. Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J. 2008, 22, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Ito, M.; Fumoto, T.; Fukuhara, R.; Ishida, J.; Fukamizu, A.; Ikeda, K. Physiological function of the angiotensin AT1a receptor in bone remodeling. J. Bone Miner. Res. 2011, 26, 2959–2966. [Google Scholar] [CrossRef]
- Li, J.; Xiao, X.; Wei, W.; Ding, H.; Yue, Y.; Tian, Y.; Nabar, N.R.; Liu, Z.; Yang, Z.; Wang, M. Inhibition of angiotensin II receptor I prevents inflammation and bone loss in periodontitis. J. Periodontol. 2019, 90, 208–216. [Google Scholar] [CrossRef]
- Patel, J.; Douglas, G.; Kerr, A.; Hale, A.B.; Channon, K. Effect of irradiation and bone marrow transplantation on angiotensin II-induced aortic inflammation in ApoE knockout mice. Atherosclerosis 2018, 276, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Nakai, K.; Kawato, T.; Morita, T.; Yamazaki, Y.; Tanaka, H.; Tonogi, M.; Oki, H.; Maeno, M. Angiotensin II suppresses osteoblastic differentiation and mineralized nodule formation via AT1 receptor in ROS17/2.8 cells. Arch. Med. Sci. 2015, 11, 628–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.-S.; Zhang, Y.; Li, X.-L.; Wu, S.-Y.; Diao, T.-Y.; Hai, R.; Deng, H.-W. Involvement of the Skeletal Renin-Angiotensin System in Age-Related Osteoporosis of Ageing Mice. Biosci. Biotechnol. Biochem. 2012, 76, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, M.; Hao, L.; Loo, W.T.; Jin, L.; Cheung, M.N.; Chow, L.W.; Ng, E.L. Angiotensin II induces mitochondrial dysfunction and promotes apoptosis via JNK signalling pathway in primary mouse calvaria osteoblast. Arch. Oral Biol. 2014, 59, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Lau, C.W.; Wong, W.T.; Xu, A.; Au, C.L.; Ng, C.F.; Ng, S.S.M.; Gollasch, M.; Yao, X.; Huang, Y. Pivotal Role of Protein Kinase Cδ in Angiotensin II–Induced Endothelial Cyclooxygenase-2 Expression: A Link to Vascular Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1169–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, P.; Schwenzer, S.; Slotta, J.; Scheuer, C.; Tami, A.; Holstein, J.; Histing, T.; Burkhardt, M.; Pohlemann, T.; Menger, M.D. Inhibition of angiotensin-converting enzyme stimulates fracture healing and periosteal callus formation–Role of a local renin-angiotensin system. Br. J. Pharmacol. 2010, 159, 1672–1680. [Google Scholar] [CrossRef] [Green Version]
- Boschmann, M.; Nussberger, J.; Engeli, S.; Danser, A.J.; Yeh, C.-M.; Prescott, M.F.; Dahlke, M.; Jordan, J. Aliskiren penetrates adipose and skeletal muscle tissue and reduces renin–angiotensin system activity in obese hypertensive patients. J. Hypertens. 2012, 30, 561–566. [Google Scholar] [CrossRef]
- Garcia-Testal, A.; Monzo, A.; Rabanaque, G.; Gonzalez, A.; Romeu, A. Evolution of the bone mass of hypertense menopausal women in treatment with fosinopril. Med. Clin. 2006, 127, 692–694. [Google Scholar]
- Kwok, T.; Leung, J.; Barrett-Connor, E. ARB users exhibit a lower fracture incidence than ACE inhibitor users among older hypertensive men. Age Ageing 2017, 46, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Uehara, K.; Takahashi, A.; Watanabe, M.; Nomura, Y. Shark protein improves bone mineral density in ovariectomized rats and inhibits osteoclast differentiation. Nutrition 2014, 30, 719–725. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Je, J.-Y. Bone health-promoting bioactive peptides. J. Food Biochem. 2019, 43, e12529. [Google Scholar] [CrossRef] [Green Version]
- Duffuler, P.; Bhullar, K.S.; de Campos Zani, S.C.; Wu, J. Bioactive Peptides: From Basic Research to Clinical Trials and Commercialization. J. Agric. Food Chem. 2022, 70, 3585–3595. [Google Scholar] [CrossRef] [PubMed]
- Di Bernardini, R.; Harnedy, P.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A.M.; Hayes, M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011, 124, 1296–1307. [Google Scholar] [CrossRef]
