Nanocomposites Based on Antiferroelectric Liquid Crystal (S)-MHPOBC Doping with Au Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
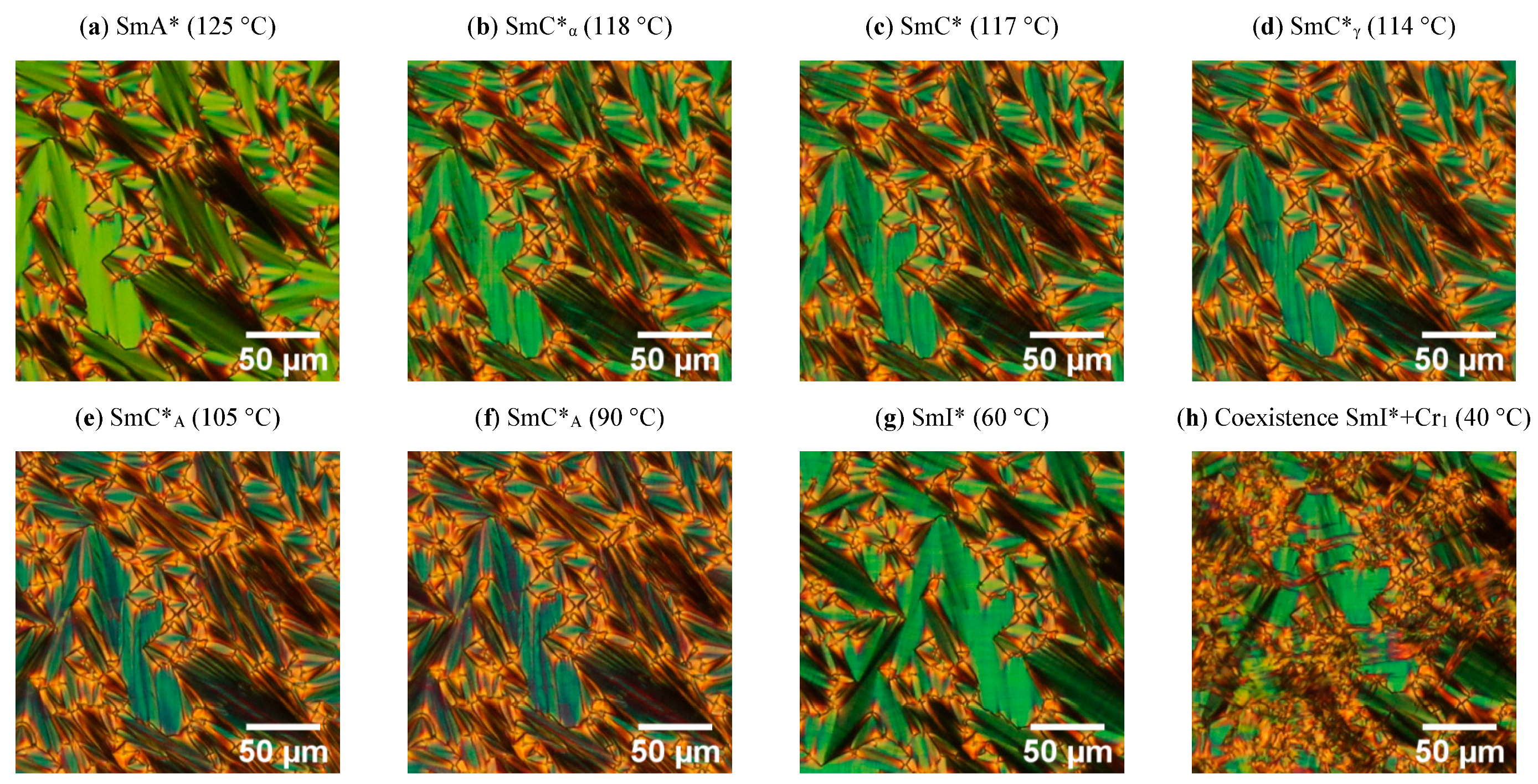
2.1. Results of Polarizing Optical Microscopy (POM) Method
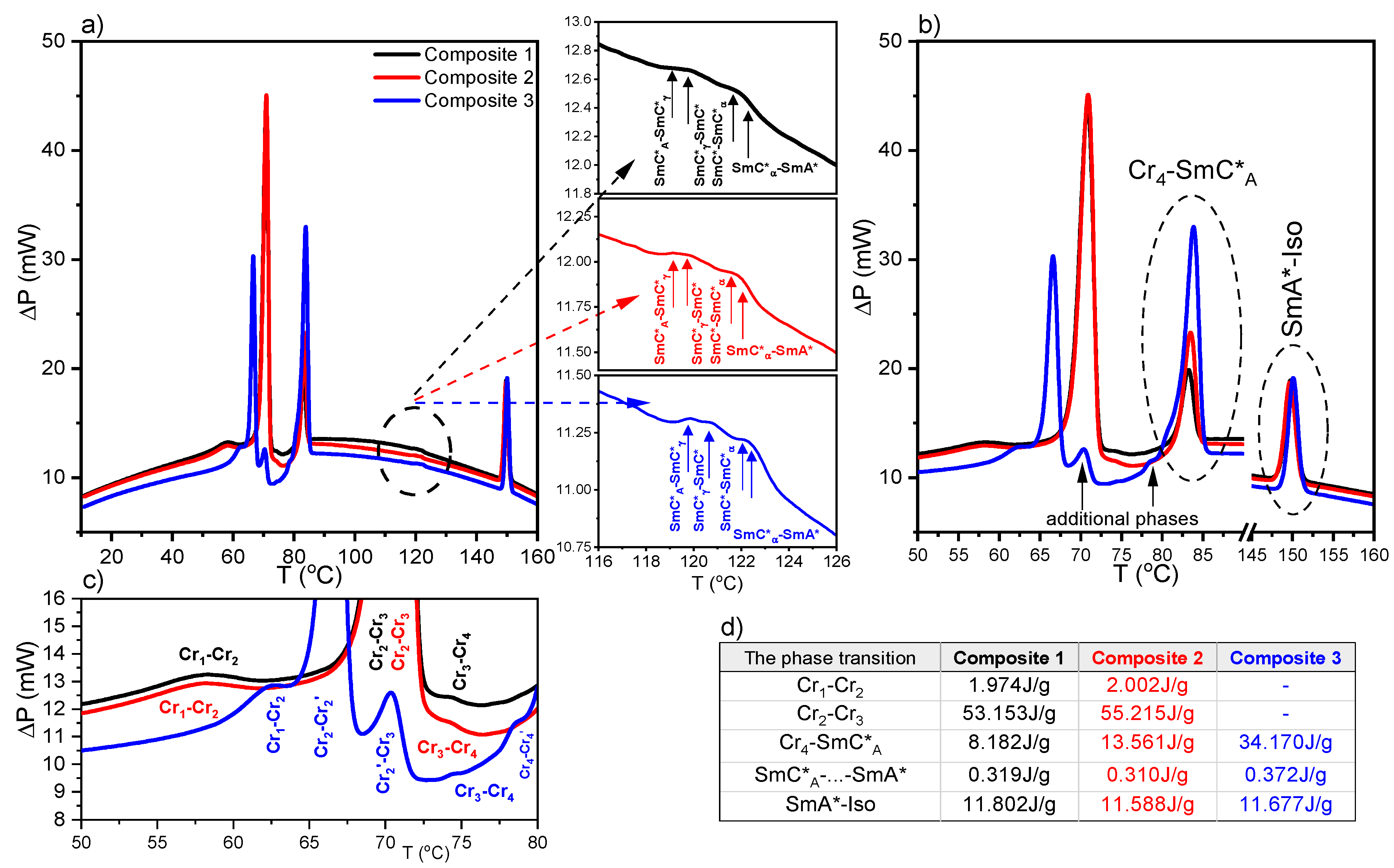
2.2. Results of Differential Scanning Calorimetry (DSC) Method
| Heating | Composite 1 |
| Cr1 (53.1 °C) Cr2 (68.8 °C) Cr3 (73.7 °C) Cr4 (81.8 °C) SmC*A (*119.1 °C) SmC*γ (*119.9 °C) SmC*/SmC*α (*122.0 °C) SmA* (148.5 °C) Iso | |
| (S)-MHPOBC—Fernandes et al. [60] | |
| SmC*A (118.2 °C) SmC*γ (119.2 °C) SmC* (120.7 °C) SmC*α (*122.0 °C) SmA* | |
| Composite 2 | |
| Cr1 (52.5 °C) Cr2 (68.9 °C) Cr3 (73.7 °C) Cr4 (81.9 °C) SmC*A (*119.1 °C) SmC*γ (*119.9 °C) SmC*/SmC*α (*122.0 °C) SmA* (148.4 °C) Iso | |
| Composite 3 | |
| Cr1 (60.0 °C) Cr2 (65.3 °C) Cr2’ (70.5 °C) Cr3 (73.8 °C) Cr4 (81.9 °C) SmC*A (*119.9 °C) SmC*γ (*120.7 °C) SmC*/SmC*α (*122.4 °C) SmA* (148.9 °C) Iso | |
| Cooling | Composite 1 |
| Iso (149.4 °C) SmA* (122.0 °C) SmC*α/SmC* (*119.5 °C) SmC*γ (*118.1 °C) SmC*A (65.0 °C) SmI* (27.3 °C) Cr1 | |
| Composite 2 | |
| Iso (149.1 °C) SmA* (121.8 °C) SmC*α/SmC* (*119.5 °C) SmC*γ (*117.6 °C) SmC*A (63.1 °C) SmI* (27.2 °C) Cr1 | |
| Composite 3 | |
| Iso (149.4 °C) SmA* (122.3 °C) SmC*α/SmC* (*119.8 °C) SmC*γ (*118.2 °C) SmC*A (64.8 °C) SmI* (27.6 °C) Cr1 |
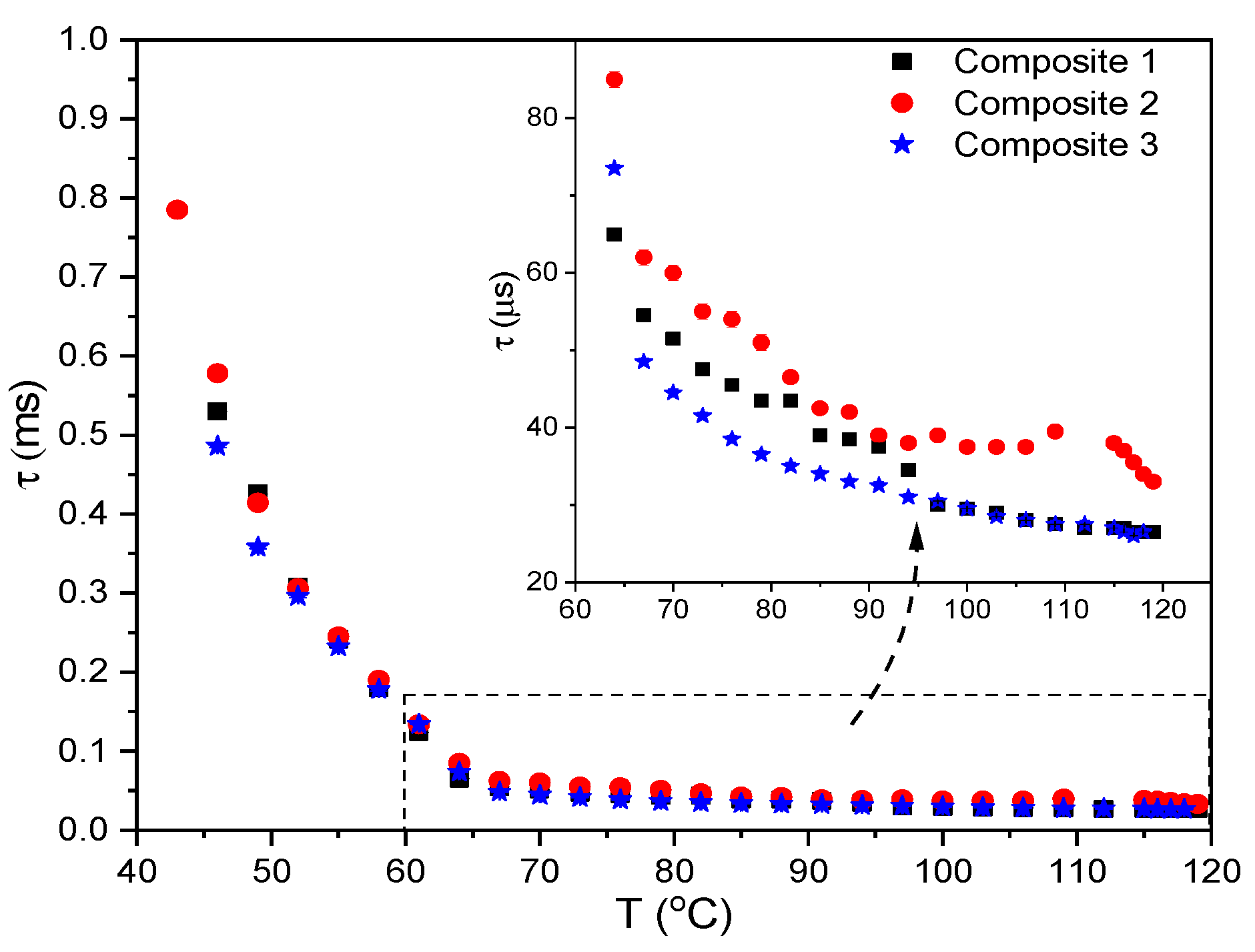
2.3. Results of Transmitted Light Intensity (TLI) Method
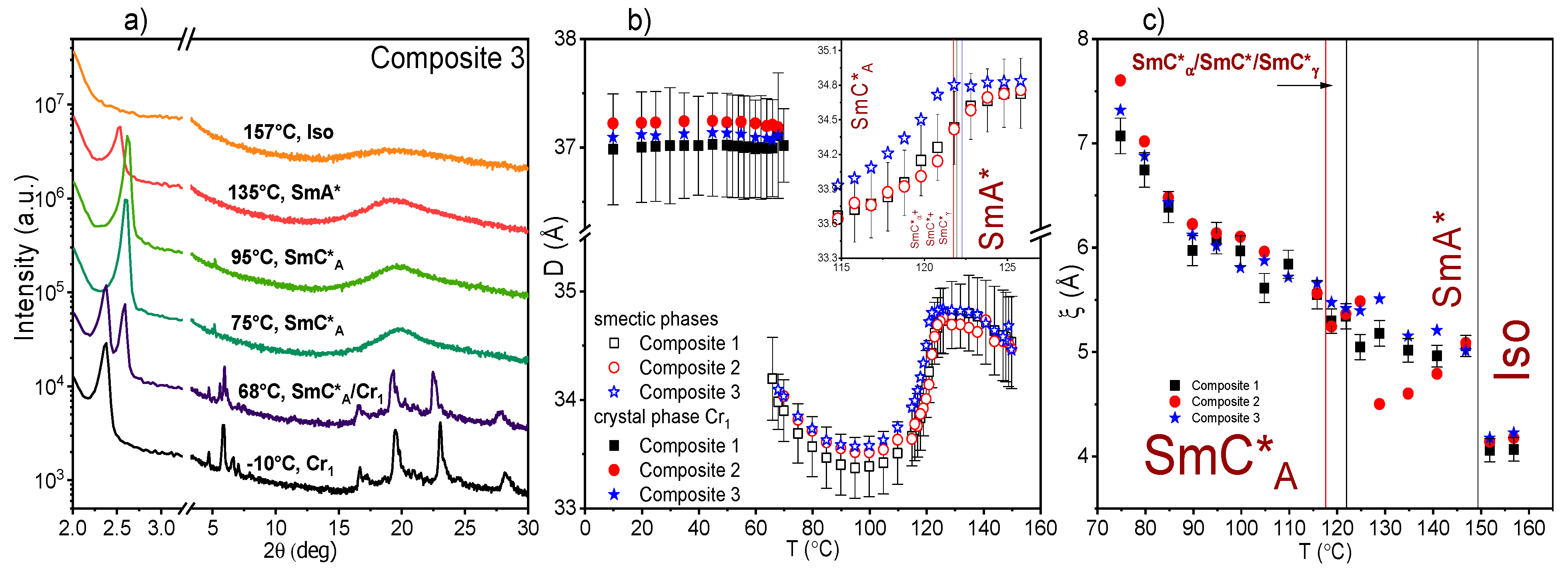