- Wu, J. A Novel Angiotensin Converting Enzyme 2 (ACE2) Activating Peptide: A Reflection of 10 Years of Research on a Small Peptide Ile-Arg-Trp (IRW). J. Agric. Food Chem. 2020, 68, 14402–14408. [Google Scholar] [CrossRef]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Structure and Activity Study of Egg Protein Ovotransferrin Derived Peptides (IRW and IQW) on Endothelial Inflammatory Response and Oxidative Stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Bhullar, K.S.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg White-Derived Tripeptide IRW (Ile-Arg-Trp) Is an Activator of Angiotensin Converting Enzyme 2. J. Agric. Food Chem. 2018, 66, 11330–11336. [Google Scholar] [CrossRef]
- Shang, N.; Bhullar, K.S.; Hubbard, B.P.; Wu, J. Tripeptide IRW initiates differentiation in osteoblasts via the RUNX2 pathway. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 1138–1146. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Son, M.; Kerek, E.; Cromwell, C.R.; Wingert, B.M.; Wu, K.; Jovel, J.; Camacho, C.J.; Hubbard, B.P.; Wu, J. Tripeptide IRW Upregulates NAMPT Protein Levels in Cells and Obese C57BL/6J Mice. J. Agric. Food Chem. 2021, 69, 1555–1566. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.-R.; Gao, P.-J. Mitochondrial division inhibitor Mdivi-1 ameliorates angiotensin II-induced endothelial dysfunction. Sheng Li Xue Bao Acta Physiol. Sin. 2016, 68, 669–676. [Google Scholar]
- Patel, V.B.; Zhong, J.-C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Dong, Z.; Gao, S.; Chen, G.; Liu, D. AT1R-Mediated Apoptosis of Bone Marrow Mesenchymal Stem Cells Is Associated with mtROS Production and mtDNA Reduction. Oxidative Med. Cell. Longev. 2019, 2019, 4608165. [Google Scholar] [CrossRef]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The Effect of Inflammation on Bone. Front. Physiol. 2021, 11, 511799. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.; Adachi, J.; Brown, J.; Juby, A.; Kovacs, C.; Duperrouzel, C.; McTavish, R.; Cameron, C.; Slatkovska, L.; Burke, N. A scorecard for osteoporosis in Canada and seven Canadian provinces. Osteoporos. Int. 2021, 32, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chhokar, V.S.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Myers, L.K.; Xing, Z.; Smith, R.A.; Gerling, I.C.; Weber, K.T. Hyperparathyroidism and the Calcium Paradox of Aldosteronism. Circulation 2005, 111, 871–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, L.; Song, Y.; Zhao, X.; Wong, M.S.; Zhang, W. Renin inhibitor aliskiren exerts beneficial effect on trabecular bone by regulating skeletal renin-angiotensin system and kallikrein-kinin system in ovariectomized mice. Osteoporos. Int. 2016, 27, 1083–1092. [Google Scholar] [CrossRef]
- Shuai, B.; Yang, Y.P.; Shen, L.; Zhu, R.; Xu, X.J.; Ma, C.; Lv, L.; Zhao, J.; Rong, J.H. Local renin-angiotensin system is associated with bone mineral density of glucocorticoid-induced osteoporosis patients. Osteoporos. Int. 2015, 26, 1063–1071. [Google Scholar] [CrossRef]
- Tsuchita, H.; Goto, T.; Shimizu, T.; Yonehara, Y.; Kuwata, T. Dietary Casein Phosphopeptides Prevent Bone Loss in Aged Ovariectomized Rats. J. Nutr. 1996, 126, 86–93. [Google Scholar] [CrossRef]
- Guillerminet, F.; Beaupied, H.; Fabien-Soulé, V.; Tomé, D.; Benhamou, C.-L.; Roux, C.; Blais, A. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: An in vitro and in vivo study. Bone 2010, 46, 827–834. [Google Scholar] [CrossRef]
- Guillerminet, F.; Fabien-Soulé, V.; Even, P.; Tomé, D.; Benhamou, C.-L.; Roux, C.; Blais, A. Hydrolyzed collagen improves bone status and prevents bone loss in ovariectomized C3H/HeN mice. Osteoporos. Int. 2012, 23, 1909–1919. [Google Scholar] [CrossRef]
- Kimira, Y.; Ogura, K.; Taniuchi, Y.; Kataoka, A.; Inoue, N.; Sugihara, F.; Nakatani, S.; Shimizu, J.; Wada, M.; Mano, H. Collagen-derived dipeptide prolyl-hydroxyproline promotes differentiation of MC3T3-E1 osteoblastic cells. Biochem. Biophys. Res. Commun. 2014, 453, 498–501. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xie, Y.; Wen, B.; Ye, M.; Liu, Y.; Imam, K.M.S.U.; Cai, H.; Zhang, C.; Wang, F.; Xin, F. Porcine bone collagen peptides promote osteoblast proliferation and differentiation by activating the PI3K/Akt signaling pathway. J. Funct. Foods 2020, 64, 103697. [Google Scholar] [CrossRef]