2.4. Results of X-ray Diffraction (XRD) Method
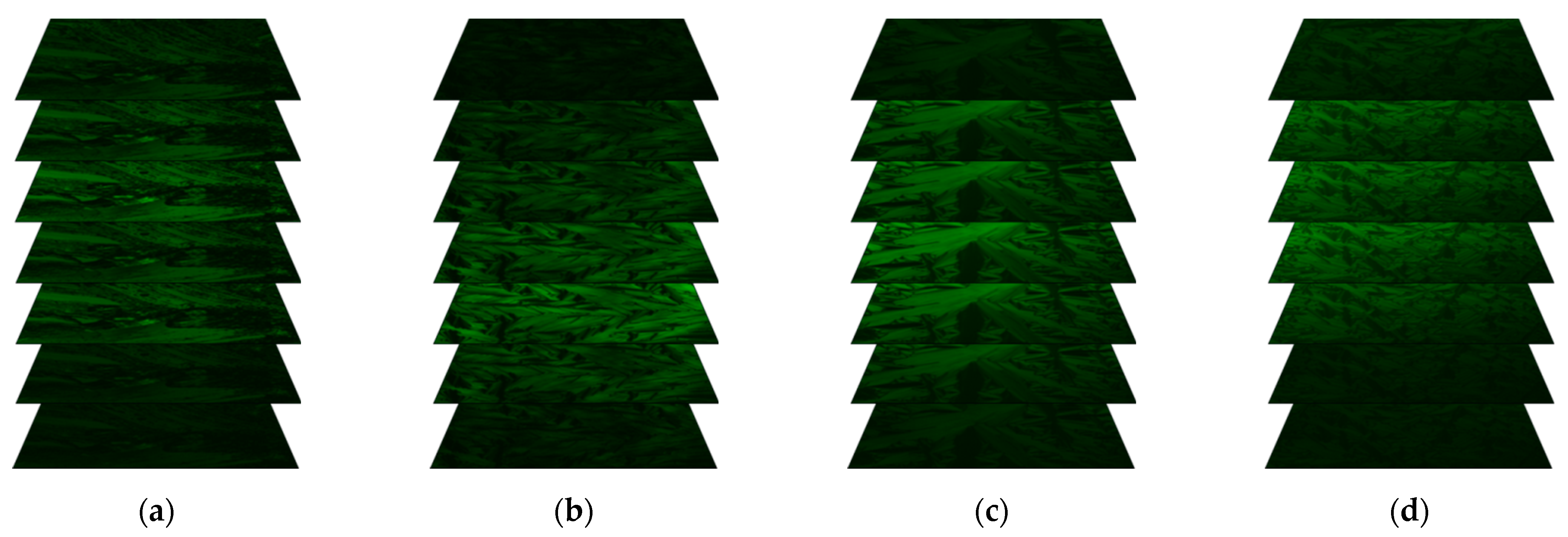
2.5. Results of Fluorescence Confocal Polarizing Microscopy (FCPM) Method
2.6. Results of Ultraviolet–Visible (UV–Vis) Spectroscopy—Free Droplet Method
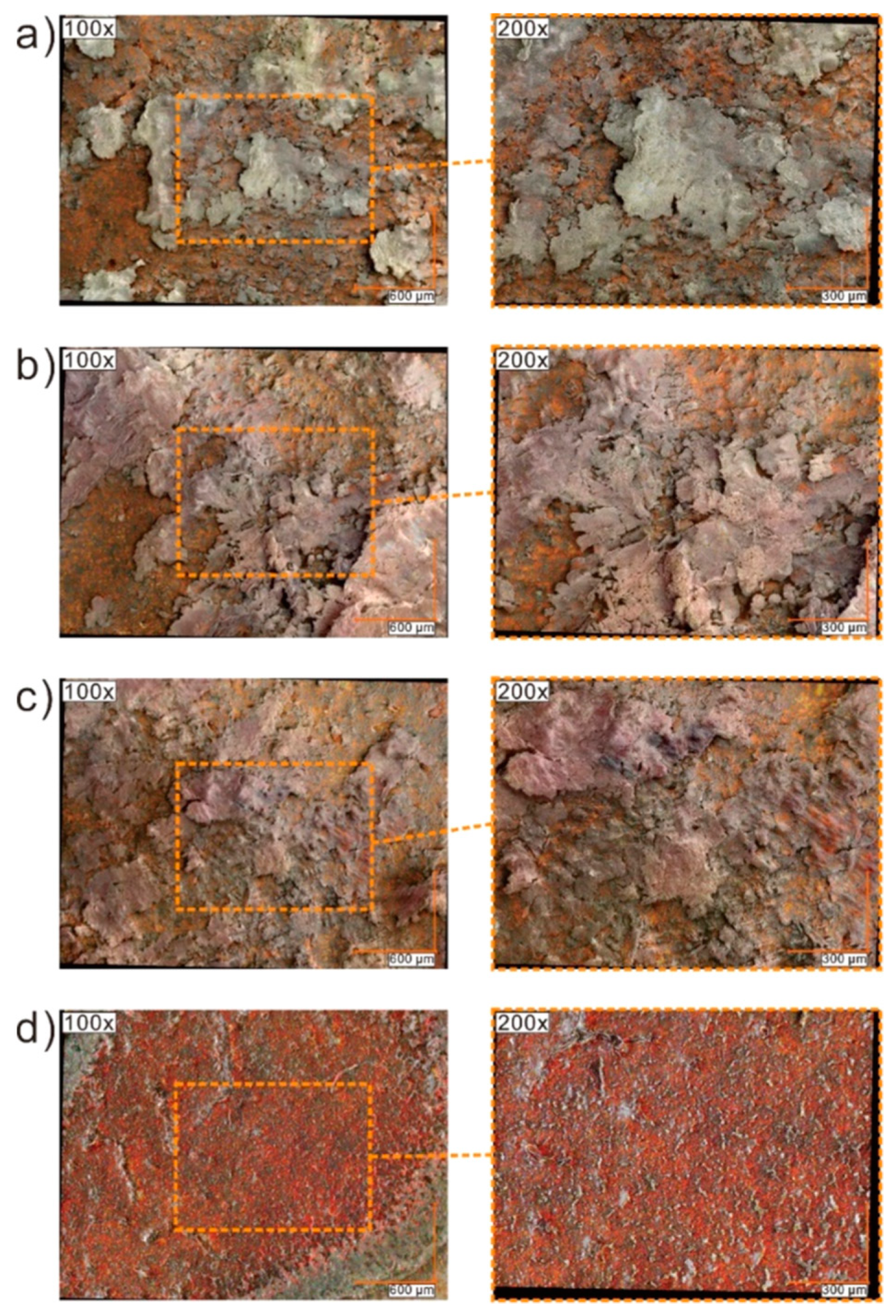
2.7. Results of Optical Microscopy (OM)/Scanning Electron Microscopy (SEM) Methods
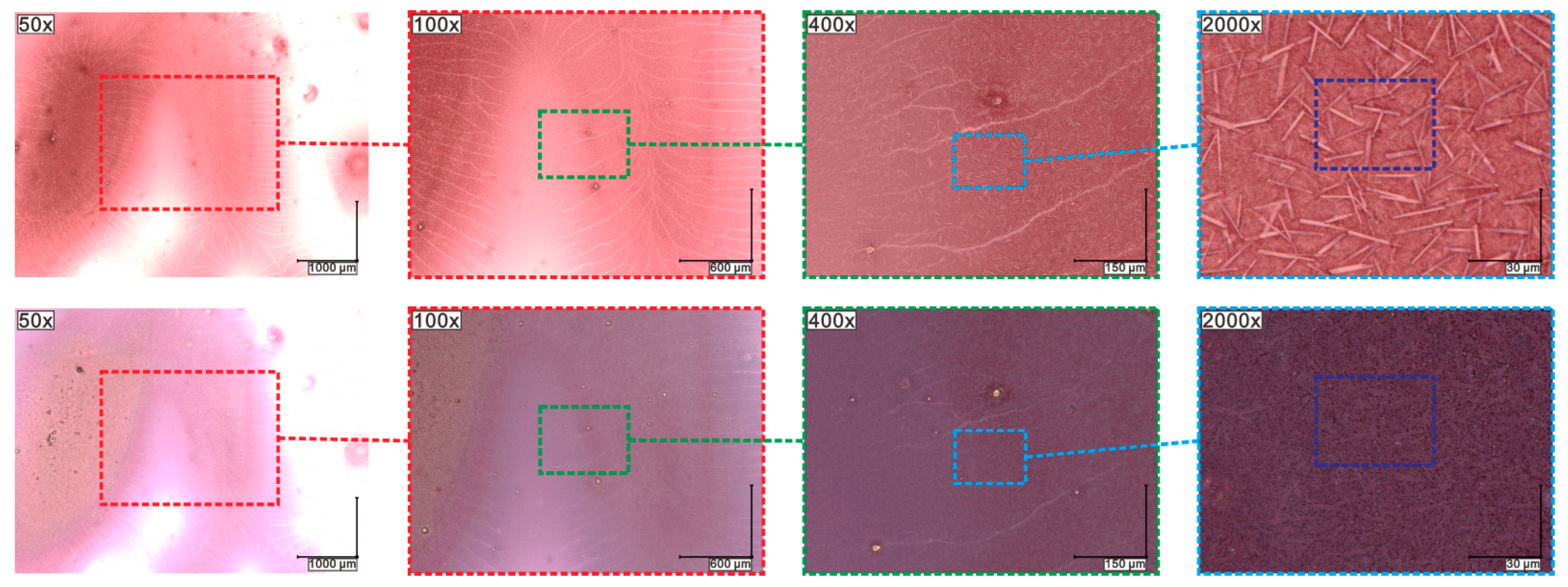
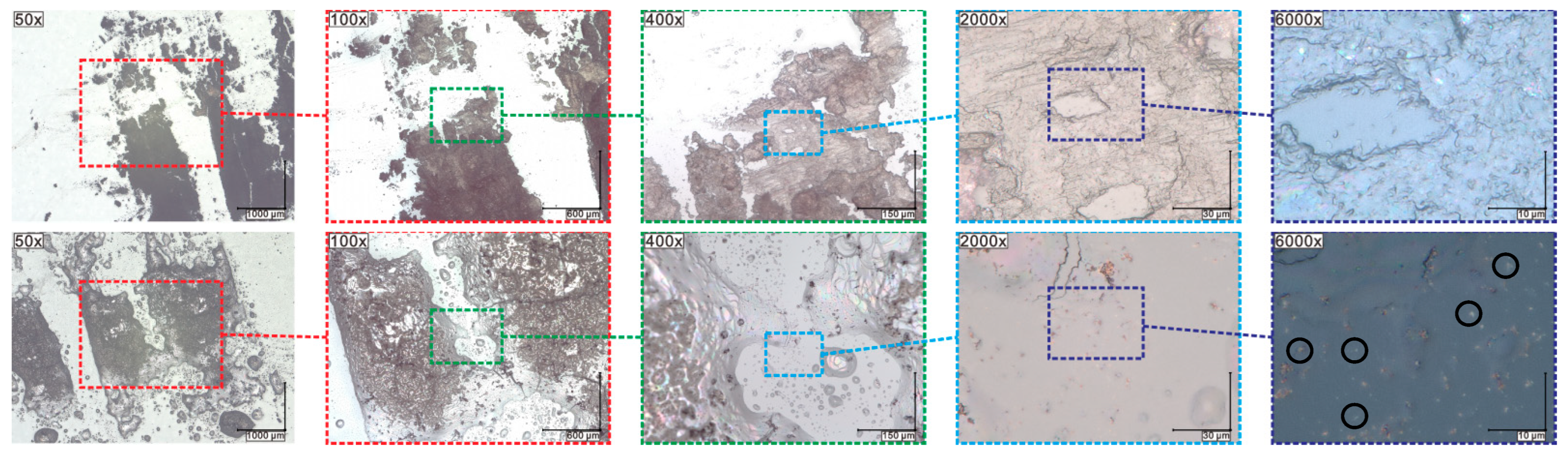
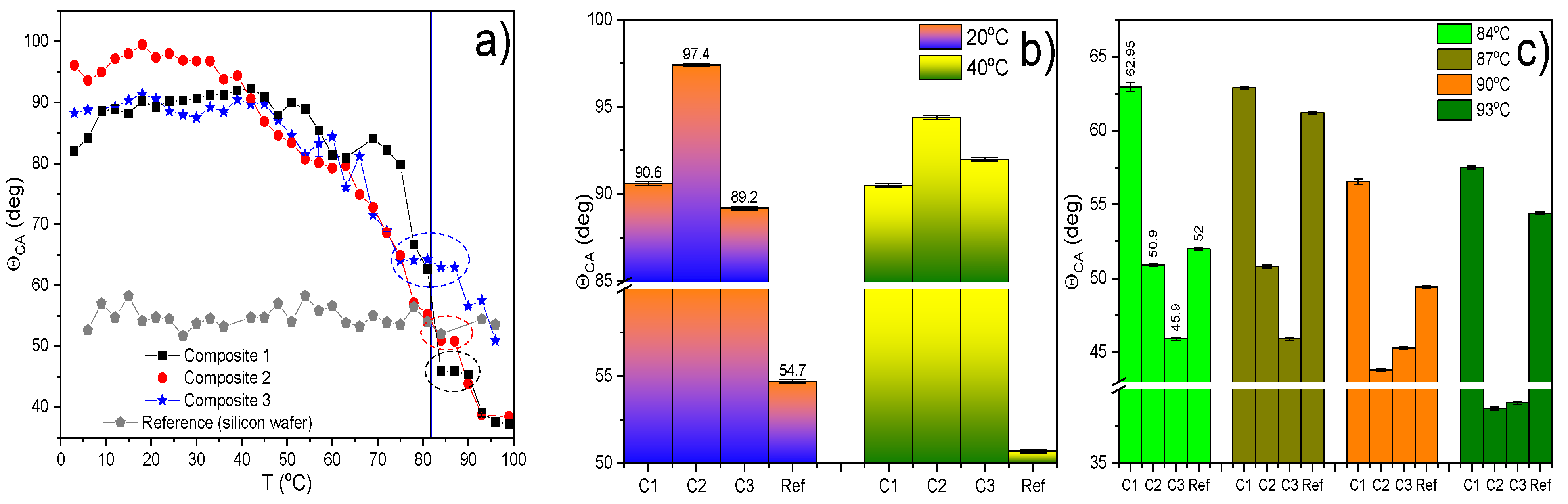
2.8. Results of Contact Angle (CA) Measurements
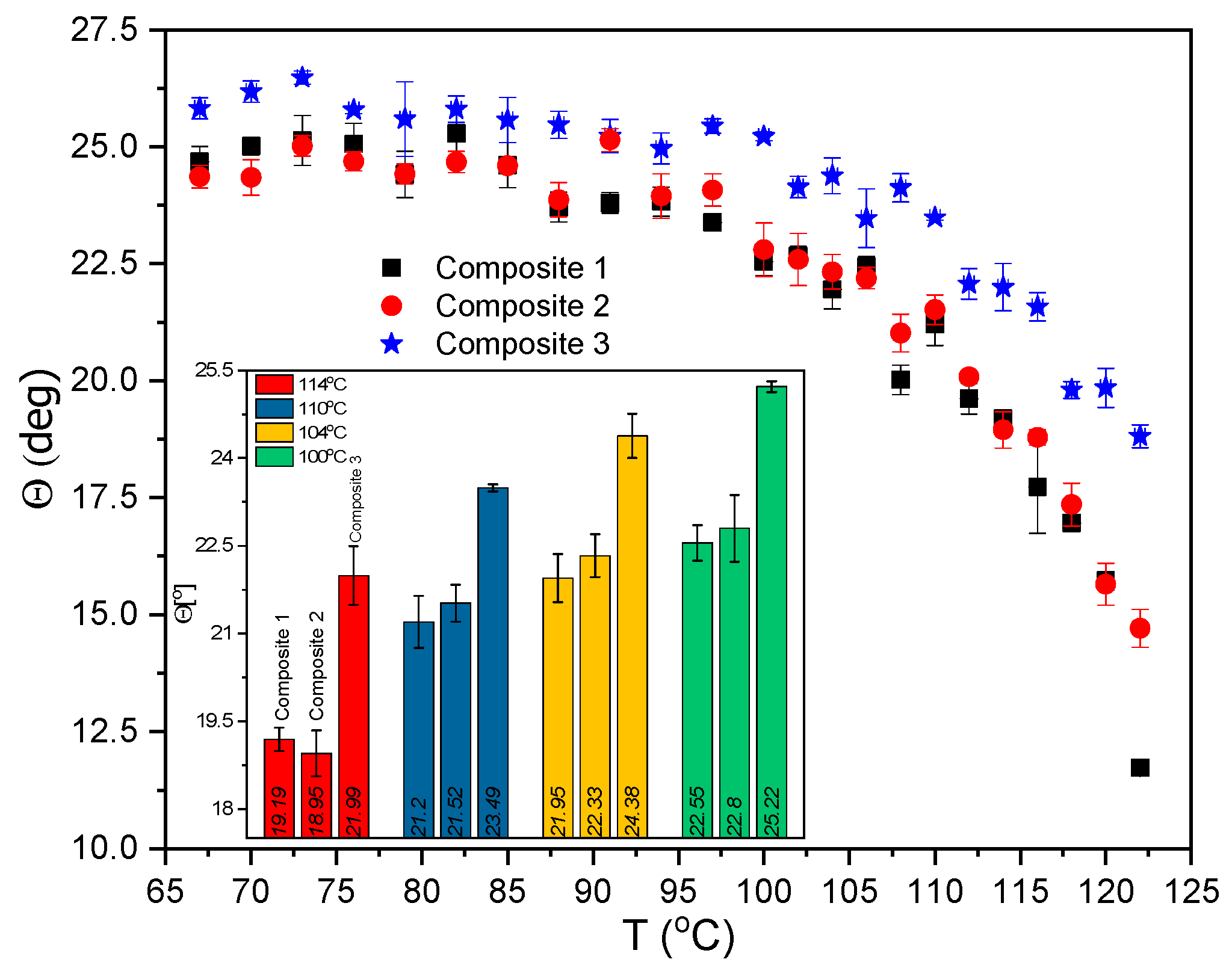
2.9. Results of Electro-Optic (EO) Measurements
3. Materials and Methods
3.1. Materials
- Appropriate amounts of pure (S)-MHPOBC were weighed and flooded with the appropriate volume of Au nanoparticles solution (see Table 2) and 2 mL of toluene was added.
- The solutions were left for a few hours (~19 h).
- The solutions were sonicated for 30 min in the 30(on)/30(off) mode with amplitude A = 40% (in the presence of ice as an external cooling agent).
- The solutions were placed on the magnetic stirrer (~100 °C, 1400 rpm) for about ~18–19 h to slowly evaporate half of the solvent volume.
- The sonication process was performed for 15 min in the 45(on)/15(off) mode with A = 40%.
- The solutions were poured (~50 μL) on cleaned microscope slides and left for solvent evaporation under ambient conditions for over 70 h.
- The prepared composites were gently scratched from a glass by metal blade and used for measurements.
3.2. Polarizing Optical Microscopy and Electro-Optic Measurements
3.3. Differential Scanning Calorimetry
3.4. X-ray Diffraction
3.5. Fluorescence Confocal Polarizing Microscopy
3.6. Ultra-Violet-Visible Spectroscopy—Free Droplets
3.7. Optical Microscopy/Scanning Electron Microscopy
3.8. Contact Angle Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mischler, S.; Guerra, S.; Deschenaux, R. Design of liquid-crystalline gold nanoparticles by click chemistry. Chem. Commun. 2012, 48, 2183–2185. [Google Scholar] [CrossRef] [Green Version]
- Kanayama, N.; Tsutsumi, O.; Kanazawa, A.; Ikeda, T. Distinct thermodynamic behaviour of a mesomorphic gold nanoparticle covered with a liquid-crystalline compound. Chem. Commun. 2001, 1, 2640–2641. [Google Scholar] [CrossRef]
- In, I.; Jun, Y.-W.; Kim, Y.J.; Kim, S.Y. Spontaneous one dimensional arrangement of spherical Au nanoparticles with liquid crystal ligands. Chem. Commun. 2005, 6, 800–801. [Google Scholar] [CrossRef]
- Yu, C.H.; Schubert, C.P.J.; Welch, C.; Tang, B.J.; Tamba, M.-G.; Mehl, G.H. Design, Synthesis, and Characterization of Mesogenic Amine-Capped Nematic Gold Nanoparticles with Surface-Enhanced Plasmonic Resonances. J. Am. Chem. Soc. 2012, 134, 5076–5079. [Google Scholar] [CrossRef]
- Wojcik, M.M.; Gora, M.; Mieczkowski, J.; Romiszewski, J.; Gorecka, E.; Pociecha, D. Temperature-controlled liquid crystalline polymorphism of gold nanoparticles. Soft Matter 2011, 7, 10561–10564. [Google Scholar] [CrossRef]
- Cseh, L.; Mehl, G.H. The Design and Investigation of Room Temperature Thermotropic Nematic Gold Nanoparticles. J. Am. Chem. Soc. 2006, 128, 13376–13377. [Google Scholar] [CrossRef]
- Wojcik, M.; Lewandowski, W.; Matraszek, J.; Mieczkowski, J.; Borysiuk, J.; Pociecha, D.; Gorecka, E. Liquid-Crystalline Phases Made of Gold Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 5167–5169. [Google Scholar] [CrossRef]
- Wolska, J.M.; Pociecha, D.; Mieczkowski, J.; Górecka, E. Control of sample alignment mode for hybrid lamellar systems based on gold nanoparticles. Chem. Commun. 2014, 50, 7975–7978. [Google Scholar] [CrossRef]
- Kanie, K.; Matsubara, M.; Zeng, X.; Liu, F.; Ungar, G.; Nakamura, H.; Muramatsu, A. Simple Cubic Packing of Gold Nanoparticles through Rational Design of Their Dendrimeric Corona. J. Am. Chem. Soc. 2012, 134, 808–811. [Google Scholar] [CrossRef]
- Wolska, J.M.; Pociecha, D.; Mieczkowski, J.; Gorecka, E. Gold nanoparticles with flexible mesogenic grafting layers. Soft Matter 2013, 9, 3005–3008. [Google Scholar] [CrossRef]
- Mallia, V.A.; Vemula, P.K.; John, G.; Kumar, A.; Ajayan, P.M. In Situ Synthesis and Assembly of Gold Nanoparticles Embedded in Glass-Forming Liquid Crystals. Angew. Chem. Int. Ed. 2007, 46, 3269–3274. [Google Scholar] [CrossRef]
- Lewandowski, W.; Constantin, D.; Walicka, K.; Pociecha, D.; Mieczkowski, J.; Górecka, E. Smectic mesophases of functionalized silver and gold nanoparticles with anisotropic plasmonic properties. Chem. Commun. 2013, 49, 7845–7847. [Google Scholar] [CrossRef]
- Zep, A.; Wojcik, M.M.; Lewandowski, W.; Sitkowska, K.; Prominski, A.; Mieczkowski, J.; Pociecha, D.; Gorecka, E. Phototunable Liquid-Crystalline Phases Made of Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 13725–13728. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Kumar, A.; Muralidhar, K.; Biradar, A.M. Nanoparticles induced multiferroicity in liquid crystal. Appl. Phys. Lett. 2016, 108, 182905. [Google Scholar] [CrossRef] [Green Version]
- Prakash, J.; Choudhary, A.; Kumar, A.; Mehta, D.S.; Biradar, A.M. Nonvolatile memory effect based on gold nanoparticles doped ferroelectric liquid crystal. Appl. Phys. Lett. 2008, 93, 112904. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, S.P.; Biradar, A.M.; Choudhary, A.; Sreenivas, K. Enhanced electro-optical properties in gold nanoparticles doped ferroelectric liquid crystals. Appl. Phys. Lett. 2007, 91, 023120. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-derivatised Gold Nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. 2000, 7, 801–802. [Google Scholar] [CrossRef]
- Hostetler, M.J.; Green, S.J.; Stokes, J.J.; Murray, R.W. Monolayers in Three Dimensions: Synthesis and Electrochemistry of ω-Functionalized Alkanethiolate-Stabilized Gold Cluster Compounds. J. Am. Chem. Soc. 1996, 118, 4212–4213. [Google Scholar] [CrossRef]
- Abdullah, L.; Khalid, F.G.; Rashad, A.A.; Jber, N.R. Improvement photoluminescence of nematic liquid crystal doped with gold nanoparticles. AIP Conf. Proc. 2019, 2190, 020082. [Google Scholar] [CrossRef]
- Kopčanský, P.; Tomašovičová, N.; Koneracká, M.; Timko, M.; Mitróová, Z.; Závišová, V.; Éber, N.; Fodor-Csorba, K.; Tóth-Katona, T.; Vajda, A.; et al. Structural Phase Transition in Liquid Crystal Doped with Gold Nanoparticles. Acta Phys. Pol. A 2010, 118, 988–989. [Google Scholar] [CrossRef]
- Hsu, L.-H.; Lo, K.-Y.; Huang, S.-A.; Huang, C.-Y.; Yang, C.-S. Irreversible redshift of transmission spectrum of gold nanoparticles doped in liquid crystals. Appl. Phys. Lett. 2008, 92, 181112. [Google Scholar] [CrossRef]
- Hsu, C.-J.; Lin, L.-J.; Huang, M.-K.; Huang, C.-Y. Electro-optical Effect of Gold Nanoparticle Dispersed in Nematic Liquid Crystals. Crystals 2017, 7, 287. [Google Scholar] [CrossRef] [Green Version]
- Inam, M.; Singh, G.; Biradar, A.M.; Mehta, D.S. Effect of gold nano-particles on switch-on voltage and relaxation frequency of nematic liquid crystal cell. AIP Adv. 2011, 1, 042162. [Google Scholar] [CrossRef] [Green Version]
- Palermo, G.; Cataldi, U.; De Sio, L.; Bürgi, T.; Tabiryan, N.; Umeton, C. Optical control of plasmonic heating effects using reversible photo-alignment of nematic liquid crystals. Appl. Phys. Lett. 2016, 109, 191906. [Google Scholar] [CrossRef]