- Huttunen, M.M.; Pekkinen, M.; Ahlström, M.E.B.; Lamberg-Allardt, C.J.E. Effects of bioactive peptides isoleucine-proline-proline (IPP), valine-proline-proline (VPP) and leucine-lysine-proline (LKP) on gene expression of osteoblasts differentiated from human mesenchymal stem cells. Br. J. Nutr. 2007, 98, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttunen, M.M.; Pekkinen, M.; Ahlström, M.E.; Lamberg-Allardt, C.J. Long-term effects of tripeptide Ile-Pro-Pro on osteoblast differentiation in vitro. J. Nutr. Biochem. 2008, 19, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-Y.; Ko, S.-C.; Nam, S.Y.; Oh, J.; Kim, Y.-M.; Kim, J.-I.; Kim, N.; Yi, M.; Jung, W.-K. Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem. Funct. 2018, 36, 137–146. [Google Scholar] [CrossRef]
- Nguyen, M.H.T.; Qian, Z.-J.; Nguyen, V.-T.; Choi, I.-W.; Heo, S.-J.; Oh, C.H.; Kang, D.-H.; Kim, G.H.; Jung, W.-K. Tetrameric peptide purified from hydrolysates of biodiesel byproducts of Nannochloropsis oculata induces osteoblastic differentiation through MAPK and Smad pathway on MG-63 and D1 cells. Process Biochem. 2013, 48, 1387–1394. [Google Scholar] [CrossRef]
- Reddi, S.; Kumar, N.; Vij, R.; Mada, S.B.; Kapila, S.; Kapila, R. Akt drives buffalo casein-derived novel peptide-mediated osteoblast differentiation. J. Nutr. Biochem. 2016, 38, 134–144. [Google Scholar] [CrossRef]
- Mada, S.B.; Reddi, S.; Kumar, N.; Kumar, R.; Kapila, S.; Kapila, R.; Trivedi, R.; Karvande, A.; Ahmad, N. Antioxidative peptide from milk exhibits antiosteopenic effects through inhibition of oxidative damage and bone-resorbing cytokines in ovariectomized rats. Nutrition 2017, 43–44, 21–31. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Ogata, S.; Ohtsuki, C.; Tanihara, M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2003, 1651, 60–67. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, J.H.; Park, D.S.; Park, K.S.; Kang, S.S.; Lee, J.S.; Jeong, M.H.; Yoon, T.R. Osteogenesis induced by a bone forming peptide from the prodomain region of BMP-7. Biomaterials 2012, 33, 7057–7063. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, J.S.; Kim, J.H.; Seon, J.K.; Park, K.S.; Jeong, M.H.; Yoon, T.R. Bone-forming peptide-2 derived from BMP-7 enhances osteoblast differentiation from multipotent bone marrow stromal cells and bone formation. Exp. Mol. Med. 2017, 49, e328. [Google Scholar] [CrossRef] [Green Version]
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendler, D.L.; Marin, F.; Zerbini, C.A.F.; Russo, L.A.; Greenspan, S.L.; Zikan, V.; Bagur, A.; Malouf-Sierra, J.; Lakatos, P.; Fahrleitner-Pammer, A.; et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2018, 391, 230–240. [Google Scholar] [CrossRef]
- Miura, M.; Chen, X.-D.; Allen, M.R.; Bi, Y.; Gronthos, S.; Seo, B.-M.; Lakhani, S.; Flavell, R.A.; Feng, X.-H.; Robey, P.; et al. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J. Clin. Investig. 2004, 114, 1704–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Lambert-Comeau, P.; Saint-Pierre, C.; Lépine, M.; Sawan, B.; Parent, J.-L. Death receptors, Fas and TRAIL receptors, are involved in human osteoclast apoptosis. Biochem. Biophys. Res. Commun. 2005, 333, 42–50. [Google Scholar] [CrossRef]
- Hughes, D.E.; Boyce, B.F. Apoptosis in bone physiology and disease. Mol. Pathol. 1997, 50, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Iz, S.G.; Çalimlioglu, B.; Gürhan, S.I.D. Using Bcl-xL anti-apoptotic protein for altering target cell apoptosis. Electron. J. Biotechnol. 2012, 15, 2. [Google Scholar]