- Chen, C.-T.; Liu, C.-C.; Wang, C.-H.; Chen, C.-W.; Chen, Y.-F. Tunable coupling between exciton and surface plasmon in liquid crystal devices consisting of Au nanoparticles and CdSe quantum dots. Appl. Phys. Lett. 2011, 98, 261918. [Google Scholar] [CrossRef]
- Hinojosa, A.; Sharma, S.C. Effects of gold nanoparticles on electro-optical properties of a polymer-dispersed liquid crystal. Appl. Phys. Lett. 2010, 97, 081114. [Google Scholar] [CrossRef]
- Tiwari, K.; Singh, A.K.; Sharma, S.C. Evidence for surface plasmons in a liquid crystal containing gold nanoparticles. Appl. Phys. Lett. 2012, 101, 253103. [Google Scholar] [CrossRef]
- Montazami, R.; Spillmann, C.M.; Naciri, J.; Ratna, B.R. Enhanced thermomechanical properties of a nematic liquid crystal elastomer doped with gold nanoparticles. Sens. Actuators A Phys. 2012, 178, 175–178. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, R.-J.; Tien, C.-L.; Yeh, S.-M. Enhanced Photoluminescence in Gold Nanoparticles Doped Homogeneous Planar Nematic Liquid Crystals. Adv. Condens. Matter Phys. 2018, 2018, 8720169. [Google Scholar] [CrossRef] [Green Version]
- Vardanyan, K.K.; Walton, R.D.; Sita, D.M.; Gurfinkiel, I.S.; Saidel, W.M. Study of pentyl-cyanobiphenyl nematic doped with gold nanoparticles. Liq. Cryst. 2012, 39, 595–605. [Google Scholar] [CrossRef]
- Vardanyan, K.K.; Walton, R.D.; Bubb, D.M. Liquid crystal composites with a high percentage of gold nanoparticles. Liq. Cryst. 2011, 38, 1279–1287. [Google Scholar] [CrossRef]
- Vardanyan, K.K.; Sita, D.M.; Walton, R.D.; Gurfinkiel, I.S.; Saidel, W.M. Liquid crystalline cyanobiphenyl homologues doped with gold nanoparticles. Liq. Cryst. 2012, 39, 1083–1098. [Google Scholar] [CrossRef]
- Tejaswi, M.; Rao, M.C.; Prasad, P.V.D.; Giridhar, G.; Pisipti, V.G.K.M.; Manepalli, R.K.N.R. Synthesis and characterization of citrate capped gold nanoparticles and their effect on liquid crystals: Optical studies. Rasayan J. Chem. 2016, 9, 697–705. [Google Scholar]
- Vardanyan, K.K.; Palazzo, E.D.; Walton, R.D. Nematic nanocomposites with enhanced optical birefringence. Liq. Cryst. 2011, 38, 709–715. [Google Scholar] [CrossRef]
- Pratibha, R.; Park, W.; Smalyukh, I. Colloidal gold nanosphere dispersions in smectic liquid crystals and thin nanoparticle-decorated smectic films. J. Appl. Phys. 2010, 107, 063511. [Google Scholar] [CrossRef] [Green Version]
- Vardanyan, K.K.; Thiel, A.; Fickas, B.; Daykin, A. Multicomponent nematic systems with doped gold nanoparticles. Liq. Cryst. 2015, 42, 445–455. [Google Scholar] [CrossRef]
- Kamaliya, B.; Kumar, M.V.; Yelamaggad, C.V.; Prasad, S.K. Enhancement of electrical conductivity of a liquid crystal-gold nanoparticle composite by a gel network of aerosil particles. Appl. Phys. Lett. 2015, 106, 083110. [Google Scholar] [CrossRef]
- Mirzaei, J.; Urbanski, M.; Kitzerow, H.-S.; Hegmann, T. Hydrophobic gold nanoparticles via silane conjugation: Chemically and thermally robust nanoparticles as dopants for nematic liquid crystals. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120256. [Google Scholar] [CrossRef]
- Kanie, K.; Muramatsu, A. Organic−Inorganic Hybrid Liquid Crystals: Thermotropic Mesophases Formed by Hybridization of Liquid-Crystalline Phosphates and Monodispersed α-Fe2O3 Particles. J. Am. Chem. Soc. 2005, 127, 11578–11579. [Google Scholar] [CrossRef]
- Urbański, M.; Kinkead, B.; Qi, H.; Hegmann, T.; Kitzerow, H. Electroconvection in nematic liquid crystals via nanoparticle doping. Nanoscale 2010, 2, 1057. [Google Scholar] [CrossRef]
- Hegmann, T.; Qi, H.; Kinkead, B.; Marx, V.; Girgis, H.; Heiney, P. Functionalized Metal and Semiconductor Nanoparticle Doped Liquid Crystals—Modifying Optical and Electro-Optical Properties. Can. Met. Q. 2009, 48, 1–9. [Google Scholar] [CrossRef]
- Meli, M.-V.; Lardner, M.J.; Taweel, A.M. Effects of gold nanoparticle film morphology on the alignment of a nematic liquid crystal. Liq. Cryst. 2015, 42, 497–505. [Google Scholar] [CrossRef]
- Yoshida, H.; Tanaka, Y.; Kawamoto, K.; Kubo, H.; Tsuda, T.; Fujii, A.; Kuwabata, S.; Kikuchi, H.; Ozaki, M. Nanoparticle-Stabilized Cholesteric Blue Phases. Appl. Phys. Express 2009, 2, 121501. [Google Scholar] [CrossRef]
- Mishra, M.; Kumar, S.; Dhar, R. Gold nanoparticles in plastic columnar discotic liquid crystalline material. Thermochim. Acta 2016, 631, 59–70. [Google Scholar] [CrossRef]
- Holt, L.A.; Bushby, R.J.; Evans, S.D.; Burgess, A.; Seeley, G. A 106-fold enhancement in the conductivity of a discotic liquid crystal doped with only 1% (w/w) gold nanoparticles. J. Appl. Phys. 2008, 103, 063712. [Google Scholar] [CrossRef]
- Jayoti, D.; Malik, P.; Singh, A. Dielectric studies in gold nanoparticles doped ferroelectric liquid crystals. AIP Conf. Proc. 2016, 1728, 20647. [Google Scholar] [CrossRef]
- Kumar, A.; Prakash, J.; Mehta, D.S.; Biradar, A.M.; Haase, W. Enhanced photoluminescence in gold nanoparticles doped ferroelectric liquid crystals. Appl. Phys. Lett. 2009, 95, 023117. [Google Scholar] [CrossRef] [Green Version]
- Salamończyk, M.; Kovářová, A.; Svoboda, J.; Pociecha, D.; Gorecka, E. Switchable fluorescent liquid crystals. Appl. Phys. Lett. 2009, 95, 171901. [Google Scholar] [CrossRef]
- Podgornov, F.V.; Ryzhkova, A.V.; Haase, W. Influence of gold nanorods size on electro-optical and dielectric properties of ferroelectric liquid crystals. Appl. Phys. Lett. 2010, 97, 212903. [Google Scholar] [CrossRef]
- Shukla, R.; Feng, X.; Umadevi, S.; Hegmann, T.; Haase, W. Influence of different amount of functionalized bulky gold nanorods dopant on the electrooptical, dielectric and optical properties of the FLC host. Chem. Phys. Lett. 2014, 599, 80–85. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Giesselmann, F. The experimental study of phases and phase transitions in antiferroelectric liquid crystals. Chiral Liq. Cryst. 2005, 2015, 147–184. [Google Scholar]
- Uehara, H.; Iino, Y.; Hatano, J. Phase Boundaries between Chiral Smectic Cα, Cβand C Phases in an Antiferroelectric Liquid Crystal, MHPOBC. Jpn. J. Appl. Phys. 1997, 36, 6118–6121. [Google Scholar] [CrossRef]
- Dierking, I. Textures of Liquid Crystals; WILEY-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2003. [Google Scholar]
- Cranton, C.W.; Fihn, M. Handbook of Visual Display Technology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1–4, pp. 1–2694. [Google Scholar] [CrossRef]
- Mikułko, A.; Douali, R.; Legrand, C.; Marzec, M.; Wrobel, S.; Dabrowski, R. Phase behaviour and dynamics of exemplary MHPOBC analogue. Phase Transit. 2005, 78, 949–955. [Google Scholar] [CrossRef]
- Labeeb, A.; Gleeson, H.F.; Hegmann, T. Polymer stabilization of the smectic C-alpha* liquid crystal phase—Over tenfold thermal stabilization by confining networks of photo-polymerized reactive mesogens. Appl. Phys. Lett. 2015, 107, 232903. [Google Scholar] [CrossRef]
- Lalik, S.; Deptuch, A.; Fryń, P.; Jaworska–Gołąb, T.; Dardas, D.; Pociecha, D.; Urbańska, M.; Tykarska, M.; Marzec, M. Systematic study of the chiral smectic phases of a fluorinated compound. Liq. Cryst. 2019, 46, 2256–2268. [Google Scholar] [CrossRef]
- Chandani, A.D.L.; Ouchi, Y.; Takezoe, H.; Fukuda, A.; Terashima, K.; Furukawa, K.; Kishi, A. Novel Phases Exhibiting Tristable Switching. Jpn. J. Appl. Phys. 1989, 28, L1261–L1264. [Google Scholar] [CrossRef]
- Tripathi, P.; Mishra, M.; Kumar, S.; Dhar, R. Thermodynamic study of a plastic columnar discotic material 2, 3, 6, 7, 10, 11-hexabutyloxytriphenylene dispersed with gold nanoparticles under elevated pressure. J. Therm. Anal. 2017, 129, 315–322. [Google Scholar] [CrossRef]
- Fernandes, P.; Barois, P.; Grelet, E.; Nallet, F.; Goodby, J.W.; Hird, M.; Micha, J.-S. Extension of the resonant scattering technique to liquid crystals without resonant element. Eur. Phys. J. E 2006, 20, 81–87. [Google Scholar] [CrossRef]
- Kim, K.H.; Takanishi, Y.; Ishikawa, K.; Takezoe, H.; Fukuda, A. Phase transitions and conformational changes in an antiferroelectric liquid crystal 4-(1-methylheptyloxycarbonyl)phenyl 4′-octyloxybiphenyl-4-carboxylate (MHPOBC). Liq. Cryst. 1994, 16, 185–202. [Google Scholar] [CrossRef]
- Blinov, L.M. Structure and Properties of Liquid Crystals; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Różycka, A.; Deptuch, A.; Jaworska-Gołąb, T.; Węgłowska, D.; Marzec, M. Evidence of monotropic hexatic tilted smectic phase in the phase sequence of ferroelectric liquid crystal. Phase Transit. 2018, 91, 159–169. [Google Scholar] [CrossRef]
- Lalik, S.; Deptuch, A.; Fryń, P.; Jaworska-Gołąb, T.; Węgłowska, D.; Marzec, M. Physical properties of new binary ferroelectric mixture. J. Mol. Liq. 2019, 274, 540–549. [Google Scholar] [CrossRef]
- Inui, S.; Kawano, S.; Saito, M.; Iwane, H.; Takanishi, Y.; Hiraoka, K.; Ouchi, Y.; Takezoe, H.; Fukuda, A. First Order Paraelectric-Antiferroelectric Phase Transition in a Chiral Smectic Liquid Crystal of a Fluorine Containing Phenyl Pyrimidine Derivative. Jpn. J. Appl. Phys. 1990, 29, L987–L990. [Google Scholar] [CrossRef]
- Milewska, K.; Drzewiński, W.; Czerwiński, M.; Dąbrowski, R.; Piecek, W. Highly tilted liquid crystalline materials possessing a direct phase transition from antiferroelectric to isotropic phase. Mater. Chem. Phys. 2016, 171, 33–38. [Google Scholar] [CrossRef]
- Czerwinski, M.; Tykarska, M. Helix parameters in bi- and multicomponent mixtures composed of orthoconic antiferroelectric liquid crystals with three ring molecular core. Liq. Cryst. 2014, 41, 850–860. [Google Scholar] [CrossRef]
- Kurp, K.; Czerwiński, M.; Tykarska, M.; Salamon, P.; Bubnov, A. Design of functional multicomponent liquid crystalline mixtures with nano-scale pitch fulfilling deformed helix ferroelectric mode demands. J. Mol. Liq. 2019, 290, 111329. [Google Scholar] [CrossRef]
- Żurowska, M.; Morawiak, P.; Piecek, W.; Czerwiński, M.; Spadło, A.; Bennis, N. A new mesogenic mixture with antiferroelectric phase only at a broad temperature range. Liq. Cryst. 2016, 43, 1365–1374. [Google Scholar] [CrossRef]
- Lapanik, A.; Rudzki, A.; Kinkead, B.; Qi, H.; Hegmann, T.; Haase, W. Electrooptical and dielectric properties of alkylthiol-capped gold nanoparticle–ferroelectric liquid crystal nanocomposites: Influence of chain length and tethered liquid crystal functional groups. Soft Matter 2012, 8, 8722–8728. [Google Scholar] [CrossRef]
- Umadevi, S.; Feng, X.; Hegmann, T. Bent-Core and Nematic Liquid Crystal Functionalized Gold Nanorods. Ferroelectrics 2012, 431, 164–175. [Google Scholar] [CrossRef]
- Hiraoka, K.; Takanishi, Y.; Skarp, K.; Takezoe, H.; Fukuda, A. Electric-Field-Induced Apparent Tilt Angle and Devil’s Staircase in SmCα* of an Antiferroelectric Chiral Smectic Liquid Crystal. Jpn. J. Appl. Phys. 1991, 30, L1819–L1822. [Google Scholar] [CrossRef]
- Glogarová, M.; Sverenyák, H.; Fukuda, A.; Takezoe, H. Electrooptic properties of chiral smectic liquid crystals with a dipolar order. Liq. Cryst. 1993, 14, 463–468. [Google Scholar] [CrossRef]
- Furukawa, K.; Terashima, K.; Ichihashi, M.; Saitoh, S.; Miyazawa, K.; Inukai, T. Chiral smectic C liquid crystals having an electronegative substituent ortho to the chiral tail group—A study of a factor determining the magnitude of spontaneous polarization. Ferroelectrics 1988, 85, 451–459. [Google Scholar] [CrossRef]
- Mikułlko, A.; Marzec, M.; Wrobel, S.; Dabrowski, R. Detection of Alpha Sub-Phase Between Para- and Ferroelectric Phase of a Fluorinated Compound. Ferroelectrics 2005, 313, 105–112. [Google Scholar] [CrossRef]
- Raszewski, Z.; Kedzierski, J.; Kutkowska, J.; Piecek, W.; Perkowski, P.; Czupryński, K.; Dabrowski, R.; Drzewiński, W.; Zieliński, J.; Zmija, J. Determination of Molecular Parameters of the mhpb(h)pbc and mhpb(f)pbc Antiferroelectric Liquid Crystals. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 2001, 366, 607–616. [Google Scholar] [CrossRef]