- Fatokun, A.; Stone, T.; Smith, R.A. Hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells: The effects of glutamate and protection by purines. Bone 2006, 39, 542–551. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Bazzaz, B.S.F.; Kerachian, M.A. Role of apoptosis in pathogenesis and treatment of bone-related diseases. J. Orthop. Surg. Res. 2015, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Plotkin, L.I.; Lezcano, V.; Thostenson, J.; Weinstein, R.S.; Manolagas, S.C.; Bellido, T. Connexin 43 Is Required for the Anti-Apoptotic Effect of Bisphosphonates on Osteocytes and Osteoblasts In Vivo. J. Bone Miner. Res. 2008, 23, 1712–1721. [Google Scholar] [CrossRef]
- Bellido, T.; Plotkin, L.I. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone 2011, 49, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and Osteoclasts in Bone Remodeling and Inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Unlap, M.; Jope, R.S. Dexamethasone attenuates NF-κ B DNA binding activity without inducing I κ B levels in rat brain in vivo. Mol. Brain Res. 1997, 45, 83–89. [Google Scholar] [CrossRef]
- Harant, H.; Andrew, P.J.; Reddy, G.S.; Foglar, E.; Lindley, I.J. 1α,25-Dihydroxyvitamin D3 and a Variety of its Natural Metabolites Transcriptionally Repress Nuclear-Factor-κB-Mediated Interleukin-8 Gene Expression. Eur. J. Biochem. 1997, 250, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.C.; Takada, Y.; Aggarwal, B.B. Curcumin (diferuloylmethane) inhibits receptor activator of NF-κB ligand-induced NF-κB activation in osteoclast precursors and suppresses osteoclastogenesis. J. Immunol. 2004, 172, 5940–5947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.N.; Fatima, N.; Ali, R.; Hussain, T. Emerging Role of Angiotensin AT2 Receptor in Anti-Inflammation: An Update. Curr. Pharm. Des. 2020, 26, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Skorska, A.; von Haehling, S.; Ludwig, M.; Lux, C.A.; Gaebel, R.; Kleiner, G.; Klopsch, C.; Dong, J.; Curato, C.; Altarche-Xifró, W.; et al. The CD4+ AT2R+ T cell subpopulation improves post-infarction remodelling and restores cardiac function. J. Cell. Mol. Med. 2015, 19, 1975–1985. [Google Scholar] [CrossRef]
- Patel, S.; Dhande, I.; Gray, E.A.; Ali, Q.; Hussain, T. Prevention of lipopolysaccharide-induced CD11b+ immune cell infiltration in the kidney: Role of AT2 receptors. Biosci. Rep. 2019, 39, BSR20190429. [Google Scholar] [CrossRef] [Green Version]
- Rompe, F.; Artuc, M.; Hallberg, A.; Alterman, M.; Ströder, K.; Thöne-Reineke, C.; Reichenbach, A.; Schacherl, J.; Dahlöf, B.; Bader, M.; et al. Direct Angiotensin II Type 2 Receptor Stimulation Acts Anti-Inflammatory through Epoxyeicosatrienoic Acid and Inhibition of Nuclear Factor κB. Hypertension 2010, 55, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Wallinder, C.; Plouffe, B.; Beaudry, H.; Mahalingam, A.; Wu, X.; Johansson, B.; Holm, M.; Botoros, M.; Karlén, A.; et al. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J. Med. Chem. 2004, 47, 5995–6008. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, C.; Liu, Y.; Tian, C.; Li, H.-H. Angiotensin II regulates dendritic cells through activation of NF-κB/p65, ERK1/2 and STAT1 pathways. Cell. Physiol. Biochem. 2017, 42, 1550–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, M.; Steinhoff, G.; Li, J. The regenerative potential of angiotensin AT2 receptor in cardiac repair. Can. J. Physiol. Pharmacol. 2012, 90, 287–293. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, N.; Bhullar, K.S.; Wu, J. Tripeptide IRW Protects MC3T3-E1 Cells against Ang II Stress in an AT2R Dependent Manner. Molecules 2022, 27, 3684. https://doi.org/10.3390/molecules27123684
Shang N, Bhullar KS, Wu J. Tripeptide IRW Protects MC3T3-E1 Cells against Ang II Stress in an AT2R Dependent Manner. Molecules. 2022; 27(12):3684. https://doi.org/10.3390/molecules27123684
Chicago/Turabian StyleShang, Nan, Khushwant S. Bhullar, and Jianping Wu. 2022. "Tripeptide IRW Protects MC3T3-E1 Cells against Ang II Stress in an AT2R Dependent Manner" Molecules 27, no. 12: 3684. https://doi.org/10.3390/molecules27123684
APA StyleShang, N., Bhullar, K. S., & Wu, J. (2022). Tripeptide IRW Protects MC3T3-E1 Cells against Ang II Stress in an AT2R Dependent Manner. Molecules, 27(12), 3684. https://doi.org/10.3390/molecules27123684