- Rutkowska, J.; Kedzierski, J.; Raszewski, Z.; Perkowski, P.; Dabrowski, R.; Czupryński, K.; Gauza, S. Properties of System with Induced SMCA* Phase. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 2007, 366, 617–628. [Google Scholar] [CrossRef]
- Chemingui, M.; Soltani, T.; Marcerou, J.; Othman, T. Effect of enantiomeric excess on the smectic-C* phases under electric field. Phase Transit. 2016, 89, 221–231. [Google Scholar] [CrossRef]
- Kašpar, M.; Bubnov, A.; Hamplova, V.; Málková, Z.; Pirkl, S.; Glogarová, M. Effect of lateral substitution by fluorine and bromine atoms in ferroelectric liquid crystalline materials containing a 2-alkoxypropanoate unit. Liq. Cryst. 2007, 34, 1185–1192. [Google Scholar] [CrossRef]
- Pramanik, A.; Das, M.K.; Das, B.; Żurowska, M.; Dąbrowski, R. Electro-optical properties of a new series of fluorinated antiferroelectric orthoconic liquid crystalline esters. Liq. Cryst. 2015, 42, 412–421. [Google Scholar] [CrossRef]
- Tripathi, S.; Prakash, J.; Chandran, A.; Joshi, T.; Kumar, A.; Dhar, A.; Biradar, A.M.; Tripathi, S.; Prakash, J.; Chandran, A.; et al. Enhanced dielectric and electro-optical properties of a newly synthesised ferroelectric liquid crystal material by doping gold nanoparticle-decorated multiwalled carbon nanotubes. Liq. Cryst. 2013, 40, 1255–1262. [Google Scholar] [CrossRef]
- Choudhary, A.; Singh, G.; Biradar, A.M. Advances in gold nanoparticle–liquid crystal composites. Nanoscale 2014, 6, 7743–7756. [Google Scholar] [CrossRef]
- Malik, P.; Chaudhary, A.; Mehra, R.; Raina, K. Electro-optic, thermo-optic and dielectric responses of multiwalled carbon nanotube doped ferroelectric liquid crystal thin films. J. Mol. Liq. 2012, 165, 7–11. [Google Scholar] [CrossRef]
- Shukla, R.; Sharma, A.; Mori, T.; Hegmann, T.; Haase, W. Effect of two different size chiral ligand-capped gold nanoparticle dopants on the electro-optic and dielectric dynamics of a ferroelectric liquid crystal mixture. Liq. Cryst. 2016, 43, 695–703. [Google Scholar] [CrossRef]
- Podgornov, F.V.; Haase, W. Chiroptic response of ferroelectric liquid crystals triggered with localized surface plasmon resonance of achiral gold nanorods. Appl. Phys. Lett. 2018, 112, 021102. [Google Scholar] [CrossRef]
- Ahmad, F.; Luqman, M.; Jamil, M. Advances in the metal nanoparticles (MNPs) doped liquid crystals and polymer dispersed liquid crystal (PDLC) composites and their applications—A review. Mol. Cryst. Liq. Cryst. 2021, 731, 1–33. [Google Scholar] [CrossRef]
- Shafiqa, A.; Aziz, A.A.; Mehrdel, B. Nanoparticle Optical Properties: Size Dependence of a Single Gold Spherical Nanoparticle. J. Phys. Conf. Ser. 2018, 1083, 012040. [Google Scholar] [CrossRef] [Green Version]
- Krueger, M.; Giesselmann, F. Laser-light diffraction studies on the electric-field response of the helical director configuration in smectic-C* liquid crystals. J. Appl. Phys. 2007, 101, 094102. [Google Scholar] [CrossRef]
- Ahuja, J.K.; Raina, K.K. Polarization Switching and Dielectric Relaxations in Ferroelectric Liquid Crystals. Jpn. J. Appl. Phys. 2000, 39, 4076–4081. [Google Scholar] [CrossRef]
- Miyasato, K.; Abe, S.; Takezoe, H.; Fukuda, A.; Kuze, E. Direct Method with Triangular Waves for Measuring Spontaneous Polarization in Ferroelectric Liquid Crystals. Jpn. J. Appl. Phys. 1983, 22, L661–L663. [Google Scholar] [CrossRef]
- Clark, N.A.; Lagerwall, S.T. Submicrosecond bistable electro-optic switching in liquid crystals. Appl. Phys. Lett. 1980, 36, 899–901. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríquez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Mater. Sci. Forum 2001, 378–381, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Lavrentovich, O.D.; Pasini, P.; Zannoni, C.; Zumer, S. (Eds.) Defects in Liquid Crystals: Computer Simulations, Theory and Experiments; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Smalyukh, I.; Shiyanovskii, S.; Lavrentovich, O. Three-dimensional imaging of orientational order by fluorescence confocal polarizing microscopy. Chem. Phys. Lett. 2001, 336, 88–96. [Google Scholar] [CrossRef]
- Kuczyński, W. Behavior of the helix in some chiral smectic-C∗liquid crystals. Phys. Rev. E 2010, 81, 021708. [Google Scholar] [CrossRef] [PubMed]



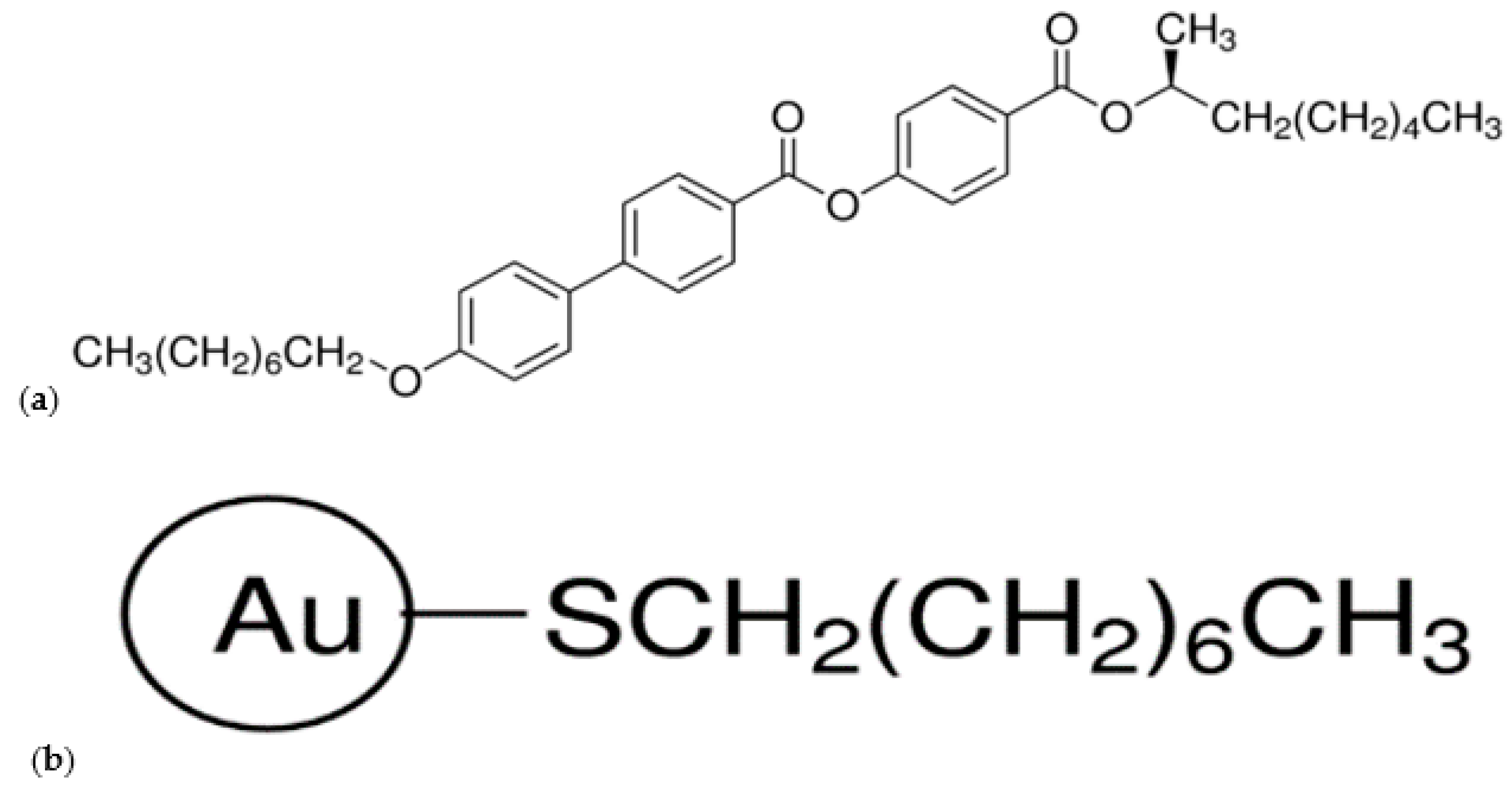
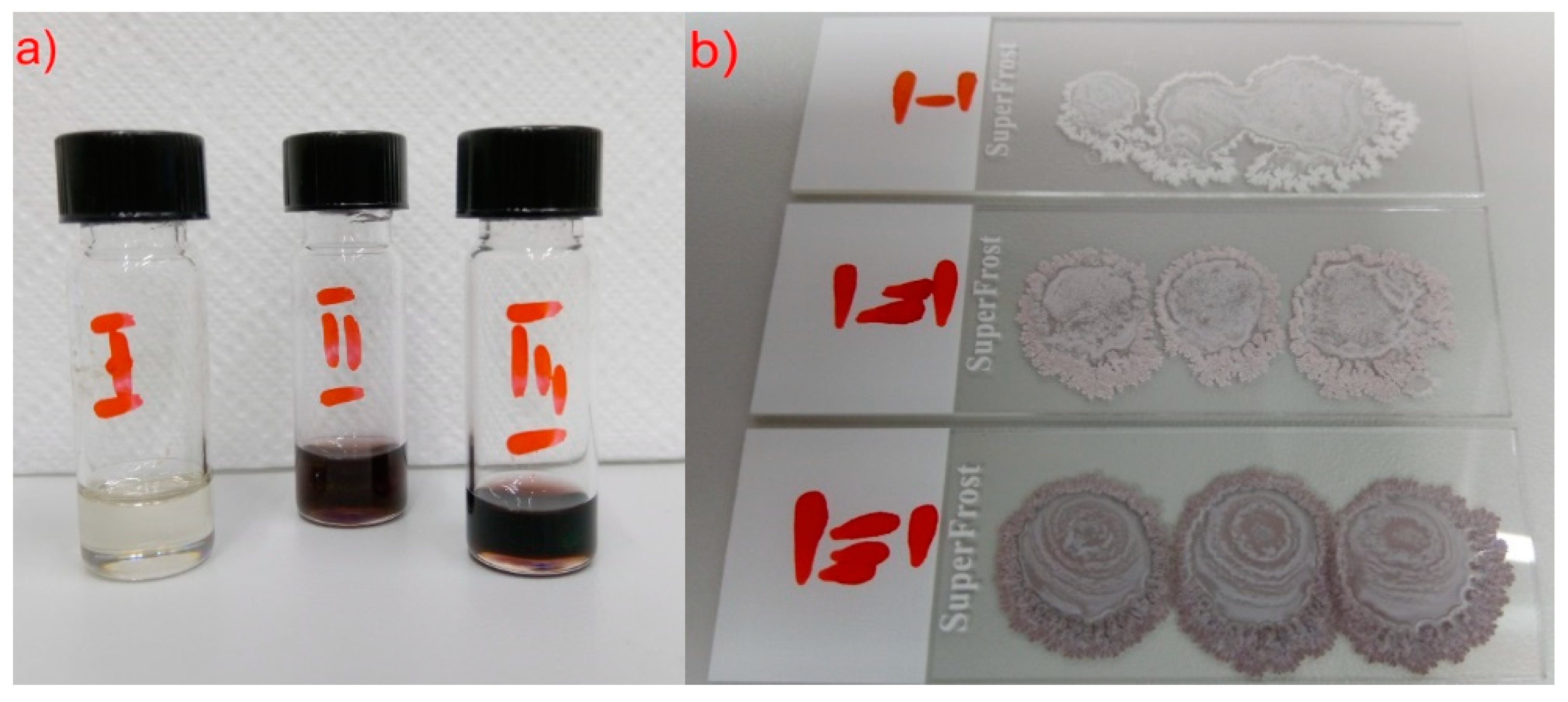
| Composite | (S)-MHPOBC | Au NPs |
| Composite 1 (0.0 wt. %) | 100.03 mg | - |
| Composite 2 (0.2 wt. %) | 100.02 mg | 10.02 μL |
| Composite 3 (0.5 wt. %) | 60.00 mg | 15.08 μL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalik, S.; Stefańczyk, O.; Dardas, D.; Deptuch, A.; Yevchenko, T.; Ohkoshi, S.-i.; Marzec, M. Nanocomposites Based on Antiferroelectric Liquid Crystal (S)-MHPOBC Doping with Au Nanoparticles. Molecules 2022, 27, 3663. https://doi.org/10.3390/molecules27123663
Lalik S, Stefańczyk O, Dardas D, Deptuch A, Yevchenko T, Ohkoshi S-i, Marzec M. Nanocomposites Based on Antiferroelectric Liquid Crystal (S)-MHPOBC Doping with Au Nanoparticles. Molecules. 2022; 27(12):3663. https://doi.org/10.3390/molecules27123663
Chicago/Turabian StyleLalik, Sebastian, Olaf Stefańczyk, Dorota Dardas, Aleksandra Deptuch, Tetiana Yevchenko, Shin-ichi Ohkoshi, and Monika Marzec. 2022. "Nanocomposites Based on Antiferroelectric Liquid Crystal (S)-MHPOBC Doping with Au Nanoparticles" Molecules 27, no. 12: 3663. https://doi.org/10.3390/molecules27123663
APA StyleLalik, S., Stefańczyk, O., Dardas, D., Deptuch, A., Yevchenko, T., Ohkoshi, S.-i., & Marzec, M. (2022). Nanocomposites Based on Antiferroelectric Liquid Crystal (S)-MHPOBC Doping with Au Nanoparticles. Molecules, 27(12), 3663. https://doi.org/10.3390/molecules27123663








